Introduction
Gastric cancer (GC) is one of the most common human
cancer types, and the second leading cause of cancer-associated
death worldwide, particularly in East Asia (1,2).
Although great effort has been made to improve its treatment, GC
remains difficult to cure, mainly due to most GC patients
presenting with advanced disease and/or metastasis at the
time-point of diagnosis (3,4). Therefore, the elucidation of the exact
mechanisms underlying GC growth and metastasis is urgently
required.
Long non-coding RNAs (lncRNAs), a class of
non-coding RNAs comprising >200 nucleotides, may exert their
functions through sponging their target microRNAs (miRs), mRNAs or
proteins, and affecting their expression (5–7). In the
last decade, accumulating evidence has indicated that lncRNAs have
important roles in physiological and pathological processes
(8–10). Furthermore, the deregulation of
lncRNAs has been implicated in human cancers, including GC
(11,12). For instance, the lncRNA X inactive
specific transcript (XIST) was reported to be significantly
upregulated in GC cells, and to promote GC progression through
transforming growth factor-β1 via targeting miR-185 (12). In addition, the lncRNA nuclear
paraspecle assembly transcript was indicated to regulate GC
development through modulating the expression of miR-506 and signal
transducer and activator of transcription 3 (13). In addition, the lncRNA small
nucleolar RNA host gene (SNHG)20 promotes GC progression by
inhibiting p21 expression and regulating the glycogen synthase
kinase (GSK)-3β/β-catenin signaling pathway (11).
The lncRNA SNHG12 is frequently upregulated in
several common human cancer types and promotes tumorigenesis by
acting as a sponge for certain miRs (14,15). For
instance, SNHG12 is significantly upregulated in osteosarcoma
tissues and cell lines, and promotes osteosarcoma cell
proliferation, invasion and migration through increasing the
expression of angiomotin, as well as Notch2 by sponging miR-195-5p
(14,15). Wang et al (16) reported that SNHG12 promotes
colorectal cancer cell growth and inhibits cell apoptosis. In
addition, C-Myc-induced upregulation of SNHG12 enhanced the
proliferation, apoptosis and migration of triple-negative breast
cancer cells (17). In addition,
upregulation of SNHG12 was identified to contribute to cervical
cancer cell proliferation and invasion by acting as a sponge for
miR-424-5p (18). Recently, Zhang
and Lu (19) reported that SNHG12
has a promoting role in GC by acting as a molecular sponge for
miR-320. However, whether SNHG12 also interacts with other miRs in
GC cells still remains to be elucidated. Therefore, the present
study aimed to explore the regulatory mechanisms of SNHG12
underlying GC cell proliferation and migration.
Materials and methods
Tissue samples
The present study was approved by the Medical Ethics
Committee of Haikou People's Hospital (Haikou, China) and complied
with the Declaration of Helsinki. A total of 56 primary GC tissues
as well as their matched adjacent non-tumor tissues were obtained
from GC patients treated at Haikou People's Hospital (Haikou,
China) between May 2011 and May 2013. The patients included 34
males and 22 females, between 38–77 years old with mean age of 65.6
years old. These GC patients did not receive any radiotherapy or
chemotherapy prior to surgical resection. Written informed consent
had been obtained from all patients. The tissues were frozen in
liquid nitrogen immediately after surgical resection and stored at
−80°C until use.
Cell culture
The AGS, HGC27, BGC823 and SGC7901 human GC cell
lines and the GES-1 normal gastric mucosa epithelial cell line and
were obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) with 10% fetal bovine serum (FBS; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere with 5% CO2.
The cells were harvested during the logarithmic growth phase for
use in the subsequent experiments.
Cell transfection
BGC823 and HGC27 cells were transfected with two
SNHG12 small interfering (si)RNAs that have different targets (100
µM; cat. nos. AM16708 and 1299001), negative control (NC) siRNA
(cat. no. 4457289; all Thermo Fisher Scientific, Inc.),
pcDNA-SNHG12 expression plasmid (cat. no. E2425; Hunan Nanhua Aishi
Pulin Biotechnology; NanHua Bio-medicine Co., Ltd., Changsha,
China), a pcDNA3.1 vector (cat. no. V79020), or were co-transfected
with SNHG12 siRNA and miR-16 inhibitor (cat. no. 4464084) or SNHG12
siRNA and NC inhibitor (cat. no. AM17010) using Lipofectamine 2000
(all Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. At 48 h after transfection, the cells were
used for the subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to extract total RNA from tissues and cells. For detection of
SNHG12 expression, 2 µg total RNA was used to synthesize
complementary DNA using SuperScript III Reverse Transcriptase
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. qPCR was then performed using Fast SYBR™ Green
Master Mix (cat. no. 4385610; Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. For
detection of miR-16 expression, the Mir-X™ miRNA qRT-PCR
SYBR® kit (Clontech Laboratories, Inc., Mountainview,
CA, USA) was applied for RT-qPCR according to the manufacturer's
protocol. GAPDH and U6 were used as internal references. The
reaction conditions were 95°C for 3 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 30 sec. The relative expression was
analyzed using the 2−ΔΔCq method (20).
Cell Counting Kit (CKK)-8 assay
BGC823 and HGC27 cells were re-suspended with DMEM
and placed into 96-well plates (5,000 cells in 200 µl per well).
After incubation at 37°C for 0, 24, 48 or 72 h, 10 µl CCK-8 reagent
(Beyotime Institute of Biotechnology, Haimen, China) was added to
each well. After incubation at 37°C for 2 h, the absorbance at 450
nm was quantitated using a Synergy™ LX Multi-Mode
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Cell migration assay
A wound healing assay was used to assess cell
migration. In brief, transfected BGC823 and HGC27 cells (500,000
cells per well) were seeded into 6-well plates and cultured for 24
h. A wound was scratched in the cell monolayer using a 200-µl
pipette tip (cat. no. 94052320; Thermo Fisher Scientific, Inc.).
Cells were washed with Dulbecco's PBS (Thermo Fisher Scientific,
Inc.) and DMEM was then added to the 6-well plates. Images of the
scraped area were captured at 0 and 24 h using an inverted
microscope (Olympus, Tokyo, Japan).
Luciferase reporter gene assay
A bioinformatics analysis was performed to determine
potential target miRs of SNHG12 by using an online prediction tool
(Starbase version 1.0; http://starbase.sysu.edu.cn/mirLncRNA.php). To
construct a luciferase reporter vector, the 3′untranslated region
(UTR) fragment of SNHG12 containing the putative binding site for
miR-16 was amplified by PCR, which was then inserted into the
multiple cloning region located downstream of the Renilla
translational stop codon in the psi-CHECK2 Luciferase reporter
vector (Promega Corp., Madison, WI, USA), named as wild-type (WT)
SNHG12 3UTR. In addition, the mutant (MT) 3UTR fragment of SNHG12
without the putative binding sites for miR-16 was generated, which
was also inserted into the multiple cloning region of psi-CHECK2
Luciferase vector, and named as MT SNHG12 3UTR. BGC823 and HGC27
cells were co-transfected with 0.5 µg WT SNHG12 3UTR or MT SNHG12
3UTR and miR-16 mimics or miR-NC using Lipofectamine 2000 according
to the manufacturer's protocol. At 48 h after transfection, a
Dual-luciferase Reporter Assay kit (cat. no. E1910; Promega Corp.)
was used to examine the luciferase activity according to the
manufacturer's protocol.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Student's t-test was used for analyzing the difference
between two groups. For comparison of more than two groups, one-way
analysis of variance was used followed by Tukey's post-hoc test.
The correlation between the SNHG12 and miR-16 expression in GC
tissues was analyzed using Pearson's correlation analysis.
Kaplan-Meier analysis with a log-rank test was performed to assess
patient survival. The chi-square test was employed to analyze the
associations between SNHG12 expression and clinicopathological
characteristics of GC patients. GraphPad Prism 6.0 software
(GraphPad Software Inc., La Jolla, CA, USA) was used to perform
statistical analyses. P<0.05 was considered to indicate
statistical significance.
Results
Upregulation of SNHG12 is associated
with GC progression
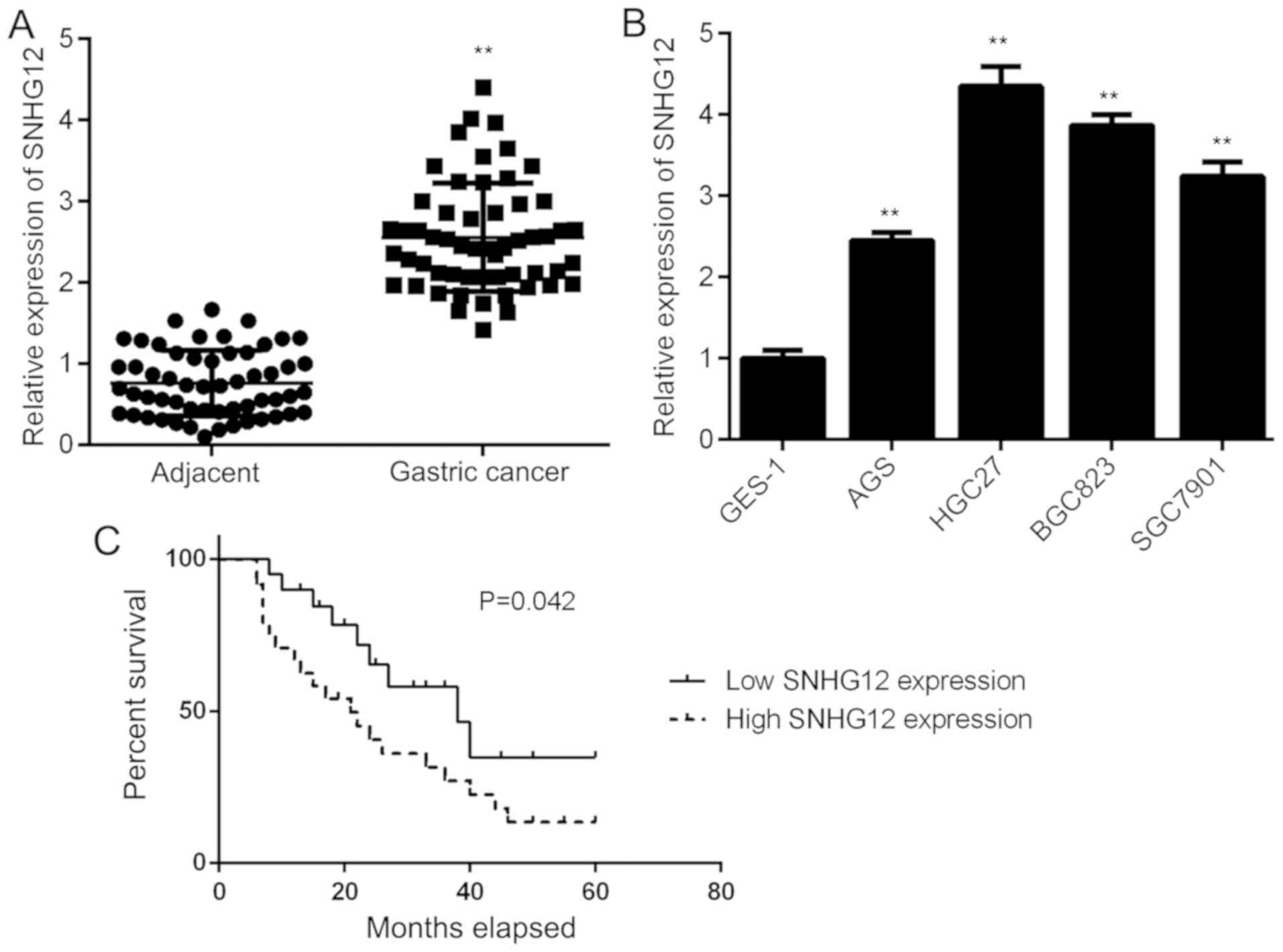
First, the expression of SNHG12 in GC tissues and
their matched adjacent normal tissues was examined by using
RT-qPCR. As shown in Fig. 1A, the
expression of SNHG12 was significantly higher in GC tissues when
compared to the adjacent tissues. Consistently, SNHG12 was also
upregulated in GC cell lines, including AGS, BGC823 HGC27 and
SGC7901, when compared with that in the normal gastric mucosa
epithelial cell line GES-1 (Fig.
1B). Thus, SNHG12 was generally upregulated in GC.
Subsequently, the clinical significance of SNHG12 expression in GC
was assessed. The GC patients were divided into a high and a low
SNHG12 expression group, based on its mean expression value in GC
tissues. The results suggested that high SNHG12 expression was
significantly associated with a larger tumor size, advanced
clinical stage and lymph node metastasis (Table I). Furthermore, those patients with
high SNHG12 expression had a shorter survival time when compared
with those with low SNHG12 expression (Fig. 1C). Therefore, upregulation of SNHG12
is associated with GC progression.
 | Table I.Association between SNHG12 expression
and clinicopathological characteristics of gastric cancer
patients. |
Table I.
Association between SNHG12 expression
and clinicopathological characteristics of gastric cancer
patients.
|
|
| SNHG12 levels |
|
|---|
|
|
|
|
|
|---|
| Parameter | Cases (n=56) | Low (n=31) | High (n=25) | P-value |
|---|
| Age (years) |
|
|
| 1.000 |
| ≤60 | 19 | 11 | 8 |
|
|
>60 | 37 | 20 | 17 |
|
| Sex |
|
|
| 0.278 |
|
Male | 34 | 21 | 13 |
|
|
Female | 22 | 10 | 12 |
|
| Tumor size
(cm) |
|
|
| 0.035 |
| ≤5 | 27 | 19 | 8 |
|
|
>5 | 29 | 12 | 17 |
|
|
Differentiation |
|
|
| 0.057 |
| Well
and moderately | 33 | 22 | 11 |
|
|
Poor | 23 | 9 | 14 |
|
| Node
metastasis |
|
|
| 0.006 |
|
Present | 48 | 23 | 25 |
|
|
Absent | 8 | 8 | 0 |
|
| Clinical stage |
|
|
| 0.045 |
|
I–II | 17 | 13 | 4 |
|
|
III–IV | 39 | 18 | 21 |
|
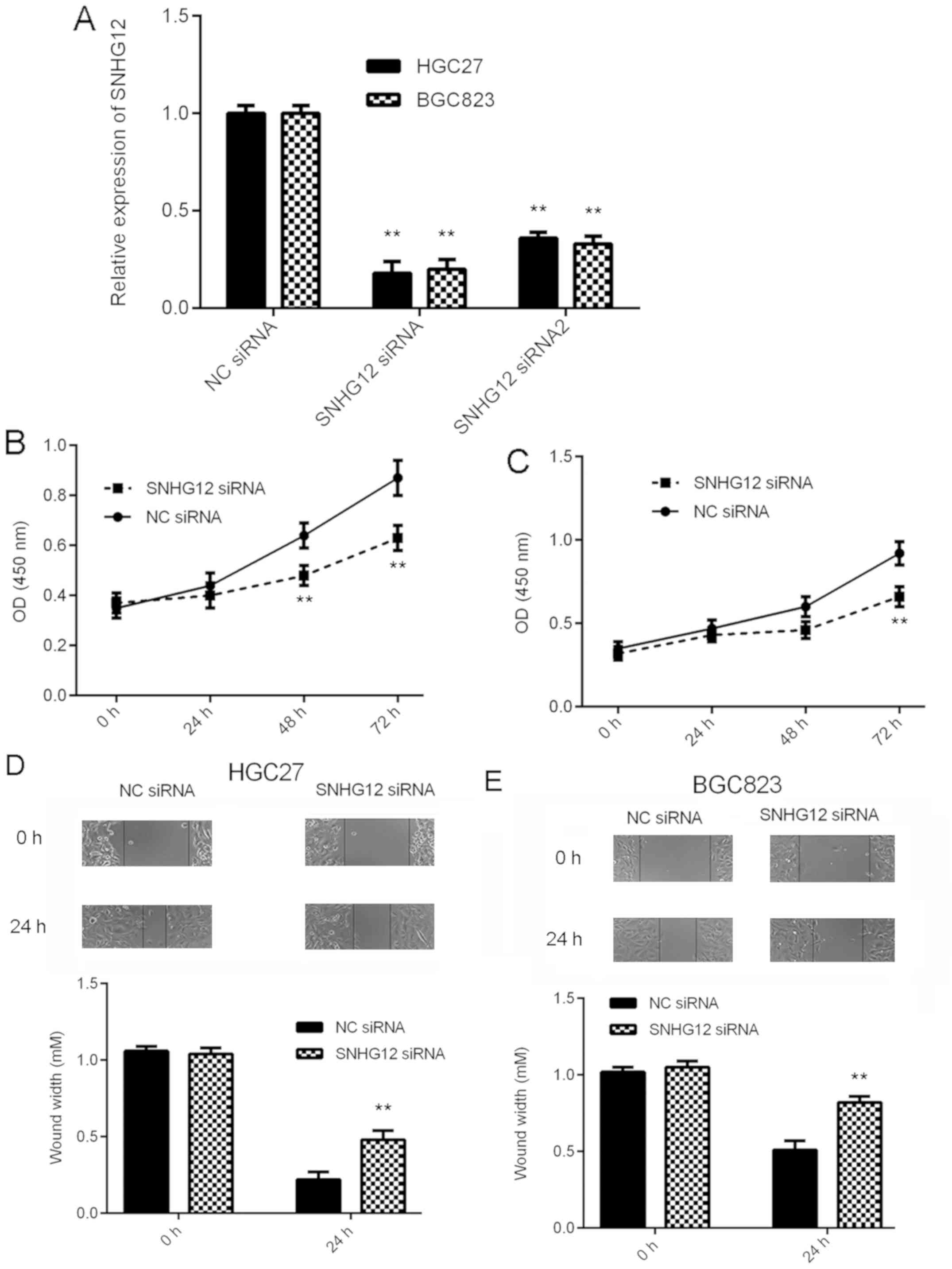
Knockdown of SNHG12 inhibits GC cell
proliferation and migration
As SNHG12 was markedly upregulated in GC, the
effects of SNHG12 downregulation on the behavior of GC cells were
then assessed in vitro. BGC823 and HGC27 cells were selected
for the subsequent experiments, as these cell lines had the highest
expression levels of SNHG12. Two SNHG12 siRNAs were used to knock
down the expression of SNHG12 in GC cells. As presented in Fig. 2A, transfection with SNHG12 siRNA and
siRNA2 significantly reduced the SNHG12 expression levels in BGC823
and HGC27 cells, when compared with those in the NC siRNA group.
SNHG12 siRNA was then selected for the subsequent experiments, as
it demonstrated a better knockdown efficiency. A CKK-8 assay and a
wound healing assay then indicated that knockdown of SNHG12
significantly reduced the proliferation and migration,
respectively, of BGC823 and HGC27 cells (Fig. 2B-E). Based on these results, SNHG12
may have a promoting role in GC growth and metastasis.
SNHG12 directly targets miR-16 in GC
cells
A Bioinformatics analysis was performed to determine
potential target miRs of SNHG12 by using an online prediction tool.
As presented in Fig. 3A, miR-16 was
predicted to be a potential target of SNHG12. To verify this
prediction, the luciferase reporter plasmids containing the WT and
MT miR-16 binding sites in SNHG12 were generated (Fig. 3A). GC cells were first transfected
with miR-16 mimics or miR-NC mimics, and it was confirmed that
after transfection, the miR-16 levels were significantly
upregulated in the miR-16 group compared with those in the miR-NC
group (Fig. 3B). Subsequently, a
luciferase reporter gene assay was performed to verify the
targeting association between miR-16 and SNHG12. The results
indicated that overexpression of miR-16 markedly inhibited the
luciferase activity of WT SNHG12 in BGC823 and HGC27 cells, but had
no effect on the luciferase activity of MT SNHG12 (Fig. 3C and D), suggesting that SNHG12
directly targets miR-16 in GC cells. The effects of SNHG12 on the
expression of miR-16 in GC cells was then assessed. As presented in
Fig. 3E, downregulation of SNHG12
significantly increased the miR-16 expression in BGC823 and HGC27
cells, suggesting that SNHG12 negatively regulates the expression
of miR-16 in GC cells. To further confirm these results, BGC823 and
HGC27 cells were transfected with SNHG12 expression plasmid to
increase its expression. As presented in Fig. 3F, transfection with SNHG12 expression
plasmid significantly enhanced its expression in GC cells, when
compared with that in the blank group. Indeed, upregulation of
SNHG12 led to a significant reduction in the expression of miR-16
in GC cells (Fig. 3G). Thus, SNHG12
negatively regulates the miR-16 expression in GC cells.
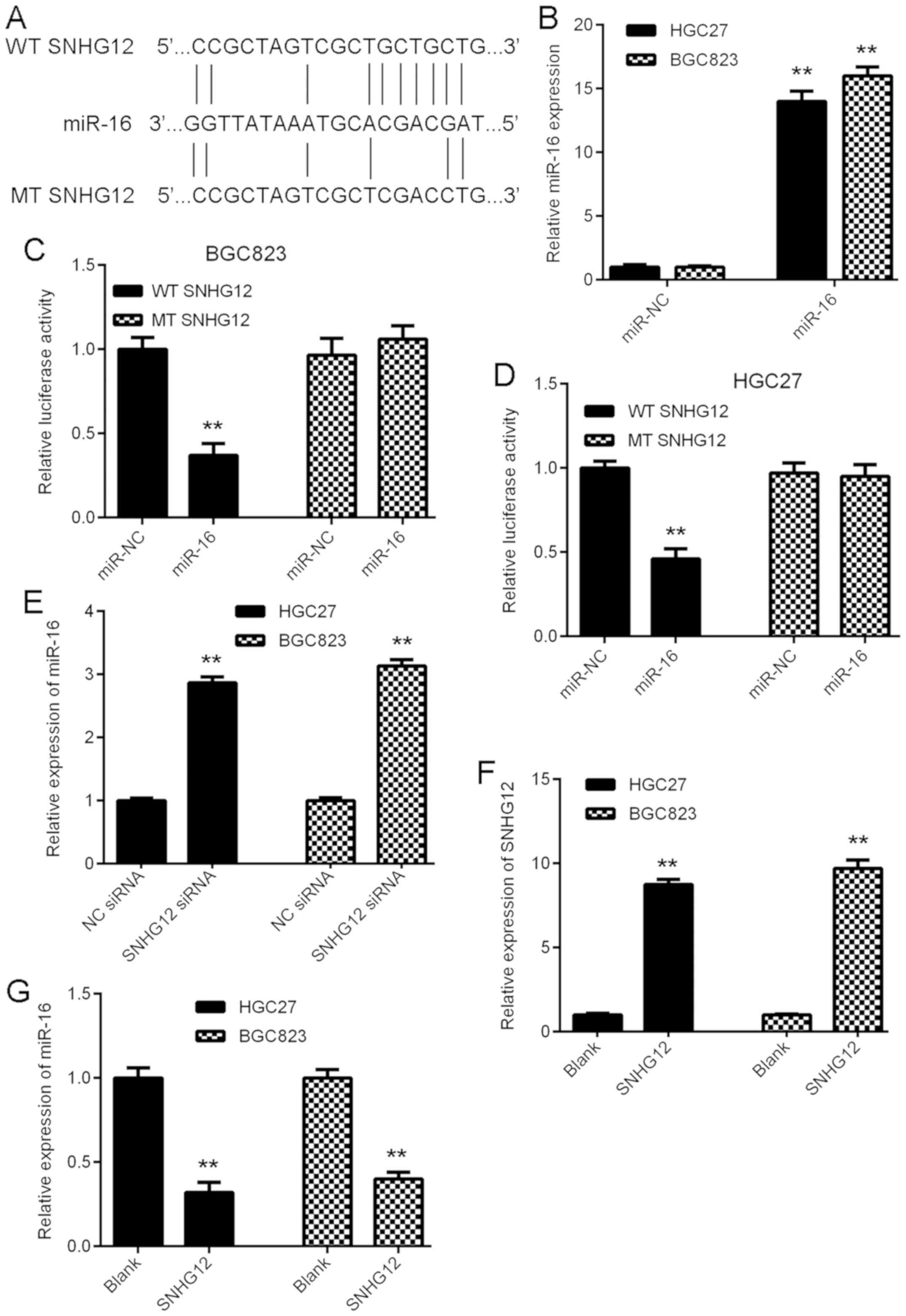 | Figure 3.SNHG12 directly targets miR-16 in GC
cells. (A) miR-16 was predicted as a potential target of SNHG12,
and luciferase reporter plasmids containing the WT and MT miR-16
binding sites in SNHG12 were generated. (B) RT-qPCR analysis was
performed to examine the miR-16 expression in GC cells after
transfection with miR-NC or miR-16 mimics. (C and D) The luciferase
reporter gene assay indicated that miR-16 mimics markedly inhibited
the luciferase activity of the reporter plasmid containing WT
SNHG12 in BGC823 and HGC27 cells, but had no effect on the
luciferase activity of the reporter plasmid containing MT SNHG12.
(E) Transfection with SNHG12 siRNA caused a significant
upregulation of the expression of miR-16 in BGC823 and HGC27 cells.
(F and G) BGC823 and HGC27 cells were transfected with SNHG12
expression plasmid or blank vector. RT-qPCR was performed to
examine the expression of (F) SNHG12 and (G) miR-16. **P<0.01
vs. miR-NC, NC siRNA or Blank. SNHG12, small nucleolar RNA host
gene 12; WT, wild-type; MT, mutated type; miR, microRNA; siRNA,
small interfering RNA; NC, negative control; GC, gastric cancer;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
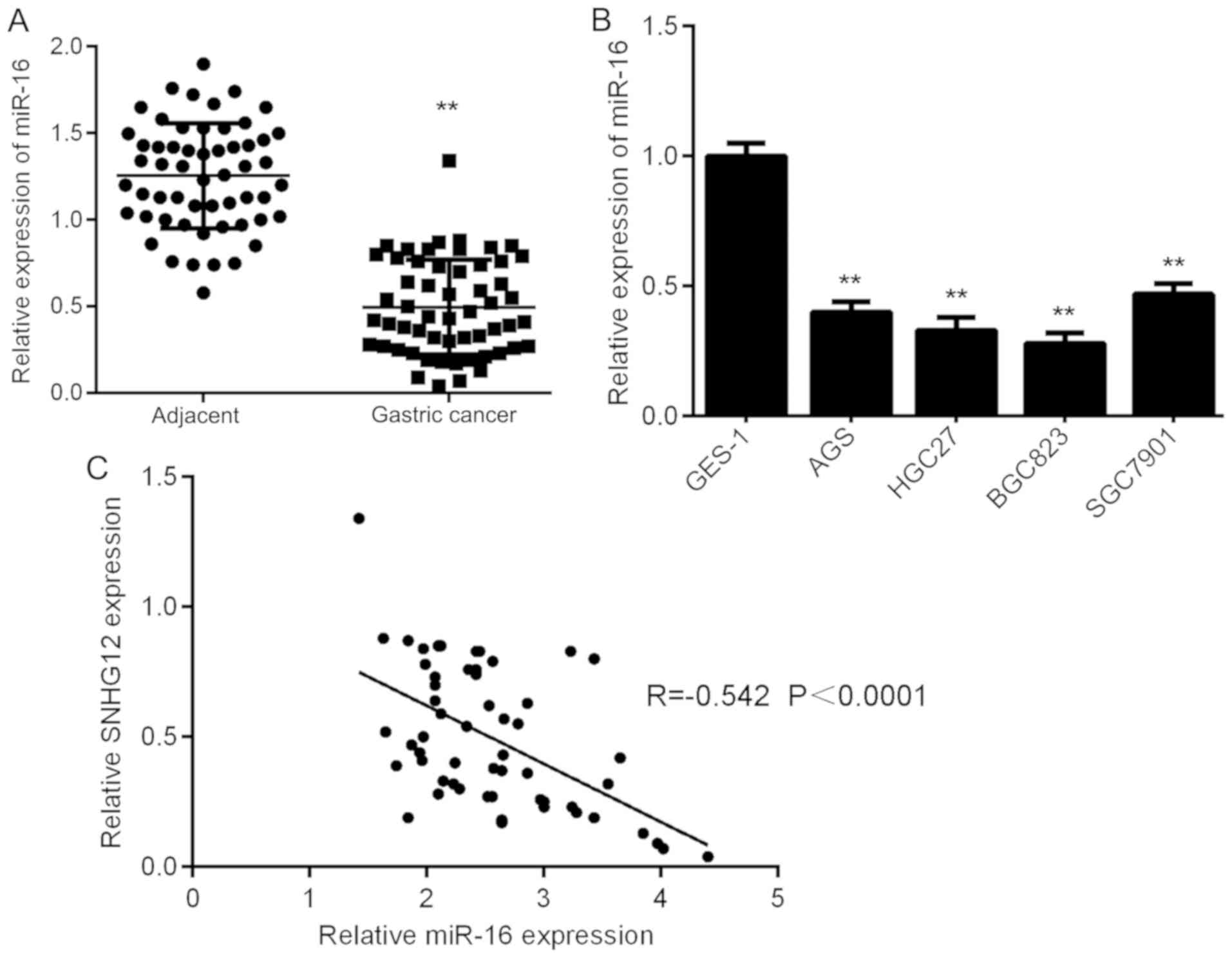
miR-16 is downregulated in GC tissues
and cell lines
The expression of miR-16 in GC tissues and cell
lines was then assessed using RT-qPCR. The results indicated that
miR-16 was markedly downregulated in GC tissues compared with that
in adjacent non-tumor tissues (Fig.
4A). In addition, it was also downregulated in GC cell lines
compared with that in GES-1 cells (Fig.
4B). Therefore, miR-16 is downregulated in GC. Subsequently, a
Pearson correlation analysis was performed to determine the
correlation between SNHG12 and miR-16 expression in GC tissues. As
provided in Fig. 4C, an inverse
association was identified between SNHG12 and miR-16 expression in
GC tissues, suggesting that upregulation of SNHG12 may contribute
to the downregulation of miR-16 in GC tissues.
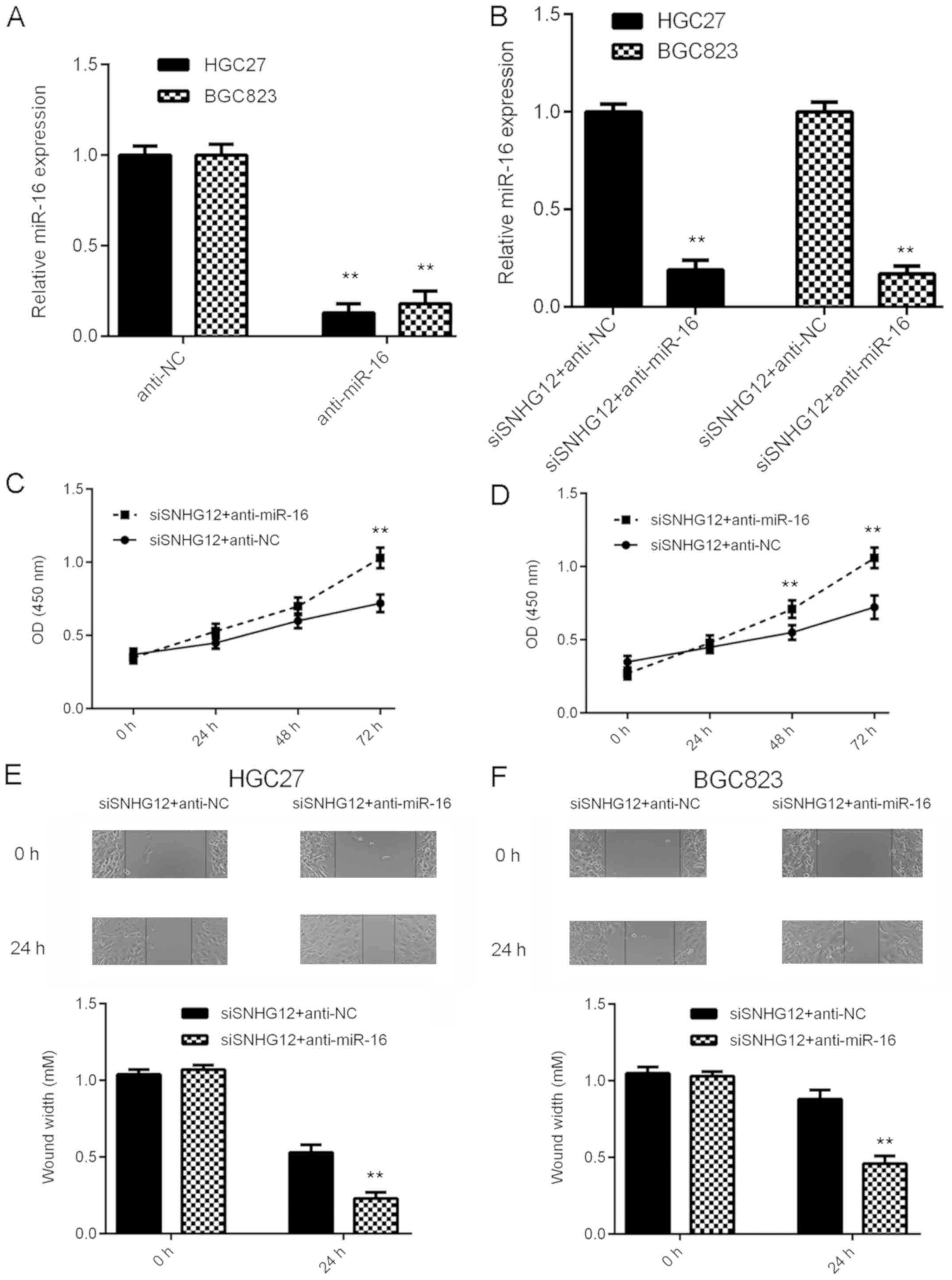
Knockdown of miR-16 impairs the
suppressive effects of SNHG12 downregulation on GC cell
proliferation and migration
Based on the above results, it was speculated that
miR-16 may be important for the biological effects of SNHG12 on GC
cells. To test this hypothesis, GC cells were transfected with NC
inhibitor or miR-16 inhibitor. After transfection, the miR-16
levels were significantly reduced in the anti-miR-16 group compared
with those in the anti-NC group (Fig.
5A). Subsequently, the SNHG12 siRNA-transfected GC cells were
transfected with miR-16 inhibitor or NC inhibitor. As provided in
Fig. 5B, the miR-16 levels were
markedly reduced in the siSNHG12+anti-miR-16 group compared with
those in the siSNHG12+anti-NC group. A CKK-8 assay and a wound
healing assay were then performed. As indicated in Fig. 5C-F, the proliferation and migration
of GC cells were significantly upregulated in the
siSNHG12+anti-miR-16 group when compared with those in the
siSNHG12+anti-NC group. These results suggest that inhibition of
miR-16 impairs the suppressive effects of SNHG12 downregulation on
GC cell proliferation and migration.
Discussion
The mechanisms of the effect of SNHG12 to promote GC
progression remains largely elusive. The present study reported
that SNHG12 was significantly upregulated in GC tissues and cell
lines, and high SNHG12 expression was associated with GC
progression and poor prognosis. Knockdown of SNHG12 markedly
inhibited the proliferation and migration of the BGC823 and HGC27
GC cell lines. miR-16 was identified as a target of SNHG12, and its
expression was negatively regulated by SNHG12 in BGC823 and HGC27
cells. Furthermore, the expression of miR-16 was significantly
decreased in GC tissues and cell lines, and inversely associated
with the expression of SNHG12 in GC tissues. In addition, knockdown
of miR-16 impaired the inhibitory effects on GC cell proliferation
and migration induced by SNHG12 knockdown.
SNHG12 has been reported to be frequently
upregulated and to have a promoting role in various common human
cancer types (14,15,18). For
instance, SNHG12 was significantly upregulated in liver cancer
tissues compared with that in the adjacent normal tissues, and
knockdown of SNHG12 effectively reduced cancer cell proliferation,
migration and invasion (21). Ding
et al (22) reported that
SNHG12 exerted promoting effects on the proliferation, migration
and invasion of papillary thyroid carcinoma cells through
regulating the Wnt/β-catenin signaling pathway. The results of the
present study indicated that SNHG12 was markedly upregulated in GC
tissues and cells compared with that in adjacent normal tissues and
GES-1 cells, respectively. It was further observed that the
expression of SNHG12 was associated with a larger tumor size, tumor
metastasis and advanced clinical stage, as well as poor prognosis
of GC patients; this was consistent with the results of a previous
study by Zhang and Lu (19), which
also demonstrated that inhibition of SNHG12 suppressed the
proliferation, colony formation and invasion of GC SGC-7901 and AGS
cells. In the present study, in vitro experiments revealed
that knockdown of SNHG12 inhibited the proliferation and migration
of the BGC823 and HGC27 GC cell lines. These present results expand
the understanding of the function of SNHG12 in GC cells.
It has been well established that lncRNAs negatively
regulate the expression of miRs through acting as sponges for them
in GC cells (7). For instance, the
lncRNA SNHG20 promotes GC progression by inhibition of p21
expression and regulating the GSK-3β/β-catenin signaling pathway
(11). The lncRNA XIST promotes GC
progression via targeting miR-185 (12). Thus, a Bioinformatics analysis and a
luciferase reporter gene assay were then performed to study the
potential target miRs of SNGH12, and the results demonstrated that
miR-16 was a potential target of SNHG12. A further experiment
confirmed that SNHG12 negatively regulated the expression of miR-16
in BGC823 and HGC27 cells. Subsequently, the correlation between
the expression of miR-16 and SNHG12 in GC tissues was examined. The
results indicated that the expression of miR-16 was significantly
reduced in GC tissues and cell lines, and inversely correlated with
the expression of SNHG12 in GC tissues, suggesting that the
increased expression of SNHG12 may contribute to the reduced
expression of miR-16 in GC.
miR-16 has been reported to have a tumor suppressive
role in several common cancer types, including GC (23–25). For
instance, miR-16 may inhibit glioma cell growth and invasion
through suppressing B-cell lymphoma 2 as well as the nuclear
factor-κB1/matrix metallopeptidase 9 signaling pathway (23). In GC, high expression of miR-16
predicates a favorable prognosis for patients (24). Furthermore, Wang et al
(25) reported that miR-16
negatively regulated Twist1 to repress GC cell invasion and
metastasis. In addition, Li et al (26) reported that overexpression of miR-16
significantly suppressed GC cell proliferation and migration by
inhibition of the hepatocyte growth factor/c-Met pathway. However,
the molecular mechanisms by which miR-16 regulates GC cell
proliferation and migration still remain to be fully elucidated.
The results of the present study indicated that knockdown of miR-16
impaired the suppressive effects on GC cell proliferation and
migration induced by SNHG12 silencing, suggesting that miR-16 is
involved in the SNHG12-induced effects on GC cells. In addition to
miR-16, several other miRs targeted by SNGH12 have also been
identified, including miR-125 (27),
miR-138 (28), miR-150 (29), miR-181 (30), miR-195 (15), miR-199 (31), miR-101 (32), miR-320 (19) and miR-195 (21). Therefore, the present study expands
the current knowledge of regulatory SNHG12/miR interactions in
human cancers.
In conclusion, the present study demonstrated that
inhibition of SNHG12 suppresses GC cell proliferation and migration
by modulation of miR-16 expression, and thus suggests that the
SNHG12/miR-16 interaction may be used as a promising target for GC
treatment.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
BP collected clinical tissues. CW performed clinical
experiments. GZ, SW and XL performed the in-vitro
experiments and statistical analysis. GZ designed the study and
wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Haikou People's Hospital (Haikou, China) and written
informed consent had been obtained from all subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 62:87–108. 2015. View Article : Google Scholar
|
|
3
|
Cheng XJ, Lin JC and Tu SP: Etiology and
prevention of gastric cancer. Gastrointest Tumors. 3:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiel A and Ristimäki A: Targeted therapy
in gastric cancer. APMIS. 123:365–372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
An J, Lv W and Zhang Y: LncRNA NEAT1
contributes to paclitaxel resistance of ovarian cancer cells by
regulating ZEB1 expression via miR-194. Onco Targets Ther.
10:5377–5390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Y, Meng X, Chen S, Li W, Li D, Singer
R and Gu W: IMP1 regulates UCA1-mediated cell invasion through
facilitating UCA1 decay and decreasing the sponge effect of UCA1
for miR-122-5p. Breast Cancer Res. 20:322018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu
Y, Qian H and Dai T: LncRNA UCA1 impacts cell proliferation,
invasion, and migration of pancreatic cancer through regulating
miR-96/FOXO3. IUBMB Life. 70:276–290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Qian W, Feng F, Cao Q, Li Y, Hou
Y, Zhang L and Fan J: Upregulated lncRNA CASC2 may inhibit
malignant melanoma development through regulating miR-18a-5p/RUNX1.
Oncol Res. 27:371–377. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang C, Wu K, Wang S and Wei G: Long
non-coding RNA XIST promotes osteosarcoma progression by targeting
YAP via miR-195-5p. J Cell Biochem. 119:5646–5656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan X: Long noncoding RNA central of
glucose homeostasis. J Cell Biochem. 117:1061–1065. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Liu L, Wan JX and Song Y: Long
noncoding RNA SNHG20 promotes gastric cancer progression by
inhibiting p21 expression and regulating the GSK-3β/β-catenin
signaling pathway. Oncotarget. 8:80700–80708. 2017.PubMed/NCBI
|
|
12
|
Zhang Q, Chen B, Liu P and Yang J: XIST
promotes gastric cancer (GC) progression through TGF-β1 via
targeting miR-185. J Cell Biochem. 119:2787–2796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan HY, Wang C, Liu G and Zhou X: Long
noncoding RNA NEAT1-modualted miR-506 regulates gastric cancer
development through targeting STAT3. J Cell Biochem. 120:4827–4836.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ruan W, Wang P, Feng S, Xue Y and Li Y:
Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes cell proliferation and migration by upregulating
angiomotin gene expression in human osteosarcoma cells. Tumour
Biol. 37:4065–4073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou S, Yu L, Xiong M and Dai G: LncRNA
SNHG12 promotes tumorigenesis and metastasis in osteosarcoma by
upregulating Notch2 by sponging miR-195-5p. Biochem Biophys Res
Commun. 495:1822–1832. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang JZ, Xu CL, Wu H and Shen SJ: LncRNA
SNHG12 promotes cell growth and inhibits cell apoptosis in
colorectal cancer cells. Braz J Med Biol Res. 50:e60792017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang O, Yang F, Liu Y, Lv L, Ma R, Chen C,
Wang J, Tan Q, Cheng Y, Xia E, et al: C-MYC-induced upregulation of
lncRNA SNHG12 regulates cell proliferation, apoptosis and migration
in triple-negative breast cancer. Am J Transl Res. 9:533–545.
2017.PubMed/NCBI
|
|
18
|
Dong J, Wang Q, Li L and Xiao-Jin Z:
Upregulation of long non-coding RNA small nucleolar RNA host gene
12 contributes to cell growth and invasion in cervical cancer by
acting as a sponge for MiR-424-5p. Cell Physiol Biochem.
45:2086–2094. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H and Lu W: LncRNA SNHG12 regulates
gastric cancer progression by acting as a molecular sponge of
miR320. Mol Med Rep. 17:2743–2749. 2018.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lan T, Ma W, Hong Z, Wu L, Chen X and Yuan
Y: Long non-coding RNA small nucleolar RNA host gene 12 (SNHG12)
promotes tumorigenesis and metastasis by targeting miR-199a/b-5p in
hepatocellular carcinoma. J Exp Clin Cancer Res. 36:112017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding S, Qu W, Jiao Y, Zhang J, Zhang C and
Dang S: LncRNA SNHG12 promotes the proliferation and metastasis of
papillary thyroid carcinoma cells through regulating wnt/β-catenin
signaling pathway. Cancer Biomark. 22:217–226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kB1/MMP9 signaling pathway. Cancer Sci. 105:265–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren C, Chen H, Han C, Fu D, Wang D and
Shen M: High expression of miR-16 and miR-451 predicating better
prognosis in patients with gastric cancer. J Cancer Res Clin Oncol.
142:2489–2496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang T, Hou J, Li Z, Zheng Z, Wei J, Song
D, Hu T, Wu Q, Yang JY and Cai JC: miR-15a-3p and miR-16-1-3p
negatively regulate twist1 to repress gastric cancer cell invasion
and metastasis. Int J Biol Sci. 13:122–134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Zhang H, Wang X, Qu Y, Duan J, Liu
R, Deng T, Ning T, Zhang L, Bai M, et al: Direct targeting of HGF
by miR-16 regulates proliferation and migration in gastric cancer.
Tumour Biol. 37:15175–15183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin XJ, Chen XJ, Zhang ZF, Hu WS, Ou RY,
Li S, Xue JS, Chen LL, Hu Y and Zhu H: Long noncoding RNA SNHG12
promotes the progression of cervical cancer via modulating
miR-125b/STAT3 axis. J Cell Physiol. 234:6624–6632. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Qi G, Zhang J, Wu J, Zhou N, Li L
and Ma J: Knockdown of long noncoding RNA small nucleolar RNA host
gene 12 inhibits cell growth and induces apoptosis by upregulating
miR-138 in nonsmall cell lung cancer. DNA Cell Biol. 36:892–900.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao M, Wang J, Xi X, Tan N and Zhang L:
SNHG12 promotes angiogenesis following ischemic stroke via
regulating miR-150/VEGF pathway. Neuroscience. 390:231–240. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Chen D, Ma H and Li Y: LncRNA
SNHG12 contributes to multidrug resistance through activating the
MAPK/Slug pathway by sponging miR-181a in non-small cell lung
cancer. Oncotarget. 8:84086–84101. 2017.PubMed/NCBI
|
|
31
|
Yin WL, Yin WG, Huang BS and Wu LX: LncRNA
SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral
ischemia/reperfusion injury through activating AMPK signaling
pathway. Neurosci Lett. 690:188–195. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun Y, Liu J, Chu L, Yang W, Liu H, Li C
and Yang J: Long noncoding RNA SNHG12 facilitates the tumorigenesis
of glioma through miR-101-3p/FOXP1 axis. Gene. 676:315–321. 2018.
View Article : Google Scholar : PubMed/NCBI
|