Introduction
Malignant melanoma has always been an alarming
health problem, with high morbidity and mortality (1). The etiology of melanoma is not
completely identified, comprising different risk factors, such as
ultraviolet radiation exposure, genetics, chemical exposure and HPV
infection (2–4). The therapeutic strategies are
individualized according to the stage of the disease and the
prognosis. The 5-year survival rate, also depends on the stage and
the prognosis (5). If the malignant
cells have reached the lymph nodes, surgical resection is the main
treatment of solid tumors. However, in the perioperative period due
to various factors, such as surgery and anesthesia, tumor cells may
enter blood circulation, lymphatic channel, bone marrow, and even
spread to all the tissues and organs. This fact can lead to
formation of micro-metastases, increase the risk of postoperative
tumor recurrence and metastasis, and also affects postoperative
survival rate (6). Thus, the
challenge in the treatment strategies, is the combination of
chemotherapy, radiation therapy, immunotherapy and targeted
therapy, in order to combat the effects of this aggressive disease.
There is a necessity of new treatment options (7–10).
In recent years, it has been found that most
anesthetics can influence the function of the immune system and
target the residual disease or make cells able to form
micro-metastasis (11). Regional
anesthesia was associated in some studies with reduced risk of
cancer recurrence and this can be associated with the
anti-inflammatory effects of local anesthetics as lidocaine that
can influence the proliferation, migration or invasion of cancer
cells (11). Cluster of
differentiation 31 (CD31) cells plays an important role in
inflammation, oxidative stress generation, cell differentiation,
angiogenesis and fibroblasts migration and might be closely related
with the skin wound healing process. Sumpio et al determined
that CD31 cells play an important role in the formation of tumor
blood vessels and its expression can accurately reflect the number
of tumor blood vessels (12).
Lidocaine directly regulates the molecular and cellular biology of
the tumor (11). It is widely
believed, that it can not only reduce the gene expression of
voltage gated sodium channels (VGSC), but it also inhibits the
migration and invasion of tumor cells in vivo. Moreover, it
can inhibit tumor growth and proliferation by demethylation of
deoxyribonucleic acid (DNA) or by mitogen-activated protein kinase
pathway (MAPK) (11). On the other
hand, a large number of studies have shown that lidocaine can
indirectly affect tumor prognosis by regulating the function of the
immune system (11).
This study investigated the effect of lidocaine by
CD31 cell modulation on would healing, skin temperature and the
infection incidence in vitro and in vivo.
Patients and methods
Patients
Sixty patients with malignant melanoma treated in
the Bishan Hospital from June 2015 to January 2017 were included in
the study. The mean age of the patients was 50±7.2 years. All the
patients included in the study were able to understand and use the
visual analogue scale (pain score), they did not have a history of
lidocaine allergy and they did not suffer from diabetes or immune
system diseases. Also, they presented normal heart, liver and
kidney function. The study was approved by the Ethics Committee of
Bishan Hospital (Bishan, China), and all the patients signed an
informed consent to participate in the study.
Inclusion criteria
The patients included qualified based on the
following criteria: The tumor was a first case surface tumor.
Primary tumor. Single lesion and tumor with a surface diameter (R)
R≤10 cm and level 1: malignant melanoma in situ, tumor
thickness ≤1.5 mm (13). No lymph
node or distal metastasis. Complete resection of tumor tissue,
i.e., the result of the first examination of frozen specimen report
after operation is malignant melanoma.
Preoperative procedures
The diameter of the tumors on the patient's body
surface was measured, recorded, and the resection area was
marked.
The effect was evaluated by comparing the sign and
symptom index before and after treatment: Curative effect index =
[(total integral before treatment - total integral after
treatment)/total integral before treatment] ×100. Grade of healing
was evaluated as following: class a healing: good healing and no
adverse reactions; class b healing: poor healing with inflammatory
reaction at the healing place, such as red swelling, hard knot,
hematoma and effusion, but not festering; class c healing: abscess
of the incision, which requires incision and drainage.
Surgery
Before the surgery, color doppler ultrasonography
and CT examination of superficial lymph nodes were performed to
diagnose the metastasis of the tumor and assist in determining the
tumor stage. The location and size of the tumor were determined
according to the diagnosis. Local anesthesia was used for the
operation and the supine position was selected for patients. The
range of resection was determined according to the preoperative
skin contrast examination and MRI. The melanoma was resected with
at least 2 cm lateral and deep surgical margins when possible or
including the deep fascia. Then the tension-free hernioplasty was
applicated to repair the wound, to prevent secondary deformity from
the wound, and maintain a good form for esthetic principles.
Postoperative wound intervention
The 60 patients included in the study were randomly
divided into 2 groups, lidocaine group and control group. The
lidocaine group received intradermal injection with 2% lidocaine
solution (approval no. H11020558; Beijing Yongkang Pharmaceutical
Co., Ltd., Beijing, China) in dose of 1.5 mg/kg and the control
group received the same quantity of 0.9% saline solution. The
lidocaine was diluted in 0.9% saline solution. The pain score
values, before and after the treatment, were recorded. After the
treatment debridement was performed in both groups.
Determination of CD4 T-cell percentage
in peripheral blood
From all the patients included in the study venous
blood was collected at 3 time-points: T0, before the surgery; T1, 4
h after the surgery; and T2, 24 h after the surgery. Lymphocyte CD4
T-cell percentage was determined using BD FACSCount analyzer (BD
Biosciences, Franklin Lakes, NJ, USA). Briefly, the reagent tubes
were brought to ambient temperature and vortexed upright for 10 sec
before using. For analysis, 50 µl of whole blood was added to the
CD4 reagent tube containing CD3/CD4 PE monoclonal antibodies (BD
Biosciences). The tube was incubated in the dark for 30 min at room
temperature and 50 µl of fixative (5% formaldehyde in PBS) was
added and vortexed before reading on Becton-Dickinson FACS machine
according to manufacturer's instructions using cell count
software.
Immunomagnetic separation to collect
CD31
The blood collected at 72 h after surgery from all
the patients was used for immunomagnetic separation of CD31 marked
immune cells. The sorted cells were added to complete cell culture
medium for endothelial cells (including EBM, 2 basal medium
enriched with recombinant human epidermal growth factor,
insulin-like growth factor, recombinant human fiber cell growth
factors, endothelial cell growth factor, fetal bovine serum,
ascorbic acid and heparin). The CD31+ cells suspended
into DMEM/F-12 medium containing 10% FBS were inoculated into a
6-well plate (2×103/cm2) containing collagen
of rat tail. The plates were incubated at 37°C, in 5%
CO2 and 95% humidity atmosphere. The culture medium was
replaced once every 2 days. The cell suspensions were then added to
24-well culture plate (0.75 ml in each well, corresponding to
3×105 cells). In the lidocaine group we used 1.5 mg/kg
2% lidocaine (0.75 ml solution included 2% lidocaine, 0.9% normal
saline and CD31 culture solution). In control group, 0.9% normal
saline solution (0.75 ml solution included 0.9% normal saline and
CD31 culture solution) was used. The cultures were incubated at
37°C in 5% CO2 for 48 h. The 24-well culture plate was
centrifuged (1,185 × g for 3 min), the supernatant was discarded,
and the CD31 expression was detected by flow cytometry according to
the method provided by the supplier.
Measurement of skin temperature
WMY-01 digital thermometer (Shanghai Xing Shuttle
Electronic Electric Appliance Co., Ltd., Shanghai, China) was used
to measure the body temperature from healthy side and surgical side
in each patient at room temperature of 19–22°C and 45–65%
humidity.
VAS pain score
Score from 0–10, score 0 point: No pain; score <3
points: Slight pain but the patient can tolerate it; score 4–6
points: The patient is in pain and the pain can affect sleep but it
still is tolerable; score 7–10 points: The pain of patient is a
growing pain and the pain is unbearable.
Statistical analysis
For statistical analysis SPSS software (version
20.0; IBM Corp., Armonk, NY, USA) was used. The data were expressed
as mean ± standard deviation (mean ± SD). For assessing the
differences between the groups, the Mann-Whitney U test was used
for numerical data and Chi-square test was used for categorical
data. P<0.05 was considered to indicate a statistically
significant difference.
Results
Recurrence of disease
All patients were followed up from 6 months to 2
years. The control group had 2 cases of local recurrence, 2 cases
of cervical lymph node metastasis, 1 case of death, and no
recurrence or metastasis. On the other hand, the lidocaine group
had 1 case of metastasis, 6 months after surgery (Table I).
 | Table I.Postoperation follow-up results. |
Table I.
Postoperation follow-up results.
| Groups | Metastases (6 months
after surgery) | Local recurrence (6
months to two years after surgery) | Cervical lymph node
metastasis (six months to two years after surgery) | Death (six months to
two years after surgery) |
|---|
| Lidocaine | 1 | 0 | 0 | 0 |
| Control | 0 | 2 | 2 | 1 |
| P-value | 0.25 | 0.76 | 0.76 | 0.25 |
Skin infection
In patients from lidocaine group no infection was
observed compared with the control group, one patient developed
infection after the surgery. The infection was caused by the
inappropriate protection of the wound and it was not associated
with the treatment (Table II).
 | Table II.Infection rate in the two groups. |
Table II.
Infection rate in the two groups.
| Groups | n | Infection |
|---|
| Lidocaine | 30 | 0 |
| Control | 30 | 1 |
| P-value |
| 0.25 |
Skin temperature
The difference in skin temperature between the
surgical side and healthy side in lidocaine group was significantly
decreased compared with the control group at every time-point. In
lidocaine group the difference between body temperature at surgery
side and body temperature at healthy side was low and this
difference decreased in time. In control group the trend was
different, increasing in the first 10 h, then slightly decreased
and continued to increase with time (Table III).
 | Table III.The difference in body temperature
between surgical side and healthy side at different times after the
surgery in the two groups. |
Table III.
The difference in body temperature
between surgical side and healthy side at different times after the
surgery in the two groups.
|
|
| Difference in body
temperature between surgical side and healthy side at different
times after surgery (°C) |
|---|
|
|
|
|
|---|
| Groups | No. of
patients/group | 4 h | 6 h | 8 h | 10 h | 12 h | 24 h | 48 h |
|---|
| Control | 30 | 2.14±0.2 | 3.12±0.74 | 3.7±0.5 | 3.1±0.1 | 2.1±0.5 | 2.74±0.2 | 2.79±0.3 |
| Lidocaine | 30 | 1.8±0.1 | 1.3±0.2 | 1.1±0.7 | 1.0±0.1 | 1.1±0.4 | 0.8±0.2 | 0.6±0.4 |
| T-value |
| 7.12 | 7.85 | 9.12 | 12.13 | 10.23 | 12.35 | 14.32 |
| P-value |
| 0.041 | 0.121 | 0.001 | 0.001 | 0.012 | 0.001 | 0.015 |
Pain score
The VAS pain score was significantly higher in
control group compared with lidocaine group in all time-points.
Regarding the VAS score variation between the time-points after the
surgery showed an increase at 4, 8 and 12 h after the surgery,
followed by a decrease at 24 h after surgery for the control group
and after 48 h from surgery for lidocaine group (Table IV).
 | Table IV.VSA pain scores at different times
after the surgery in the two groups. |
Table IV.
VSA pain scores at different times
after the surgery in the two groups.
|
|
| VAS pain score at
different times after surgery |
|---|
|
|
|
|
|---|
| Groups | No. of
patients/group | 4 h | 8 h | 12 h | 24 h | 48 h |
|---|
| Control | 30 | 1.84±0.52 | 4.12±0.74 | 7.17±1.5 | 5.74±0.2 | 5.79±1.3 |
| Lidocaine | 30 | 0.8±0.59 | 1.71±0.21 | 2.1±0.7 | 2.8±0.2 | 2.6±0.4 |
| T-value |
| 4.12 | 9.85 | 12.12 | 12.45 | 13.32 |
| P-value |
| 0.043 | 0.001 | 0.001 | 0.001 | 0.001 |
Adverse drug reaction
Regarding the incidence of adverse reactions to the
treatment, no drug allergic reaction was observed in either
group.
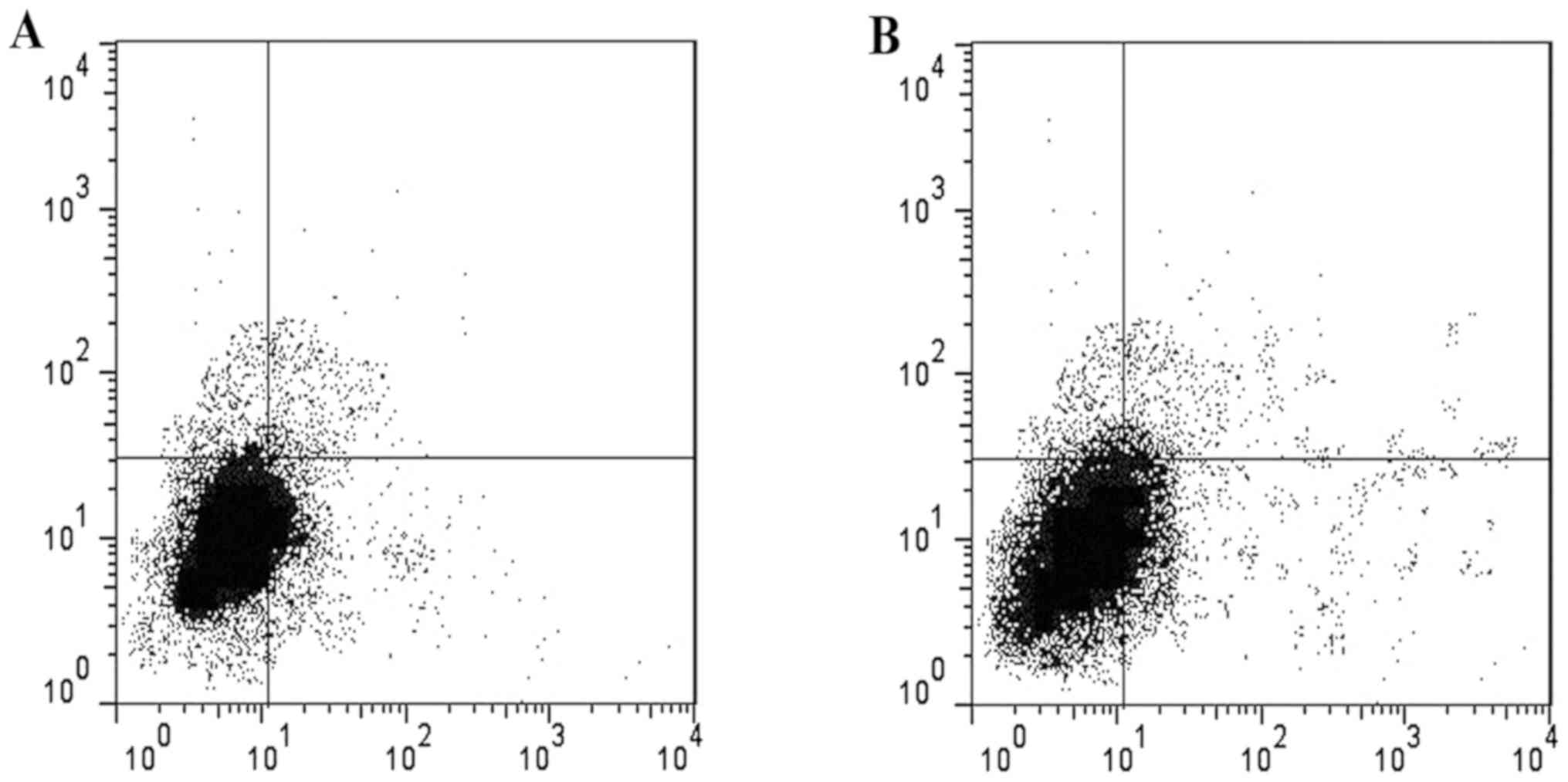
In vitro experiment on cell culture
for CD31 cell induction
After induction of CD31 cells in the two groups,
their levels were significantly increased in lidocaine group
compared to control group. The ratio of CD31 in lidocaine group was
45.54±0.03% and the ratio of CD31 in the control group was
28.37±0.02% (P<0.05) (Fig.
1).
Wound healing time
The wound healing time in the experimental group was
4–7 days according to the clinical examination records. The control
group healing time averaged from 6 to 10 days. According to the
value distribution, the treatment time was not normally
distributed, so the rank-sum test was adopted in both groups. The
difference between the two groups was statistically significant,
and the wound healing time in the experimental group was
significantly shorter than that in the control group (Table V).
 | Table V.Wound healing time in the two
groups. |
Table V.
Wound healing time in the two
groups.
| Groups | No. of
patients/group | Wound healing time
(day) |
|---|
| Lidocaine | 30 | 7.61±2.30 |
| Control | 30 | 10.93±2.29 |
| Z-value |
| 2.92 |
| P-value |
| 0.003 |
CD4 T-cell percentage in peripheral
blood
At T0 before the surgery there were no differences
in the CD4 T-cell percentage in the two groups. We observed that in
both groups the percentage of CD4 T-cell decreased after surgery.
In comparison with the control group, in lidocaine group the CD4
T-cell percentage was significantly increased at T1 and T2
(P<0.05) (Table VI).
 | Table VI.Percentages of CD4 T-cells in
peripheral blood leucocytes in the groups at different
time-points. |
Table VI.
Percentages of CD4 T-cells in
peripheral blood leucocytes in the groups at different
time-points.
|
|
| Percentages of
lymphocytes |
|---|
|
|
|
|
|---|
| Groups | No. of
patients/group | T0 | T1 | T2 |
|---|
| Control | 30 | 30.859±9.690% | 15.665±8.102% | 7.482±3.936% |
| Lidocaine | 30 | 30.215±9.871% | 20.204±9.886% | 13.111±7.365% |
| Z-value |
| 1.12 | 3.14 | 3.21 |
| P-value |
| 0.25 | 0.005 | 0.001 |
Discussion
According to bibliographic data, the incidence of
melanoma in China is lower than in Europe, with different clinical
features and prognosis (11).
Studies have found that in domestic settings the ratio between
men's and women's incidence ratio is 0.87:1 in China, in Brazil
1:1.4 and in Scotland 1:1.6 in 50 patients with boundary (12). In this study, patients were divided
into two groups and it was shown that the difference of average
survival time in melanoma patients treated with lidocaine was not
significant.
Lidocaine is a type Ib anti-arrhythmic agent and
sodium channel antagonist commonly utilized in the cardiac and pain
conditions (14). Via its sodium
channel blocking the neural conduction is reduced and impeded,
leading to its anti-arrhythmic and anesthetic properties. Unlike
other sodium channel blocking AEDs, such as phenytoin (also a class
Ib anti-arrhythmic), its structure includes an aromatic and amine
chain motif allowing the binding to the sodium channel via both the
channels pore-lining phenyl binding site, or via the external amine
chain site, both of which lead to the reduction of ion transport
across the cellular membrane. Lidocaine hydrochloride has the
advantages of quick action, safety, strong penetration, and
possibility of repeatable use. Lidocaine can more rapidly reduce
harmful stimulation of peripheral nerve excitability, alleviate
nerve dysfunction, achieve the goal of relieving itching, improve
local blood flow into the body's normal regulating function
recovery (15,16).
Pain is the fifth most important inducer of changes
in body temperature, pulse, breathing and blood pressure. In this
study, except for physical factors, pain may be caused by related
psychological stress, such as the fear of disease recurrence, speed
of skin recovery, spouse attitude and worries about the children.
In order to improve postoperative quality of life, painless
treatment is advocated clinically. According to relevant
literature, the appropriate method for anesthesia management is
direct application to the wound as surface anesthesia (17). Similar to previous studies, this
study used lidocaine directly in post-operative skin monitoring and
found that it can reduce the pain of debridement. Lidocaine can
effectively treat a wide range of wound pain, depending on the
scope and drug safety criteria. In clinical practice, medical staff
can calculate the dosage based according to the size and area of
the wound, and accounting for the safe range of medication. Zhou
et al pointed out that CD31 expression level in tumor cells
could be used as an indicator to monitor the disease progression
and tumor recurrence risk (18).
Recently, some researchers tried to add lidocaine to MCF-7 breast
cancer cells and then added normal breast epithelium MCF-10, to
intervene. They observed the cell vitality by immunofluorescence
staining, DNA fragments and WB analysis, and the results showed
that lidocaine can inhibit cancer cell activity (19). Interestingly, in the process of cell
induction, we found that lidocaine interfered with CD31 cells by
stimulating the proliferation and apoptosis. Therefore, we
hypothesized that the application of lidocaine on the wound could
have some effect on the proliferation of tumor cells.
The lymphocytes have significant influence on the
postoperative recovery of patients with melanoma, and a good
prognosis is correlated with the content of intratumoral
inflammatory infiltrate and the levels of circulatory immune cells
(20,21). Previous studies have shown that
surgical stress can inhibit lymphocyte proliferation and accelerate
apoptosis, leading to lower lymphocyte numbers in the blood
circulation (22,23). Studies showed that lidocaine through
its anti-inflammatory effects regulation of HPA axis, affect the
inflammatory changes that are immune-induced, further affecting
lymphocyte proliferation and apoptosis, thus reducing the
postoperative immunosuppressive state (11). At T1 and T2 time-points in both
groups of patients the proportions of lymphocytes were lower than
at T0 time-point (P<0.05). These results are consistent with
previous research conclusions, that lidocaine reduce the healing
time. Some researchers found that lidocaine through its
anti-inflammatory effects on regulation of HPA axis can improve the
perioperative activity of lymphocytes, which is confirmed by our
study in which we showed an increase of the number of
CD4+ lymphocytes at T2. However, the molecular mechanism
of interaction between lidocaine and the lymphocyte is not yet
fully understood, and the relevant separation mechanism needs to be
investigated.
Skin temperature, skin tone, tension and capillary
recirculation time are the main indications of blood circulation in
the body, and skin temperature is the only objective indicator from
these indications.
In conclusion, the local application of lidocaine
can promote wound healing to a certain extent, reduce pain, and
promote postoperative skin reconstruction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KX and YW contributed to the conception and design
of the study. JW and LY were responsible for the collection and
assembly of data. LW completed data analysis and interpretation. KX
and YW contributed to writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Bishan Hospital (Bishan, China), and all the patients signed an
informed consent to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Felcht M and Thomas M: Angiogenesis in
malignant melanoma. J Dtsch Dermatol Ges. 13:125–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zurac S, Neagu M, Constantin C, Cioplea M,
Nedelcu R, Bastian A, Popp C, Nichita L, Andrei R, Tebeica T, et
al: Variations in the expression of TIMP1, TIMP2 and TIMP3 in
cutaneous melanoma with regression and their possible function as
prognostic predictors. Oncol Lett. 11:3354–3360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruszkiewicz JA, Pinkas A, Ferrer B, Peres
TV, Tsatsakis A and Aschner M: Neurotoxic effect of active
ingredients in sunscreen products, a contemporary review. Toxicol
Rep. 4:245–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boda D, Docea AO, Calina D, Ilie MA,
Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE,
Voiculescu V, et al: Human papilloma virus: Apprehending the link
with carcinogenesis and unveiling new research avenues (Review).
Int J Oncol. 52:637–655. 2018.PubMed/NCBI
|
|
5
|
Danciu C, Oprean C, Coricovac DE, Andreea
C, Cimpean A, Radeke H, Soica C and Dehelean C: Behaviour of four
different B16 murine melanoma cell sublines: C57BL/6J skin. Int J
Exp Pathol. 96:73–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma Y, Gui Q and Lang S: Intracranial
malignant melanoma: A report of 7 cases. Oncol Lett. 10:2171–2175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caunii A, Oprean C, Cristea M, Ivan A,
Danciu C, Tatu C, Paunescu V, Marti D, Tzanakakis G, Spandidos DA,
et al: Effects of ursolic and oleanolic on SK-MEL-2 melanoma cells:
In vitro and in vivo assays. Int J Oncol.
51:1651–1660. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SH, Tsatsakis AM, Tzanakakis G, Kim
HS, Le B, Sifaki M, Spandidos DA, Tsukamoto C, Golokhvast KS,
Izotov BN, et al: Soyasaponin Ag inhibits α-MSH-induced
melanogenesis in B16F10 melanoma cells via the downregulation of
TRP-2. Int J Mol Med. 40:631–636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chalkiadaki G, Nikitovic D, Katonis P,
Berdiaki A, Tsatsakis A, Kotsikogianni I, Karamanos NK and
Tzanakakis GN: Low molecular weight heparin inhibits melanoma cell
adhesion and migration through a PKCa/JNK signaling pathway
inducing actin cytoskeleton changes. Cancer Lett. 312:235–244.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sevastre B, Sárpataki O, Stan RL, Taulescu
M, Sevastre-Berghian AC, Olah NK, Furtuna F, Hanganu D, Hangan AC,
Cenariu M, et al: Anticancer activity of Euonymus europaeus
fruits extract on human melanoma cells. Farmacia. 65:56–62.
2017.
|
|
11
|
Chamaraux-Tran TN and Piegeler T: The
amide local anesthetic lidocaine in cancer surgery - Potential
antimetastatic effects and preservation of immune cell function? A
Narrative Review. Front Med (Lausanne). 4:2352017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumpio BE, Yun S, Cordova AC, Haga M,
Zhang J, Koh Y and Madri JA: MAPKs (ERK1/2, p38) and AKT can be
phosphorylated by shear stress independently of platelet
endothelial cell adhesion molecule-1 (CD31) in vascular endothelial
cells. J Biol Chem. 280:11185–11191. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keohane SG, Proby CM, Newlands C, Motley
RJ, Nasr I, Mohd Mustapa MF and Slater DN; British Association of
Dermatologists (Squamous Basal Cell Carcinoma Guideline Development
Groups); Royal College of Pathologists (Skin Cancer Lead), : The
new 8th edition of TNM staging and its implications for skin
cancer: a review by the British Association of Dermatologists and
the Royal College of Pathologists, U.K. Br J Dermatol. 179:824–828.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hamano S, Sugiyama N, Yamashita S, Tanaka
M, Hayakawa M, Minamitani M, Yoshinari S and Eto Y: Intravenous
lidocaine for status epilepticus during childhood. Dev Med Child
Neurol. 48:220–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang YC, Huang CS and Kuo CC: Lidocaine,
carbamazepine, and imipramine have partially overlapping binding
sites and additive inhibitory effect on neuronal Na+
channels. Anesthesiology. 113:160–174. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aggarwal P and Wali JP: Lidocaine in
refractory status epilepticus: A forgotten drug in the emergency
department. Am J Emerg Med. 11:243–244. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HJ, Cho YJ, Gong HS, Rhee SH, Park HS
and Baek GH: The effect of buffered lidocaine in local anesthesia:
A prospective, randomized, double-blind study. J Hand Surg Am.
38:971–975. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Peng G, Song Y, Zhang C and Wang
J: Expression of CD31 in the serum of hepatocellular carcinoma
patients and the effects of cancer cell migration and
proliferation. China J Mod Med. 31:5–9. 2015.
|
|
19
|
Chamaraux-Tran TN, Mathelin C, Aprahamian
M, Joshi GP, Tomasetto C, Diemunsch P and Akladios C: Antitumor
effects of lidocaine on human breast cancer cells: An in vitro and
in vivo experimental trial. Anticancer Res. 38:95–105.
2018.PubMed/NCBI
|
|
20
|
Neagu M, Constantin C and Tanase C:
Immune-related biomarkers for diagnosis/prognosis and therapy
monitoring of cutaneous melanoma. Expert Rev Mol Diagn. 10:897–919.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neagu M, Constantin C and Zurac S: Immune
parameters in the prognosis and therapy monitoring of cutaneous
melanoma patients: Experience, role, and limitations. Biomed Res
Int. 2013:1079402013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colucci R and Moretti S: The role of
stress and beta-adrenergic system in melanoma: Current knowledge
and possible therapeutic options. J Cancer Res Clin Oncol.
142:1021–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Bucur). 10:545–558.
2014. View Article : Google Scholar
|