Introduction
With the development of new carrier technologies,
the use of carbon and carbon-related materials has gained the
attention of numerous pharmaceutical researchers (1–5). Carbon
materials include active carbon, multi-walled carbon nanotubes
(MWCNTs) and fullerenes (6–10). Compared to traditional drug carriers,
carbon materials possess several unique properties, which render
them especially suitable for pharmaceutical research. For example,
it is well recognized that active carbon has a strong adsorption,
which enables it to have high loading capacity. Also, carbon
nanotubes have remarkable advantages due to their nanoscale channel
structure, stability, adsorption property and surface
functionalization (11,12), which make them suitable materials for
drug delivery systems. Carbon nanotubes are widely used as drug
carriers in the process of loading drugs (13). MWCNTs have been successfully used as
drug delivery systems for loading Doxorubicin, Dorzolamide and
Carbamazepine (14).
Dipyridamole (DDM) is a non-nitrate coronary
vasodilator that inhibits platelet aggregation and can be used in
combination with other anticoagulant drugs, such as warfarin, to
prevent thrombosis in patients with valvular or vascular disease.
DDM is also used in myocardial perfusion imaging, and as an
antiplatelet agent, DDM's application, in combination with aspirin,
has been reported in stroke prophylaxis (15,16). DDM
belongs to class II of the Biopharmaceutical Classification System
(BCS), and it is poorly soluble in water. Thus, the dissolution
process limits its rate and degree of oral absorption. In the
present study, DDM was selected as a model drug in an attempt to
improve its oral bioavailability by enhancing its solubility. In
the past, various carriers have been employed to solve the
solubility problem of DDM, such as liposomes, solid dispersion, and
nanospheres (17–20). Unfortunately, there are very few
carriers which possess advantages, such as high drug loading,
system stability and drug release controllability. Therefore,
identifying a suitable carrier for DDM is the research focus of the
current study. Although MWCNTs have been recently used for loading
other drugs, they have not been studied as drug delivery systems
for DDM (21–23). Multiple studies have proposed
carriers, including MWCNTs, with delineation of pore volume,
surface area and surface modification (24–26).
Among the many factors that play a role in the process of drug
delivery, drug-loading efficiency is particularly critical for
MWCNTs. In order to characterize the relationship between
drug-loading efficiency and drug release, it is necessary to
perform a systematic study to explore the ability of MWCNTs with
different drug loading rates in enhancing the dissolution rate of
poorly water-soluble drugs.
The current study evaluated the suitability of
MWCNTs as carriers for DDM and elucidated whether they can improve
the water solubility of DDM. DDM was incorporated into MWCNTs using
the solvent deposition method at different ratios (10, 25 and 50%)
(27,28). The obtained samples were examined by
scanning electron microscopy (SEM), transmission electron
microscopy (TEM), Fourier transform-infrared (FT-IR) spectroscopy,
X-ray diffraction (XRD) and differential scanning calorimetry
(DSC). Subsequently, the behavior of DDM released from MWCNT with
different drug-loading rates was compared and analyzed.
Materials and methods
Materials
DDM (purity >99.0%) was purchased from Wuhan Yuan
Cheng Gong Chuang Technology Co., Ltd. (Wuhan, China). MWCNTs were
purchased from Nanjing Xianfeng Nano Material Technology Co., Ltd.
(Nanjing, China). All other chemical reagents were used in
accordance with the requirements of analytical/HPLC grade.
Synthesis of MWCNT-DDM system
DDM was incorporated into MWCNTs at different
percentages (10, 25 and 50%) using the solvent deposition method,
which included soaking equilibrium and solvent evaporation.
Briefly, DDM was dissolved in methanol to obtain a concentrated
solution (5 mg/ml). Next, different amounts of MWCNTs were added to
the drug solution to obtain a mixture. The ratio of drug/MWCNTs in
the loading solution was 10, 25 and 50%, respectively. Then, the
mixture was gently stirred for 24 h at room temperature in a closed
container to complete the adsorption equilibrium operation.
Finally, the products were dried at 40°C in air to remove the
organic solvent. Drug-loaded samples were labeled DM-10, DM-25 and
DM-50, respectively.
Characterization techniques
SEM observation
A field emission scanning electron microscope
(Hitachi S-4300; Hitachi, Ltd., Tokyo, Japan) was employed to
characterize the morphology of the obtained samples (20). A small number of samples were fixed
on metal stubs and sputtered with gold under vacuum conditions.
TEM observation
The internal structure of the samples was observed
using a transmission electron microscope (HT7700; Hitachi, Ltd.)
(21). Subsequently, minute
quantities of the samples were fixed on the copper network for
characterization.
FT-IR study
The chemical bonding and functional groups of the
samples were examined using an FT-IR spectrometer (Nicolet 380;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) (22). The samples were mixed with potassium
bromide with a 1-2:200 ratio. Then, the mixture was pressed into a
round cake and characterized in the scanning range of 400–3,500
cm−1.
Raman spectroscopy analysis
Raman spectroscopy (Thermo U-LH100L-3; Thermo Fisher
Scientific, Inc.) was employed to examine the functional groups of
the samples. After the slides were wrapped with tin foil, the
samples were coated in foil. The samples were characterized in the
scanning range of 50–3,300 cm−1, and scanning was
concluded using the mode of area sweep.
Nitrogen adsorption analysis
Nitrogen adsorption-desorption analysis was
performed using an adsorption analyzer (SA3100; Beckman Coulter,
Inc., Brea, CA, USA). The physically adsorbed water of samples was
removed by degasification at 50°C for 24 h. Then, the container
carrying the sample was immersed in liquid nitrogen. Subsequently,
the specific surface area and the pore size of the samples were
determined using the surface area analyzer.
XRD and DSC analysis
The crystalline characteristics of samples were
analyzed using an X-ray diffractometer (PW3040/60 PANALYII CALB.V,
Almelo, The Netherlands) and the data were recorded (27). The scanned angle range of XRD
patterns was 10°-50°. DSC analysis was performed using a
differential scanning calorimeter (HCR-1; Beijing Hengjiu
Experimental Equipment Co., Ltd., Beijing, China). The temperature
range for the DSC analysis was 50–200°C with a heating rate of
10°C/min.
In vitro drug release study
Dissolution experiments were conducted using a USP
II paddle method with a dissolution instrument (RCZ-8B; Shanghai
Huanghai Drug Testing Instrument Co., Ltd., Shanghai, China).
Hydrochloric acid solution (pH 1.2) and phosphate buffer (pH 6.8)
were employed as dissolution media. The dissolution operation was
as follows: dissolution media (900 ml) was added to the basket and
maintained at 37°C. The added samples were stirred for 1 h at a
rate of 100 rpm. In addition, 5 ml samples were extracted at fixed
time intervals (5, 10, 15, 20, 30, 45 and 60 min). Subsequently, an
ultraviolet spectrophotometer (UV-2000; Unico, Franksville, WI,
USA) at an excitation wavelength of 283 nm (n=6) was used to
determine the amount of DDM obtained from the samples. All
measurements were repeated 6 times. The measurement data of the
dissolution were expressed as the mean ± standard deviation,
n=6.
Results
SEM observation
DDM was effectively loaded into MWCNTs using the
solvent deposition method, which is a suitable method for loading
drugs into MWCNTs (29,30). It is well documented that long narrow
channels play an important role in the release of drugs from MWCNTs
(31,32). SEM was employed to characterize the
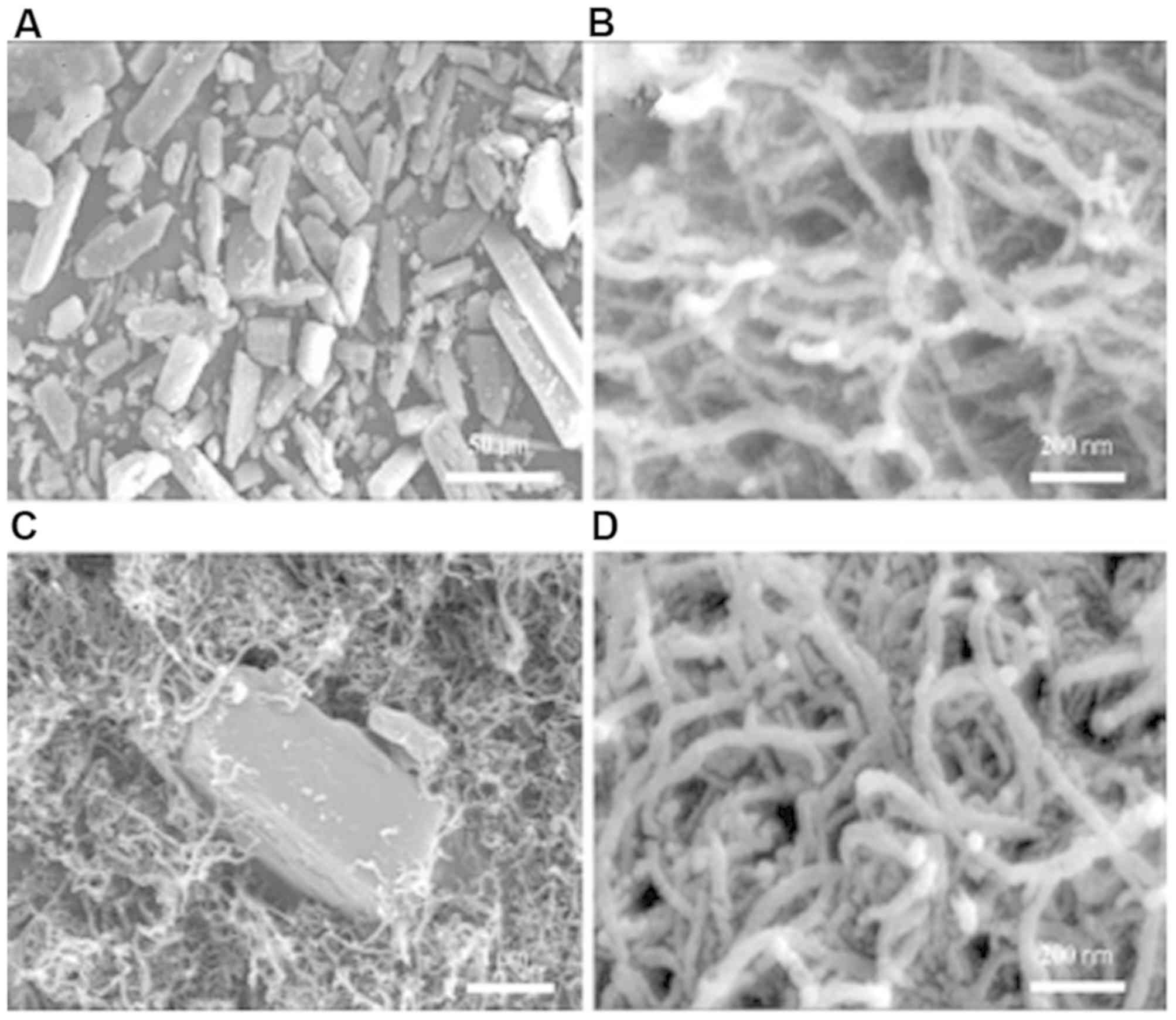
external morphology of the samples. As shown in Fig. 1A, the raw drug substance was observed
to be strip-shaped and had no fixed length. MWCNTs were tubular in
shape with a three-dimensional structure (Fig. 1B). The diameter of the MWCNTs was
determined to be <50 nm and MWCNTs were longer than 500 nm. The
cross-sectional width of MWCNTs was ~25 nm and the pore diameter of
MWCNTs was ~2–10 nm (Figs. 1B and
2C). Fig.
1C demonstrates that MWCNTs surrounded the raw drug substance.
This could be attributed to the fact that the raw drug was left
untreated, and was directly mixed with the MWCNTs. In addition, the
drug had not been loaded into the channels of the MWCNTs. It was
apparent that MWCNTs surrounded the raw drug substance. After the
DDM was incorporated into MWCNTs using the solvent deposition
method, the bulk of the raw drug substance could not be found,
indicating that a large number of raw drugs were loaded into the
channels of the MWCNTs or attached to the surface of MWCNTs.
Fig. 1D presents the SEM image of
DM-25. Also, the size of the occupying space was observed to be not
regular because of the random distribution of the drug inside or on
the surface of the channels.
TEM observation
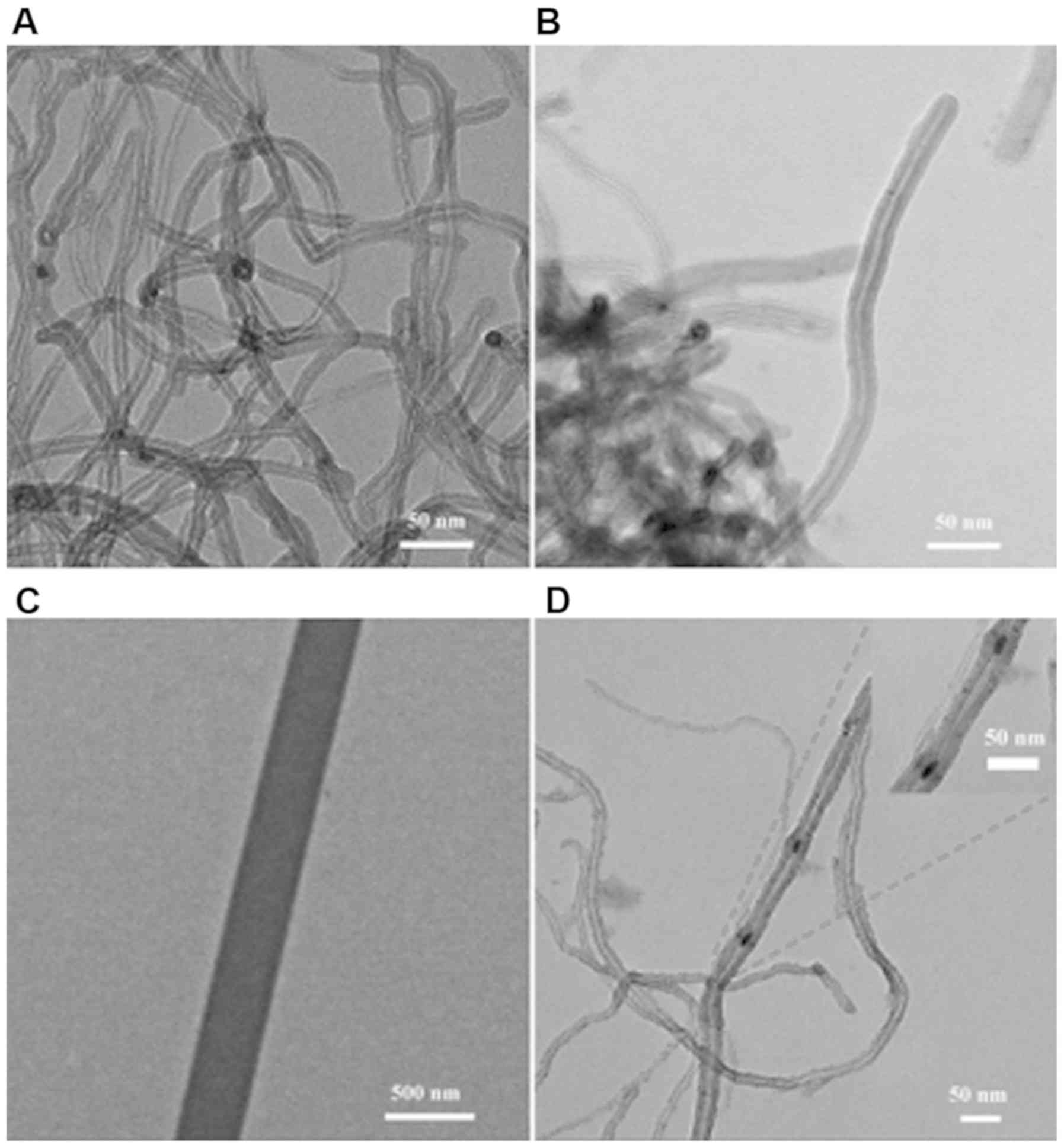
TEM was applied to identify the morphology of the
samples. As shown in Fig. 2A and B,
the long tubular structures of MWCNTs were visible and intertwined
with each other. The tube wall structure was visible in the
cross-section of the MWCNTs. The channels were sandwiched between
the long and narrow pipe walls of the MWCNTs. This specific
structure of the MWCNTs played important role in the release of
drugs. The channel structure of a MWCNT is enlarged in Fig. 2C in order to observe the channel
structure distinctly. It is clearly revealed that the inner cavity
of the tunnel was hollow, which could potentially be used for drug
loading. As seen in Fig. 2D, in
contrast to unloaded MWCNTs, the channels of the MWCNTs samples
were blocked by some particles, indicating that drugs were loaded
into the channels of the MWCNTs.
Nitrogen adsorption analysis
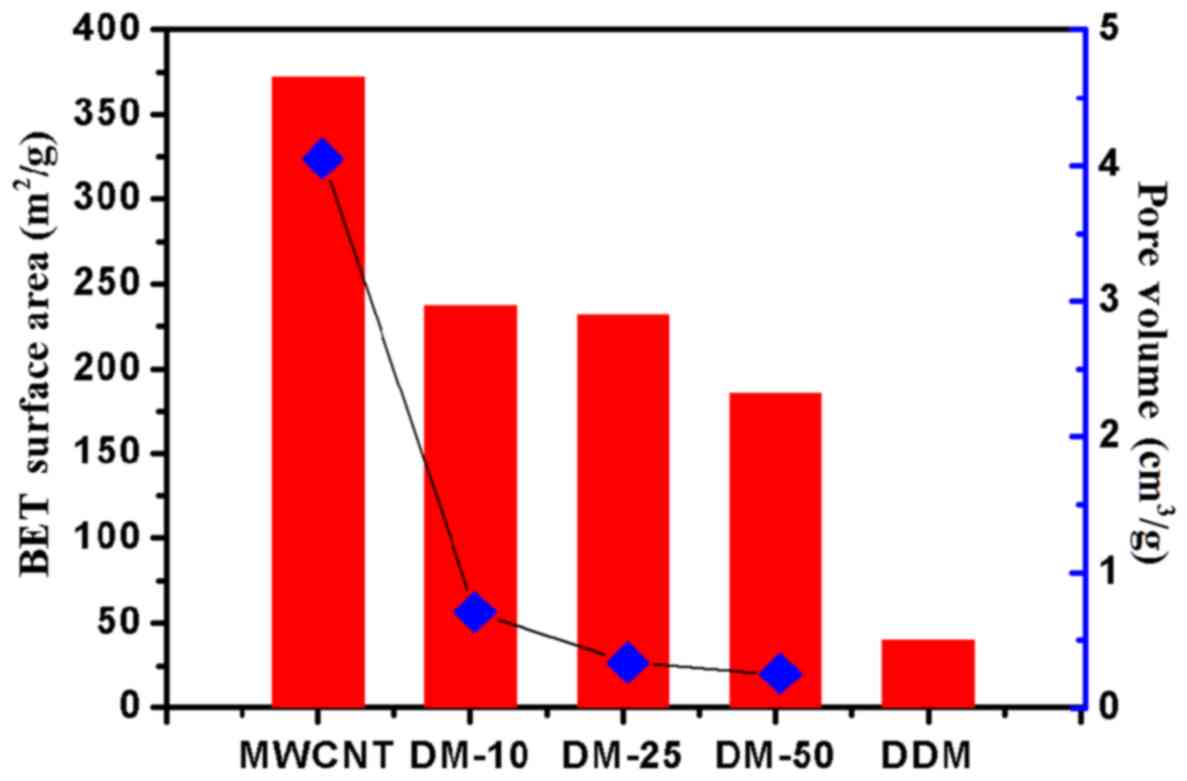
In the current study we further assessed the
progress of drug loading using nitrogen adsorption measurements. As
shown in Fig. 3, the pore volume and
the surface area of DM-10, DM-25 and DM-50 were reduced compared
with that of the MWCNTs (unloaded drugs).
FT-IR analysis
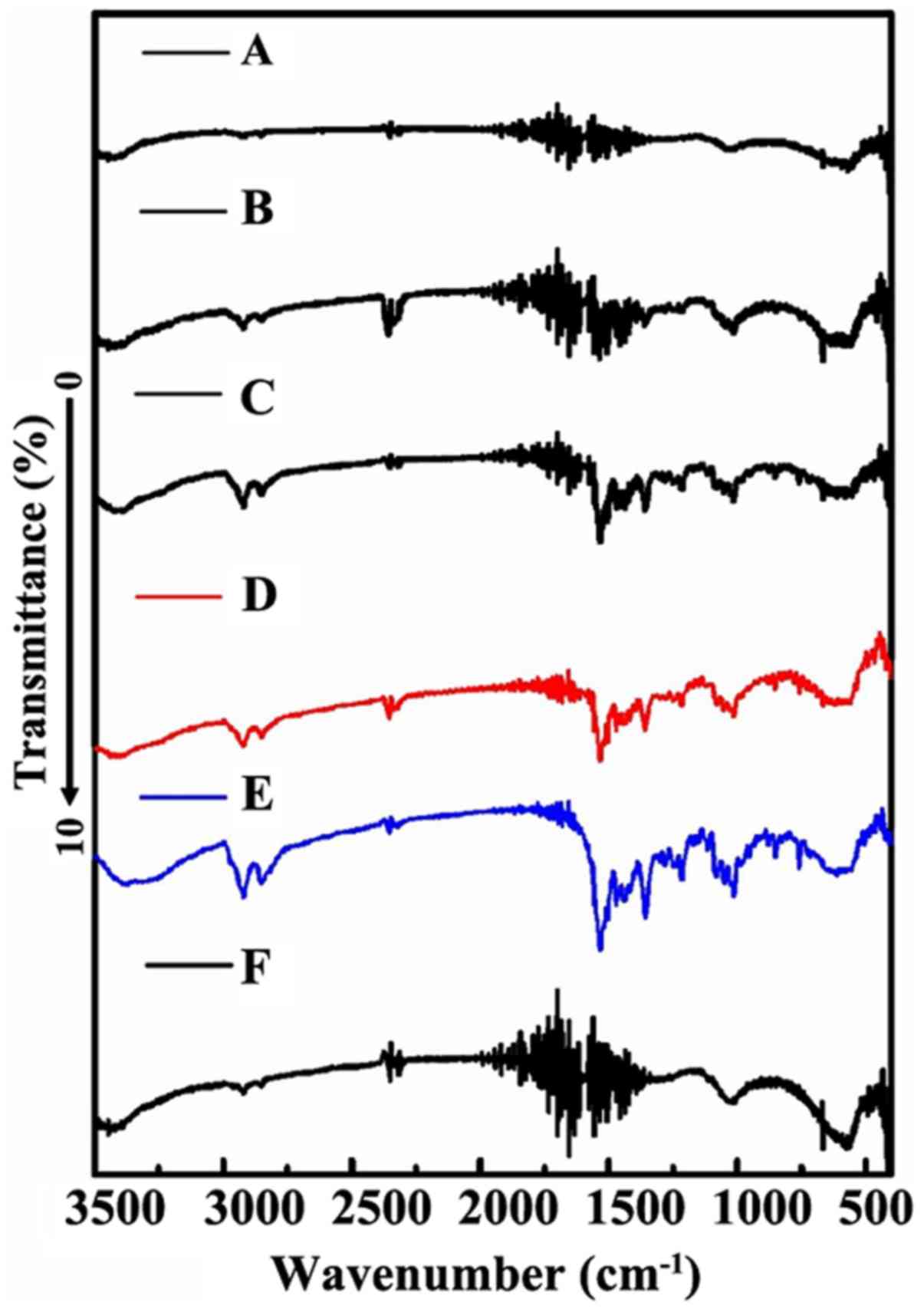
FT-IR method was used to further confirm the
progress of loading DDM into the channel of MWCNT. As shown in
Fig. 4D, DDM presented a
characteristic peak at ~3,500–3,000 cm−1, which reflects
the characteristics of hydroxyl peaks. The existence of the
hydroxyl peak was also supported by the vibration peak at
1,500–1,260 cm−1. In addition, the MWCNT exhibited peaks
at 1,600–1,700 cm−1, which corresponds to the C=C
expansion vibration absorption peaks (Fig. 4F). This absorption peak arose from
the skeleton of MWCNT. After the completion of the drug-loading
progress, the enol-type structure is possible to be formed as a
result of the association between the C=C double bond with the
hydroxyl group. Therefore, as expected, DM-10, DM-25 and DM-50
showed the characteristic peaks of the enol structure at ~1,700
cm−1 (Fig. 4A-C). The
characteristic peak was not obvious, which may be attributed to the
instability of the enol-type structure.
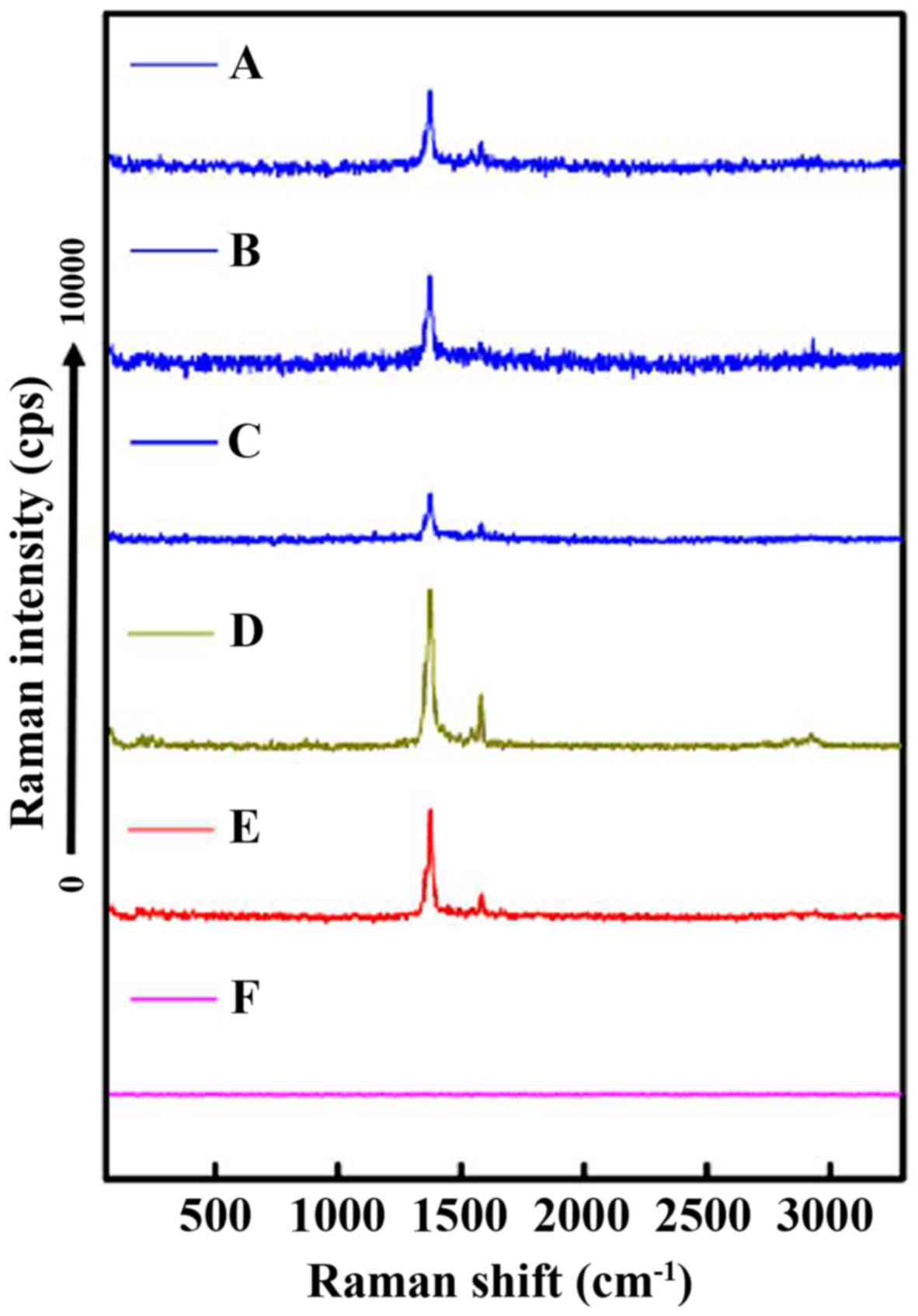
Raman spectrum analysis
Raman spectra proved to be very beneficial for
monitoring the drug-loading process. As shown in Fig. 5E, the Raman spectra had a main band
with a maximum at 1,375 cm−1 and a low frequency band
with a maximum near 1,580 cm−1. For DM-10, DM-25 and
DM-50, the characteristic peaks of the DDM were apparent for all,
indicating the existence of DDM in the MWCNT-DDM delivery system.
Moreover, the results illustrated that DDM was successfully loaded
into the MWCNTs. The MWCNT exhibited no peaks in the range of
50–3,300 cm−1 (Fig. 5F).
The characteristic peaks of MWCNT with DDM (DM-10, DM-25 and DM-50)
were observed at 1,375 and 1,580 cm−1.
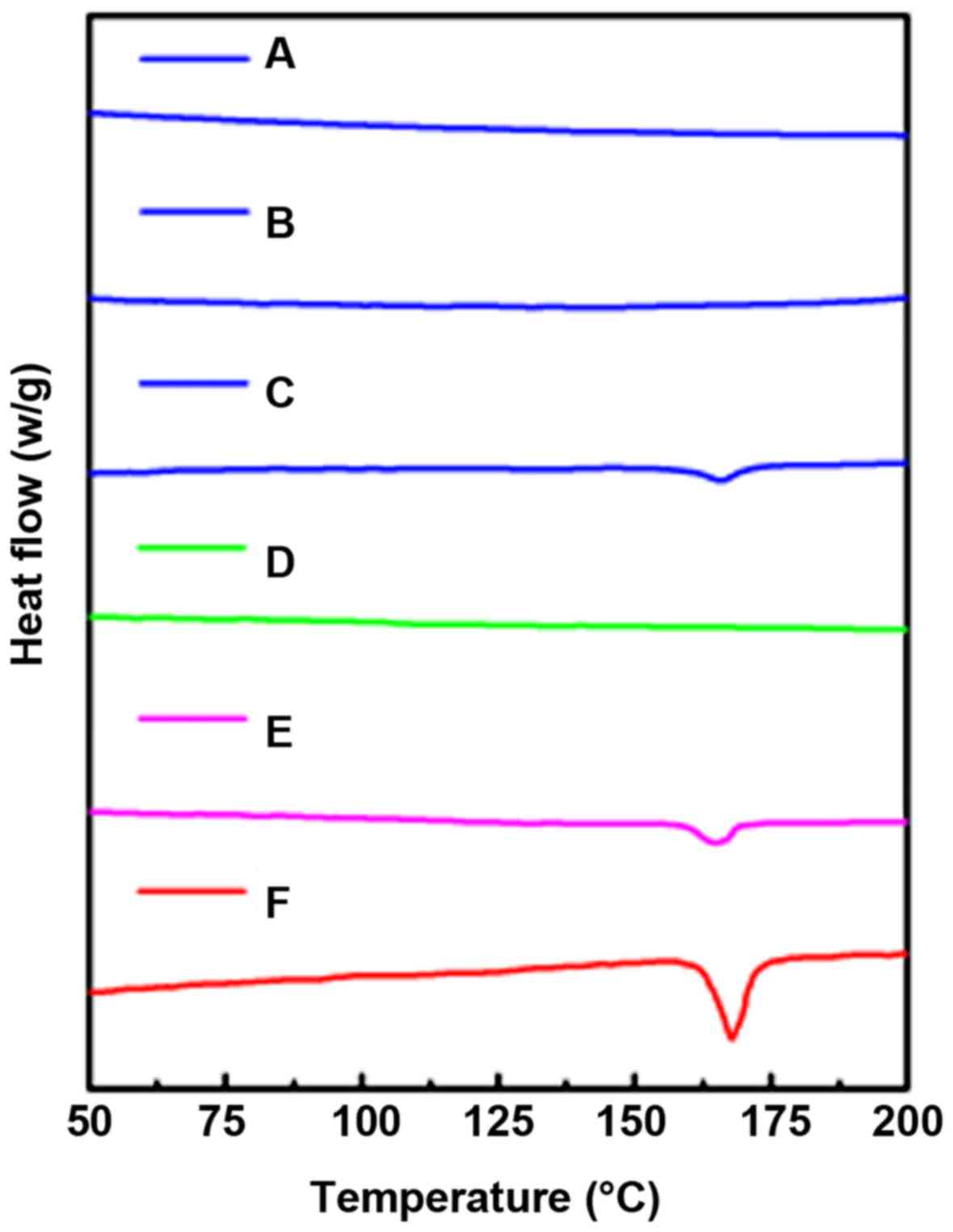
Solid state study by DSC and XRD
DSC was employed to confirm the crystal
characteristics of the samples (28). As shown in Fig. 6, the strong endothermic melting peak
was observed at 165.6°C, which is the melting point of DDM.
Conversely, MWCNT did not exhibit any peak at the corresponding
temperature. The endothermic melting peak of DM-50 was still
present. However, the inverted peak of DM-25 and DM-50 were not
observed at the corresponding melting point.
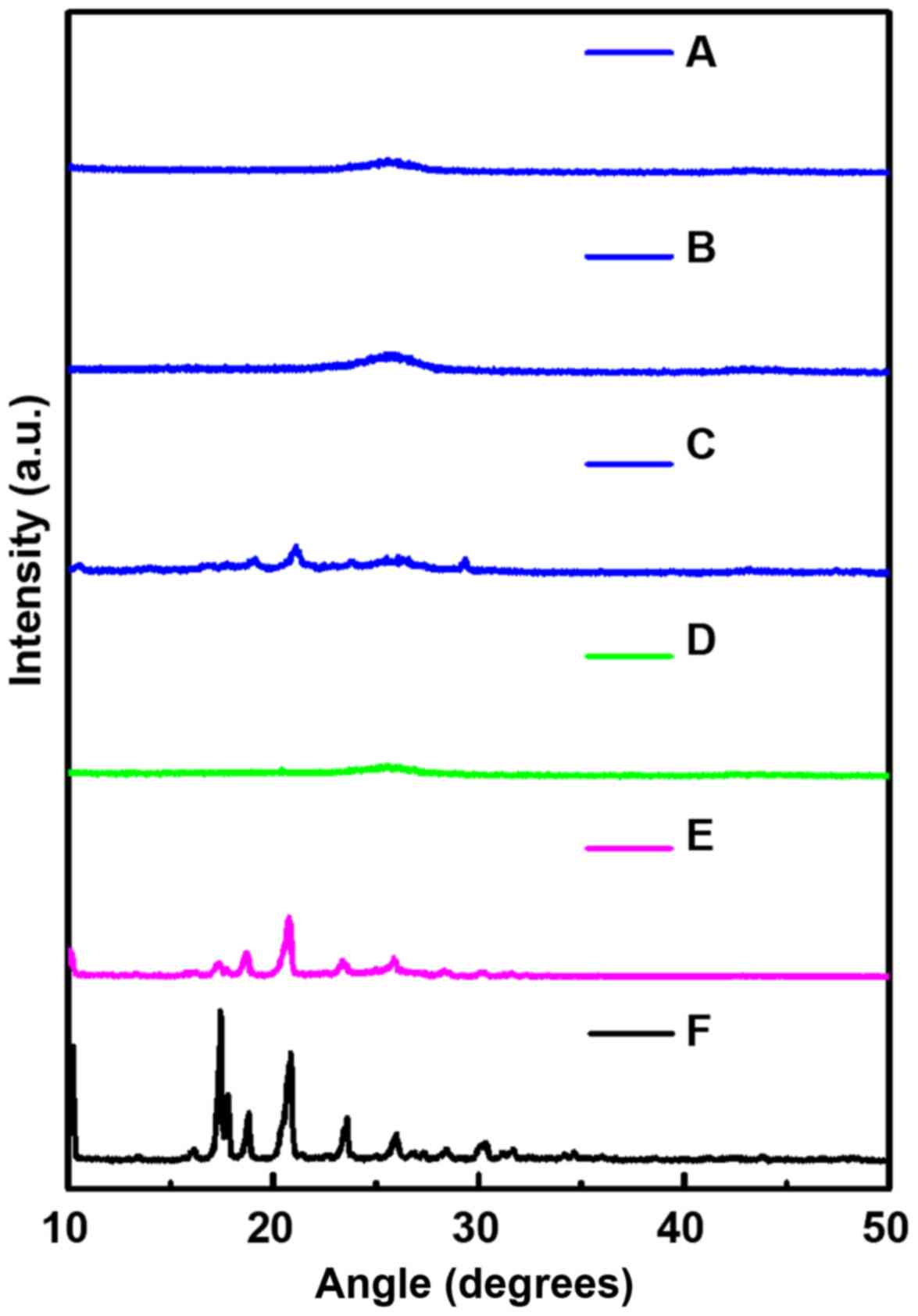
XRD study was used to evaluate the crystal
properties of the samples with a fixed angle ranging from 10° to
50°. As shown in Fig. 7, three
intense and characteristic peaks of pure DDM were observed at
17.4°, 17.8° and 20.8°. However, the same peaks were not found in
the corresponding position for MWCNT. In fact, a completely
different diffraction peak at 24.7° was noted for MWCNT. When the
DDM was physically mixed with MWCNT, the diffraction peak of the
DDM was found to be slightly reduced.
In vitro drug dissolution
Dissolution experiments were performed in order to
explore the application of MWCNTs as carriers for DDM and to
elucidate the effect of different drug-loading rates on the
dissolution behavior of DDM. As shown in Fig. 8, the 1 h cumulative release of DDM
was found to be ~50% in acid medium (pH 1.5). However, the
dissolution rates of DM-10 and DM-25 were observed to be higher
than DM-50. The results revealed that the dissolution behavior of
the DM-50 curve was very close to that of the DDM in acidic medium
(pH 1.5), indicating that the effect of the MWCNT on DDM
dissolution behavior was not significant. Notably, it showed good
solubility in acidic medium due to the chemical nature of DDM. DDM
is easily soluble in acidic medium (pH 1.5) because of its chemical
properties. So, the 1 h cumulative release of DDM was found to be
~50% in acidic medium (pH 1.5).
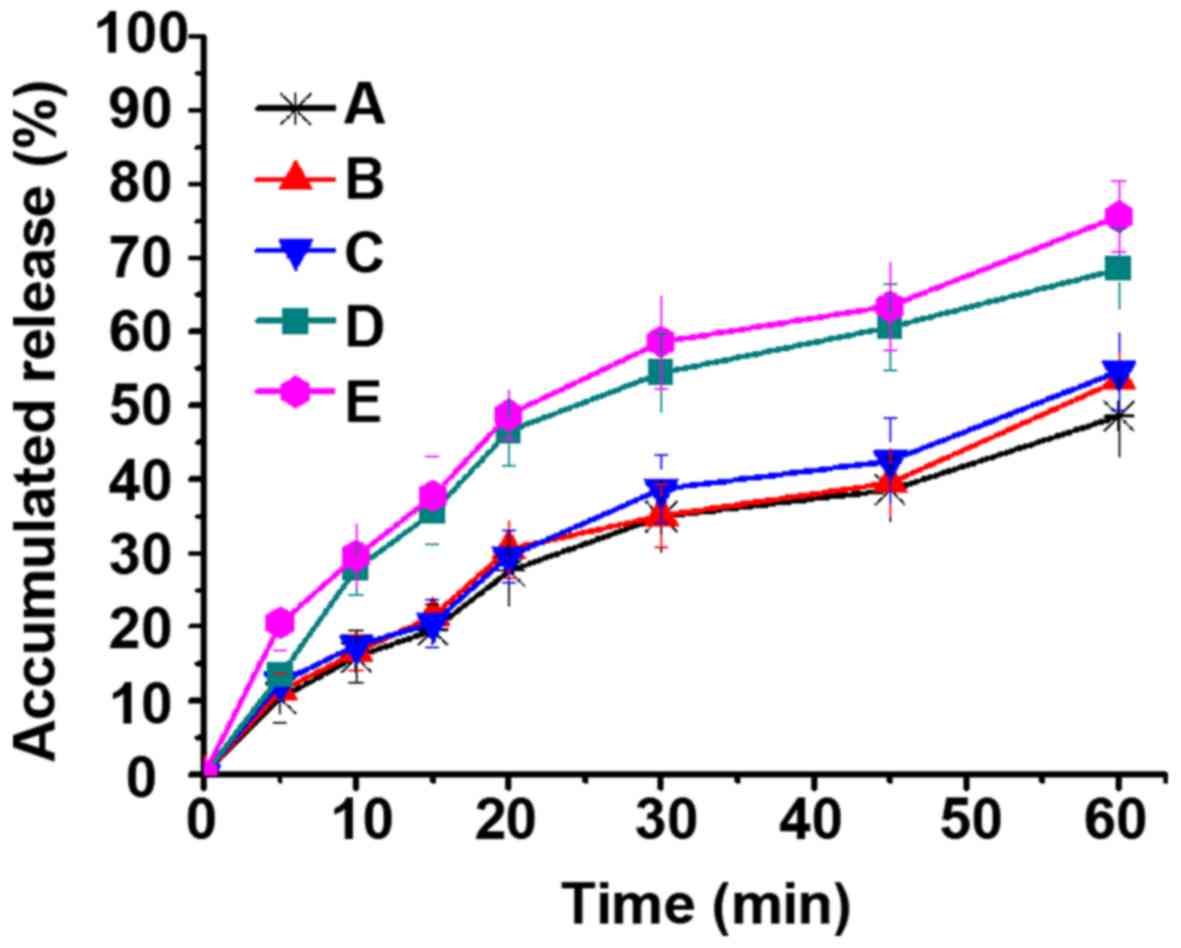
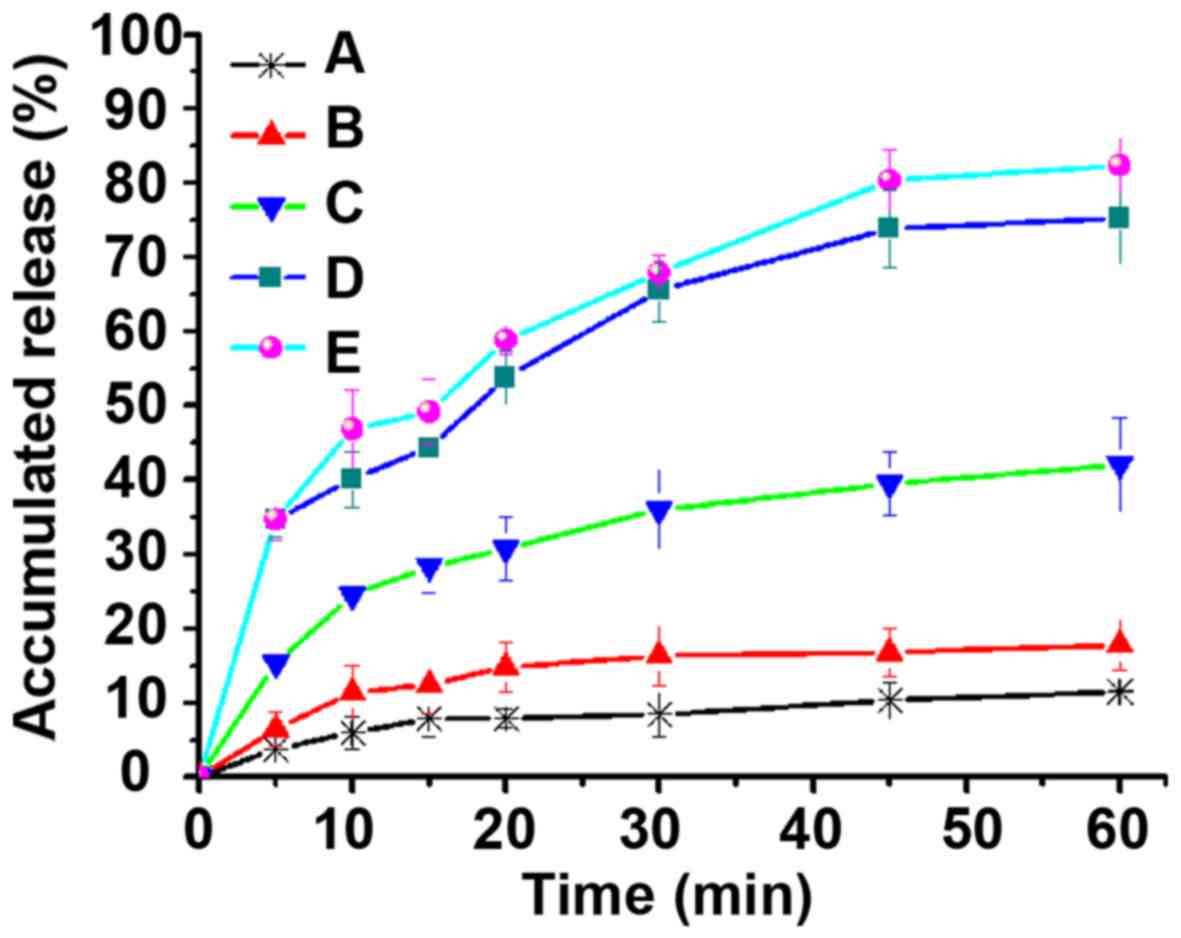
As shown in Fig. 9,
the cumulative release amount of DDM was just 10% in weak-basic
medium (pH 6.8), while the cumulative release amounts of DM-10,
DM-25 and DM-50 were all especially higher than that of the DDM.
Moreover, some drugs on the surface of the DM-50 showed a similar
release behavior to that of DDM, which is also consistent with the
results of DSC and XRD. Interestingly, both DM-50 and DDM showed
the same crystalline characteristics. This could be explained by
the fact that some drugs of DM-50 might have been gathered on the
surface of the MWCNTs, leading to similar dissolution behavior as
pure DDM. However, the dissolution rates of DM-10 and DM-25 were
higher than that of DM-50 in acidic medium because DM-10 and DM-25
drugs were primarily localized in the interior of the MWCNTs,
resulting in relatively more uniform distribution (Figs. 8 and 9). This particular finding demonstrated
that MWCNTs covered up the crystalline form of DDM when drug
loading was 10 and 25%. Furthermore, MWCNTs did not entirely change
the crystalline form of DDM into an amorphous form when the drug
loading was up to 50%. In the weak-basic medium (pH 6.8, Fig. 9), the cumulative release amount of
DDM was found to be merely 10%, which was attributable to DDM that
is a BCS class II drug and has poor dissolution. However, the
cumulative release amounts of DM-10, DM-25 and DM-50 were all
especially higher than that of the DDM. This suggested that MWCNTs
had improved the dissolution of crude DDM as a result of dispersion
of the drug. In addition, MWCNTs limited the crystal growth of the
DDM. So, it could be inferred that the MWCNTs reduced the degree of
DDM crystallization or maintained DDM in a non-crystalline state.
It is well known that the drag solubility in a non-crystalline
state is higher than that in the crystalline state (33–35). The
results of DSC and XRD showed that the crystallinities of DM-10,
DM-25 and DM-50 were all decreased, and even became amorphous, in
contrast to that of DDM. Moreover, the dissolution behavior of the
samples was also found to be affected by the drug-loading rate.
Increasing the drug-loading rate led to slight decrease in the
dissolution rate of samples. DDM in the DM-10 and DM-25 was in a
non-crystalline state. In contrast, DDM in the DM-50 was in the
original crystalline state. This may be due to the drugs being
completely loaded into the channels of the MWCNT, when drug-loading
rates were relatively low (10 and 25%). Accordingly, DM-10 and
DM-25 were both in amorphous forms. However, drugs may not have
been completely loaded into the channels of the MWCNTs and some
drugs were still deposited on the surface of the MWCNT, when drug
loading was relatively higher (50%). This was also the reason why
the drugs in the DM-50 were in crystalline form and the crystalline
form was different from the DM-10 and DM-25. Moreover, the
dissolution rate of DM-10 was found to be a little higher than that
of DM-25, whereas the dissolution rate of DM-50 was slower than
that of DM-10 and DM-25. These findings were highly suggestive that
the dissolution rate of DDM-MWCNT system decreased upon increasing
the drug-loading rate. The drugs were in different positions of the
MWCNTs for different drug-loading rates, which led to different
crystalline forms of the drugs. This particular finding is also in
line with the results of DSC. The results of DSC showed that the
drugs in the DM-50 were in crystal state, but the drugs in the
DM-10 and DM-25 were amorphous, explaining that the solubility of
drugs was affected by the crystalline form. Similarly, it has been
reported that the solubility of the crystal drugs is lower than
that of the amorphous drugs (36,37). To
further explore the causes, the drug-loading rate determined the
crystal form of drugs in the MWCNT system, and also affected their
dissolution rates. Drug loading is a key factor in the MWCNT
system, which may affect the crystal morphology of drugs and the
dissolution of drugs. Therefore, the aforementioned results
indicate that the drug dissolution rate in MWCNTs is directly
affected by the drug-loading rate.
Discussion
Studies have shown that MWCNT is a suitable carrier
for poorly soluble drugs (24,38). On
the one hand, the current study developed the application range of
MWCNTs in the field of pharmacy, on the other hand, it attempted to
develop a new platform for the delivery of poorly soluble drugs. In
the present study MWCNTs were presented to effectively solve the
problem of low solubility of DDM. The improvement of the
dissolution rate is of great significance for the oral absorption
of DDM. The screening of drug-loading methods for MWCNTs and the
optimization of drug-loading processes are not perfect. MWCNTs as
drug carriers still have some limitations. There are many problems,
such as poor controllability of the length of the pipe and the
intertwining of the pipes, that affect the drug-loading processes.
The effect of the carrier structure on the drug-loading processes
will be the aim of our future research.
Acknowledgements
Not applicable.
Funding
This study was supported by the Science and
Technology General Program of Heilongjiang Educational Committee
(no. 2016-KYYWF-0857).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ drafted the manuscript. WZ and HH were mainly
devoted to the synthesis of the MWCNT-DDM system. YD and CH
assisted with the characterization techniques. XS and BJ were
responsible for the in vitro drug release study. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
D'Souza SL, Deshmukh B, Bhamore JR, Rawat
KA, Lenka N and Kailasa SK: Synthesis of fluorescent nitrogen-doped
carbon dots from dried shrimps for cell imaging and boldine drug
delivery system. Rsc Adv. 6:12169–12179. 2016. View Article : Google Scholar
|
|
2
|
Zhang H, Hou L, Jiao X, Ji Y, Zhu X and
Zhang Z: Transferrin-mediated fullerenes nanoparticles as
Fe(2+)-dependent drug vehicles for synergistic anti-tumor efficacy.
Biomaterials. 37:353–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Huang X, Long Y, Wang X, Zhang H,
Zhu R, Liang L, Teng P and Zheng H: Hollow luminescent carbon dots
for drug delivery. Carbon. 59:192–199. 2013. View Article : Google Scholar
|
|
4
|
Potter C, Tian Y, Walker G, McCoy C,
Hornsby P, Donnelly C, Jones DS and Andrews GP: Novel supercritical
carbon dioxide impregnation technique for the production of
amorphous solid drug dispersions: A comparison to hot melt
extrusion. Mol Pharm. 12:1377–1390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Muzi L, Ménard-Moyon C, Russier J, Li J,
Chin CF, Ang WH, Pastorin G, Risuleo G and Bianco A:
Diameter-dependent release of a cisplatin pro-drug from small and
large functionalized carbon nanotubes. Nanoscale. 7:5383–5394.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao MZ, Huang-Fu MY, Liu HN, Wang XR,
Sheng X and Gao JQ: Fabrication and characterization of drug-loaded
nano-hydroxyapatite/polyamide 66 scaffolds modified with carbon
nanotubes and silk fibroin. Int J Nanomedicine. 11:6181–6194. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sciortino N, Fedeli S, Paoli P, Brandi A,
Chiarugi P, Severi M and Cicchi S: Multiwalled carbon nanotubes for
drug delivery: Efficiency related to length and incubation time.
Int J Pharm. 521:69–72. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhirde AA, Patel V, Gavard J, Zhang G,
Sousa AA, Masedunskas A, Leapman RD, Weigert R, Gutkind JS and
Rusling JF: Targeted killing of cancer cells in vivo and in vitro
with EGF-directed carbon nanotube-based drug delivery. ACS Nano.
3:307–316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajima K, Yudasaka M, Murakami T, Maigné A,
Shiba K and Iijima S: Carbon nanohorns as anticancer drug carriers.
Mol Pharm. 2:475–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heister E, Neves V, Lamprecht C, Silva
SRP, Coley HM and McFadden J: Drug loading, dispersion stability,
and therapeutic efficacy in targeted drug delivery with carbon
nanotubes. Carbon. 50:622–632. 2012. View Article : Google Scholar
|
|
11
|
Cheng Y, Lu S, Zhang H, Varanasi CV and
Liu J: Synergistic effects from graphene and carbon nanotubes
enable flexible and robust electrodes for high-performance
supercapacitors. Nano Lett. 12:4206–4211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan L, Lu XH, Xiao X, Zhai T, Dai J,
Zhang F, Hu B, Wang X, Gong L, Chen J, et al: Flexible solid-state
supercapacitors based on carbon nanoparticles/MnO2
nanorods hybrid structure. ACS Nano. 6:656–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park H, Afzali A, Han SJ, Tulevski GS,
Franklin AD, Tersoff J, Hannon JB and Haensch W: High-density
integration of carbon nanotubes via chemical self-assembly. Nat
Nanotechnol. 7:787–791. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ncibi MC and Sillanpää M: Optimizing the
removal of pharmaceutical drugs Carbamazepine and Dorzolamide from
aqueous solutions using mesoporous activated carbons and
multi-walled carbon nanotubes. J Mol Liq. 238:379–388. 2017.
View Article : Google Scholar
|
|
15
|
Lee G, Choong H, Chiang G and Woo K:
Three-year randomized controlled trial of dipyridamole and low-dose
warfarin in patients with IgA nephropathy and renal impairment.
Nephrology (Carlton). 3:117–121. 2010. View Article : Google Scholar
|
|
16
|
Tang Y, Liu SY, Armes SP and Billingham
NC: Solubilization and controlled release of a hydrophobic drug
using novel micelle- forming ABC triblock copolymers.
Biomacromolecules. 4:1636–1645. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Waard H, Hessels MJT, Boon M, Sjollema
KA, Hinrichs WLJ, Eissens AC and Frijlink HW: CLSM as quantitative
method to determine the size of drug crystals in a solid
dispersion. Pharm Res. 28:2567–2574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zecevic DE, Meier R, Daniels R and Wagner
KG: Site specific solubility improvement using solid dispersions of
HPMC-AS/HPC SSL-mixtures. Eur J Pharm Biopharm. 87:264–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakai T and Thommes M: Investigation into
mixing capability and solid dispersion preparation using the DSM
Xplore Pharma Micro Extruder. J Pharm Pharmacol. 66:218–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Gray L, Leonardi-Bee J, Weaver CS,
Heptinstall S and Bath PM: Effect of aspirin, clopidogrel and
dipyridamole on soluble markers of vascular function in normal
volunteers and patients with prior ischaemic stroke. Platelets.
17:100–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan YD, Sung JH, Kim KK, Kim DW, Kim JO,
Lee BJ, Yong CS and Choi HG: Novel valsartan-loaded solid
dispersion with enhanced bioavailability and no crystalline
changes. Int J Pharm. 422:202–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahmoodi M, Arjmand M, Sundararaj U and
Park S: The electrical conductivity and electromagnetic
interference shielding of injection molded multi-walled carbon
nanotube/polystyrene composites. Carbon. 50:1455–1464. 2012.
View Article : Google Scholar
|
|
23
|
Gupta TK, Singh BP, Mathur RB and Dhakate
SR: Multi-walled carbon nanotube-graphene-polyaniline multiphase
nanocomposite with superior electromagnetic shielding
effectiveness. Nanoscale. 6:842–851. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pippa N, Chronopoulos DD, Stellas D,
Fernández-Pacheco R, Arenal R, Demetzos C and Tagmatarchis N:
Design and development of multi-walled carbon nanotube-liposome
drug delivery platforms. Int J Pharm. 528:429–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bottini M, Bruckner S, Nika K, Bottini N,
Bellucci S, Magrini A, Bergamaschi A and Mustelin T: Multi-walled
carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett.
160:121–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabizon AA, Barenholz Y and Bialer M:
Prolongation of the circulation time of doxorubicin encapsulated in
liposomes containing a polyethylene glycol-derivatized
phospholipid: Pharmacokinetic studies in rodents and dogs. Pharm
Res. 10:703–708. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu W, Zhao Q, Sun C, Zhang Z, Jiang T,
Sun J, Li Y and Wang S: Mesoporous carbon with spherical pores as a
carrier for celecoxib with needle-like crystallinity: Improve
dissolution rate and bioavailability. Mater Sci Eng C. 39:13–20.
2014. View Article : Google Scholar
|
|
28
|
Zhu WQ, Wan L, Zhang C, Gao YK, Zheng X,
Jiang TY and Wang SL: Exploitation of 3D face-centered cubic
mesoporous silica as a carrier for a poorly water soluble drug:
Influence of pore size on release rate. Mater Sci Eng C. 34:78–85.
2014. View Article : Google Scholar
|
|
29
|
Zhang X, Hui Z, Wan D, Huang H, Huang J,
Yuan H and Yu J: Alginate microsphere filled with carbon nanotube
as drug carrier. Int J Biol Macromol. 47:389–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao W, Paul A, Zhao B, Lee C, Rodes L and
Prakash S: Carbon nanotube lipid drug approach for targeted
delivery of a chemotherapy drug in a human breast cancer xenograft
animal model. Biomaterials. 34:10109–10119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wong BS, Yoong SL, Jagusiak A, Panczyk T,
Ho HK, Ang WH and Pastorin G: Carbon nanotubes for delivery of
small molecule drugs. Adv Drug Deliv Rev. 65:1964–2015. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan J, Zeng F, Xu J and Wu S: Targeted
anti-cancer prodrug based on carbon nanotube with photodynamic
therapeutic effect and pH-triggered drug release. J Nanopart Res.
15:19112013. View Article : Google Scholar
|
|
33
|
Chen J, Sarma B, Evans JMB and Myerson AS:
Pharmaceutical crystallization. Cryst Growth Des. 11:887–895. 2011.
View Article : Google Scholar
|
|
34
|
Weimann PA, Hajduk DA, Chu C, Chaffin KA,
Brodil JC and Bates FS: Crystallization of tethered polyethylene in
confined geometries. J Polym Sci Pol Phys37. 2053–2068. 2015.
|
|
35
|
Leon RAL, Wan WY, Badruddoza AZM, Hatton
TA and Khan SA: Simultaneous spherical crystallization and
co-formulation of drug(s) and excipient from microfluidic double
emulsions. Cryst Growth Des. 14:140–146. 2014. View Article : Google Scholar
|
|
36
|
Löbmann K, Laitinen R, Grohganz H, Gordon
KC, Strachan C and Rades T: Coamorphous drug systems: Enhanced
physical stability and dissolution rate of indomethacin and
naproxen. Mol Pharm. 8:1919–1928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shegokar R and Müller RH: Nanocrystals:
Industrially feasible multifunctional formulation technology for
poorly soluble actives. Int J Pharm. 399:129–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ezzati Nazhad Dolatabadi J, Omidi Y and
Losic D: Carbon nanotubes as an advanced drug and gene delivery
nanosystem. Curr Nanosci. 7:297–314. 2011. View Article : Google Scholar
|