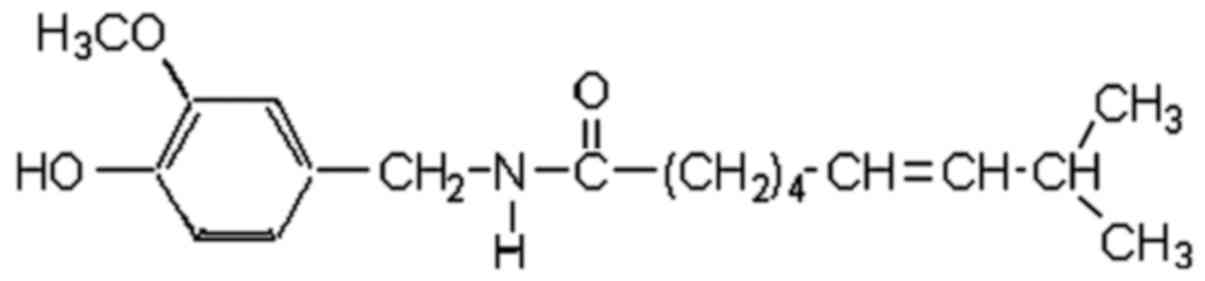
|
1
|
Dubin AE and Patapoutian A: Nociceptors:
The sensors of the pain pathway. J Clin Invest. 120:3760–3772.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Căruntu C, Negrei C, Ghiţă MA, Căruntu A,
Bădărău AI, Buraga I, Boda D, Albu A and Brănişteanu D: Capsaicin,
a hot topic in skin pharmacology and physiology. Farmacia.
63:487–491. 2015.
|
|
3
|
du Jardin KG, Gregersen LS, Røsland T,
Uggerhøj KH, Petersen LJ, Arendt-Nielsen L and Gazerani P:
Assessment of pain response in capsaicin-induced dynamic mechanical
allodynia using a novel and fully automated brushing device. Pain
Res Manag. 18:6–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caruntu C, Boda D, Musat S, Caruntu A,
Poenaru E, Calenic B, Savulescu-Fiedler I, Draghia A, Rotaru M and
Badarau AI: Stress effects on cutaneous nociceptive nerve fibers
and their neurons of origin in rats. Rom Biotechnol Lett.
19:9517–9530. 2014.
|
|
5
|
Szallasi A and Blumberg PM: Vanilloid
(Capsaicin) receptors and mechanisms. Pharmacol Rev. 51:159–212.
1999.PubMed/NCBI
|
|
6
|
Derry S, Rice AS, Cole P, Tan T and Moore
RA: Topical capsaicin (high concentration) for chronic neuropathic
pain in adults. Cochrane Database Syst Rev.
1:CD0073932017.PubMed/NCBI
|
|
7
|
Ständer S, Moormann C, Schumacher M,
Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz
BM, Luger TA, et al: Expression of vanilloid receptor subtype 1 in
cutaneous sensory nerve fibers, mast cells, and epithelial cells of
appendage structures. Exp Dermatol. 13:129–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nolano M, Simone DA, Wendelschafer-Crabb G
and Kennedy WR: Decreased sensation and loss of epidermal nerve
fibers following repeated topical application of capsaicin in
humans. Soc Neurosci Abstr. 22:18021996.
|
|
9
|
Simone DA, Nolano M, Johnson T,
Wendelschafer-Crabb G and Kennedy WR: Intradermal injection of
capsaicin in humans produces degeneration and subsequent
reinnervation of epidermal nerve fibers: Correlation with sensory
function. J Neurosci. 18:8947–8959. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mankowski C, Poole CD, Ernault E, Thomas
R, Berni E, Currie CJ, Treadwell C, Calvo JI, Plastira C,
Zafeiropoulou E, et al: Effectiveness of the capsaicin 8% patch in
the management of peripheral neuropathic pain in European clinical
practice: The ASCEND study. BMC Neurol. 17:802017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burness CB and McCormack PL: Capsaicin 8%
patch: A review in peripheral neuropathic pain. Drugs. 76:123–134.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haanpää M, Cruccu G, Nurmikko TJ, McBride
WT, Docu Axelarad A, Bosilkov A, Chambers C, Ernault E and
Abdulahad AK: Capsaicin 8% patch versus oral pregabalin in patients
with peripheral neuropathic pain. Eur J Pain. 20:316–328. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Giménez-Milà M, Videla S, Navarro MA,
Faulí A, Ojeda A, Bogdanovich A, Moreno LA, Hernández-Cera C and
Busquets C: Assessment of the feasibility of high-concentration
capsaicin patches in the pain unit of a tertiary hospital for a
population of mixed refractory peripheral neuropathic pain
syndromes in non-diabetic patients. BMC Anesthesiol. 14:1202014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zis P, Apsokardos A, Isaia C, Sykioti P
and Vadalouca A: Posttraumatic and postsurgical neuropathic pain
responsive to treatment with capsaicin 8% topical patch. Pain
Physician. 17:E213–E218. 2014.PubMed/NCBI
|
|
15
|
Serrano A, Torres D, Veciana M, Caro C,
Montero J and Mayoral V: Quantitative thermal testing profiles as a
predictor of treatment response to topical capsaicin in patients
with localized neuropathic pain. Pain Res Treat.
2017:74259072017.PubMed/NCBI
|
|
16
|
Bauchy F, Mouraux A, Deumens R, Leerink M,
Ulpiano Trillig A, le Polain de Waroux B, Steyaert A, Joëlle QL and
Forget P: Feasibility of topical applications of natural
high-concentration capsaicinoid solutions in patients with
peripheral neuropathic pain: A retrospective analysis. Pain Res
Manag. 2016:97030362016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baranidharan G, Das S and Bhaskar A: A
review of the high-concentration capsaicin patch and experience in
its use in the management of neuropathic pain. Ther Adv Neurol
Disorder. 6:287–297. 2013. View Article : Google Scholar
|
|
18
|
Yong YL, Tan LT, Ming LC, Chan KG, Lee LH,
Goh BH and Khan TM: The effectiveness and safety of topical
capsaicin in postherpetic neuralgia: A systematic review and
meta-analysis. Front Pharmacol. 7:5382017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boyd K, Shea SM and Patterson JW: The role
of capsaicin in dermatology. In: Capsaicin as a Therapeutic
Molecule. Springer; Basel: pp. 293–306. 2014, PubMed/NCBI
|
|
20
|
Ostrovsky DA: Single treatment with
capsaicin 8% patch may reduce pain and sleep interference up to 12
weeks in patients with painful diabetic peripheral neuropathy.
Explore (NY). 13:351–353. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gálvez R, Navez ML, Moyle G, Maihöfner C,
Stoker M, Ernault E, Nurmikko TJ and Attal N: Capsaicin 8% patch
repeat treatment in nondiabetic peripheral neuropathic pain: A
52-week, open-label, single-arm, safety study. Clin J Pain.
33:921–931. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kiani J, Ahmad Nasrollahi S, Esna-Ashari
F, Fallah P and Sajedi F: Amitriptyline 2% cream vs. capsaicin
0.75% cream in the treatment of painful diabetic neuropathy (Double
blind, randomized clinical trial of efficacy and safety). Iran J
Pharm Res. 14:1263–1268. 2015.PubMed/NCBI
|
|
23
|
Kulkantrakorn K, Lorsuwansiri C and
Meesawatsom P: 0.025% capsaicin gel for the treatment of painful
diabetic neuropathy: A randomized, double-blind, crossover,
placebo-controlled trial. Pain Pract. 13:497–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown S, Simpson DM, Moyle G, Brew BJ,
Schifitto G, Larbalestier N, Orkin C, Fisher M, Vanhove GF and
Tobias JK: NGX-4010, a capsaicin 8% patch, for the treatment of
painful HIV-associated distal sensory polyneuropathy: Integrated
analysis of two phase III, randomized, controlled trials. AIDS Res
Ther. 10:52013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simpson DM, Brown S, Tobias JK and Vanhove
GF; NGX-4010 C107 Study Group, : NGX-4010, a capsaicin 8% dermal
patch, for the treatment of painful HIV-associated distal sensory
polyneuropathy: Results of a 52-week open-label study. Clin J Pain.
30:134–142. 2014.PubMed/NCBI
|
|
26
|
Feller L, Fourie J, Bouckaert M, Khammissa
RAG, Ballyram R and Lemmer J: Burning mouth syndrome:
Aetiopathogenesis and principles of management. Pain Res Manag.
2017:19262692017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campbell BK, Fillingim RB, Lee S, Brao R,
Price DD and Neubert JK: Effects of high-dose capsaicin on TMD
subjects: A randomized clinical study. JDR Clin Trans Res. 2:58–65.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Filipczak-Bryniarska I, Krzyzewski RM,
Kucharz J, Michalowska-Kaczmarczyk A, Kleja J, Woron J, Strzepek K,
Kazior L, Wordliczek J, Grodzicki T, et al: High-dose 8% capsaicin
patch in treatment of chemotherapy-induced peripheral neuropathy:
Single-center experience. Med Oncol. 34:1622017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Casanueva B, Rodero B, Quintial C, Llorca
J and González-Gay MA: Short-term efficacy of topical capsaicin
therapy in severely affected fibromyalgia patients. Rheumatol Int.
33:2665–2670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deal CL, Schnitzer TJ, Lipstein E, Seibold
JR, Stevens RM, Levy MD, Albert D and Renold F: Treatment of
arthritis with topical capsaicin: A double-blind trial. Clin Ther.
13:383–395. 1991.PubMed/NCBI
|
|
31
|
Laslett LL and Jones G: Capsaicin for
osteoarthritis pain. In: Capsaicin as a Therapeutic Molecule.
Springer; Basel: pp. 277–291. 2014, PubMed/NCBI
|
|
32
|
Caselli A, Spallone V, Marfia GA, Battista
C, Pachatz C, Veves A and Uccioli L: Validation of the nerve axon
reflex for the assessment of small nerve fibre dysfunction. J
Neurol Neurosurg Psychiatry. 77:927–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Căruntu C and Boda D: Evaluation through
in vivo reflectance confocal microscopy of the cutaneous neurogenic
inflammatory reaction induced by capsaicin in human subjects. J
Biomed Opt. 17:0850032012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Căruntu C, Negrei C, Boda D, Constantin C,
Căruntu A and Neagu M: Biotechnological advances for diagnosis of
peripheral diabetic neuropathy. Rom Biotechnol Lett. 19:9846–9858.
2014.
|
|
35
|
Adriana Ghita M, Caruntu C, Lixandru D,
Pitea A, Batani A and Boda D: The quest for novel biomarkers in
early diagnosis of diabetic neuropathy. Curr Proteomics. 14:86–99.
2017. View Article : Google Scholar
|
|
36
|
Fattori V, Hohmann MS, Rossaneis AC,
Pinho-Ribeiro FA and Verri WA: Capsaicin: Current understanding of
its mechanisms and therapy of pain and other pre-clinical and
clinical uses. Molecules. 21:8442016. View Article : Google Scholar
|
|
37
|
Rollyson WD, Stover CA, Brown KC, Perry
HE, Stevenson CD, McNees CA, Ball JG, Valentovic MA and Dasgupta P:
Bioavailability of capsaicin and its implications for drug
delivery. J Control Release. 196:96–105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reyes-Escogido ML, Gonzalez-Mondragon EG
and Vazquez-Tzompantzi E: Chemical and pharmacological aspects of
capsaicin. Molecules. 16:1253–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bode AM and Dong Z: The two faces of
capsaicin. Cancer Res. 71:2809–2814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
North H: Colorimetric determination of
capsaicin in oleoresin of capsicum. Anal Chem. 21:934–936. 1949.
View Article : Google Scholar
|
|
41
|
Hartman KT: A rapid gas-liquid
chromatographic determination for capsaicin in capsicum spices. J
Food Sci. 35:543–547. 1970. View Article : Google Scholar
|
|
42
|
Cooper TH, Guzinski JA and Fisher C:
Improved high-performance liquid chromatography method for the
determination of major capsaicinoids in capsicum oleoresins. J
Agric Food Chem. 39:2253–2256. 1991. View Article : Google Scholar
|
|
43
|
Iwai K, Suzuki T, Fujiwake H and Oka S:
Simultaneous microdetermination of capsaicin and its four analogues
by using high-performance liquid chromatography and gas
chromatography - mass spectrometry. J Chromatogr A. 172:303–311.
1979. View Article : Google Scholar
|
|
44
|
Nyberg NT, Baumann H and Kenne L:
Application of solid-phase extraction coupled to an NMR flow-probe
in the analysis of HPLC fractions. Magn Reson Chem. 39:236–240.
2001. View Article : Google Scholar
|
|
45
|
Nikolaeva DA: Spectrophotometric
determination of capsaicin in peppers (Capsicum annuum L.).
Biokhim. Metody Analiza Plodov; Kishinev: pp. 99–102. 1984
|
|
46
|
Pryakhin OR, Tkach VI, Golovkin VA,
Gladyshev VV and Kuleshova ND: Method for determination of the
total amount of capsaicinoids in thick red pepper extract by
amperometric titration. U.S.S.R. 90:48803301992.
|
|
47
|
Laskaridou-Monnerville A: Determination of
capsaicin and dihydrocapsaicin by micellar electrokinetic capillary
chromatography and its application to various species of
Capsicum, Solanaceae. J Chromatogr A. 838:293–302. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Korel F, Baǧdatlioǧlu N, Balaban MÖ and
Hişil Y: Ground red peppers: Capsaicinoids content, Scoville
scores, and discrimination by an electronic nose. J Agric Food
Chem. 50:3257–3261. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Way RM: Official Analytical Methods of the
American SpiceTrade Association. 3. American Spice Trade
Association; Washington, DC: pp. 51–52. 1985
|
|
50
|
Stipcovich T, Barbero GF,
Ferreiro-González M, Palma M and Barroso CG: Fast analysis of
capsaicinoids in Naga Jolokia extracts (Capsicum
chinense) by high-performance liquid chromatography using fused
core columns. Food Chem. 239:217–224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fan Y, Lu YM, Yu B, Tan CP and Cui B:
Extraction and purification of capsaicin from capsicum oleoresin
using an aqueous two-phase system combined with chromatography. J
Chromatogr B Analyt Technol Biomed Life Sci. 1063:11–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Darré L and Domene C: Binding of capsaicin
to the TRPV1 ion channel. Mol Pharm. 12:4454–4465. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Srinivasan K: Biological activities of red
pepper (Capsicum annuum) and its pungent principle
capsaicin: A review. Crit Rev Food Sci Nutr. 56:1488–1500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Clapham DE: TRP channels as cellular
sensors. Nature. 426:517–524. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Szolcsányi J and Jancsó-Gábor A: Sensory
effects of capsaicin congeners I. Relationship between chemical
structure and pain-producing potency of pungent agents.
Arzneimittelforschung. 25:1877–1881. 1975.PubMed/NCBI
|
|
56
|
Montell C, Birnbaumer L and Flockerzi V:
The TRP channels, a remarkably functional family. Cell.
108:595–598. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ferrer-Montiel A, García-Martínez C,
Morenilla-Palao C, García-Sanz N, Fernández-Carvajal A,
Fernández-Ballester G and Planells-Cases R: Molecular architecture
of the vanilloid receptor. Insights for drug design. Eur J Biochem.
271:1820–1826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
García-Sanz N, Fernández-Carvajal A,
Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E,
Fernández-Ballester G and Ferrer-Montiel A: Identification of a
tetramerization domain in the C terminus of the vanilloid receptor.
J Neurosci. 24:5307–5314. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song S, Ayon RJ, Yamamura A, Yamamura H,
Dash S, Babicheva A, Tang H, Sun X, Cordery AG, Khalpey Z, et al:
Capsaicin-induced Ca2+ signaling is enhanced via
upregulated TRPV1 channels in pulmonary artery smooth muscle cells
from patients with idiopathic PAH. Am J Physiol Lung Cell Mol
Physiol. 312:L309–L325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Caterina MJ and Julius D: The vanilloid
receptor: A molecular gateway to the pain pathway. Annu Rev
Neurosci. 24:487–517. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morenilla-Palao C, Planells-Cases R,
García-Sanz N and Ferrer-Montiel A: Regulated exocytosis
contributes to protein kinase C potentiation of vanilloid receptor
activity. J Biol Chem. 279:25665–25672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kárai LJ, Russell JT, Iadarola MJ and Oláh
Z: Vanilloid receptor 1 regulates multiple calcium compartments and
contributes to Ca2+-induced Ca2+ release in
sensory neurons. J Biol Chem. 279:16377–16387. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Marshall IC, Owen DE, Cripps TV, Davis JB,
McNulty S and Smart D: Activation of vanilloid receptor 1 by
resiniferatoxin mobilizes calcium from inositol
1,4,5-trisphosphate-sensitive stores. Br J Pharmacol. 138:172–176.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Vrechi TA, Crunfli F, Costa AP and Torrão
AS: Cannabinoid receptor type 1 agonist ACEA protects neurons from
death and attenuates endoplasmic reticulum stress-related apoptotic
pathway signaling. Neurotox Res. 33:846–855. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Van Der Stelt M and Di Marzo V:
Endovanilloids. Putative endogenous ligands of transient receptor
potential vanilloid 1 channels. Eur J Biochem. 271:1827–1834. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim SR, Lee DY, Chung ES, Oh UT, Kim SU
and Jin BK: Transient receptor potential vanilloid subtype 1
mediates cell death of mesencephalic dopaminergic neurons in vivo
and in vitro. J Neurosci. 25:662–671. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Smart D, Gunthorpe MJ, Jerman JC, Nasir S,
Gray J, Muir AI, Chambers JK, Randall AD and Davis JB: The
endogenous lipid anandamide is a full agonist at the human
vanilloid receptor (hVR1). Br J Pharmacol. 129:227–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Marinelli S, Di Marzo V, Florenzano F,
Fezza F, Viscomi MT, van der Stelt M, Bernardi G, Molinari M,
Maccarrone M and Mercuri NB: N-arachidonoyl-dopamine tunes synaptic
transmission onto dopaminergic neurons by activating both
cannabinoid and vanilloid receptors. Neuropsychopharmacology.
32:298–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ,
Jung J, Cho S, Min KH, Suh YG, Kim D, et al: Direct activation of
capsaicin receptors by products of lipoxygenases: Endogenous
capsaicin-like substances. Proc Natl Acad Sci USA. 97:6155–6160.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Eberhardt MJ, Schillers F, Eberhardt EM,
Risser L, de la Roche J, Herzog C, Echtermeyer F and Leffler A:
Reactive metabolites of acetaminophen activate and sensitize the
capsaicin receptor TRPV1. Sci Rep. 7:127752017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Smutzer G and Devassy RK: Integrating
TRPV1 receptor function with capsaicin psychophysics. Adv Pharmacol
Sci. 2016:15124572016.PubMed/NCBI
|
|
72
|
Elokely K, Velisetty P, Delemotte L,
Palovcak E, Klein ML, Rohacs T and Carnevale V: Understanding TRPV1
activation by ligands: Insights from the binding modes of capsaicin
and resiniferatoxin. Proc Natl Acad Sci USA. 113:E137–E145. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nagy I, Friston D, Valente JS, Torres
Perez JV and Andreou AP: Pharmacology of the capsaicin receptor,
transient receptor potential vanilloid type-1 ion channel. Prog
Drug Res. 68:39–76. 2014.PubMed/NCBI
|
|
74
|
Tominaga M, Caterina MJ, Malmberg AB,
Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI and Julius
D: The cloned capsaicin receptor integrates multiple pain-producing
stimuli. Neuron. 21:531–543. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Moreira FA, Aguiar DC, Terzian AL,
Guimarães FS and Wotjak CT: Cannabinoid type 1 receptors and
transient receptor potential vanilloid type 1 channels in fear and
anxiety-two sides of one coin? Neuroscience. 204:186–192. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ryu S, Liu B and Qin F: Low pH potentiates
both capsaicin binding and channel gating of VR1 receptors. J Gen
Physiol. 122:45–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chuang HH, Prescott ED, Kong H, Shields S,
Jordt SE, Basbaum AI, Chao MV and Julius D: Bradykinin and nerve
growth factor release the capsaicin receptor from
PtdIns(4,5)P2-mediated inhibition. Nature. 411:957–962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Moriyama T, Higashi T, Togashi K, Iida T,
Segi E, Sugimoto Y, Tominaga T, Narumiya S and Tominaga M:
Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive
mechanism of prostaglandins. Mol Pain. 1:32005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang X, Huang J and McNaughton PA: NGF
rapidly increases membrane expression of TRPV1 heat-gated ion
channels. EMBO J. 24:4211–4223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Nakagawa H and Hiura A: Four possible
itching pathways related to the TRPV1 channel, histamine, PAR-2 and
serotonin. Malays J Med Sci. 20:5–12. 2013.PubMed/NCBI
|
|
81
|
Bertrand H, Kyriazis M, Reeves KD, Lyftogt
J and Rabago D: Topical mannitol reduces capsaicin-induced pain:
Results of a pilot-level, double-blind, randomized controlled
trial. PM R. 7:1111–1117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Luvisetto S, Vacca V and Cianchetti C:
Analgesic effects of botulinum neurotoxin type A in a model of
allyl isothiocyanate- and capsaicin-induced pain in mice. Toxicon.
94:23–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matak I, Rossetto O and Lacković Z:
Botulinum toxin type A selectivity for certain types of pain is
associated with capsaicin-sensitive neurons. Pain. 155:1516–1526.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Arout CA, Perrino AC Jr, Ralevski E,
Acampora G, Koretski J, Limoncelli D, Newcomb J and Petrakis IL:
Effect of intravenous ethanol on capsaicin-induced hyperalgesia in
human subjects. Alcohol Clin Exp Res. 40:1425–1429. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Filippi A, Caruntu C, Gheorghe RO, Deftu
A, Amuzescu B and Ristoiu V: Catecholamines reduce transient
receptor potential vanilloid type 1 desensitization in cultured
dorsal root ganglia neurons. J Physiol Pharmacol. 67:843–850.
2016.PubMed/NCBI
|
|
86
|
Tominaga M, Wada M and Masu M:
Potentiation of capsaicin receptor activity by metabotropic ATP
receptors as a possible mechanism for ATP-evoked pain and
hyperalgesia. Proc Natl Acad Sci USA. 98:6951–6956. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Amadesi S, Nie J, Vergnolle N, Cottrell
GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes
H, et al: Protease-activated receptor 2 sensitizes the capsaicin
receptor transient receptor potential vanilloid receptor 1 to
induce hyperalgesia. J Neurosci. 24:4300–4312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Matta JA, Miyares RL and Ahern GP: TRPV1
is a novel target for omega-3 polyunsaturated fatty acids. J
Physiol. 578:397–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sowa NA, Street SE, Vihko P and Zylka MJ:
Prostatic acid phosphatase reduces thermal sensitivity and chronic
pain sensitization by depleting phosphatidylinositol
4,5-bisphosphate. J Neurosci. 30:10282–10293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Premkumar LS and Ahern GP: Induction of
vanilloid receptor channel activity by protein kinase C. Nature.
408:985–990. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bhave G, Zhu W, Wang H, Brasier DJ, Oxford
GS and Gereau RW IV: cAMP-dependent protein kinase regulates
desensitization of the capsaicin receptor (VR1) by direct
phosphorylation. Neuron. 35:721–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhang X, Wu J, Fang L and Willis WD: The
effects of protein phosphatase inhibitors on the duration of
central sensitization of rat dorsal horn neurons following
injection of capsaicin. Mol Pain. 2:232006. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Por ED, Samelson BK, Belugin S, Akopian
AN, Scott JD and Jeske NA: PP2B/calcineurin-mediated
desensitization of TRPV1 does not require AKAP150. Biochem J.
432:549–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Numazaki M, Tominaga T, Takeuchi K,
Murayama N, Toyooka H and Tominaga M: Structural determinant of
TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci
USA. 100:8002–8006. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Pecze L, Blum W and Schwaller B: Mechanism
of capsaicin receptor TRPV1-mediated toxicity in pain-sensing
neurons focusing on the effects of Na(+)/Ca(2+) fluxes and the
Ca(2+)-binding protein calretinin. Biochim Biophys Acta.
1833:1680–1691. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kobayashi K, Fukuoka T, Obata K, Yamanaka
H, Dai Y, Tokunaga A and Noguchi K: Distinct expression of TRPM8,
TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with
adelta/c-fibers and colocalization with trk receptors. J Comp
Neurol. 493:596–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lumpkin EA and Caterina MJ: Mechanisms of
sensory transduction in the skin. Nature. 445:858–865. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hong S, Morrow TJ, Paulson PE, Isom LL and
Wiley JW: Early painful diabetic neuropathy is associated with
differential changes in tetrodotoxin-sensitive and -resistant
sodium channels in dorsal root ganglion neurons in the rat. J Biol
Chem. 279:29341–29350. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Michael GJ and Priestley JV: Differential
expression of the mRNA for the vanilloid receptor subtype 1 in
cells of the adult rat dorsal root and nodose ganglia and its
downregulation by axotomy. J Neurosci. 19:1844–1854. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chung MK and Campbell JN: Use of capsaicin
to treat pain: Mechanistic and therapeutic considerations.
Pharmaceuticals (Basel). 9:662016. View Article : Google Scholar
|
|
101
|
Davis JB, Gray J, Gunthorpe MJ, Hatcher
JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson
K, et al: Vanilloid receptor-1 is essential for inflammatory
thermal hyperalgesia. Nature. 405:183–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Julius D and Basbaum AI: Molecular
mechanisms of nociception. Nature. 413:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Mezey E, Tóth ZE, Cortright DN, Arzubi MK,
Krause JE, Elde R, Guo A, Blumberg PM and Szallasi A: Distribution
of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like
immunoreactivity, in the central nervous system of the rat and
human. Proc Natl Acad Sci USA. 97:3655–3660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Fernandes ES, Fernandes MA and Keeble JE:
The functions of TRPA1 and TRPV1: Moving away from sensory nerves.
Br J Pharmacol. 166:510–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Roosterman D, Goerge T, Schneider SW,
Bunnett NW and Steinhoff M: Neuronal control of skin function: The
skin as a neuroimmunoendocrine organ. Physiol Rev. 86:1309–1379.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Southall MD, Li T, Gharibova LS, Pei Y,
Nicol GD and Travers JB: Activation of epidermal vanilloid
receptor-1 induces release of proinflammatory mediators in human
keratinocytes. J Pharmacol Exp Ther. 304:217–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kim SJ, Lee SA, Yun SJ, Kim JK, Park JS,
Jeong HS, Lee JH, Moon SJ and Won YH: Expression of vanilloid
receptor 1 in cultured fibroblast. Exp Dermatol. 15:362–367. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Treede RD, Meyer RA, Raja SN and Campbell
JN: Peripheral and central mechanisms of cutaneous hyperalgesia.
Prog Neurobiol. 38:397–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Southall MD and Vasko MR: Prostaglandin
receptor subtypes, EP3C and EP4, mediate the prostaglandin
E2-induced cAMP production and sensitization of sensory neurons. J
Biol Chem. 276:16083–16091. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gábor M and Rázga Z: Development and
inhibition of mouse ear oedema induced with capsaicin. Agents
Actions. 36:83–86. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lee YM, Kim YK and Chung JH: Increased
expression of TRPV1 channel in intrinsically aged and photoaged
human skin in vivo. Exp Dermatol. 18:431–436. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lee YM, Kang SM and Chung JH: The role of
TRPV1 channel in aged human skin. J Dermatol Sci. 65:81–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee YM, Kim YK, Kim KH, Park SJ, Kim SJ
and Chung JH: A novel role for the TRPV1 channel in UV-induced
matrix metalloproteinase (MMP)-1 expression in HaCaT cells. J Cell
Physiol. 219:766–775. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bíró T, Maurer M, Modarres S, Lewin NE,
Brodie C, Acs G, Acs P, Paus R and Blumberg PM: Characterization of
functional vanilloid receptors expressed by mast cells. Blood.
91:1332–1340. 1998.PubMed/NCBI
|
|
115
|
Căruntu C, Boda D, Musat S, Căruntu A and
Mandache E: Stress-induced mast cell activation in glabrous and
hairy skin. Mediators Inflamm. 2014:1059502014. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo
JY, Lee CH, Kim M and Oh U: TRPV1 mediates histamine-induced
itching via the activation of phospholipase A2 and 12-lipoxygenase.
J Neurosci. 27:2331–2337. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bodó E, Bíró T, Telek A, Czifra G, Griger
Z, Tóth BI, Mescalchin A, Ito T, Bettermann A, Kovács L, et al: A
hot new twist to hair biology: Involvement of vanilloid receptor-1
(VR1/TRPV1) signaling in human hair growth control. Am J Pathol.
166:985–998. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Holzer P: Local effector functions of
capsaicin-sensitive sensory nerve endings: Involvement of
tachykinins, calcitonin gene-related peptide and other
neuropeptides. Neuroscience. 24:739–768. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Richardson JD and Vasko MR: Cellular
mechanisms of neurogenic inflammation. J Pharmacol Exp Ther.
302:839–845. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Birklein F and Schmelz M: Neuropeptides,
neurogenic inflammation and complex regional pain syndrome (CRPS).
Neurosci Lett. 437:199–202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Maggi CA and Meli A: The sensory-efferent
function of capsaicin-sensitive sensory neurons. Gen Pharmacol.
19:1–43. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Botchkarev VA, Eichmüller S, Peters EM,
Pietsch P, Johansson O, Maurer M and Paus R: A simple
immunofluorescence technique for simultaneous visualization of mast
cells and nerve fibers reveals selectivity and hair cycle-dependent
changes in mast cell - nerve fiber contacts in murine skin. Arch
Dermatol Res. 289:292–302. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ansel JC, Brown JR, Payan DG and Brown MA:
Substance P selectively activates TNF-alpha gene expression in
murine mast cells. J Immunol. 150:4478–4485. 1993.PubMed/NCBI
|
|
124
|
Kowalski ML and Kaliner MA: Neurogenic
inflammation, vascular permeability, and mast cells. J Immunol.
140:3905–3911. 1988.PubMed/NCBI
|
|
125
|
Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ,
Li D, Deng HW and Li YJ: Transient receptor potential vanilloid
1-mediated expression and secretion of endothelial cell-derived
calcitonin gene-related peptide. Regul Pept. 150:66–72. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Price RC, Gandhi W, Nadeau C, Tarnavskiy
R, Qu A, Fahey E, Stone L and Schweinhardt P: Characterization of a
novel capsaicin/heat ongoing pain model. Eur J Pain. 22:370–384.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Szolcsányi J: Capsaicin and sensory
neurones: A historical perspective. In: Capsaicin as a Therapeutic
Molecule. Springer; Basel: pp. 1–37. 2014
|
|
128
|
Simone DA, Ngeow JY, Putterman GJ and
LaMotte RH: Hyperalgesia to heat after intradermal injection of
capsaicin. Brain Res. 418:201–203. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
LaMotte RH, Shain CN, Simone DA and Tsai
EF: Neurogenic hyperalgesia: Psychophysical studies of underlying
mechanisms. J Neurophysiol. 66:190–211. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Torebjörk HE, Lundberg LE and LaMotte RH:
Central changes in processing of mechanoreceptive input in
capsaicin-induced secondary hyperalgesia in humans. J Physiol.
448:765–780. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Simone DA and Ochoa J: Early and late
effects of prolonged topical capsaicin on cutaneous sensibility and
neurogenic vasodilatation in humans. Pain. 47:285–294. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Carpenter SE and Lynn B: Vascular and
sensory responses of human skin to mild injury after topical
treatment with capsaicin. Br J Pharmacol. 73:755–758. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Schmelz M, Schmid R, Handwerker HO and
Torebjörk HE: Encoding of burning pain from capsaicin-treated human
skin in two categories of unmyelinated nerve fibres. Brain.
123:560–571. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Simone DA, Baumann TK and LaMotte RH:
Dose-dependent pain and mechanical hyperalgesia in humans after
intradermal injection of capsaicin. Pain. 38:99–107. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Serra J, Campero M and Ochoa J: Flare and
hyperalgesia after intradermal capsaicin injection in human skin. J
Neurophysiol. 80:2801–2810. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kinnman E, Nygårds EB and Hansson P:
Peripheral α-adrenoreceptors are involved in the development of
capsaicin induced ongoing and stimulus evoked pain in humans. Pain.
69:79–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ma XL, Zhang FX, Dong F, Bao L and Zhang
X: Experimental evidence for alleviating nociceptive
hypersensitivity by single application of capsaicin. Mol Pain.
11:222015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
White JPM, Urban L and Nagy I: TRPV1
function in health and disease. Curr Pharm Biotechnol. 12:130–144.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Amaya F, Shimosato G, Nagano M, Ueda M,
Hashimoto S, Tanaka Y, Suzuki H and Tanaka M: NGF and GDNF
differentially regulate TRPV1 expression that contributes to
development of inflammatory thermal hyperalgesia. Eur J Neurosci.
20:2303–2310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Urban L, White JPM and Nagy I: Molecular
structure of transient receptor potential vanilloid type 1 ion
channel (TRPV1). Curr Pharm Biotechnol. 12:115–121. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tympanidis P, Casula MA, Yiangou Y,
Terenghi G, Dowd P and Anand P: Increased vanilloid receptor VR1
innervation in vulvodynia. Eur J Pain. 8:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Yilmaz Z, Renton T, Yiangou Y, Zakrzewska
J, Chessell IP, Bountra C and Anand P: Burning mouth syndrome as a
trigeminal small fibre neuropathy: Increased heat and capsaicin
receptor TRPV1 in nerve fibres correlates with pain score. J Clin
Neurosci. 14:864–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Haanpää M and Treede RD: Capsaicin for
neuropathic pain: Linking traditional medicine and molecular
biology. Eur Neurol. 68:264–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ji RR, Samad TA, Jin SX, Schmoll R and
Woolf CJ: p38 MAPK activation by NGF in primary sensory neurons
after inflammation increases TRPV1 levels and maintains heat
hyperalgesia. Neuron. 36:57–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Jordt SE, Tominaga M and Julius D: Acid
potentiation of the capsaicin receptor determined by a key
extracellular site. Proc Natl Acad Sci USA. 97:8134–8139. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang J, Zhang X and McNaughton PA:
Inflammatory pain: The cellular basis of heat hyperalgesia. Curr
Neuropharmacol. 4:197–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Szallasi A and Blumberg PM: Specific
binding of resiniferatoxin, an ultrapotent capsaicin analog, by
dorsal root ganglion membranes. Brain Res. 524:106–111. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Bleakman D, Brorson JR and Miller RJ: The
effect of capsaicin on voltage-gated calcium currents and calcium
signals in cultured dorsal root ganglion cells. Br J Pharmacol.
101:423–431. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Docherty RJ, Robertson B and Bevan S:
Capsaicin causes prolonged inhibition of voltage-activated calcium
currents in adult rat dorsal root ganglion neurons in culture.
Neuroscience. 40:513–521. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dray A, Bettaney J and Forster P: Actions
of capsaicin on peripheral nociceptors of the neonatal rat spinal
cord-tail in vitro: Dependence of extracellular ions and
independence of second messengers. Br J Pharmacol. 101:727–733.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Anand P, Bloom SR and McGregor GP: Topical
capsaicin pretreatment inhibits axon reflex vasodilatation caused
by somatostatin and vasoactive intestinal polypeptide in human
skin. Br J Pharmacol. 78:665–669. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Bjerring P and Arendt-Nielsen L:
Inhibition of histamine skin flare reaction following repeated
topical applications of capsaicin. Allergy. 45:121–125. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Tóth-Kása I, Jancsó G, Bognár A, Husz S
and Obál F Jr: Capsaicin prevents histamine-induced itching. Int J
Clin Pharmacol Res. 6:163–169. 1986.PubMed/NCBI
|
|
154
|
Winter J, Bevan S and Campbell EA:
Capsaicin and pain mechanisms. Br J Anaesth. 75:157–168. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Hartel M, di Mola FF, Selvaggi F, Mascetta
G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Büchler
MW, et al: Vanilloids in pancreatic cancer: Potential for
chemotherapy and pain management. Gut. 55:519–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Shin CY, Shin J, Kim BM, Wang MH, Jang JH,
Surh YJ and Oh U: Essential role of mitochondrial permeability
transition in vanilloid receptor 1-dependent cell death of sensory
neurons. Mol Cell Neurosci. 24:57–68. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Athanasiou A, Smith PA, Vakilpour S,
Kumaran NM, Turner AE, Bagiokou D, Layfield R, Ray DE, Westwell AD,
Alexander SP, et al: Vanilloid receptor agonists and antagonists
are mitochondrial inhibitors: How vanilloids cause non-vanilloid
receptor mediated cell death. Biochem Biophys Res Commun.
354:50–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Buck SH and Burks TF: The
neuropharmacology of capsaicin: Review of some recent observations.
Pharmacol Rev. 38:179–226. 1986.PubMed/NCBI
|
|
159
|
Chung K, Klein CM and Coggeshall RE: The
receptive part of the primary afferent axon is most vulnerable to
systemic capsaicin in adult rats. Brain Res. 511:222–226. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wood JN, Coote PR, Minhas A, Mullaney I,
McNeill M and Burgess GM: Capsaicin-induced ion fluxes increase
cyclic GMP but not cyclic AMP levels in rat sensory neurones in
culture. J Neurochem. 53:1203–1211. 1989. View Article : Google Scholar : PubMed/NCBI
|