Introduction
The rhythm of life in modern society is constantly
accelerating with endless social conflicts, fighting, traffic
accidents, natural disasters and man-made disasters which have led
to an increased number of trauma patients (1). Among them, the shock that is caused by
violent trauma has become a critical clinical emergency (2). Compared with ordinary traumatic
hemorrhage, traumatic shock is more complicated and has a more
rapid disease progression (3).
Accurately determining the severity of a patient's trauma at an
early stage and prompt treatment of patients in a timely manner
have become very important for the prognosis of patients.
Procalcitonin (PCT) is a protein that reflects the
activity of the body's inflammatory response (4). High expression occurs in sepsis, shock,
and multiple organ dysfunctions (5,6).
C-reactive protein (CRP), an acute protein, is sensitive to tissue
damage and microbial infections (7).
Both are inflammatory indicators commonly used in the diagnosis and
treatment of clinically acute diseases. Interleukin-6 (IL-6) is an
inflammatory factor that can coordinate with the body's immune
response after injury (8). Studies
have shown that IL-6 can reflect the degree of tissue damage in the
body to some extent (9). The current
clinical diagnosis and treatment of patients with traumatic shock
mainly depend on the monitoring of hemodynamic parameters, however,
these judgments on the degree of traumatic shock in patients are
still insufficient (10). Therefore,
in this study we focused on the analysis of PCT, CRP and IL-6
expression levels in the serum of traumatic shock patients, and
explored the value of the three in the assessment of the condition
in traumatic shock patients.
Patients and methods
General information
A total of 80 cases of traumatic shock patients who
were in the emergency surgery in Zhangzhou Municipal Hospital of
Fujian Province (Zhangzhou, China) from January 2014 to December
2017 were retrospectively collected as the experimental group,
including 42 males and 38 females, aged 51–72 years, average age
52.78±4.67 years. Further 80 patients with general trauma who did
not suffer from shock during the same period were selected as the
control group, including 45 males and 35 females, aged 48–73 years,
average age 53.68±4.95 years. According to the prognosis outcome of
the patients, the experimental components were divided into good
prognosis and poor prognosis group. There were 56 cases in the good
prognosis and 24 cases in the poor prognosis group.
Inclusion criteria were: i) All subjects were over
18 years old, have not been traumatized recently, and have not
undergone surgery; ii) patients in the experimental group met the
diagnostic criteria for traumatic shock (11); iii) patients in control group had no
disturbance of consciousness and their hemodynamic parameters were
normal, with no signs of shock; iv) patients in the good prognosis
group showed a significant improvement in their disease condition
after admission, and can be discharged within 30 days, no adverse
events occurred such as death, multiple organ dysfunction syndrome;
and v) adverse events such as death or multiple organ dysfunction
syndrome occurred to patients in the poor prognosis group. Total
treatment in the intensive care unit took more than 30 days.
Exclusion criteria were: i) Subjects with history of
hypertension, chronic disease of diabetes or coagulopathy; ii)
patients with heart and blood vessel diseases, hepatic and renal
dysfunction and adverse reaction during transfusion; and iii)
patients with incomplete medical data.
The study was approved by the Ethics Committee of
Zhangzhou Municipal Hospital of Fujian Province. Patients who
participated in this study had complete clinical data. Signed
informed consents were obtained from the patients or the
guardians.
Experimental reagents and
instruments
Ultra-low temperature refrigerator (Premium U410;
Suzhou Beirui Instrument Technology Co., Ltd., Suzhou, China);
Human PCT ELISA kit (FK-pk0812; Shanghai Fanke Biotechnology Co.,
Ltd., Shanghai, China); IL-6 ELISA kit [JK-(a)-0023; Shanghai
Jingkang Bioengineering Co., Ltd., Shanghai, China]; Automatic
microplate reader (26262002; Beijing Anmaige Trading Co., Ltd.,
Beijing, China); High-speed refrigerated centrifuge [AVANTI J-15R;
Beckman Coulter Trading (China) Co., Ltd., Shanghai, China];
Ultra-micro spectrophotometer (NP80-Touch; Guangzhou Haohan
Instrument Co., Ltd., Guangzhou, China); CRP assay kit (XFSW245B;
Shanghai Xinfan Biotechnology Co., Ltd., Shanghai, China);
Micro-adjustable pipette (F144565; Shenzhen Kangchuyuan Co., Ltd.,
Shenzhen, China).
Experimental methods
Collection of blood samples
During the period of fasting, the patients' elbow
venous blood (2 ml) were collected with a disposable blood
collection needle immediately after the admission (T1), and on the
first (T2), third (T3), seventh (T4) day after admission. The blood
was naturally coagulated for 10–20 min at room temperature, then
centrifuged at 3,000 × g for 20 min at 4°C. After the serum was
naturally precipitated, the supernatant was carefully pipetted into
another clean tube and the tube was place at −80°C in a freezer for
further testing.
Determination of PCT and IL-6
expression levels in serum by enzyme-linked immunosorbent assay
(ELISA)
The expression levels of PCT and IL-6 in patients'
serum by ELISA were tested by double antibody sandwich assay.
Standard, blank and sample wells were set on an enzyme plate. The
standards were diluted to 1.0–2.0 µg/ml with diluent, and each well
was added with 50 µl dilution. Then 40 µl sample dilution was added
to the sample wells of the enzyme plate, and 10 µl of samples was
added for further testing. Avoiding touching the surrounding walls
loading was done, and then gently shaken. Then the enzyme plate was
sealed with a sealing membrane, and incubated at 37°C for 30 min.
Next, the sealing membrane was uncovered and the liquid discarded
in the well and each well was washed 5 times with the diluted
washing solution. Except the blank wells, 50 µl of the enzyme
standard reagent was added to each well, the plate was sealed with
a sealing membrane, and incubated for 30 min, then the liquid was
discarded in each well and washed 5 times. Then 50 µl of
chromogenic reagent A and 50 µl of chromogenic reagent B was added
to each well. After shaking, protected from light stained at a
temperature of 37°C for 15 min. Finally, 50 µl of stop solution was
added to each well, the blank well was zero in value, the OD value
in each well was measured at a wavelength of 450 nm in 15 min.
Determination of CRP expression levels
in patient serum by immunoturbidimetry
A sample of 100 µl was mixed with reagent (R1-3),
adding R4, and centrifuged at 3,000 × g for 10 min at 4°C, then the
supernatant was pipetted into another tube, adding chromogenic
reagent, and R6, then left at room temperature for 2 min. After 5
min the OD value was measured at a wavelength of 636 nm.
Observation of indices
The expression levels of PCT, CRP and IL-6 in the
serum in the control group with the good and the poor prognosis
were recorded at the T1-T4 time periods. Also the injury severity
score (ISS) (12) between the good
and the poor prognosis before admission, and the score of acute
pathologic and chronic health evaluation (APACHE II) during the
period of T1-T4 were recorded (13).
Statistical analysis
The experimental data were statistically analyzed by
SPSS 19.0 statistical software (SPSS, Inc., Chicago, IL, USA), the
data counting was expressed as percentages (%), the Chi-square test
was used for comparison between groups, measurement data was
expressed by mean ± standard deviation, the t-test was used for
comparison between groups, repeated measures analysis of variance
was used for comparison of multiple time points between groups with
Dunnett's test. One-way ANOVA was used for comparison between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of general information
As shown in Table I,
48 cases of trauma in the experimental group were caused by traffic
accidents, 19 cases were caused by fall injury, 10 cases were
caused by sharp injury and 3 cases were caused by other factors.
Trauma time (30 sec-1.2 h), average 1.17±0.16 h. In the control
group, there were 46 cases of traffic accidents, 18 cases of fall
injuries, 9 cases of sharp injuries and 7 cases of others. Trauma
time (50 sec-1.3 h), average 1.12±0.21 h. There were no differences
in age, body mass index, sex, cause of injury and time of trauma
between the experimental and the control group (P>0.05).
 | Table I.Comparison of general clinical basic
information [n (%)](mean ± standard deviation). |
Table I.
Comparison of general clinical basic
information [n (%)](mean ± standard deviation).
| Clinical factors | Experiment group
(n=80) | Control group
(n=80) | t/χ2 | P-value |
|---|
| Age (years) | 52.78±4.67 | 53.68±4.95 | 1.183 | 0.239 |
| BMI
(kg/m2) | 23.67±2.82 | 23.79±2.53 | 0.283 | 0.777 |
| Sex |
|
| 0.227 | 0.634 |
| Male | 42 | 45 |
|
|
|
Female | 38 | 35 |
|
|
| Causes of injury |
|
| 1.722 | 0.632 |
| Traffic
accident | 48 | 46 |
|
|
| Fall
injury | 19 | 18 |
|
|
| Sharp
injury | 10 | 9 |
|
|
|
Others | 3 | 7 |
|
|
| Blood sugar
(mmol/l) |
|
| 0.526 | 0.468 |
|
<6.11 | 75 | 77 |
|
|
|
≥6.11 | 5 | 3 |
|
|
| Triglyceride
(mmol/l) |
|
| 0.278 | 0.598 |
|
<1.70 | 73 | 71 |
|
|
|
≥1.70 | 7 | 9 |
|
|
| Trauma time (h) | 1.17±0.16 | 1.12±0.21 | 1.694 | 0.092 |
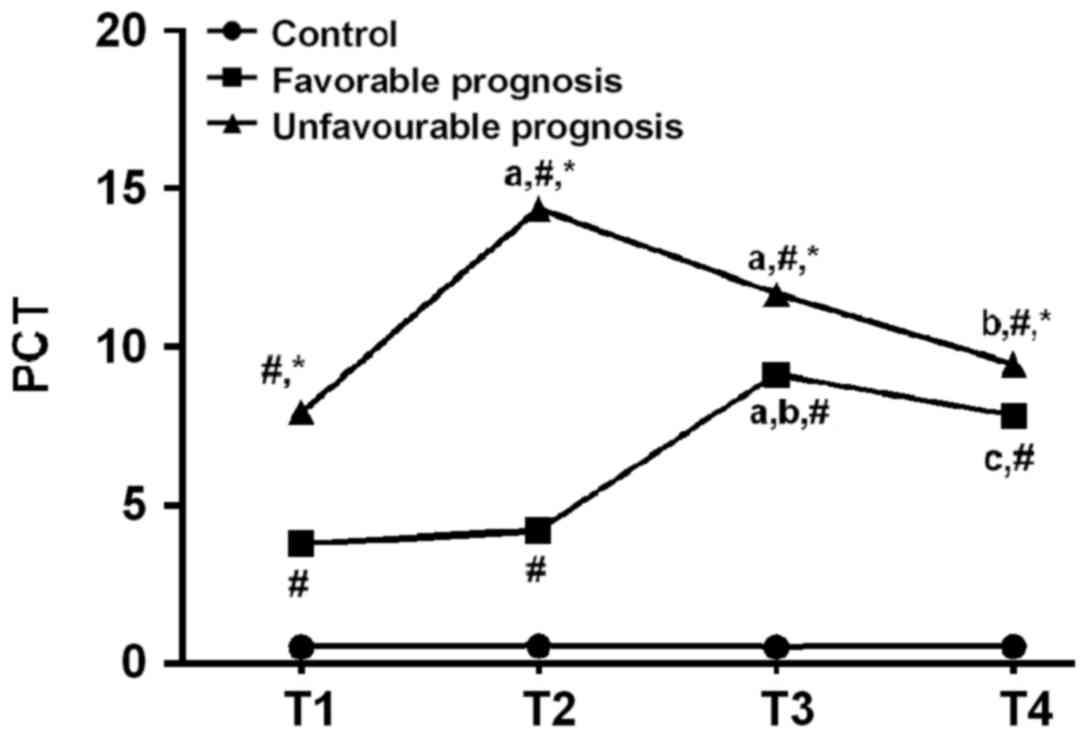
Comparison of PCT in serum among the
experimental, the good and poor prognosis group
There was no significant difference in serum PCT in
the control group at the period of T1-T4 (P>0.05). There was a
difference in serum PCT between the good prognosis and the poor
prognosis group at the period of T1-T4 (P<0.05). Serum PCT
levels of T2, T3 were significantly increased compared with the
period of T1 in the poor prognosis group (P<0.05). Compared with
the period of T2, T3 was significantly increased in good prognosis
group, and the period of T4 was significantly decreased in poor
prognosis group (P<0.05). Compared with the period of T3, T4 was
significantly decreased in good prognosis group (P<0.05). At the
same time point, both good prognosis and poor prognosis groups were
higher than the control group. The poor prognosis group was higher
than the good prognosis group (P<0.05; Table II and Fig. 1).
 | Table II.Comparison of PCT in serum. |
Table II.
Comparison of PCT in serum.
| Variables | T1 | T2 | T3 | T4 | F | P-value |
|---|
| Control group
(n=80) | 0.52±0.06 | 0.55±0.12 | 0.53±0.08 | 0.54±0.11 | 1.461 | 0.225 |
| Good prognosis
group (n=56) |
3.79±1.83d |
4.21±2.36d |
9.14±3.74a,b,d |
4.83±1.24c,d | 55.86 | <0.001 |
| Poor prognosis
group (n=24) |
7.93±2.49d,e |
14.38±3.58a,d,e |
11.68±5.26a,d,e |
9.46±3.73b,d,e | 12.250 | <0.001 |
| F | 264.600 | 461.300 | 198.700 | 215.400 |
|
|
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
|
|
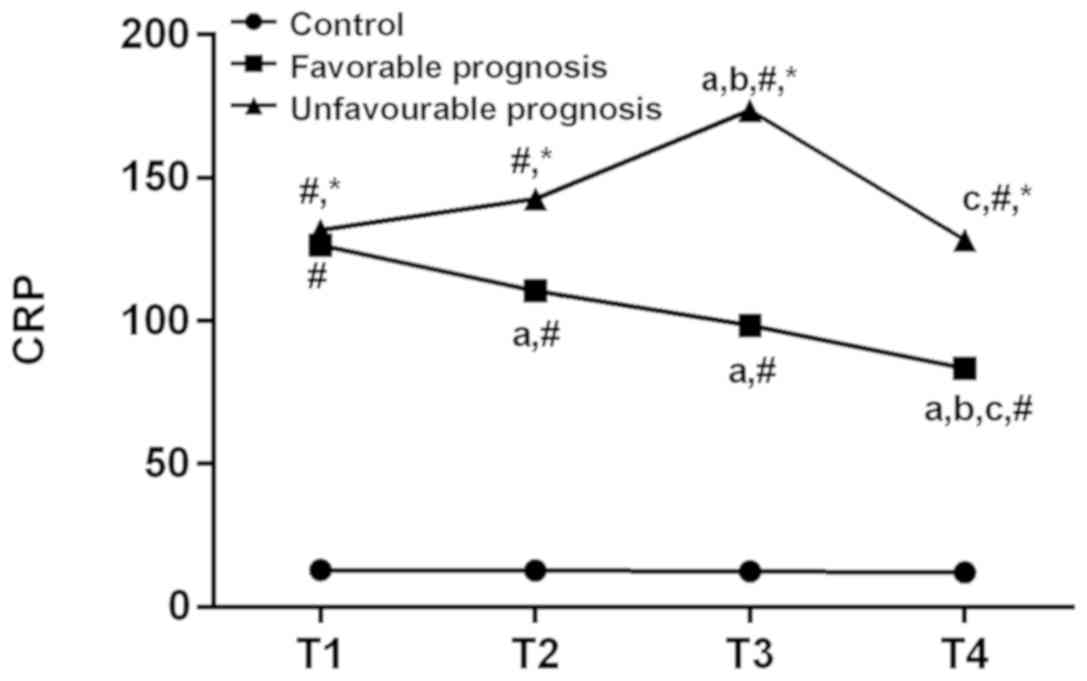
Comparison of serum CRP among the
experimental, the good prognosis and poor prognosis groups
There was no significant difference in serum CRT in
the control group at the period of T1-T4 (P>0.05). There was a
difference in serum CRP between the good prognosis and the poor
prognosis groups at the period of T1-T4 (P<0.05). Compared with
T1, T3 was significantly increased in the poor prognosis group
(P<0.05). The poor prognosis has significantly decreased at
T2-T4 time periods (P<0.05). Compared with the period of T2, T4
was significantly decreased in the good prognosis group, and the
period of T3 was significantly increased in the poor prognosis
group (P<0.05). Compared with the period of T3, T4 was
significantly decreased in both good prognosis and poor prognosis
groups (P<0.05). At the same time point, both the good prognosis
and the poor prognosis groups were higher than the control group.
Serum CRP in the poor prognosis group was higher than the good
prognosis group at T2-T4 (P<0.05; Table III and Fig. 2).
 | Table III.Comparison of CRP in serum. |
Table III.
Comparison of CRP in serum.
| Variables | T1 | T2 | T3 | T4 | F | P-value |
|---|
| Control group
(n=80) | 12.88±1.41 | 12.78±1.23 | 12.47±1.13 |
12.13±1.12a,b |
6.078 |
0.001 |
| Good prognosis
group (n=56) |
126.37±31.73d |
110.42±30.24a,d |
98.45±28.63a,d |
83.34±24.82a–d | 24.190 | <0.001 |
| Poor prognosis
group (n=24) |
131.68±29.37d,e |
142.58±35.41d,e |
173.60±36.19a,b,d,e |
128.32±31.23c–e |
9.275 | <0.001 |
| F | 565.500 | 473.300 | 590.000 | 446.700 |
|
|
| P-value |
<0.001 |
<0.001 | <0.001 | <0.001 |
|
|
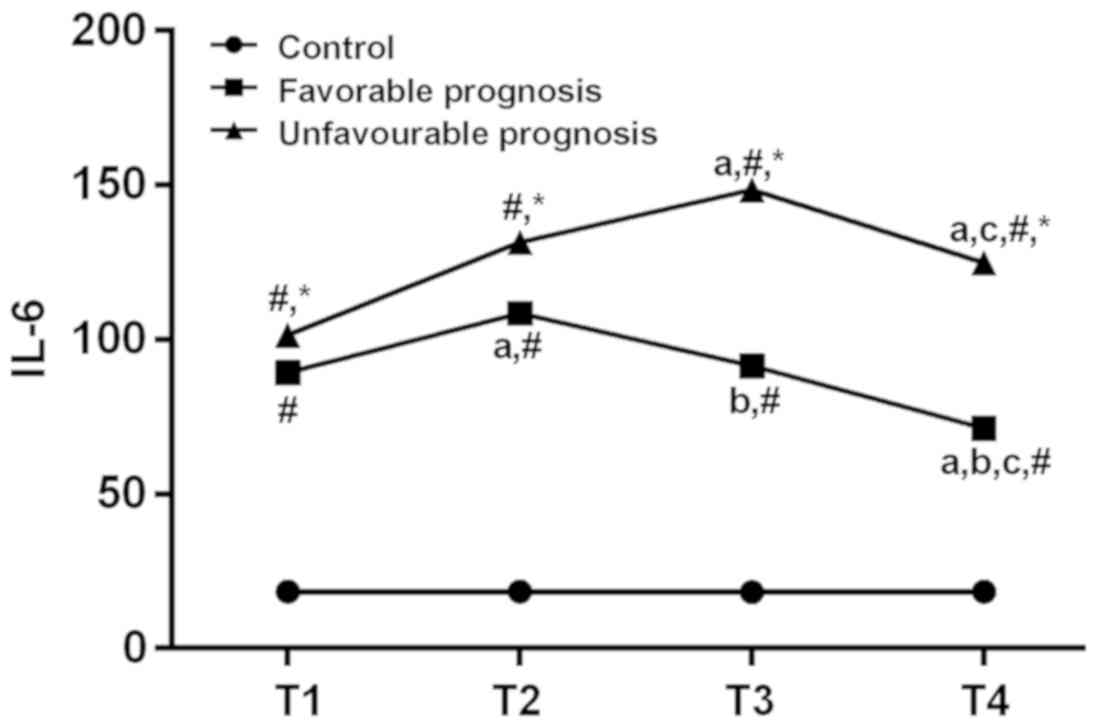
Comparison of serum IL-6 among the
experimental group with the good prognosis and poor prognosis
group
There was no significant difference in serum IL-6
between the control group at T1-T4 time period (P>0.05). There
was a difference in serum IL-6 between the good prognosis and the
poor prognosis groups at the time of T1-T4 (P<0.05). Compared
with the period of T1, T2 in good prognosis group and T3 in poor
prognosis group increased significantly (P<0.05). Both good and
poor prognosis groups were decreased significantly at the T4 time
period (P<0.05). Compared with the the period of T2, T4 was
significantly decreased in good prognosis group (P<0.05).
Compared with the period of T3, T4 was significantly decreased in
both good and poor prognosis groups (P<0.05). At the same time
point, both good and poor prognosis groups were higher than the
control group. Also poor prognosis group was higher than good
prognosis group (P<0.05; Table
IV and Fig. 3).
 | Table IV.Comparison of IL-6 in serum. |
Table IV.
Comparison of IL-6 in serum.
| Variables | T1 | T2 | T3 | T4 | F | P-value |
|---|
| Control group
(n=80) | 18.38±3.25 | 18.49±3.16 | 18.21±3.21 | 18.13±3.22 | 0.206 | 0.892 |
| Good prognosis
group (n=56) |
89.30±17.91d |
108.46±12.43a,d |
91.35±10.62b,d |
71.25±6.21a–d | 82.770 | <0.001 |
| Poor prognosis
group (n=24) |
101.38±21.64d,e |
131.48±31.56d,e |
148.44±38.12a,d,e |
124.76±28.52a,c–e | 9.780 | <0.001 |
| F | 603.200 | 937.00 | 739.000 | 868.500 |
|
|
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
|
|
Comparison of ISS score and APACHE II
score of patients in the good prognosis and the poor prognosis
groups
As shown in Table V,
there were differences in pre-admission ISS scores and APACHE II
scores between the good prognosis and the poor prognosis groups.
The pre-admission ISS score and APACHE II score in the good
prognosis group were lower than in the poor prognosis group
(P<0.05).
 | Table V.Comparison of ISS score and APACHE II
score. |
Table V.
Comparison of ISS score and APACHE II
score.
| Variables | Good prognosis
group (n=56) | Poor prognosis
group (n=24) | t | P-value |
|---|
| Pre-admission ISS
scores | 15.82±4.24 | 30.23±7.38 | 11.020 | <0.001 |
| APACHE II
scores |
| T1 | 18.38±5.21 | 24.45±8.29 | 3.963 | <0.001 |
| T2 | 16.24±4.63 | 26.36±5.52 | 5.657 | <0.001 |
| T3 | 15.37±3.94 | 29.74±5.83 | 12.860 | <0.001 |
| T4 | 13.45±3.21 | 31.39±6.38 | 16.750 | <0.001 |
Discussion
Traumatic shock is a common emergency with rapid
progression, the effective circulation of blood will decrease
sharply after the body suffers from violence, this can lead to a
syndrome of hypoperfusion of the microcirculation (14). During the progress of trauma
treatment, if not treated in a timely manner, can lead to systemic
inflammatory response syndrome or multiple organ dysfunction
syndrome, which further cause the failure of multiple organs or
systems, and patients can die within 10 min (15).
There is a clear standard for clinical diagnosis of
whether a patient suffer from shock or not (16), however, there is still no clear
indicator for the judgment of the shock severity in patients, only
relying on physical examination and changes in hemodynamic
parameters (17). Timely assessment
of the patient's inflammatory response and infection status is
important for the survival of traumatic shock patients (18), currently, PCT, CRP and IL-6 are
widely used acute wound proteins and cellular immune factors with
high sensitivity in the body's inflammatory response (19). However, there is scarce research on
the application value of PCT, CRP and IL-6 in assessing the
condition of patients with traumatic shock, therefore, this topic
is focused on in our present study.
The results of this study have shown that the
changes of PCT and IL-6 in serum at the time period of T1-T4 were
not significant in the control group. The serum CRP at the T4 time
period was lower than the T1 and T2 time periods. There were
significant differences in serum PCT, CRP and IL-6 between the good
prognosis and the poor prognosis groups during the T1-T4 time
period. At the same time points, both the good prognosis and the
poor prognosis groups were higher than the control group and the
poor prognosis group was higher than the good prognosis group. Also
the pre-admission ISS score and APACHE II score of patients in the
good prognosis group were lower than in the poor prognosis group.
In the early stages of trauma, the PCT, CRP and IL-6 in the serum
of traumatic shock patients were higher than ordinary trauma
patients, also the higher the expression levels of PCT, CRP and
IL-6, the worse the prognosis condition. Moreover, CRP has been
showing a downward trend in patients with good prognosis. PCT and
IL-6 have a short-term rise in the treatment process, and decline
subsequently. PCT, CRP, and IL-6 in patients with poor prognosis
show an upward trend at the early stage. After maintaining at a
higher level, the expression level decreases subsequently.
PCT is a protein secreted by macrophages in the
liver after severe infection and major trauma (20), some documents have shown that high
expression of PCT in serum is closely related to the occurrence of
shock disease (21), monitoring of
PCT in patient serum can even predict adverse complications in
trauma patients (22). CRP is also a
non-specific acute protein synthesized in the liver (23). It is often difficult to detect in
serum of ordinary humans, but occurs when the body has an
inflammatory reaction (24), it can
extremely sensitively reflect the damage of the body tissues and
the severity of the infection (25).
However, IL-6 can induce acute protein responses and mediate body
damage in a variety of infectious or acute diseases (26). Some researchers have shown that IL-6
can directly reflect the degree of damage to the body and determine
the prognosis of patients (27).
Therefore, it is speculated that the high level of serum PCT, CRP
and IL-6 in traumatic shock patients may indicate a worse
prognosis. This may reflect the activity of inflammation in the
body, suggested that there may be an occurrence of systemic
inflammatory response syndrome or multiple organ dysfunction
syndrome. Increased expression of PCT, CRP, and IL-6 in the serum
of patients during the treatment progress indicates that patients
may require higher intensity clinical interventions, and require to
be highly valued by clinicians.
Due to the limitations of experimental conditions
and experimental time of this study, there are still some
shortcomings in the experimental design. The sample size of the
experiment was not large enough. The molecular mechanism for the
specific changes of PCT, CRP and IL-6 expression levels in
patients' serum is still unclear. So animal models need to be
constructed for further exploration.
Overall, the higher the expression levels of PCT,
CRP and IL-6 in serum of traumatic shock patients, the worse
prognosis it may indicate. It has an important reference value for
the assessment of traumatic shock patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL wrote the manuscript. YL and LC analyzed and
interpreted the patient data. WF collected blood samples. HC and YL
performed ELISA. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhangzhou Municipal Hospital of Fujian Province (Zhangzhou, China).
Patients who participated in this study had complete clinical data.
Signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Benjet C, Bromet E, Karam EG, Kessler RC,
McLaughlin KA, Ruscio AM, Shahly V, Stein DJ, Petukhova M, Hill E,
et al: The epidemiology of traumatic event exposure worldwide:
results from the World Mental Health Survey Consortium. Psychol
Med. 46:327–343. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunitatsu K, Ueda K, Iwasaki Y, Yamazoe S,
Yonemitsu T, Kawazoe Y, Kawashima S, Shibata N and Kato S: Outcomes
of abdominal trauma patients with hemorrhagic shock requiring
emergency laparotomy: efficacy of intra-aortic balloon occlusion.
Acute Med Surg. 3:345–350. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grote S, Böcker W, Mutschler W, Bouillon B
and Lefering R: Diagnostic value of the Glasgow Coma Scale for
traumatic brain injury in 18,002 patients with severe multiple
injuries. J Neurotrauma. 28:527–534. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaroustovsky M, Rogalskaya E, Plyushch M,
Klimovich L, Samsonova N and Abramyan M: The level of oxidative
neutrophil response when determining endotoxin activity assay: a
new biomarker for defining the indications and effectiveness of
intensive care in patients with sepsis. Int J Inflam.
2017:34952932017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Müller B, Christ-Crain M and Schuetz P:
Meta-analysis of procalcitonin for sepsis detection. Lancet Infect
Dis. 7:498–499; author reply 502–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zurek J and Vavrina M: Procalcitonin
biomarker kinetics to predict multiorgan dysfunction syndrome in
children with sepsis and systemic inflammatory response syndrome.
Iran J Pediatr. 25:e3242015.PubMed/NCBI
|
|
7
|
Harrison M: Erythrocyte sedimentation rate
and C-reactive protein. Aust Prescr. 38:93–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hunter CA and Jones SA: IL-6 as a keystone
cytokine in health and disease. Nat Immunol. 16:448–457. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Máca J, Peteja M, Reimer P, Jor O,
Šeděnková V, Panáčková L, Ihnát P, Burda M and Ševčík P: Surgical
injury: comparing open surgery and laparoscopy by markers of tissue
damage. Ther Clin Risk Manag. 14:999–1006. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lou X, Lu G, Zhao M and Jin P:
Preoperative fluid management in traumatic shock: a retrospective
study for identifying optimal therapy of fluid resuscitation for
aged patients. Medicine (Baltimore). 97:e99662018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nieboer P, van der Werf TS, Beentjes JA,
Tulleken JE, Zijlstra JG and Ligtenberg JJ: Catecholamine
dependency in a polytrauma patient: relative adrenal insufficiency?
Intensive Care Med. 26:125–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Korać Z, Krajacić I, Hancević J and
Marusić Z: Multiple injuries in peacetime and wartime estimate of
severity of injury by the Injury Severity Score and
Polytraumaschlüssel. Eur J Surg. 164:563–567. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Hu J, Xiao L, Zhang Y, Zhao W,
Zheng W and Shang H: Adverse drug reactions of Shenmai injection: a
systematic review. J Evid Based Med. 3:177–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okonkwo KC, Wong KG, Cho CT and Gilmer L:
Testicular trauma resulting in shock and systemic inflammatory
response syndrome: a case report. Cases J. 1:42008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Punyadeera C, Schneider EM, Schaffer D,
Hsu HY, Joos TO, Kriebel F, Weiss M and Verhaegh WF: A biomarker
panel to discriminate between systemic inflammatory response
syndrome and sepsis and sepsis severity. J Emerg Trauma Shock.
3:26–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seymour CW and Rosengart MR: Septic shock:
Advances in diagnosis and treatment. JAMA. 314:708–717. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hofmann N, Zipperle J, Jafarmadar M,
Ashmwe M, Keibl C, Penzenstadler C, Ponschab M, Jafarmadar B, Redl
H, Bahrami S, et al: Experimental models of endotheliopathy: impact
of shock severity. Shock. 49:564–571. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White NJ, Martin EJ, Brophy DF and Ward
KR: Examining platelet-fibrin interactions during traumatic shock
in a swine model using platelet contractile force and clot elastic
modulus. Blood Coagul Fibrinolysis. 22:379–387. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gür A, Oguzturk H, Köse A, Turtay MG,
Ersan V, Bayindir Y, Ince V, Gurbuz S and Yucel N: Prognostic value
of procalcitonin, CRP, serum amyloid A, lactate and IL-6 markers in
liver transplant patients admitted to ED with suspected infection.
In Vivo. 31:1179–1185. 2017.PubMed/NCBI
|
|
20
|
Rule JA, Hynan LS, Attar N, Sanders C,
Korzun WJ and Lee WM; Acute Liver Failure Study Group, :
Procalcitonin identifies cell injury, not bacterial infection, in
acute liver failure. PLoS One. 10:e01385662015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Togari H, Sugiyama S, Ogino T, Suzuki S,
Ito T, Ichiki T, Kamiya K, Watanabe I, Ogawa Y, Wada Y, et al:
Interactions of endotoxin with cortisol and acute phase proteins in
septic shock neonates. Acta Paediatr Scand. 75:69–74. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed AI, Soliman RA and Samir S: Cell
free DNA and procalcitonin as early markers of complications in ICU
patients with multiple trauma and major surgery. Clin Lab.
62:2395–2404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chirapongsathorn S, Bunraksa W,
Chaiprasert A, Punpanich D, Supasyndh O and Kamath PS: Adding
C-reactive protein and procalcitonin to the model of end-stage
liver disease score improves mortality prediction in patients with
complications of cirrhosis. J Gastroenterol Hepatol. 33:726–732.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexandrov PN, Kruck TP and Lukiw WJ:
Nanomolar aluminum induces expression of the inflammatory systemic
biomarker C-reactive protein (CRP) in human brain microvessel
endothelial cells (hBMECs). J Inorg Biochem. 152:210–213. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braig D, Nero TL, Koch HG, Kaiser B, Wang
X, Thiele JR, Morton CJ, Zeller J, Kiefer J, Potempa LA, et al:
Transitional changes in the CRP structure lead to the exposure of
proinflammatory binding sites. Nat Commun. 8:141882017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang R, Han X, Uchiyama T, Watkins SK,
Yaguchi A, Delude RL and Fink MP: IL-6 is essential for development
of gut barrier dysfunction after hemorrhagic shock and
resuscitation in mice. Am J Physiol Gastrointest Liver Physiol.
285:G621–G629. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu FM, Li QL, Gao Q, Jiang JH, Zhu K,
Huang XY, Pan JF, Yan J, Hu JH, Wang Z, et al: IL-17 induces
AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in
hepatocellular carcinoma. Mol Cancer. 10:1502011. View Article : Google Scholar : PubMed/NCBI
|