Introduction
Diabetic cardiomyopathy (DCM) is one of the most
common complications of diabetes mellitus and is closely associated
with the increasing incidence of heart failure (1). Although the duration and severity of
hyperglycaemia are considered major risk factors for the
development of DCM (2), the
molecular mechanisms underlying DCM remain unknown. Cardiac
hypertrophy is one of the key structural changes in DCM and is
often preceded by the pathological phenotype of DCM (3). Calpain-1, a Ca2+-dependent
cysteine protease, is implicated in a number of pathological
conditions associated with cardiovascular diseases including
cardiac hypertrophy (4–7). It has been previously reported that
calpain-1 is upregulated in the heart tissue of rats treated with
isoproterenol (8), highlighting the
important role of calpain-1 in the development of sympathetic
hypertrophic cardiomyopathy. The involvement of calpain-1 in
hypertrophic cardiomyopathy largely depends on its mediation of
oxidative stress and inflammation, which are the most important
contributors to the onset and progression of diabetic cardiac
hypertrophy (8,9). In addition, a previous study has
revealed that the inhibition of the reactive oxygen species
(ROS)-/NF-κB pathway protects cardiomyocytes from hypertrophy
(10). There has been increasing
evidence that inflammation and adhesion molecules are involved in
diabetes and the progression of cardiac hypertrophy in diabetes
(11,12). NF-κB serves an important role in the
regulation of pro-inflammatory genes leading to the overproduction
of inflammatory mediators, including tumor necrosis factor (TNF)-α,
interleukin-6 (IL-6) and inducible nitric oxide synthase (iNOS) in
the hearts of isoproterenol treated rats (13). It has been reported that the activity
of calpain-1 increases in high glucose-treated cardiomyocytes and
streptozotocin (STZ)-induced diabetes (2,8). In
addition, calpain-1 has been reported to be involved in acute
inflammatory processes via the activation of the NF-κB pathway
(7,14). In conclusion, these results indicate
that the improvement of cardiac hypertrophy may be achieved by
inhibiting the expression of calpain-1 expression and the
activation of NF-κB.
Lycium barbarum, a member of the Solanaceae
family, produces red-colored fruits, commercially known as Goji
berries and has been used as a traditional Chinese herbal medicine
for thousands of years (15).
Lycium barbarum polysaccharide (LBP) is the major active
ingredient extracted from Lycium barbarum and has a number
of important bioactivities, including anti-oxidation (16), immunomodulation (17) and anti-inflammation (18). In STZ-induced diabetic animals, LBP
has been indicated to attenuate testicular dysfunction (19), protect peripheral neuropathy
(20), improve male sexual
dysfunction and fertility impairments in males (21), enhance spermatogenesis (22) and inhibit diabetic nephropathy
(23). However, to the best of our
knowledge, the protective effect of LBP on cardiac hypertrophy in
diabetic rats has not yet been reported. Further investigation is
required on whether this potential protective effect is targeted on
calpain-1 expression and NF-κB pathway.
The current study hypothesized that LBP may protect
diabetic rats from cardiac hypertrophy with the following taken
into consideration: Calpain-1 mediates activation of the NF-κB
pathway, which leads to oxidative stress and inflammation, serving
an essential role in the development of cardiac hypertrophy, and
LBP possesses antioxidative and anti-inflammatory effects (7,9). The
present study also assessed the underlying mechanism of this
protected effect by targeting calpain-1 expression and the NF-κB
pathway.
Materials and methods
Chemicals and reagents
LBP with >98% purity was obtained from Ningxia
Qiyuan Pharmaceutical Co., Ltd. The immunohistochemical kit was
purchased from OriGene Technologies, Inc. Antibodies against NF-κB
subunit (p65), inhibitory protein кB (IкB)-α, laminB and GAPDH were
obtained from Abcam. Antibodies against iNOS, TNF-α, intercellular
adhesion molecule (ICAM)-1, vascular adhesion molecule (VCAM)-1,
Toll-Like Receptor 4 (TLR-4) and horseradish peroxidase goat
anti-rabbit IgG (H+L) were obtained from ABclonal Biotechnology
Co., Ltd. Endogenous nitric oxide synthase (eNOS), IL-6 and
calpain-1 were obtained from Cell Signaling Technology Inc. STZ was
obtained from Sigma-Aldrich (Merck KGaA).
STZ-induced diabetic model in
rats
A total of 60 adult male Sprague-Dawley rats
(180–200 g) were obtained from the Laboratory Animal Center of the
Jinzhou Medical University, (Liaoning, China). The present study
was approved by the Ethics Committee of Animal Experiments of the
Jinzhou Medical University (approval number: LMU-2016-138;
Liaoning, China). Animal procedures were performed in accordance
with the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (24).
Rats were housed at a temperature of 20–22°C, a relative humidity
of 50–60%, a 12 h light/dark cycle and with a free access to food
and water. Rats were considered diabetic if they exhibited
hyperglycemia (≥15 mmol/l) 72 h following the one-time intra
peritoneal injection of STZ (50 mg/kg). Diabetic rats were divided
into three groups: An STZ group (n=10), an STZ+LBP (60 mg/kg/d)
group (n=10) and an STZ+LBP (30 mg/kg/d) group (n=10). The groups
were administered saline solution [intragastric (i.g.)] and/or 60
and 30 mg/kg/d LBP (i.g.) for 12 weeks. An additional 10 healthy
non-diabetic rats were used as the control group (n=10) and were
administered the vehicle. Cardiac hypertrophy was defined by the
following: Dysfunction of the cardiac hemodynamics, an increase in
the ratios of left ventricular weight/body weight and heart
weight/body weight and the increased expressions of atrial
natriuretic peptide (ANP) and brain natriuretic peptide (BNP),
which serve as hypertrophic markers in cardiac tissue.
Hemodynamics and heart weight index
measurement
Hemodynamics was conducted after the rats were
anesthetized with intraperitoneal injection of sodium pentobarbital
(0.04 g/kg). Overdose of 20% urethane (1 g/kg) followed by
exsanguination were used to sacrifice the rats following the
hemodynamics. Hemodynamics and heart weight index was calculated
according to previous reported methods (25). The BL-420S polygraph (Chengdu TME
Technology Co. Ltd.) was used to record the left ventricular
end-diastolic pressure (LVEDP), left ventricular systolic pressure
(LVSP) and the maximal rate of left ventricular systolic and
diastolic pressure (±dp/dtmax). The indexes of HW/BW and
LVW/BW were defined as heart weight/body weight, and left
ventricular weight/body weight, respectively.
Histological analysis
Heart tissues (thickness 5 µm) were fixed in 10%
neutral formaldehyde buffer at 25°C for 24 h and embedded in
paraffin. Tissues were subsequently stained with hematoxylin-eosin
(HE; H staining for 5 min at 25°C, 1% E staining for 3 min at 25°C)
to evaluate morphological changes.
mRNA expression with reverse
transcription-quantitative PCR (RT-qPCR)
Heart tissue was homogenized and total mRNA was
isolated using a TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). According to the manufacturer's protocol, total
RNA (500 ng) was reverse-transcribed to cDNA using the
HiScript® II One Step RT-PCR kit (Vazyme). The mRNA
expression of atrial natriuretic peptide (ANP) and brain
natriuretic peptide (BNP) was examined using a ChamQ SYBR qPCR
Master Mix kit (Vazyme) with a BioRad iQ5 Real Time PCR system
(Bio-Rad Laboratories, Inc.) according to the manufacturer's
protocol. The sequences of the primers were as follows: ANP
forward, 5′-CAGCACAATAGAGCCGCTGA-3′ and reverse,
5′-GGGCAGGAGCTTGAACACG-3′; BNP forward, 5′-GCAGAAGCTGCTGGAGCTGA-3′
and reverse, 5′-ATCCGGAAGGCGCTGTCTTG-3′; GAPDH forward,
5′-GAGACAGCCGCATCTTCTTG-3′ and reverse, 5′-ATACGGCCAAATCCGTTCAC-3′.
The thermocycling conditions were as follows: Initial denaturation
at 95°C for 30 sec; 40 cycles of annealing at 95°C for 10 sec and
extension at 60°C for 30 sec. mRNA levels were normalized to the
internal reference gene GAPDH according to the 2−ΔΔCq
method (26).
Serum IL-6 and TNF-α
determination
Serum contents of IL-6 and TNF-α were determined
using IL-6 ELISA kit (cat. no. R6000B) and TNF-α ELISA kit (cat.
no. RTA00) obtained from R&D Systems, Inc. according to the
manufacturer's protocols.
Detection of ROS in heart tissues
The oxidation-sensitive fluorescent dye
dihydroethidium (DHE) was used to assess ROS content in
situ. Heart tissues were embedded in tissue freezing medium
(O.C.T. Compound 4583; Sakura Finetek USA, Inc.) and cut in a
cryostat (LeicaCM1850; Leica Microsystems GmbH). Transverse heart
tissue sections (5 µm) were disposed on glass slides, in order to
reach the equilibrium for 30–60 min at 37°C in PBS. The sections
were then incubated with DHE at 37°C for 30 min in the dark and
control sections received an identical volume of PBS. Fluorescent
images were subsequently obtained with an optical microscope
(magnification, ×200; LeicaDFC 300FX: Leica Microsystems GmbH).
Total and background pixel intensity were measured and used to
correct the DHE intensity of each image. Quantification of DHE
intensity was performed using ImageJ software (version 1.4.3.67,
National Institutes of Health).
Immunohistochemical analysis
Tissue samples were treated with endogenous
peroxidase blocker (cat. no. PV-6001; OriGene Technologies, Inc.)
at 25°C for 10 min, and incubated with anti-p65 antibodies (1:100,
cat. no. ab16502; Abcam) overnight at 4°C. This was followed by an
incubation with horseradish peroxidase-labeled goat anti-rabbit IgG
polymer (cat. no. PV-6001; OriGene Technologies, Inc.) at 25°C for
20 min. Tan or brown pigmented cell nuclei were considered to
indicate a positive expression. Images were captured using a
fluorescence microscope (Leica DMI300B). The results were analyzed
using the Image-Pro Plus 6.0 (Media Cybernetics, Inc.) image
analysis system. In each section, five positive colored regions
were randomly selected and the average intensity of nuclear
staining was determined.
Western blot analysis
Heart tissues were homogenized in phenylmethane
sulfonyl fluoride and RIPA lysis buffer (cat. no. P0013B, Beyotime
Institute of Biotechnology) and centrifuged at 12,000 × g for 20
min at 4°C. Nuclear proteins were separated using Active Motif's
Nuclear and Cytoplasm Extraction kit (Dakewe Biotech Co., Ltd.)
according to the manufacturer's protocol. A total of 40 µg of
protein/lane from all samples was loaded onto 10% SDS-PAGE,
transferred to polyvinylidene fluoride membranes. The membranes
were then blocked for 1.5 h at 25°C with 10% bovine serum albumin
(cat. no. 10735108001; Roche Applied Science) and incubated with
anti-calpain-1 (1:1,000, cat. no. 2556S), anti-p65 (1:500, cat. no.
ab86299), IкB-α (1:2,000, cat. no. ab7217), GAPDH (1:500, cat. no.
ab9483), IL-6 (1:1,000, cat. no. 3732S), TNF-α (1:1,500, cat. no.
A0277), ICAM-1 (1:1,000, cat. no. A559), VCAM-1 (1:1,000, cat. no.
A0279), TLR-4 (1:1,500, cat. no. A5258), eNOS (1:1,000, cat. no.
9572S), iNOS (1:1,000, cat. no. A0312) and Lamin B (1:500, cat. no.
ab8982) overnight at 4°C. Following extensive washing (TBST),
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:1,000, cat. no. AS014; ABclonal
Biotechnology Co., Ltd) for 2 h at 25°C and the immunoreactivity in
protein bands was visualized via enhanced chemiluminescence (Gaomin
ECL Chemiluminescence kit; Wanleibio Co., Ltd.). Western blotting
results were quantified with Wright Cell Imaging Facility ImageJ
Launcher software (version 1.4.3.67, National Institute of Health).
Each experiment was performed at least in triplicate.
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Statistical analysis of data was performed using a
one-way analysis of variance with Bonferroni's correction for
multiple group comparisons. A Student's t-test was used for
comparisons between two groups with SPSS 17.0 software (SPSS,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
LBP attenuates cardiac hypertrophy in
STZ-induced diabetic rats
In the control group, HE staining of the heart
tissue revealed that the morphology of cells was normal and that
myocardial fibers were arranged in neat rows (Fig. 1A). However, STZ-induced diabetic rats
demonstrated wide myocardial fibers and disarranged myocytes
(Fig. 1A). The subsequent
administration of LBP (30 and 60 mg/kg/d) improved the observed
pathological changes, indicating that LBP prevents effects of STZ
in heart tissue. The results of the current study also indicated
that STZ increased the ratios of HW/BW (Fig. 1B) and LVW/BW (Fig. 1C), and upregulated mRNA and protein
expression of various hypertrophic markers, including ANP and BNP
(Fig. 1D-G) when compared with the
control. However, LBP treatment reduced the ratios of HW/BW and
LVW/BW, and downregulated the mRNA and protein expression of ANP
and BNP compared with STZ alone. The aforementioned results
indicate that LBP treatment attenuates cardiac hypertrophy induced
by STZ.
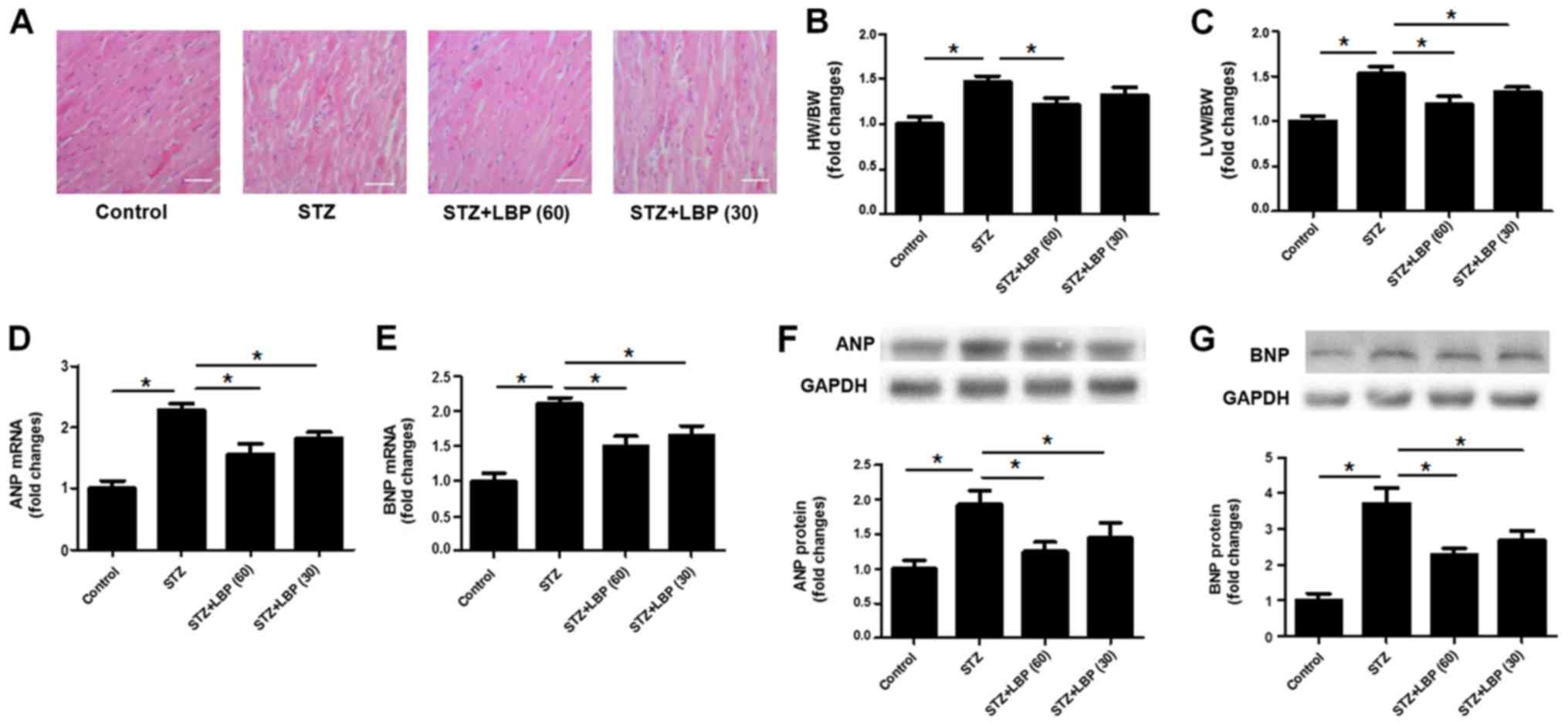 | Figure 1.LBP attenuates cardiac hypertrophy in
STZ-induced diabetic rats. (A) Histological changes of heart tissue
(scale bar, 40 µm). (B) HW/BW ratios. (C) LVW/BW ratio. (D) mRNA
expression of ANP. (E) mRNA expression of BNP. (F) Protein
expression of ANP. (G) Protein expression of BNP. Data are
expressed as the mean ± SEM (A, n=4, B and C, n=10, D-G, n=4).
*P<0.05. LBP, lycium barbarum polysaccharide; STZ,
streptozotocin; HW/BW, heart weight/body weight; LVW/BW, left
ventricle weight/body weight; ANP, atrial natriuretic peptide; BNP,
brain natriuretic peptide. |
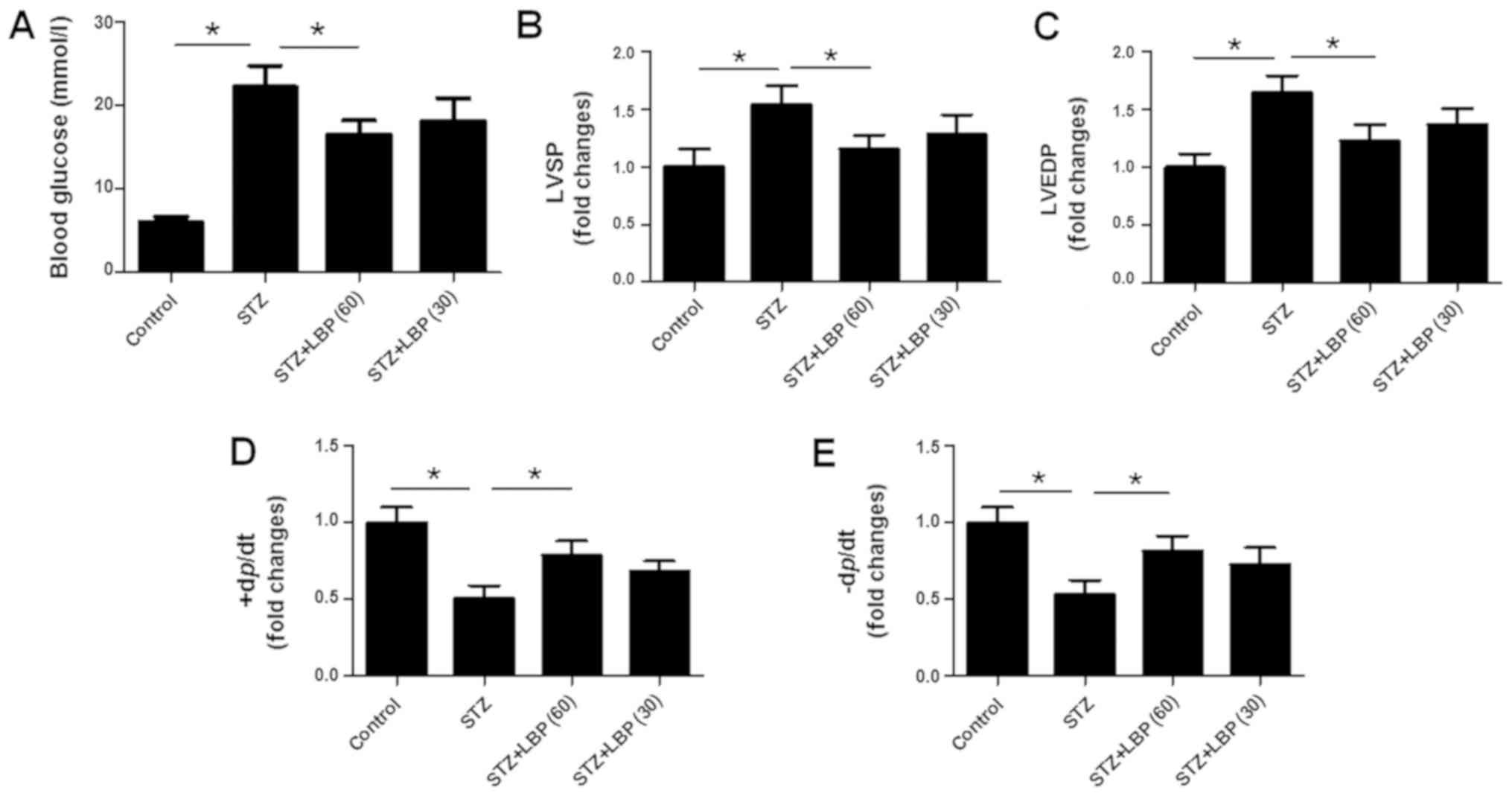
LBP lowers blood glucose levels of
STZ-induced diabetic rats
Compared with the control group, it was revealed
that there was nearly a two-fold increase in fasting blood glucose
levels following STZ injection (Fig.
2A). However, blood glucose levels were significantly decreased
in the STZ+LBP (60) group, but not in the STZ+LBP (30) group when compared with the STZ
group.
LBP improves cardiac hemodynamics in
STZ-induced diabetic rats
STZ treatment was revealed to increase LVSP
(Fig. 2B) and LVEDP (Fig. 2C), and decrease +dp/dtmax
(Fig. 2D) and-dp/dtmax
(Fig. 2E) compared with the control
group. However, LBP treatment was demonstrated to decrease LVSP and
LVEDP in a dose-dependent manner. Furthermore, LBP treatment and
increased ±dp/dtmax compared with STZ group.
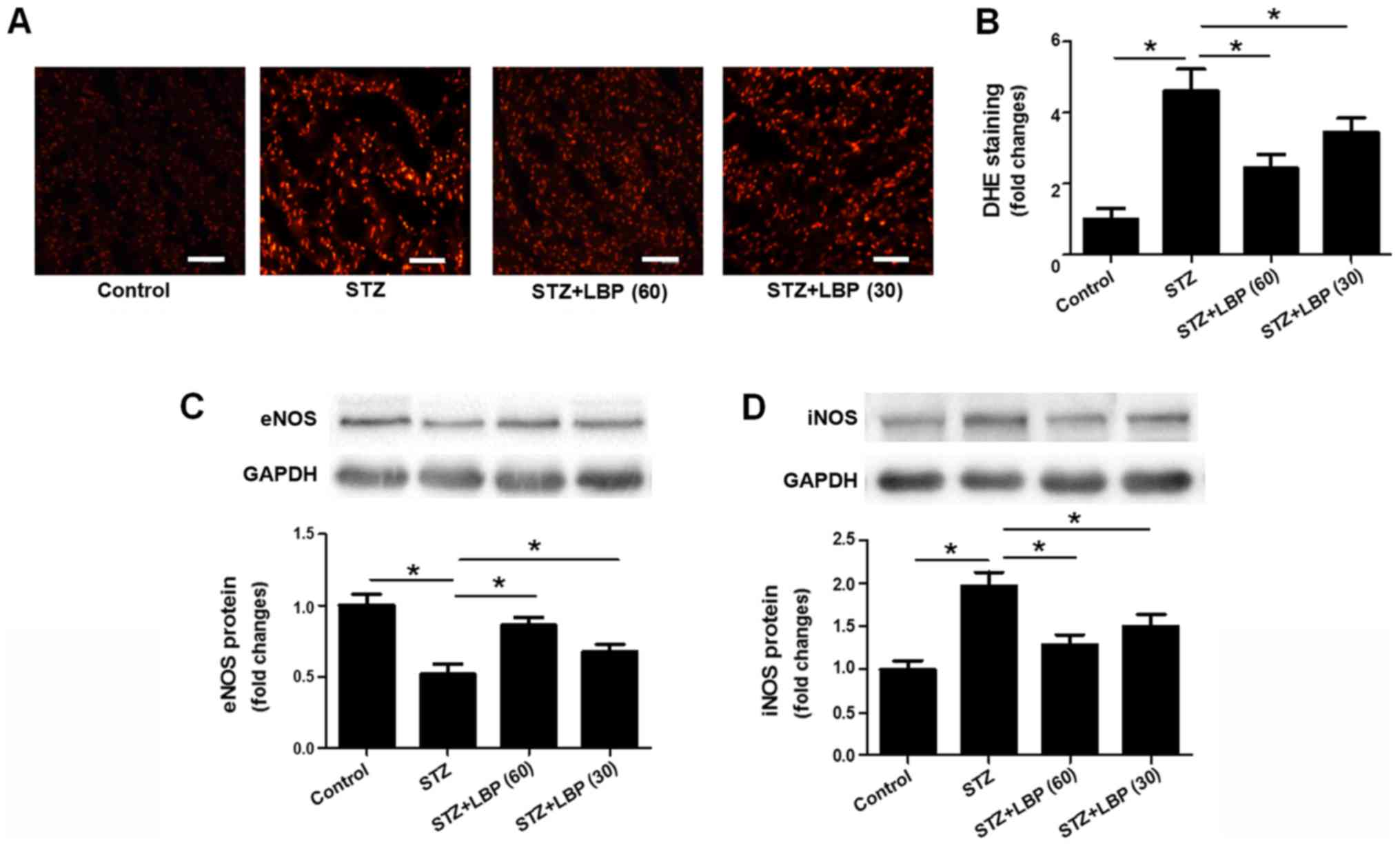
LBP reduces superoxide anion
generation and regulates the protein expression of eNOS and iNOS in
STZ-induced diabetic rats
DHE staining indicated a significant increase in
superoxide anion generation in heart tissue following STZ treatment
when compared with the control. However, LBP treatment was revealed
to reduce superoxide anion generation in a dose-dependent manner
compared with the STZ group (Fig. 3A and
B). STZ treatment was also revealed to downregulate the protein
expression of eNOS and upregulate the expression of iNOS compared
with the control group. Furthermore, the effect of STZ was reversed
when introducing LBP treatment (Fig. 3C
and D).
LBP decreases inflammatory cytokine
production in STZ-induced diabetic rats
Compared with the control group, STZ increased IL-6
(Fig. 4A) and TNF-α (Fig. 4B) content in rat serum, and
upregulated the protein expression of IL-6 (Fig. 4C), TNF-α (Fig. 4D), ICAM-1 (Fig. 4E), VCAM-1 (Fig. 4F) and TLR-4 (Fig. 4G) in rat heart tissue. However,
subsequent LBP treatment decreased serum IL-6 and TNF-α in a
dose-dependent manner and downregulated the protein expression of
IL-6, TNF-α, ICAM-1, VCAM-1 and TLR-4 compared with the STZ group.
These results indicate that the inhibition of cardiac hypertrophy
by LPB may be potentially attributed to its anti-inflammatory
properties.
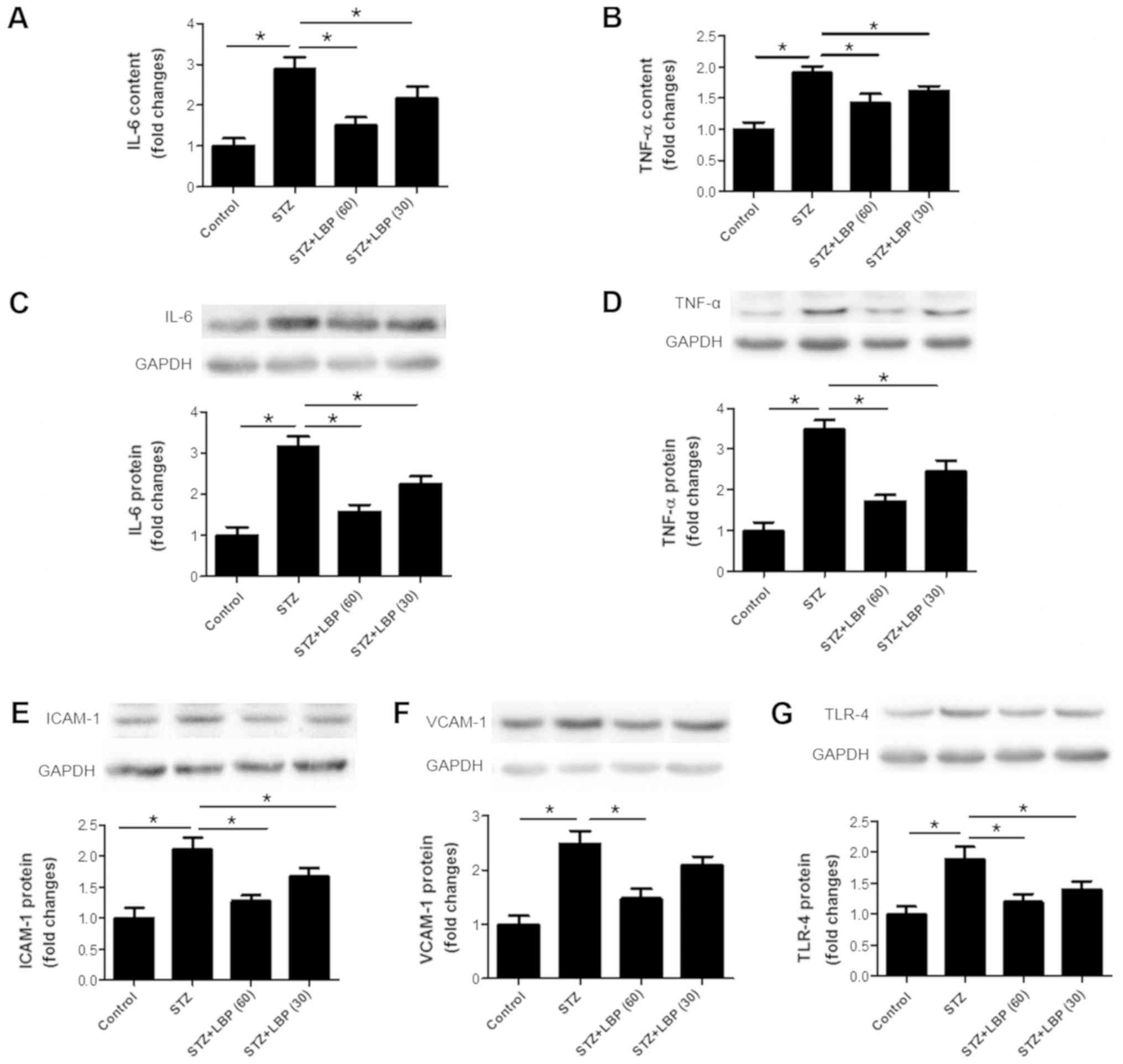 | Figure 4.LBP downregulates inflammatory
cytokine production in STZ-induced diabetic rats. Serum (A) IL-6
and (B) TNF-α content in STZ-induced rats. The expression of (C)
IL-6, (D) TNF-α, (E) ICAM-1, (F) VCAM-1 and (G) TLR-4 as determined
via western blotting. Data are expressed as the mean ± SEM (A and
B, n=10; C-G, n=4). *P<0.05. LBP, lycium barbarum
polysaccharide; STZ, streptozotocin; IL-6, interleukin-6; TNF-α,
tumor necrosis factor-α; ICAM-1, intercellular adhesion molecule-1;
VCAM-1, vascular adhesion molecule-1; TLR-4, Toll like
receptor-4. |
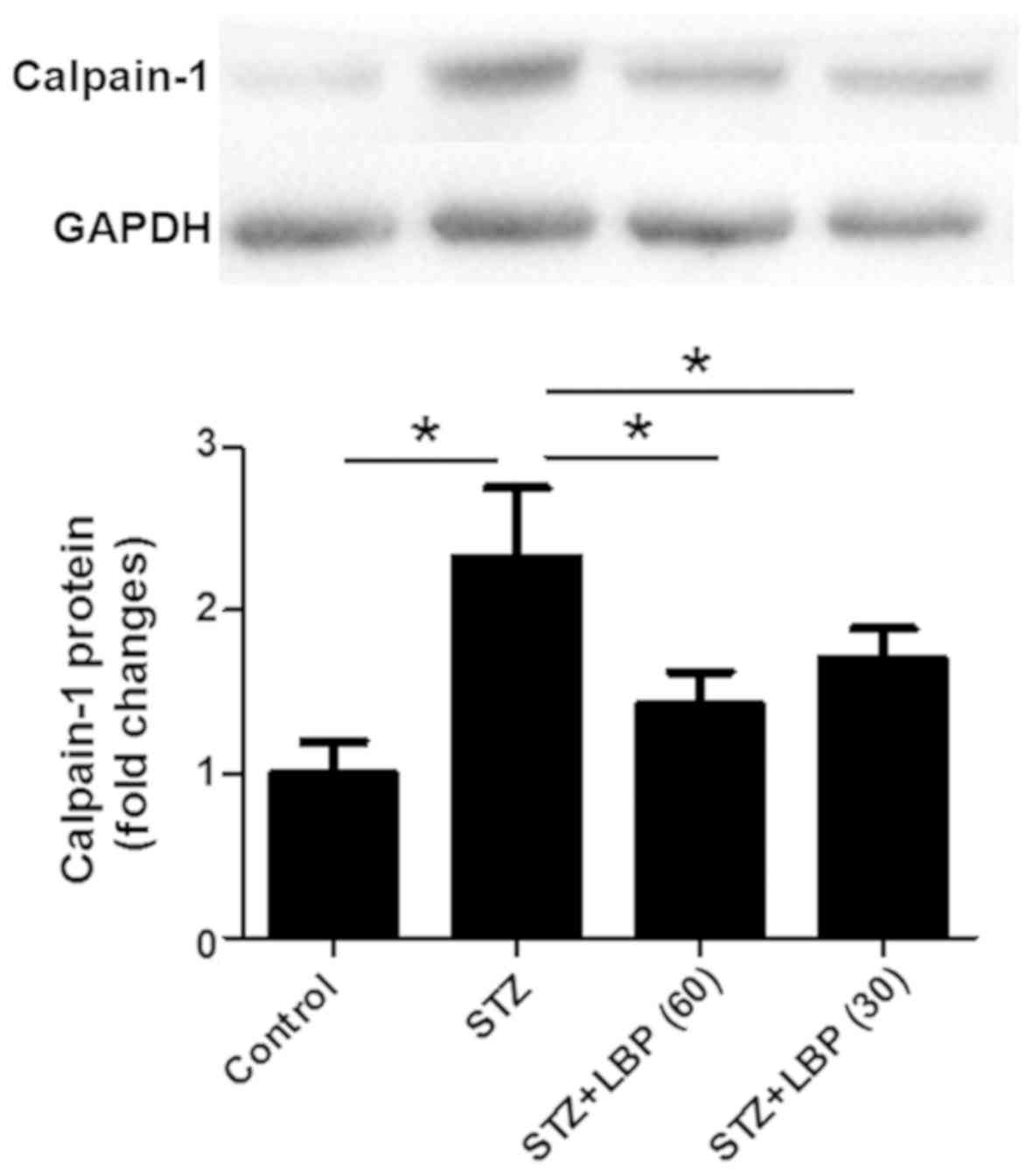
LBP downregulates the protein
expression of calpain-1 in STZ-induced diabetic rats
STZ increased the protein expression of calpain-1 in
rat heart tissue compared with the control group (Fig. 5). However, subsequent LBP treatment
dose-dependently downregulated the protein expression of calpain-1
when compared with the STZ group.
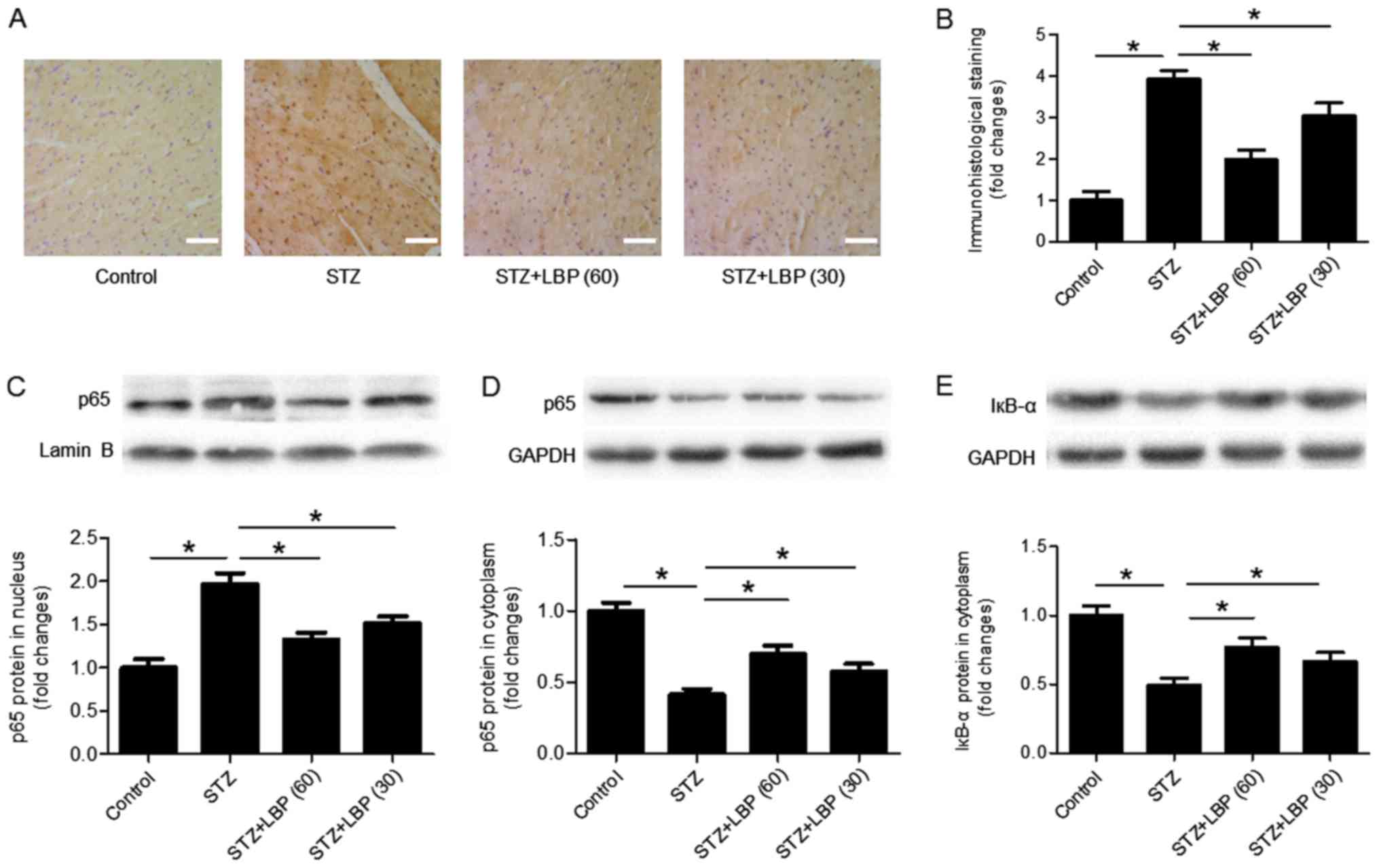
LBP inhibits the nuclear translocation
of NF-κB in STZ-induced diabetic rats
Immunohistological staining of the p65 antibody
indicated that STZ increased the number of p65 nuclear inputs
compared with the control, which was decreased following LBP
treatment (Fig. 6A and B). Western
blot analysis revealed that compared with the control, STZ
increased the protein expression of p65 in the nucleus (Fig. 6C), and decreased the expression of
p65 (Fig. 6D) and IкB-α (Fig. 6E) in the cytoplasm of heart tissues.
However, LBP treatment reversed the effect of STZ in a
dose-dependent manner.
Discussion
In the present study, it was revealed that
STZ-induced diabetic rats exhibited cardiac hypertrophy represented
by upregulation of mRNA and protein expression levels of ANP and
BNP, two hypertrophic markers (27),
accompanied by an increased protein expression of calpain-1 and
NF-κB activation in hypertrophic heart tissue. The results
indicated that an increased protein expression of calpain-1 and the
activation of NF-κB may serve key roles in the development of
cardiac hypertrophy in diabetic rats. It was also demonstrated that
the intragastrical administration of LBP attenuated cardiac
hypertrophy, as it decreased the expression of calpain-1 and
inhibited the activation of the NF-κB pathway. These results
indicate that the improvement of cardiac hypertrophy by LBP
treatment may be associated with the inhibition of calpain-1
expression and NF-κB pathway activation.
Lycium barbarum has been used as a
traditional Chinese herbal medicine for thousands of years
(15). LBP is the major active
ingredient extracted from Lycium barbarum. In STZ-induced
diabetic animals, LBP has been indicated to attenuate testicular
dysfunction (19), protect
peripheral neuropathy (20), improve
male sexual dysfunction and fertility impairments (21), enhance spermatogenesis (22) and inhibit diabetic nephropathy
(23). The results of the present
study indicated that LBP improves cardiac hypertrophy in
STZ-induced diabetic rats. The results of the aforementioned
studies and present study indicate that LBP may effectively
attenuate diabetic complications (19,22,23).
These results also provide experimental evidence for the effective
use of LBP in patients with diabetes. In terms of practical
applications, Cai et al (28)
reported that LBP may serve as a potential treatment aided-agent
for patients with diabetes.
Increasing evidence has indicated that inflammation
and oxidative stress serve key roles in the pathogenesis of cardiac
hypertrophy (2,25,29). The
present study also demonstrated that LBP inhibited the expression
of various inflammatory molecules including IL-6, TNF-α, ICAM-1,
VCAM-1 and TLR-4 in the serum and/or heart tissue of STZ-induced
rats. In addition, LBP reduced the production of ROS and regulated
the expression of iNOS and eNOS in diabetic heart tissue. These
results indicate that the protective effect of LBP on cardiac
hypertrophy in diabetes may be partly associated with the
inhibition of inflammation and oxidative stress. Similarly, Du
et al (18) reported that LBP
reduces oxidative stress and inflammation, subsequently leading to
anti-diabetic and anti-nephritis effects in a high-fat diet (12%
protein, 5% fat, 67% carbohydrate, 5% cholesterol, and 5% other
additives) and STZ-induced diabetic rats. In conclusion, these
results indicated that anti-oxidation and anti-inflammation may
serve as key underlying mechanisms for the improvement of diabetic
complications by LBP.
Guo et al (29) reported that NF-κB activation is an
important underlying mechanism of diabetic cardiac hypertrophy in
STZ-induced type 1 diabetic mice and high glucose-treated H9c2
cardiomyocytes. The aforementioned study supports those of the
present study as the protein expression of calpain-1 was increased
and NF-κB was activated in the hypertrophic heart tissue of
diabetic rats. In addition, it has been reported that calpain-1
accumulates in the mitochondria and promotes diabetic
cardiomyopathy (30). It has been
previously demonstrated that calpain-1 is upregulated in the
hypertrophic heart tissue of rats treated with isoproterenol
(8). In conclusion, the results of
the current study revealed that calpain-1 and NF-κB serve important
roles in the pathogenesis of cardiac hypertrophy. In accordance
with the present results indicating that LBP inhibits the
activation of NF-κB, Du et al (18) also reported that LBP mediates
anti-diabetic and anti-nephritic effects in a high-fat diet and
STZ-induced diabetic rats by regulating the NF-κB pathway.
The main limitation of the present study is that the
cause-effect relations among parameters including calpain-1
expression, NF-κB activation, inflammatory molecules and oxidation
indicators cannot be precisely determined. It is possible in the
future to overcome this limitation by conducting animal experiments
and/or by in vitro studies, using inhibitors of calpain-1
and NF-κB.
In conclusion, the present study demonstrated that
LBP treatment inhibited cardiac hypertrophy in STZ-induced diabetic
rats, an effect that may be associated with the inhibition of
calpain-1 expression and NF-κB activation. These results provide an
insight into the underlying mechanism of LBP and an experimental
basis for the potential therapeutic use of LBP in diabetic cardiac
hypertrophy.
Acknowledgements
Not applicable.
Funding
The current study was supported by the National
Natural Science Foundation of China (grant no. 81870329), the
Talent Fund of Liaoning Medical University (grant no. 2014-18) and
the Natural Science Foundation of Liaoning Province (grant no.
2018020325).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL, QH and ML performed the experiments, analyzed
the data and prepared the manuscript. FT and HW wrote and revised
the manuscript, and designed the experiments.
Ethical approval and consent to
participate
The experimental protocols were approved by the
Committee on the Ethics of Animal Experiments of Jinzhou Medical
University, China (Approval number: LMU-2016-138).
Patient consent for publication
Not applicable.
Conflict of interest
The authors declare that there is no conflict of
interest associated with this work.
References
|
1
|
Zhang J, Qiu H, Huang J, Ding S, Huang B,
Wu Q and Jiang Q: Naringenin exhibits the protective effect on
cardiac hypertrophy via EETs-PPARs activation in
streptozocin-induced diabetic mice. Biochem Biophys Res Commun.
502:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Ma J, Zhu H, Singh M, Hill D, Greer
PA, Arnold JM, Abel ED and Peng T: Targeted inhibition of calpain
reduces myocardial hypertrophy and fibrosis in mouse models of type
1 diabetes. Diabetes. 60:2985–2994. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Negishi K: Echocardiographic feature of
diabetic cardiomyopathy: Where are we now? Cardiovasc Diagn Ther.
8:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan M, Chen K, He L, Li S, Huang D and Li
J: Uric acid induces cardiomyocyte apoptosis via activation of
calpain-1 and endoplasmic reticulum stress. Cell Physiol Biochem.
45:2122–2135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hua Y and Nair S: Proteases in
cardiometabolic diseases: Pathophysiology, molecular mechanisms and
clinical applications. Biochim Biophys Acta. 1852:195–208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen B, Zhao Q, Ni R, Tang F, Shan L,
Cepinskas I, Cepinskas G, Wang W, Schiller PW and Peng T:
Inhibition of calpain reduces oxidative stress and attenuates
endothelial dysfunction in diabetes. Cardiovasc Diabetol.
13:882014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu L, Yin M, Yang X, Lu M, Tang F and Wang
H: Calpain inhibitor I attenuates atherosclerosis and inflammation
in atherosclerotic rats through eNOS/NO/NF-κB pathway. Can J
Physiol Pharmacol. 96:60–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mei M, Tang F, Lu M, He X, Wang H, Hou X,
Hu J, Xu C and Han R: Astragaloside IV attenuates apoptosis of
hypertrophic cardiomyocyte through inhibiting oxidative stress and
calpain-1 activation. Environ Toxicol Pharmacol. 40:764–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu T, Zhang B, Yang F, Cai C, Wang G, Han
Q and Zou L: HSF1 and NF-κB p65 participate in the process of
exercise preconditioning attenuating pressure overload-induced
pathological cardiac hypertrophy. Biochem Biophys Res Commun.
460:622–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu C, Tang F, Lu M, Yang J, Han R, Mei M,
Hu J, Zhou M and Wang H: Astragaloside IV improves the
isoproterenol-induced vascular dysfunction via attenuating eNOS
uncoupling-mediated oxidative stress and inhibiting ROS-NF-κB
pathways. Int Immunopharmacol. 33:119–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo G, Ren X, Qian X, Ye P, Luo J, Gao X,
Zhang J and Chen S: Inhibition of JNK and p38 MAPK-mediated
inflammation and apoptosis by ivabradine improves cardiac function
in streptozotocin-induced diabetic cardiomyopathy. J Cell Physiol.
234:1925–1936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Huang X, Ma Z, Wang Y, Chen X and
Gao Y: Ophiopogonin D alleviates cardiac hypertrophy in rat by
upregulating CYP2J3 in vitro and suppressing inflammation in vivo.
Biochem Biophys Res Commun. 503:1011–1019. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yun JM, Jialal I and Devaraj S: Epigenetic
regulation of high glucose-induced proinflammatory cytokine
production in monocytes by curcumin. J Nutr Biochem. 22:450–458.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou J, Li H, Chen X, Zeng S, Ye J, Zhou C,
Liu M, Zhang L, Yu N, Gan X, et al: C/EBPβ knockdown protects
cardiomyocytes from hypertrophy via inhibition of p65-NFκssssB. Mol
Cell Endocrinol. 390:18–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang HL, Chen C, Wang SK and Sun GJ:
Biochemical analysis and hypoglycemic activity of a polysaccharide
isolated from the fruit of Lycium barbarum L. Int J Biol Macromol.
77:235–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang DM, Zhang JQ and Fei YF: Lycium
barbarum polysaccharide attenuates chemotherapy-induced ovarian
injury by reducing oxidative stress. J Obstet Gynaecol Res.
43:1621–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen S, Liang L, Wang Y, Diao J, Zhao C,
Chen G, He Y, Luo C, Wu X and Zhang Y: Synergistic
immunotherapeutic effects of Lycium barbarum polysaccharide and
interferon-α2b on the murine Renca renal cell carcinoma cell line
in vitro and in vivo. Mol Med Rep. 12:6727–6737.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du M, Hu X, Kou L, Zhang B and Zhang C:
Lycium barbarum polysaccharide mediated the antidiabetic and
antinephritic effects in diet-streptozotocin-induced diabetic
sprague dawley rats via regulation of NF-κB. Biomed Res Int.
2016:31402902016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi GJ, Zheng J, Han XX, Jiang YP, Li ZM,
Wu J, Chang Q, Niu Y, Sun T, Li YX, et al: Lycium barbarum
polysaccharide attenuates diabetic testicular dysfunction via
inhibition of the PI3K/Akt pathway-mediated abnormal autophagy in
male mice. Cell Tissue Res. 374:653–666. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu SY, Chen L, Li XC, Hu QK and He LJ:
Lycium barbarum polysaccharide protects diabetic peripheral
neuropathy by enhancing autophagy via mTOR/p70S6K inhibition in
Streptozotocin-induced diabetic rats. J Chem Neuroanat. 89:37–42.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi GJ, Zheng J, Wu J, Qiao HQ, Chang Q,
Niu Y, Sun T, Li YX and Yu JQ: Protective effects of Lycium
barbarum polysaccharide on male sexual dysfunction and fertility
impairments by activating hypothalamic pituitary gonadal axis in
streptozotocin-induced type-1 diabetic male mice. Endocr J.
64:907–922. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi GJ, Zheng J, Wu J, Qiao HQ, Chang Q,
Niu Y, Sun T, Li YX and Yu JQ: Beneficial effects of Lycium
barbarum polysaccharide on spermatogenesis by improving antioxidant
activity and inhibiting apoptosis in streptozotocin-induced
diabetic male mice. Food Funct. 8:1215–1226. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao R, Li QW, Li J and Zhang T:
Protective effect of Lycium barbarum polysaccharide 4 on kidneys in
streptozotocin-induced diabetic rats. Can J Physiol Pharmacol.
87:711–719. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals, 8th
edition. National Academies Press (US); Washington, DC: 2011
|
|
25
|
Tang F, Lu M, Yu L, Wang Q, Mei M, Xu C,
Han R, Hu J, Wang H and Zhang Y: Inhibition of TNF-α-mediated NF-κB
Activation by Ginsenoside Rg1 Contributes the Attenuation of
Cardiac Hypertrophy Induced by Abdominal Aorta Coarctation. J
Cardiovasc Pharmacol. 68:257–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alvarez BV, Quon AL, Mullen J and Casey
JR: Quantification of carbonic anhydrase gene expression in
ventricle of hypertrophic and failing human heart. BMC Cardiovasc
Disord. 13:22013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai H, Liu F, Zuo P, Huang G, Song Z, Wang
T, Lu H, Guo F, Han C and Sun G: Practical application of
antidiabetic efficacy of Lycium barbarum polysaccharide in patients
with type 2 diabetes. Med Chem. 11:383–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo Y, Zhuang X, Huang Z, Zou J, Yang D,
Hu X, Du Z, Wang L and Liao X: Klotho protects the heart from
hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated
inflammation both in vitro and in vivo. Biochim Biophys Acta Mol
Basis Dis. 1864:238–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ni R, Zheng D, Xiong S, Hill DJ, Sun T,
Gardiner RB, Fan GC, Lu Y, Abel ED, Greer PA and Peng T:
mitochondrial calpain-1 disrupts atp synthase and induces
superoxide generation in type 1 diabetic hearts: A novel mechanism
contributing to diabetic cardiomyopathy. Diabetes. 65:255–268.
2016.PubMed/NCBI
|