Introduction
Nerve cells are non-renewable cells. Due to ageing
and damage of external environment, nerve cells are injured,
leading to apoptosis. A variety of related diseases are induced
when the number of nerve cells gradually declines (1,2), such as
Alzheimers disease, Parkinsons disease, cerebellar atrophy and
other neurodegenerative diseases (3). At present, maintenance treatment
prevails in the drug therapy for neurodegenerative diseases in
clinic, but no radical efficacy is obtained (4). Relatively speaking, neural stem cells
have such characteristics as self-amplification and constant
updating, as well as the potential of directional differentiation
into neuronal cells, which are considered as the biomaterial that
is able to cure neurodegenerative diseases (5). However, the premise is that the number
of neural stem cells in the body is sufficient and they are not
damaged or reduced (6). Anesthetics
are currently drugs with the greatest direct impact on the nerves,
and are applied most frequently. As a commonly-used anesthetic,
sevoflurane used to be considered as a landmark drug in inhalation
anesthesia (7), which is
characterized by low dose of muscle relaxant and rapid
postoperative recovery, and it is often used in anesthesia for
children (8,9). However, there have been different
reports on the effect of sevoflurane on nerves so far. In the
present study, therefore, the effect of sevoflurane on neural stem
cells was investigated with neural stem cells and deoxyribonucleic
acid (DNA) methylation as entry points.
Materials and methods
Experimental materials
A total of 10 healthy specific pathogen-free
Sprague-Dawley (SD) rats aged 6–8 weeks (Beijing Vital River
Laboratory Animal Technology Co., Ltd., Beijing, China) and
sevoflurane (Beijing Baitaike Medical Co., Ltd., Beijing, China)
were used. The rats were kept in cages with controlled temperature
and light/dark cycles (24°C and 12:12-h light/dark cycles),
humidity (60±10%) and free access to food and water.
Main reagents
Neural stem cell culture medium (R&D Systems,
Inc., Minneapolis, MN, USA), Cell Counting Kit-8 (CCK-8; Dojindo
China Co., Ltd., Beijing, China), primary and secondary antibodies
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and primers (Sangon
Biotech Co., Ltd., Shanghai, China).
Methods
Isolation and culture of neural stem
cells (10)
The brain tissues were peeled off from
Sprague-Dawley rats (male, 200±20 g) sacrificed in advance on a
super clean bench under sterile conditions, the hippocampus was
isolated, and the meninges and visible blood vessels were removed.
The remaining hippocampal tissues were cut into pieces with sterile
scissors and added with 0.25% trypsin, placed in a water bath at
37°C and centrifuged for 5 min (800 × g/min) at 4°C after 15 min.
After the supernatant was discarded, the mixture was filtered
through a 400-mesh filter, added with neural stem cell culture
medium, pipetted and mixed evenly, followed by counting. Then cells
were inoculated into a 75-cm2 culture flask
(5×106/cm2) and cultured in an incubator with
CO2 at 37°C. The medium was replaced once every 3 days,
observed and recorded.
The present study was approved by the Ethics
Committee of Central South University Xiangya School of Medicine
Affiliated Haikou Hospital (Haikou, China).
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR) detection
Ribonucleic acid (RNA) was extracted from neural
stem cells using TRIzol, and 1 µg RNA was reverse transcribed to
obtain complementary DNA (cDNA). The cDNA concentration was
adjusted, and the messenger RNA (mRNA) level was detected using the
Bio-Rad CFX96 PCR instrument (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The reaction conditions were as follows: 95°C for 2 min,
94°C for 15 sec, 50°C for 25 sec, a total of 40 cycles. The
corresponding primer sequences are shown in Table I. The results were analyzed using the
2−ΔΔCq method (11).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Gene name | Primer sequences |
|---|
| Nestin | Forward:
5′-CCTCCACCCTTGCCTGCTACCCT-3′ |
|
| Reverse:
5′-ACGGAGCCTGTTTCCTCCCACC-3′ |
| Glial fibrillary
acidic portein (GFAP) | Forward:
5′-TAGACAGGAAGCATGAAGCCACC3′ |
|
| Reverse:
5′-TGCAAACTTGGAGCGGTACCACTCT-3′ |
| β-tubulin | Forward:
5′-TGGGCCAAGGGTCACTACAC-3′ |
|
| Reverse:
5′-CTGATGCGGTCGGGATACTC-3′ |
|
Microtubule-associated protein-2
(MAP-2) | Forward:
5′-GCACGGCGGACCACCAGGTC-3′ |
|
| Reverse:
5′-TGGCGACCTTCTTCTCACTC-3′ |
| β-actin | Forward:
5′-GTGGACATCCGCAAAGAC-3′ |
|
| Reverse:
5′-GAAAGGGTGTAACGCAACTA-3′ |
Western blot analysis
Cells were ground in liquid nitrogen, lysed on ice
for 30 min and centrifuged at 3,000 × g for 10 min to obtain the
supernatant. A portion of supernatant was taken, the protein
content the protein was extracted with ProteoPrep®
protein extraction kit (Sigma St. Louis, MO, USA) and protein
content was detected with BCA protein quantitative kit and adjusted
to 100 µg, and the 5X reduced loading buffer was added and boiled
for 10 min. A total of 9 µl sample solution was added slowly with a
microsyringe into the 10% polyacrylamide gel loading well, followed
by sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) at 80 V. After electrophoresis, the target protein in
the gel was transferred onto a nitrocellulose membrane at 40 V for
0.5 h, washed with eluant at least 3 times (10 min/time) and
applied with the antibody. The protein was blocked with skim milk
powder at 4°C overnight, incubated with the primary rabbit anti-rat
antibody Nestin, GFAP, β-tubulin, MAP-2, β-acin polyclonal
antibodies (diluted at 1:500; cat. nos. 19483-1-AP, 16825-1-AP,
10068-1-AP, 17490-1-AP, 20536-1-AP; Proteintech, Wuhan, China) at
room temperature for 2 h and incubated with the secondary goat
anti-rabbit polyclonal antibody (diluted at 1:1,000; cat. no.
SA00001-2; Proteintech) at room temperature for 1 h, and the ECL
substrate was added (Sigma, St. Louis), followed by tabletting and
imaging in the dark. The imaging results were quantified using
ImageJ software (National Institutes of Health, Bethesda, MD,
USA).
In vitro cell assay using the 96-well
plate
A 96-well plate was selected and added with
1×105 neural stem cells/well, followed by culture for 24
h. Three wells were only added with the normal medium as the
control group (C0), 3 wells were added with the
low-concentration sevoflurane (0.2 g/ml) prepared by the medium as
the low-concentration group (C1), 3 wells were added
with the moderate-concentration of sevoflurane (0.5 g/ml) as the
moderate-concentration group (C2), and 3 wells were
added with the high-concentration sevoflurane (1 g/ml) as the
high-concentration group (C3). After action for 12 h,
the medium was replaced with CCK-8 and placed in the incubator with
CO2 for 2 h. The absorbance was measured at 480 nm.
Apoptosis rate = (C0 - Cx)/C0. The
experimental processes at 24 and 36 h were the same as that at 12
h.
Detection of overall methylation
content in cells via high-performance liquid chromatography
(HPLC)
The sample was added into the Hypersil BDS C18
column of a high-performance liquid chromatographic instrument
(Shimadzu, Corp., Kyoto, Japan) using a microsyringe. Elution was
performed with the mixed solution of methanol, sodium
pentanesulfonate and triethylamine as the mobile phase at low
temperature at a flow rate of 1 l/min (ultraviolet wavelength, 273
nm; sensitivity, 0.01 AUPS). With deoxycytosine and
methyldeoxycytosine standard samples as controls, the DNA
methylation content in samples was detected. Each sample was
detected 3 times, and the average was taken.
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (IBM Corp., Armonk, NY, USA) was used for processing
of research data. Measurement data were expressed as (mean ± SD),
and t-test and one-way analysis of variance (ANOVA) with Least
Significant Difference post hoc test was used for the comparison of
mean. Pearsons correlation analysis was used for correlation
between methylation content and sevoflurane concentration.
P<0.05 indicated that the difference was statistically
significant.
Results
Isolation and identification of neural
stem cells
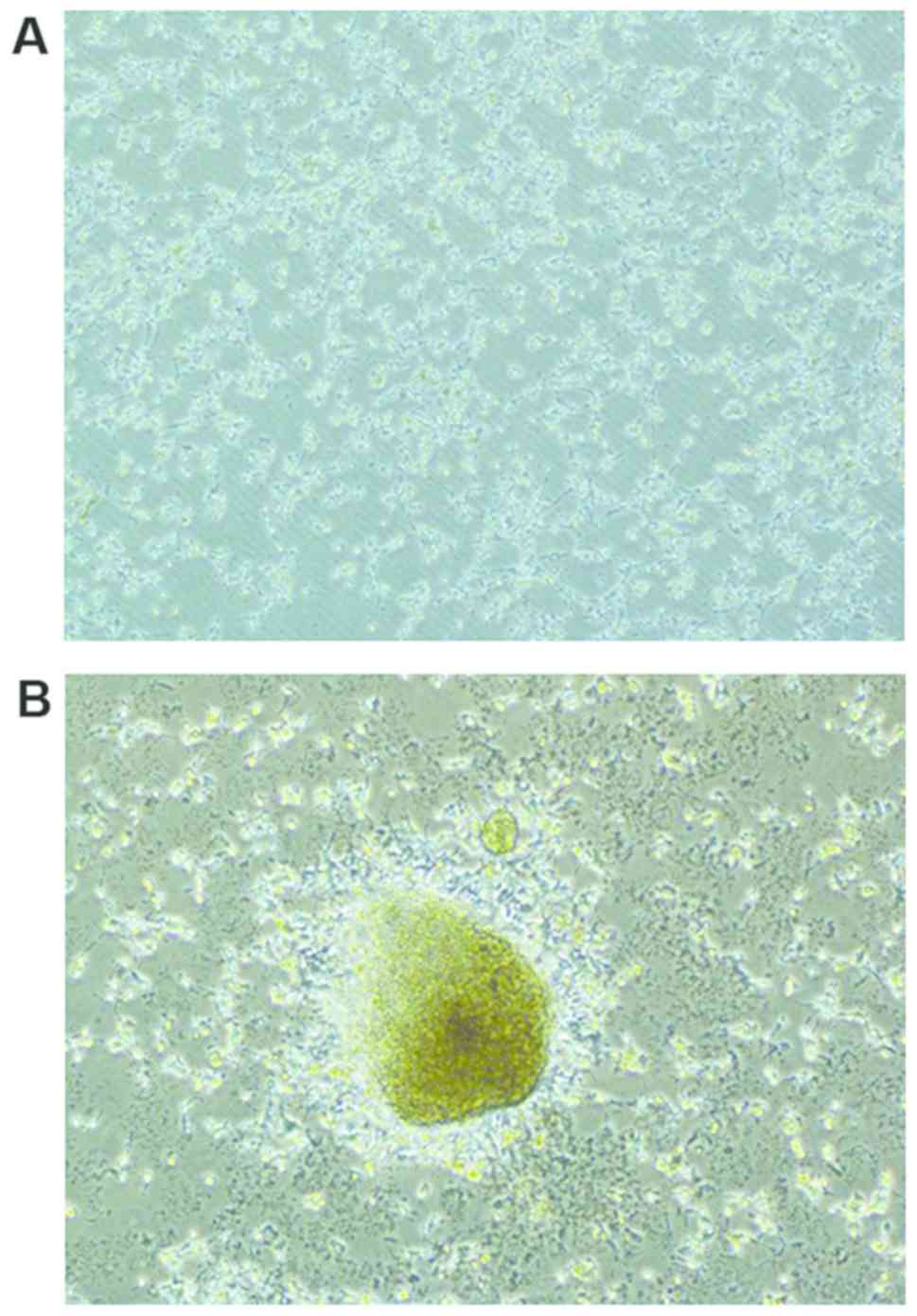
Primary neural stem cells had good transmittance and
were distributed densely like stars in the visual field of an
inverted microscope (Olympus, Tokyo, Japan) (Fig. 1A). At 5 days after culture, a large
number of cells gathered, forming a ball structure with a strong
stereoscopic sense, which was similar to a waxberry (Fig. 1B). At 3 days after induced
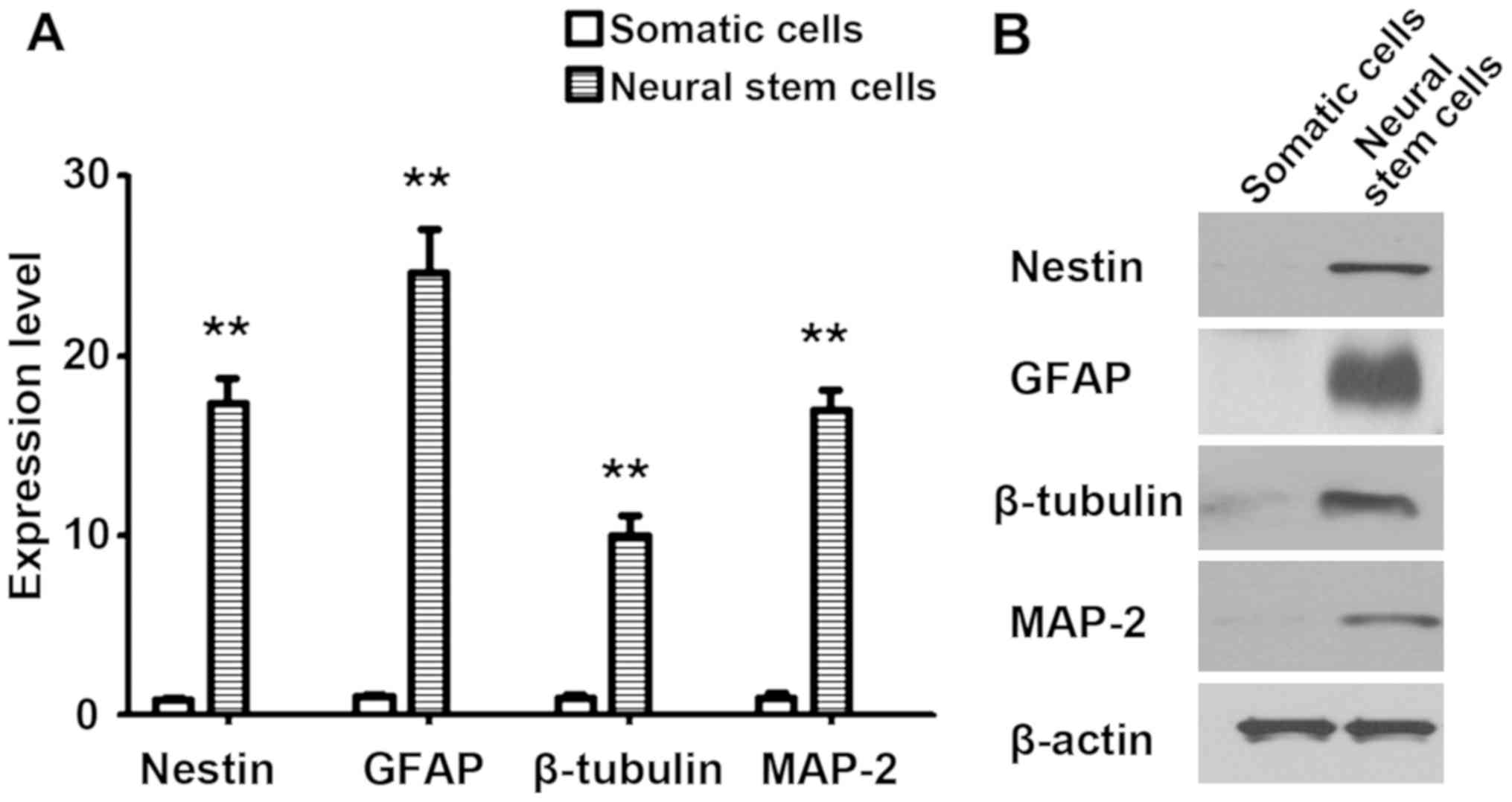
differentiation, neural markers were detected via RT-qPCR and
western blot analysis, and the results revealed that the expression
levels of Nestin, GFAP, β-tubulin and MAP-2 were significantly
higher than those in somatic cells (P<0.01) (Fig. 2).
Effects of sevoflurane in different
concentrations on apoptosis of neural stem cells
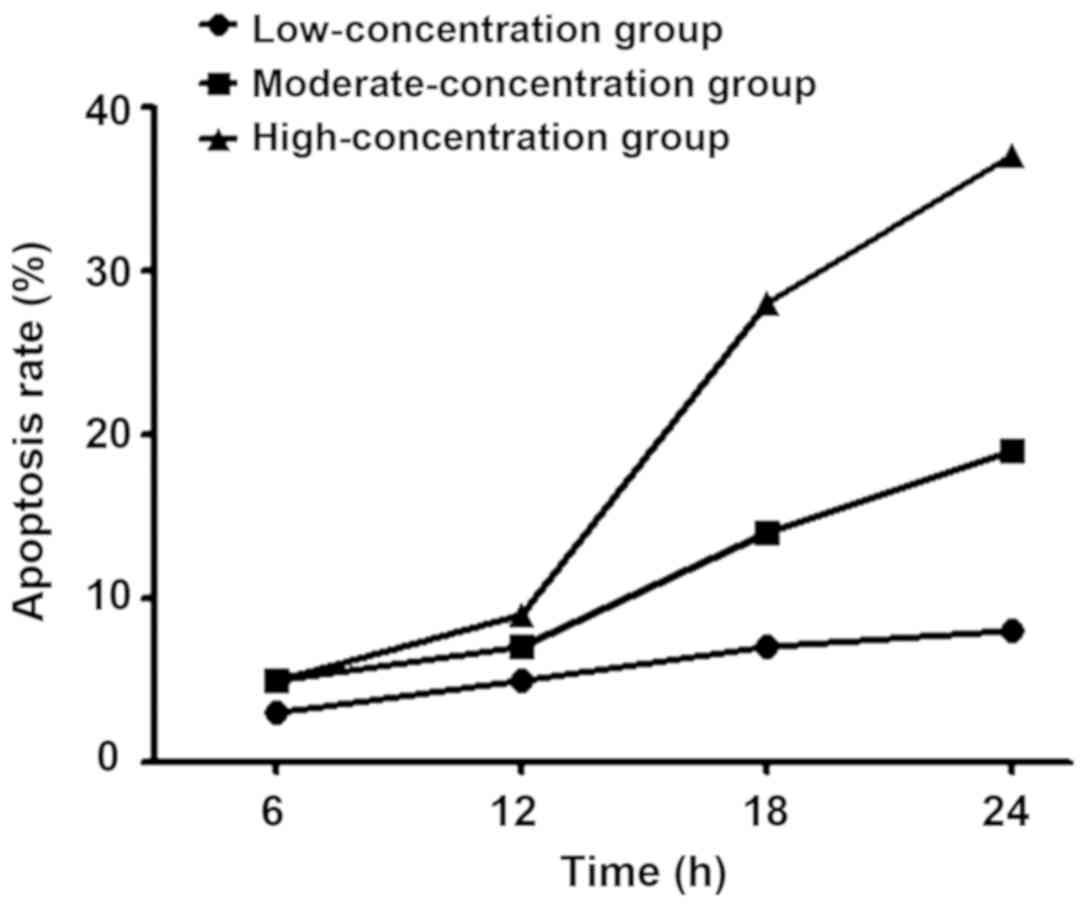
Results of CCK-8 assay using the 96-well plate
showed that with the increase of sevoflurane concentration, the
apoptosis rate of stem cells was gradually increased, which was
also progressively increased with the prolongation of time
(Fig. 3).
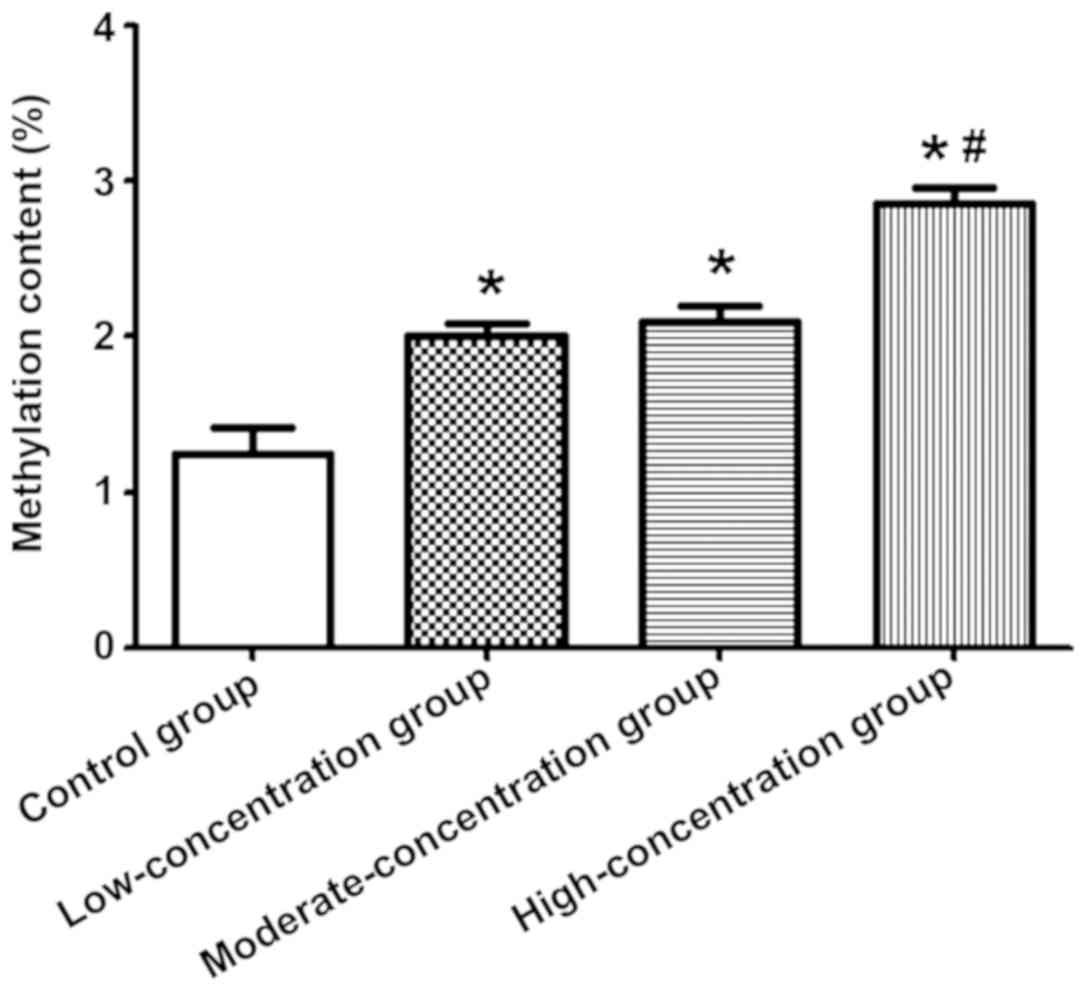
The genomic DNA methylation content in the 4 groups
of neural stem cells was detected via HPLC. The methylation content
in the 3 groups of cells treated with sevoflurane was higher than
that in the control group (P<0.05), and it was also higher in
the high-concentration group than those in the
moderate-concentration and low-concentration groups (P<0.05)
(Fig. 4).
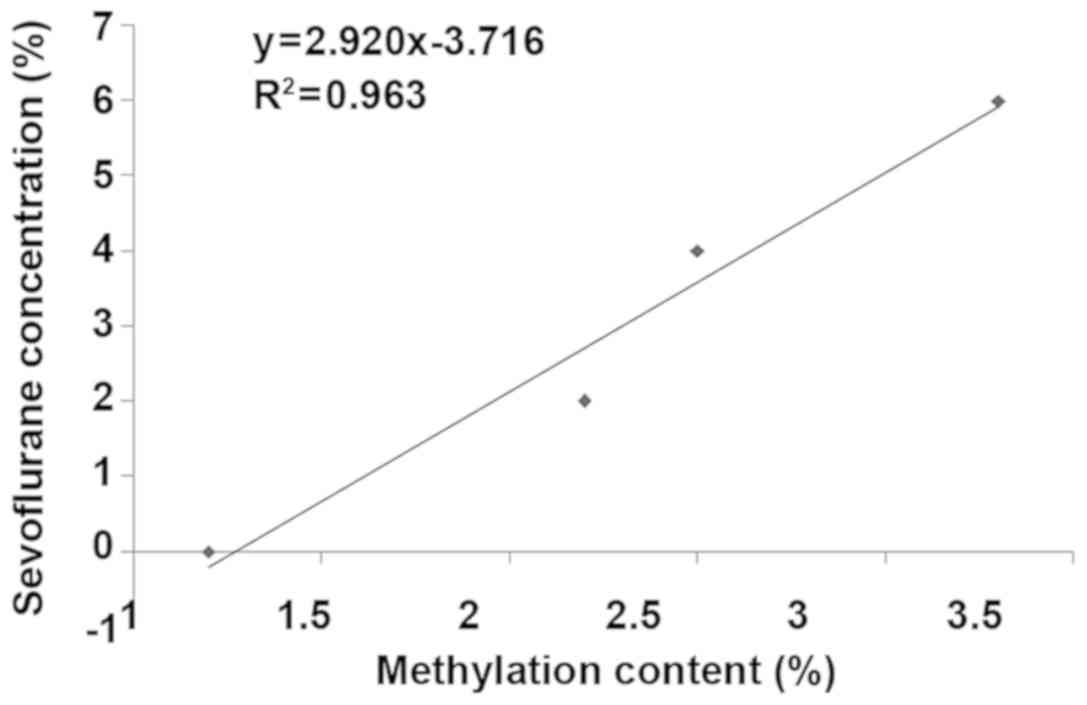
Correlation analysis between
methylation content and sevoflurane concentration
According to the Pearsons correlation analysis, the
methylation content in neural stem cells was closely correlated
with the sevoflurane concentration, and the overall methylation
content was increased with the increase of sevoflurane
concentration (Fig. 5).
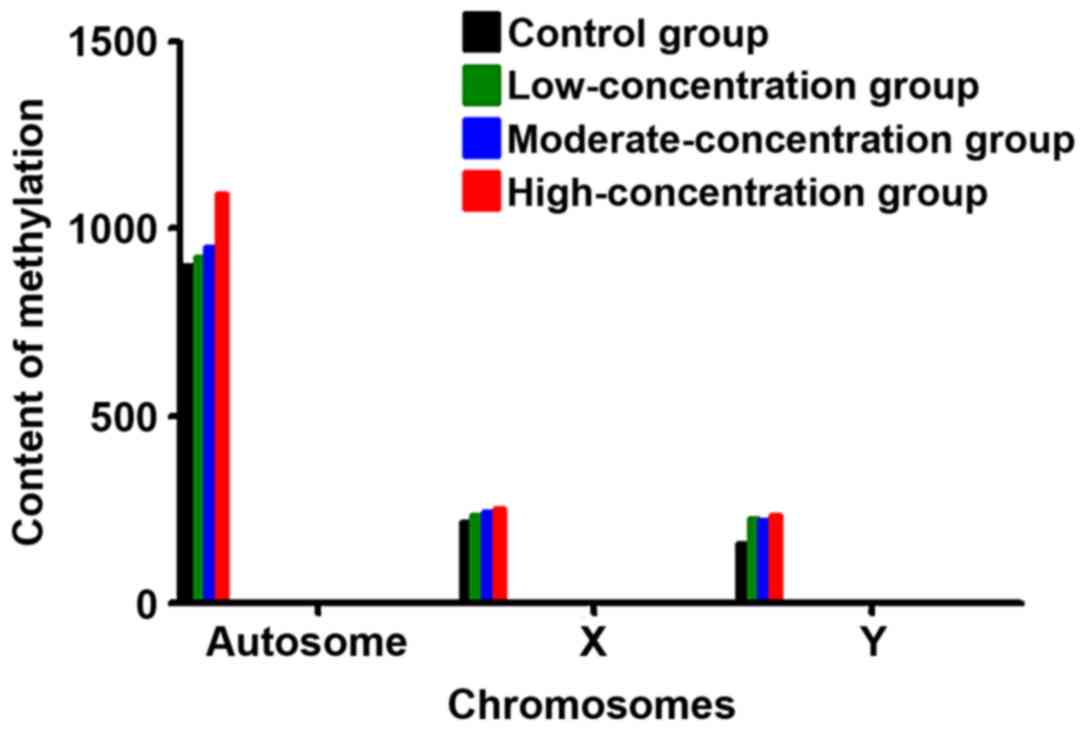
Comparison of distribution of
methylation in the chromosome in each group
Methylation mostly occurred in the autosome, and the
content of methylation in the high-concentration group was higher
than that in the moderate-concentration, low-concentration and
control groups (P<0.05). The degree of methylation on the sex
chromosome had no significant differences among the four groups
(Fig. 6).
Discussion
Neurodegenerative diseases lead to incurable pain
for the patients worldwide. There is a lack of self-amplification
and self-repairing capacity in nerve cells, so it difficult to make
up for the loss of nerve cells once damaged (12). Moreover, nerve-related diseases
gradually emerge with the decrease of neurons. Many neurologists
worldwide have made unremitting efforts in the research on
neurodegenerative diseases, and developed a variety of drugs and
therapeutic methods successively, such as dopamine drugs, which,
however, can mostly cure the symptom, instead of the underlying
problem, and relieve symptoms in different degrees. Besides, the
efficacy of drugs also gradually declines with the progression of
the disease (13,14). As a new therapeutic material, neural
stem cells are highly valued currently, they possess self-repairing
capacity and differentiate into neuronal cells or neuroglial cells
required in the body, thus supplementing and repairing damaged
nerve cells and enabling normal neuronal function (15,16).
Cornelissen et al (17)
peeled off the hippocampal tissues of mice, hen screened, purified
and cultured to obtain the ball-shaped nerve cells at 7 days, and
added with inducer for neural induction. The immunofluorescence
assay revealed that the neural markers are all highly expressed. In
the present study, the hippocampus was also peeled off from rats
and digested with trypsin to obtain primary neural stem cells.
After culture for 5 days and induced differentiation for 3 days,
the cellular morphology and surface markers met criteria for nerve
cells, which were roughly consistent with the above study.
Great attention is required to the fact that the
number of neural stem cells in the brain is small, and they can be
easily damaged by external environment. One of the external
environment factors with greater direct impact on the nerve is
anesthetics that are often used in clinic and not easily detectable
(18). Due to various advantages,
sevoflurane is often used in the facial surgery for children.
According to the study on clinical symptoms, sevoflurane has little
effect on neural evaluation indexes. However, some clinical
phenomena are obscured due to the strong metabolism of children.
From a microscopic perspective, neural stem cells were cultured
in vitro and fully treated with sevoflurane in different
concentrations in the present study. It was found that the
apoptosis rate of stem cells was increased with the increase of
sevoflurane concentration and the prolongation of time, indicating
that sevoflurane has a certain toxic effect on neural stem cells,
and approximately 40% of cells are damaged after action under high
concentration for 24 h.
DNA methylation is a kind of DNA modification, and
different degrees of methylation affect the structure and function
of the whole gene. Therefore, methylation is an important index in
the study on various clinical symptoms from the perspective of gene
molecules (19,20). Currently, there are many studies on
methylation and tumorigenesis, and it was found that there is a
certain correlation between them (21). There are few studies in the
neurological field. In this study, results showed that the
methylation content in cells treated with sevoflurane was higher
than that in the control group, and it was also higher in the
high-concentration group than that in the moderate-concentration
and low-concentration groups. The methylation content in neural
stem cells was closely correlated with the sevoflurane
concentration, and the overall methylation content was increased
with the increase of sevoflurane concentration. Methylation mostly
occurred in the autosome, and the content of methylation in the
high-concentration group was higher than that in the
moderate-concentration, low-concentration and control groups. The
degree of methylation on the sex chromosome had no significant
difference among the four groups. The above results suggest that
the higher the degree of methylation is, the higher the toxicity on
neural stem cells may be from the perspective of molecular
mechanism, so the degree of methylation can serve as an important
index for the determination of neurotoxicity. However, the in
vitro environment in the present study was different from the
complex internal environment in the human body, so whether in
vivo and in vitro results are consistent remains to be
further verified.
In conclusion, the concentration of sevoflurane can
affect the degree of methylation in neural stem cells of rats and
produce certain cytotoxicity.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Hainan Province (no. 20168314).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors’ contributions
KW drafted the manuscript. KW and YT helped with the
isolation and culture of neural stem cells. YZ and XL performed PCR
and western blot analysis. XW, HH and SX contributed to CCK-8 assay
and the high-performance liquid chromatography. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Central South University Xiangya School of Medicine
Affiliated Haikou Hospital (Haikou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lie PPY and Nixon RA: Lysosome trafficking
and signaling in health and neurodegenerative diseases. Neurobiol
Dis. 22:94–105. 2019. View Article : Google Scholar
|
|
2
|
Gerhardt S and Mohajeri MH: Changes of
colonic bacterial composition in Parkinsons disease and other
neurodegenerative diseases. Nutrients. 10:7082018. View Article : Google Scholar
|
|
3
|
Maiti P and Dunbar GL: Use of curcumin, a
natural polyphenol for targeting molecular pathways in treating
age-related neurodegenerative diseases. Int J Mol Sci.
19:E16372018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaneko N and Sawamoto K: Go with the flow:
Cerebrospinal fluid flow regulates neural stem cell proliferation.
Cell Stem Cell. 22:783–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Colle D, Farina M, Ceccatelli S and Raciti
M: Paraquat and maneb exposure alters rat neural stem cell
proliferation by inducing oxidative stress: New insights on
pesticide-induced neurodevelopmental toxicity. Neurotox Res.
34:820–833. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Q, Li Y, Zhou L, Li Y, Xu P, Liu X, Lv
Q, Li J, Guo H, Cai H, et al: GRP78 promotes neural stem cell
antiapoptosis and survival in response to oxygen-glucose
deprivation (OGD)/reoxygenation through PI3K/Akt, ERK1/2, and
NF-κB/p65 pathways. Oxid Med Cell Longev. 3541807:20182018.
|
|
7
|
Petrella RA, Mollica PA, Zamponi M, Reid
JA, Xiao S, Bruno RD and Sachs PC: 3D bioprinter applied picosecond
pulsed electric fields for targeted manipulation of proliferation
and lineage specific gene expression in neural stem cells. J Neural
Eng. 15:0560212018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang G, Chen L, Chen W, Li B, Yu Y, Lin
F, Guo X, Wang H, Wu G, Gu B, et al: Neural stem cells alleviate
inflammation via neutralization of IFN-γ negative effect in
ischemic stroke model. J Biomed Nanotechnol. 14:1178–1188. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu L, Shen J, Yu L, Sun J, McQuillan PM,
Hu Z and Yan M: Role of autophagy in sevoflurane-induced
neurotoxicity in neonatal rat hippocampal cells. Brain Res Bull.
140:291–298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iliescu DA, Ciubotaru A, Ghiţă MA, Dumitru
A and Zăgrean L: Effect of sevoflurane preconditioning on
light-induced retinal damage in diabetic rats. Rom J Ophthalmol.
62:24–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salilew-Wondim D, Saeed-Zidane M, Hoelker
M, Gebremedhn S, Poirier M, Pandey HO, Tholen E, Neuhoff C, Held E,
Besenfelder U, et al: Genome-wide DNA methylation patterns of
bovine blastocysts derived from in vivo embryos subjected to in
vitro culture before, during or after embryonic genome activation.
BMC Genomics. 19:4242018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Heijden AG, Mengual L,
Ingelmo-Torres M, Lozano JJ, van Rijt-van de Westerlo CCM, Baixauli
M, Geavlete B, Moldoveanud C, Ene C, Dinney CP, et al: Urine
cell-based DNA methylation classifier for monitoring bladder
cancer. Clin Epigenetics. 10:712018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiao S, Liu Y, Yao Y and Teng J: miR-124
promotes proliferation and neural differentiation of neural stem
cells through targeting DACT1 and activating Wnt/β-catenin
pathways. Mol Cell Biochem. 449:305–314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nguyen H, Kerimoglu C, Pirouz M, Pham L,
Kiszka KA, Sokpor G, Sakib MS, Rosenbusch J, Teichmann U, Seong RH,
et al: Epigenetic regulation by BAF complexes limits neural stem
cell proliferation by suppressing Wnt signaling in late embryonic
development. Stem Cell Rep. 10:1734–1750. 2018. View Article : Google Scholar
|
|
16
|
Petrik D, Myoga MH, Grade S, Gerkau NJ,
Pusch M, Rose CR, Grothe B and Götz M: Epithelial sodium channel
regulates adult neural stem cell proliferation in a flow-dependent
manner. Cell Stem Cell. 22:865–878.e8. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cornelissen L, Kim SE, Lee JM, Brown EN,
Purdon PL and Berde CB: Electroencephalographic markers of brain
development during sevoflurane anaesthesia in children up to 3
years old. Br J Anaesth. 120:1274–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Murahata Y, Hikasa Y, Hayashi S,
Shigematsu K, Akashi N, Osaki T, Tsuka T, Okamoto Y and Imagawa T:
The effect of remifentanil on the minimum alveolar concentration
(MAC) and MAC derivatives of sevoflurane in dogs. J Vet Med Sci.
80:1086–1093. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng C, Dong J, Chang W, Cui M and Xu T:
The progress of methylation regulation in gene expression of
cervical cancer. Int J Genomics. 2018:82606522018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng J, Wei D, Ji Y, Chen L, Yang L, Li
G, Wu L, Hou T, Xie L, Ding G, et al: Integrative analysis of DNA
methylation and gene expression reveals hepatocellular
carcinoma-specific diagnostic biomarkers. Genome Med. 10:422018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slieker RC, Relton CL, Gaunt TR, Slagboom
PE and Heijmans BT: Age-related DNA methylation changes are
tissue-specific with ELOVL2 promoter methylation as exception.
Epigenetics Chromatin. 11:252018. View Article : Google Scholar : PubMed/NCBI
|