Introduction
Neonatal hypoxic-ischemic brain damage (HIBD) can
cause a variety of serious irreversible neurological sequelae,
including cerebral palsy, epilepsy, delayed growth and development,
cognitive impairment, intelligence disturbance, and even neonatal
death (1). According to a large
number of studies, the incidence rate of asphyxia in neonates
during delivery is as high as 5% in China. Hypoxia ischemia
encephalopathy occurs in approximately 100,000 neonates every year
due to severe asphyxia during delivery (1,2).
However, there have been no effective means to improve a variety of
severe neurological sequelae caused by perinatal hypoxia ischemia.
Therefore, research and investigation of neonatal HIBD is of
profound significance.
The pathogenesis of HIBD involves a wide range of
pathological mechanism, which is the result of a series of lesions
caused by various factors (3). It
has been found that neuronal apoptosis plays an important role in
the pathologic process of HIBD, which is closely related to the
pathologic degree of HIBD. A large number of studies have confirmed
that neuronal apoptosis is involved and plays an important role in
the development process of immature brain and in the process of
nervous system injury, such as cerebral hypoxia, ischemia and
trauma (4,5). Apoptosis involves a variety of factors,
in which the caspase family plays a dominant role. Caspase-3, also
known as the executing molecule that exerts an apoptotic function
in various apoptotic pathways, is studied the most and clarified
the most clearly (6–8). In this study, the mouse model of HIBD
was constructed to study neuronal apoptosis and caspase-3
expression in brain tissues in HIBD, so as to lay an experimental
foundation for further study on the mechanism of drugs.
Materials and methods
Materials
A total of 25 neonatal CD1 mice aged 1 week were
purchased from Nanjing Jiancheng Laboratory Animal Co., Ltd.
(Nanjing, China). Terminal deoxynucleotidyl transferase-mediated
dUTP nick end labeling (TUNEL) apoptosis assay kit (S7165; Merck
KGaA, Darmstadt, Germany), rabbit anti-mouse Fas ligand (FasL),
caspase-3 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
primary antibodies and horseradish peroxidase-labeled secondary
antibody (Proteintech, Wuhan, China), reverse
transcription-polymerase chain reaction (RT-PCR) kit (Ambion;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), DNA polymerase
(Sigma-Aldrich, St. Louis, MO, USA), immunohistochemical staining
kit SP-9001 (Beijing Zhongshan Goldenbridge Biotechnology Co.,
Ltd., Beijing, China), FasL and caspase-3 primers (synthesized by
Shanghai GenePharma Co., Ltd., Shanghai, China), and Leica DMi8
fluorescence microscope (Leica Microsystems GmbH, Wetzlar, Germany)
and ImageJ analysis software (NIH, Bethesda, MD, USA).
The present study was approved by the Ethics
Committee of The Fifth Affiliated Hospital of Guangzhou Medical
University (Guangzhou, China).
Construction of mouse model of newborn
hypoxia ischemia encephalopathy (NHIE)
Neonatal male CD1 mice (70–90 g) were randomly
divided into sham-operation group (n=8) and NHIE model group
(n=17). Mice in NHIE model group were treated as follows: First,
after anesthesia via inhalation of isoflurane, a cervical median
incision was made, the right common carotid artery was correctly
separated and closed via electrocoagulation, and then the incision
was sutured. After operation, mice were revived at room
temperature, normally fed with breast milk and treated under
hypoxia: NHIE mice were placed into a closed chamber at 37°C
injected with nitrogen gas containing 5% CO2 and 8%
O2. After 100 min, mice were taken and continued to be
normally fed with breast milk. In sham-operation group, the
incision was sutured after operation without hypoxia treatment and
closure of common carotid artery.
Detection of caspase-3 and FasL mRNA
expression levels in brain tissues
The total RNA was extracted from brain tissues using
TRIzol (Beyotime, Shanghai, China). After that, 1 µg RNA was taken
from each group and reversely transcribed into complementary DNA
(cDNA) according to instructions of the kit. Then caspase-3 and
FasL primers were added, and caspase-3 and FasL mRNA expression
levels were quantitatively detected via PCR, with GAPDH as the
internal reference. Primer sequences of caspase-3, FasL and GAPDH
are shown in Table I. Reaction
conditions were as follows: at 94°C for 5 min, at 94°C for 30 sec,
at 57°C for 30 sec, at 72°C for 30 sec, amplification for 30
cycles, and at 72°C for 5 min.
 | Table I.RT-PCR primer sequences and gene
silencing sequences. |
Table I.
RT-PCR primer sequences and gene
silencing sequences.
| Genes | Primer sequence |
|---|
| Caspase-3 | F:
5′-TGCGTGTGGAGTATTTGGATG-3′ |
|
| R:
5′-TGGTACAGTCAGAGCCAACCTC-3′ |
| FasL | F:
5′-TGGGATGCCTTTGTGGAAC-3′ |
|
| R:
5′-CATATTTGTTTGGGGCAGGTC-3′ |
| GAPDH | F:
5′-ATGGCACCGTCAAGGCTGAG-3′ |
|
| R:
5′-GCAGTGATGGCATGGACTGT-3′ |
Detection of caspase-3 and FasL
protein expression levels in brain tissues via western
blotting
Brain tissues were taken to extract the total
protein according to instructions of the protein extraction kit,
and the protein concentration was determined using bicinchoninic
acid (BCA) method, followed by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE). After
electrophoresis, the protein was transferred onto a polyvinylidene
fluoride (PVDF) membrane using the wet method, sealed with skim
milk, and incubated with primary rabbit anti-mouse caspase-3, FasL
and GAPDH monoclonal antibodies (dilution, 1:1,000; cat. nos.
19677-1-AP, 13098-1-AP and 10494-1-AP; Proteintech) at 4°C for 16
h. After the membrane was washed with TTBS, the HRP-labeled
secondary goat anti-rabbit polyclonal antibody (dilution, 1:1,200;
cat. nos. SA00001-2; Proteintech) was added and the mixture was
shaken at 20°C for 2 h, followed by image development via
electrochemiluminescence (ECL) in a darkroom and photography.
Finally, the protein band was analyzed using Image Lab 4.0.1
software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Immunohistochemical detection of
caspase-3 protein expression in brain tissues
The detection was performed as follows according to
instructions of the immunohistochemical staining kit SP-9001: After
the paraffin-embedded sections were deparaffinized, the endogenous
peroxidase activity was eliminated using hydrogen peroxide, and the
citrate buffer was used for antigen retrieval. Then sections were
sealed with 10% goat serum and added with primary antibody (diluted
at 1:100) in a refrigerator at 4°C overnight. After sections were
washed with phosphate-buffered saline (PBS), they were incubated
with secondary antibody and washed again with PBS 15 min later,
followed by color development via diaminobenzidine (DAB),
hematoxylin counterstaining and photography under the
microscope.
Detection of apoptosis in brain
tissues
At 3 days after modeling, mice were anesthetized
with 4% isoflurane, and the brain was immediately taken from 3 mice
in each group. The brain was fixed in 4% paraformaldehyde solution,
washed with PBS and sliced into 30 µm sections, and then sections
were fixed in 1% paraformaldehyde solution to be used in subsequent
experiments. Brain tissue sections (3–5) were
randomly taken from each group and treated according to
instructions of the TUNEL assay kit, followed by observation and
photography under the fluorescence microscope. Red-labeled cells
were TUNEL-positive cells, namely apoptotic cells.
Statistical analysis
Data in this study are presented as mean ± standard
deviation (mean ± SD). SPSS 17.0 software (SPSS Inc., Chicago, IL,
USA) and one-way analysis of variance (ANOVA) were adopted for data
processing. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Caspase-3 and FasL mRNA expression
levels in brain tissues
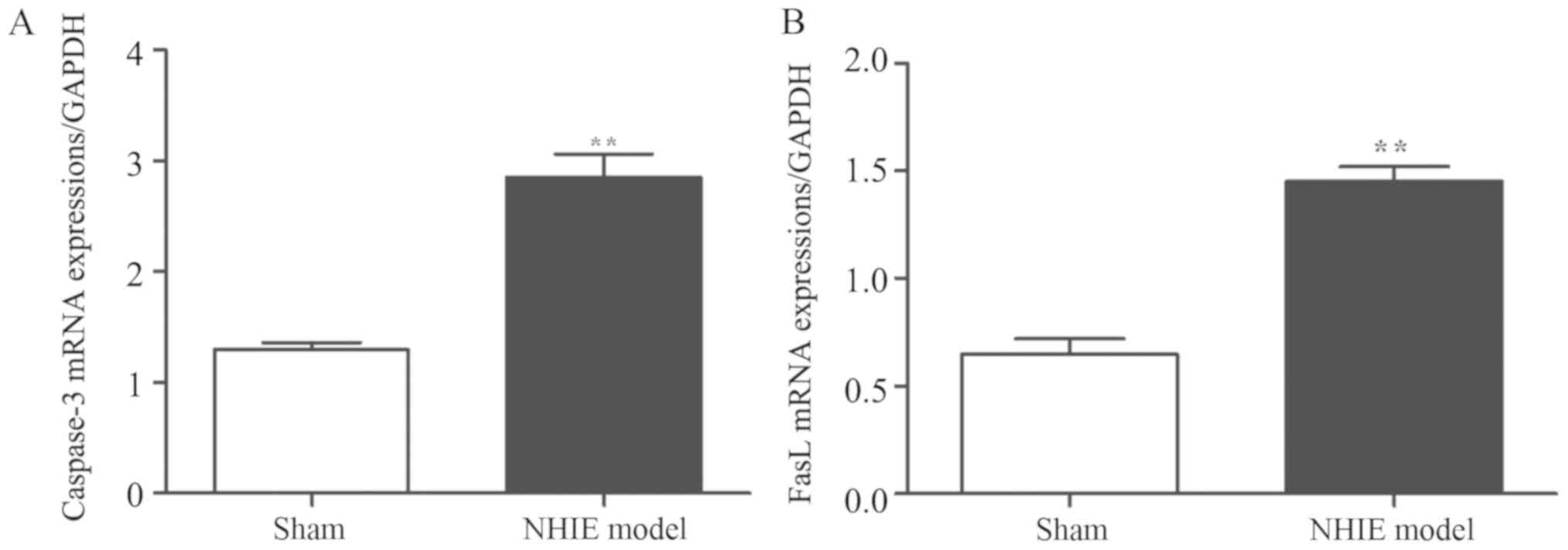
Results of RT-PCR revealed that compared with those
in sham-operation group, the caspase-3 and FasL mRNA expression
levels in NHIE model group were significantly increased
(P<0.01), indicating that caspase-3 and FasL mRNA expression
levels have a certain association with hypoxia ischemia in brain
tissues (Fig. 1).
Caspase-3 and FasL protein expression
levels in brain tissues
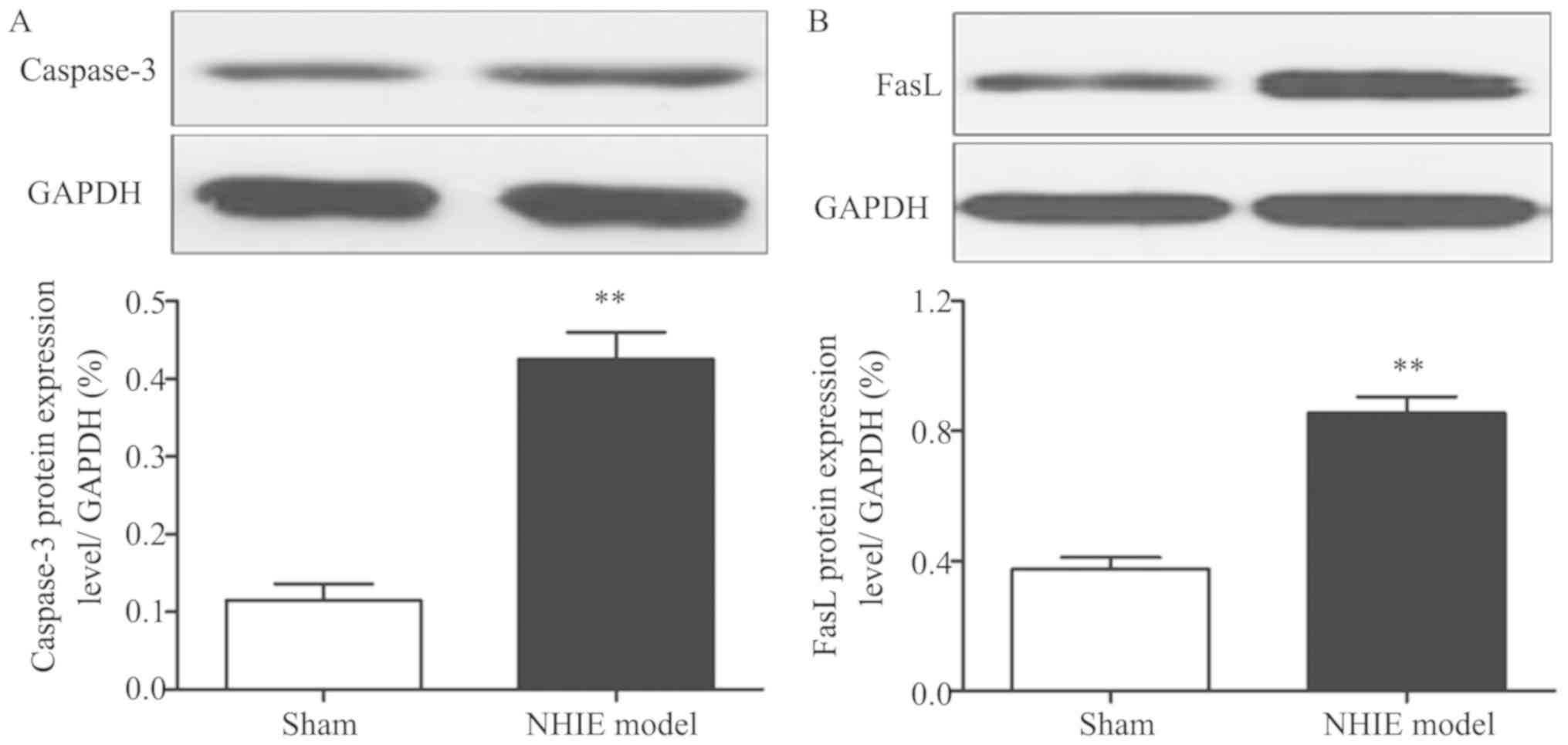
Results of western blotting showed that caspase-3
and FasL protein expression levels in NHIE model group were
obviously increased compared with those in sham-operation group
(P<0.01), suggesting that caspase-3 and FasL protein expression
levels have a certain association with hypoxia ischemia in brain
tissues (Fig. 2).
Detection of neuronal apoptosis in
brain tissues via TUNEL
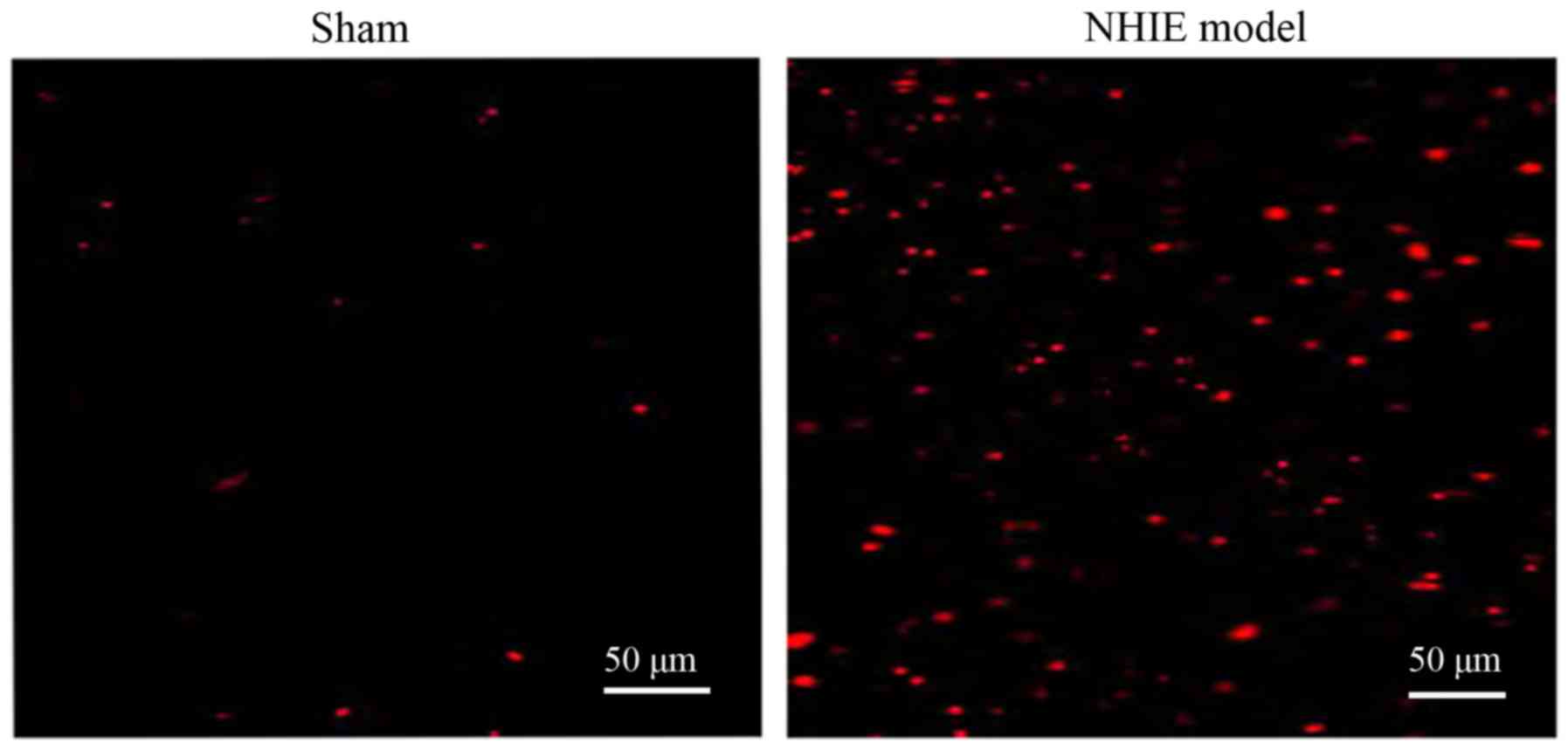
The number of TUNEL-positive cells in brain tissues
in NHIE model group was obviously increased compared with that in
sham-operation group, indicating that hypoxia ischemia can lead to
neuronal apoptosis (Fig. 3).
Immunohistochemical detection of
caspase-3 protein expression in brain tissues
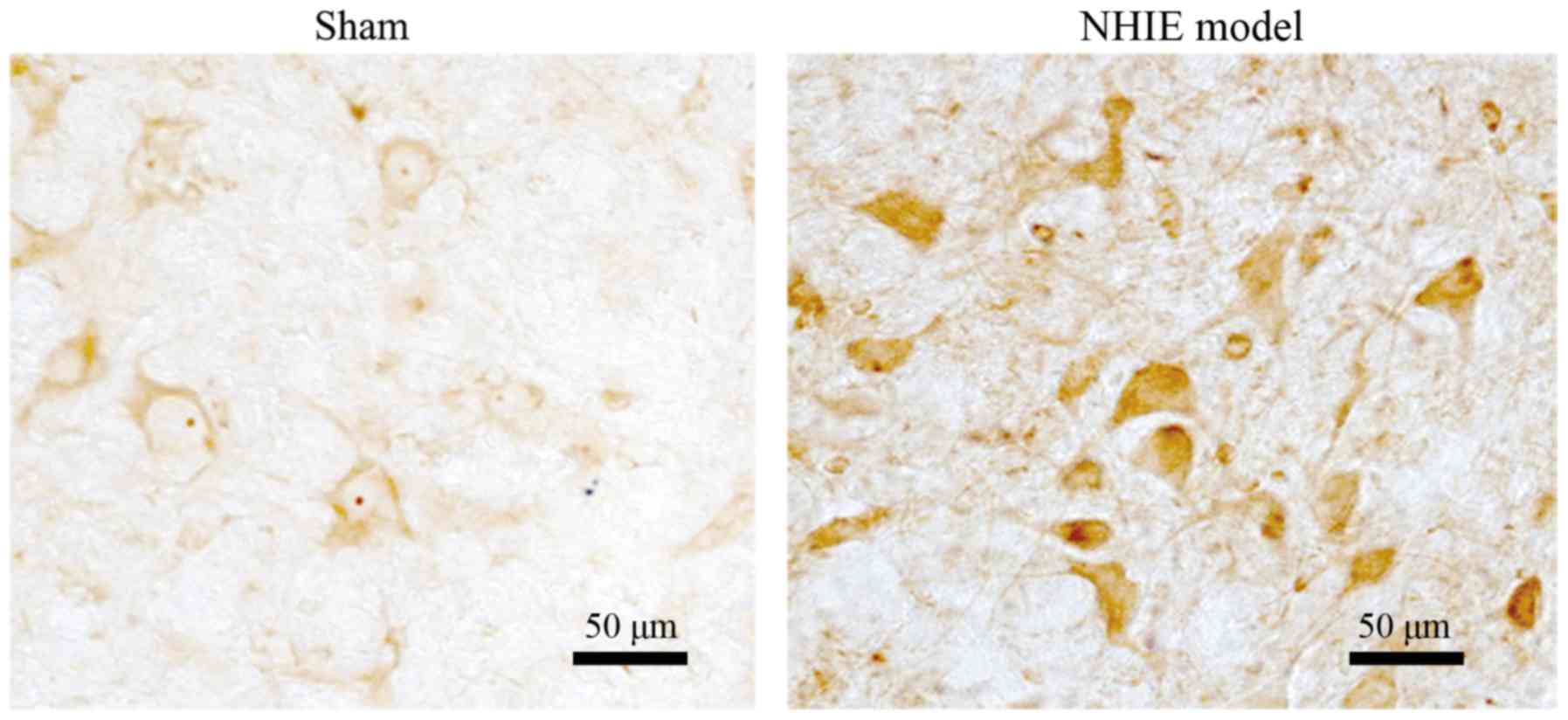
Compared with that in sham-operation group, brain
tissues in NHIE model group were stained significantly and the
caspase-3 protein expression was remarkably increased, suggesting
that hypoxia ischemia can lead to the significant upregulation of
caspase-3 protein expression (Fig.
4).
Discussion
At present, neonatal HIBD is still the main cause of
neonatal death and a variety of severe neurological sequelae, whose
pathogenesis involves various factors and pathological changes.
Therefore, there is still a lack of effective prevention and
treatment means in clinic.
In the pathological mechanism of neonatal HIBD,
important factors leading to neuronal damage include accumulation
of excitatory amino acid, oxidative stress and apoptosis. The most
serious damage in the process of HIBD is neuronal apoptosis, and
caspase-3 and Bcl-2 are important proteins executing apoptosis,
which have significantly increased expression levels in the process
of hypoxia ischemia and play important roles in pathogenesis
(9,10). The final result of various pathways
leading to cell damage is irreversible apoptosis, so protecting
neurons and inhibiting apoptosis are the most effective treatment
means for neonatal HIBD (11).
Reports have demonstrated that the apoptosis process includes
transmission of apoptotic signals and execution of apoptosis
command. During the signal transmission, the activation of caspase
family is realized through the following pathways. In the
endogenous pathway, also known as the mitochondrial pathway,
cytochrome c in the mitochondria is released into the
cytoplasm and interacts with activator-1 to activate caspase-9. In
the exogenous pathway, also known as the death receptor pathway,
the signaling molecules will activate the death receptor on the
surface of cytoplasmic membrane to activate caspase-8. In the
endoplasmic reticulum pathway, endoplasmic reticulum stress will be
produced when cells are stimulated, ultimately activating caspase-9
(12,13). The caspase family is the core in the
apoptosis process. Caspase is stored in the cytoplasm in the form
of zymogen after being synthesized, and a chain reaction occurs
after caspase receives related signal commands, so that caspase is
cleaved and activated, further leading to signaling cascade
amplification and activating a variety of downstream proteases,
thereby initiating multiple apoptotic pathways. Cleavage of caspase
is a reversible process, while activation of caspase-3 is an
irreversible process, and caspase-3 is the executor of the final
apoptotic signal (14). Therefore,
inhibiting activation or reversible stage of caspase-3 can
effectively inhibit apoptosis, thereby alleviating the pathological
changes of HIBD. In the Fas signaling pathway, Fas and its ligand
FasL exert the function, the latter of which, as a transmembrane
protein, mediates apoptosis when its expression is increased
through binding to Fas (15).
In this study, the mRNA and protein expression
levels of caspase-3 and FasL in neonatal mice in model group were
significantly increased. TUNEL results manifested that the number
of apoptotic neurons in model group was significantly increased.
Results of immunohistochemical detection showed that the caspase-3
expression in model group was obviously increased. Similarly, there
are reports that the activation and expression of caspase-3 are
remarkably increased within a short period of time after
intracerebral and extracerebral injury. The activity and protein
expression of caspase-3 were downregulated obviously in the
experiment about the effect of endogenous caspase inhibitor or
exogenous caspase inhibitor on the transcription level (16,17). In
addition, some studies have demonstrated that the activity and
expression of caspase-3 in brain tissues are significantly
increased in neonatal HIBD mice, and inhibiting the caspase-3
expression level can prevent neuronal apoptosis. Therefore,
inhibiting caspase-3 expression plays a role in protecting nerve
cells (18–20).
In conclusion, the mouse model of neonatal HIBD was
constructed in this study to investigate apoptosis and caspase-3
expression in brain tissues, and it was found that hypoxia ischemia
could lead to significant increase of caspase-3 expression and
increase of neuronal apoptosis in the brain of neonatal mice, thus
laying an experimental foundation for further study on the
mechanism of drugs.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CD wrote the manuscript. CD and JL performed PCR. LL
and FS assisted with animal model construction. JX was responsible
for immunohistochemical detection. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Fifth Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gluckman PD, Wyatt JS, Azzopardi D,
Ballard R, Edwards AD, Ferriero DM, Polin RA, Robertson CM,
Thoresen M, Whitelaw A, et al: Selective head cooling with mild
systemic hypothermia after neonatal encephalopathy: Multicentre
randomised trial. Lancet. 365:663–670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng H, Dai T, Zhou B, Zhu J, Huang H,
Wang M and Fu G: SDF-1alpha/CXCR4 decreases endothelial progenitor
cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway.
Atherosclerosis. 201:36–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller JT, Bartley JH, Wimborne HJ, Walker
AL, Hess DC, Hill WD and Carroll JE: The neuroblast and angioblast
chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated
by reactive astrocytes in brain following neonatal hypoxic-ischemic
injury. BMC Neurosci. 6:632005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Northington FJ, Ferriero DM, Flock DL and
Martin LJ: Delayed neurodegeneration in neonatal rat thalamus after
hypoxia-ischemia is apoptosis. J Neurosci. 21:1931–1938. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stumm RK, Rummel J, Junker V, Culmsee C,
Pfeiffer M, Krieglstein J, Höllt V and Schulz S: A dual role for
the SDF-1/CXCR4 chemokine receptor system in adult brain:
Isoform-selective regulation of SDF-1 expression modulates
CXCR4-dependent neuronal plasticity and cerebral leukocyte
recruitment after focal ischemia. J Neurosci. 22:5865–5878. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC,
Wang HJ and Li H: Stromal cell-derived factor-1 alpha promotes
neuroprotection, angiogenesis, and mobilization/homing of bone
marrow-derived cells in stroke rats. J Pharmacol Exp Ther.
324:834–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andiné P, Thordstein M, Kjellmer I,
Nordborg C, Thiringer K, Wennberg E and Hagberg H: Evaluation of
brain damage in a rat model of neonatal hypoxic-ischemia. J
Neurosci Methods. 35:253–260. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnston MV: Neurotransmitter alterations
in a model of perinatal hypoxic-ischemic brain injury. Ann Neurol.
13:511–518. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren C, Du A, Li D, Sui J, Mayhan WG and
Zhao H: Dynamic change of hydrogen sulfide during global cerebral
ischemia-reperfusion and its effect in rats. Brain Res.
1345:197–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kilicdag H, Daglioglu YK, Erdogan S and
Zorludemir S: Effects of caffeine on neuronal apoptosis in neonatal
hypoxic-ischemic brain injury. J Matern Fetal Neonatal Med.
27:1470–1475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu M, Lu M, Li QJ, Zhang Z, Wu ZZ, Li J,
Qian L, Xu Y and Wang ZY: Hyperbaric oxygen suppresses
hypoxic-ischemic brain damage in newborn rats. J Child Neurol.
30:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rice JE III, Vannucci RC and Brierley JB:
The influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levine S: Anoxic-ischemic encephalopathy
in rats. Am J Pathol. 36:1–17. 1960.PubMed/NCBI
|
|
14
|
Cowan CM, Thai J, Krajewski S, Reed JC,
Nicholson DW, Kaufmann SH and Roskams AJ: Caspases 3 and 9 send a
pro-apoptotic signal from synapse to cell body in olfactory
receptor neurons. J Neurosci. 21:7099–7109. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berger R and Garnier Y: Perinatal brain
injury. J Perinat Med. 28:261–285. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheng Y, Deshmukh M, D'Costa A, Demaro JA,
Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM and Holtzman DM:
Caspase inhibitor affords neuroprotection with delayed
administration in a rat model of neonatal hypoxic-ischemic brain
injury. J Clin Invest. 101:1992–1999. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Van De Water TR, Bonny C, de
Ribaupierre F, Puel JL and Zine A: A peptide inhibitor of c-Jun
N-terminal kinase protects against both aminoglycoside and acoustic
trauma-induced auditory hair cell death and hearing loss. J
Neurosci. 23:8596–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han W, Sun Y, Wang X, Zhu C and Blomgren
K: Delayed, long-term administration of the caspase inhibitor
Q-VD-OPh reduced brain injury induced by neonatal hypoxia-ischemia.
Dev Neurosci. 36:64–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang X, Guo S, Lu S, Zhou J, Li J and Xia
S: Ultrasound-induced release of GDNF from lipid coated
microbubbles injected into striatum reduces hypoxic-ischemic injury
in neonatal rats. Brain Res Bull. 88:495–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao A, Kang M, Zhang H and Gong L:
Effects of moxibustion pretreatment on caspase-3 expression in
cortex of rats with cerebral ischemia-reperfusion injury. Tianjin
Zhong Yi Yao. 42:550–552. 2014.(In Chinese).
|