Introduction
Neuropathic pain, a complex pathological change, is
stimulated or caused by the primary lesion and dysfunction of the
nervous system, which is mainly related to the plasticity changes
of the peripheral and central nervous systems (1,2). The
pathogenesis of the disease remains currently unclear. It is
relatively recognized that neurological deficits lead to the
sensitization of primary sensory neurons, and the enhancement of
excitatory synaptic transmission in the brainstem, spinal cord and
cerebral cortex, thereby resulting in chronic pain (3,4).
Neuropathic pain is a great challenge for clinical treatment due to
its complex mechanism, and there is little specific medicine for
its treatment, so it is of great significance to study the
mechanism of the disease and find new therapeutic targets.
Belonging to the histone deacetylase (HDAC) family,
HDAC2, widely present in eukaryotes, is important for cell
proliferation and homeostasis (5,6).
Acetylation is irreplaceable during inflammation of chronic pain
and neuronal sensitization, which opens chromatin structure,
activates transcription sites and increases gene expression.
Deacetylation of HDAC2 causes chromatin condensation, inhibits
transcription of related genes, and results in neuropathic pain.
HDACs mainly target K5, K9 and K13 sites on H2A; K5, K12, K15 and
K20 sites on H2B; K9, K14, K18 and K23 sites on H3; K5, K8, K12 and
K16 sites on H4 (7,8). Inositol polyphosphate-5-phosphatase F
(Inpp5f) has the SAC phosphatase domain, so it exerts the activity
of SAC phosphatase, inhibits the conversion of phosphatidylinosital
biphosphate (PIP2) to phosphatidylinosital triphosphate (PIP3) and
promotes the conversion of PIP2 to phosphatidylinosital phosphate
(PIP), thus inhibiting PI3K/AKT signal pathway (9). According to a study, pregabalin
effectively relieves neuropathic pain in rats with the disease, and
its efficacy is related to downregulation of HDAC2 and upregulation
of Inpp5f (10). Studies have also
reported that the knockout of mouse HDAC2 increases Inpp5f
expression, and makes the heart tolerant to the stimulation of
hypertrophy, and HDAC2 is a potential target for the treatment of
myocardial hypertrophy (11,12). These studies indicate a close
relationship among HDAC2, Inpp5f and PI3K/AKT signal pathway, which
may also be the case in the occurrence and progression of
neuropathic pain.
Therefore, a rat model of neuropathic pain was
established in this study to explore the relationship among HDAC2,
Inpp5f and PI3K/Akt/GSK-3β signal pathway, in order to provide an
experimental basis for further understanding the mechanism of
neuropathic pain in clinic.
Materials and methods
Research objects
Eighty SPF mature male SD rats were purchased from
Guangdong Medical Laboratory Animal Center, fed with SPF fortified
rat feeds (Jiangsu Xietong Organism Co., Ltd.). The age of the rats
was 42–50 days with an average age of 46.2±3.4 days; the weight was
216–250 g with an average weight of 233.6±4.8 g; the temperature
was maintained at 22±3°C and the humidity was 45–60%. The rats were
separately fed in the vivarium lighted with fluorescent lamps, free
to eat and drink water, with the cage and water bottle changed once
to twice weekly. The rats were randomly divided into the sham
operation, the model, the HDAC2 intervention (group A) and the
Inpp5f intervention (group B) groups (n=20).
The study was approved by the Ethics Committee of
Fifth Hospital in Wuhan (Wuhan, China).
SD rat modeling
Morris water maze was carried out for one week,
twice daily. The rats found the third quadrant security platform
within 2 min and stayed on it for 45 sec, and those who failed were
guided manually to complete the training. Thirty minutes later, the
rat models of neuropathic pain were established in the model and
intervention groups according to Chen et al (13). The rats were fixed on a larminar flow
and anesthetized with an intraperitoneal injection of 10% chloral
hydrate (Wuhan Yuancheng Technology Development Co., Ltd.) (300
µg/g body weight). Then, skin incision was performed and the
sciatic nerve was bluntly dissected. After that, 4-0 chromic catgut
(Henan Zeyuan Medical Device Sales Co., Ltd.) was used to ligate
the sciatic nerve trunk 3 times at intervals no more than 2 mm, and
Johnson absorbable sutures (Shanghai Hanfei Medical Instrument Co.,
Ltd.) to suture the incision layer by layer, with lincomycin
hydrochloride (0.2 ml, 10 mg/ml) injected into the lateral lower
limb muscles for diminishing inflammation. The ligature ensured
that the nerve was compressed within 7 days while not affecting the
blood transportation of the epineurium. The calf muscle of the rats
slightly vibrated during ligation.
Intervention
Interference vector of HDAC2 and overexpression
vector of Inpp5f, namely pCDsRed2-HDAC2-shRNA and
pCDsRed2-Inpp5f-mimic, were constructed and synthesized by Takara
Biotechnology Co., Ltd. At the 15th day after operation, all rats
were generally anesthetized with an intraperitoneal injection of
pentobarbital sodium (50 mg/kg), and received lumbar puncture at
the L5-L6 intervertebral space with a puncture needle (Nanjing
Jiancheng Bioengineering Institute). Rats showing lateral tail whip
or cerebrospinal fluid flowing from the end of the needle tubing
indicated successful puncture. A microinjector with 50 µl was used
to extract normal saline (10 µg/g), bubbles (1 µl) and vectors or
normal saline (2.0 µl) in sequence, connected with the puncture
needle to inject evenly and slowly into the spinal canal.
Observational indexes
Rats in the four groups were observed before
modeling, after modeling/before intervention and 3 days after
intervention in terms of paw thermal withdrawal latency (PWL), paw
withdrawal mechanical threshold (PWT, measured through Von Frey
filaments) and changes in cognitive function (Morris water maze and
passive avoidance task). Then the rats were sacrificed. RT-qPCR and
western blot analysis were used to detect the levels of HDAC2 mRNA,
Inpp5f mRNA, phosphorylated PI3K (p-PI3K), phosphorylated AKT
(p-AKT), phosphorylated GSK-3β (p-GSK-3β) in rat brain tissue,
Correlation of HDAC2 mRNA with Inpp5f mRNA expression levels was
detected by Pearsons correlation analysis.
PWL
When the rats placed in the cage were quiet, the
light was gathered in the middle of the bottom of the toe, and the
upper limit of PWL was 20 sec in order to avoid scalding. PWL was
the time from irradiation to the rats raising the leg or escaping,
the average value was obtained for 3 times with an interval of 10
min each time. PWL measured the day before modeling was used as a
baseline value.
PWT
The rats' feet were stimulated through Von Frey
filaments with different thresholds with the highest one of 26 g.
Rapid paw withdrawal within the stimulation time or when the
filaments were removed was considered as positive. When the
withdrawal was positive for 3 times within 5 consecutive tests with
a certain Von Frey filament, the threshold was regarded as PWT. The
interval between each experiment was 10 sec.
RT-qPCR
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) was used to extract total RNA in the brain
tissue, with the steps carried out according to manufacturers
instructions, an ultraviolet spectrophotometer (Mettler Toledo) to
analyze the concentration and purity, 3% agarose gel
electrophoresis to analyze the integrity, a micro nucleic acid
spectrometer to detect the purity, with A260/A280 value between 1.8
and 2.1 considered to meet the experimental requirements. After
that, RT-qPCR reaction was carried out. The reverse transcription
reaction system was 1.0 µl of DTT (0.1 M), 2.0 µl of dNTP mixture
(10 M), 1.0 µl of M-MLV reverse transcriptase, 2 µg of total RNA,
4.0 µl of 5X Buffer, RNAse Free ddH2O added to 20 µl,
incubated at 75°C for 5 min and at 37°C for 2 h. Then, PCR
amplification was carried out, and the system was 2 µl of cDNA
template, 10 µl of SYBR Premix Ex Taq II (2X), each 1 µl of
upstream and downstream primers, double distilled water added to 20
µl, at 95°C for 3 min, at 95°C for 5 sec, at 60°C for 34 sec, for
40 cycles. Melting curve analysis was performed after the
experiment. With glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
as a reaction internal reference, 3 identical wells were set for
each sample, and the results were analyzed by 2−ΔΔCq
(14). QuantScript RT kit was
purchased from Tiangen Biotech Co., Ltd. with an item no. KR103-04,
RT-qPCR detection kit from Takara Biotechnology Co., Ltd. Primer
sequences were designed and synthesized by HePeng Biology (Table I).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Variables | Upstream primers | Downstream
primers |
|---|
| HDAC2 |
5′-TGACATTGTGCTTGCTGTCC-3′ |
5′-CCCTCAAGTCTCCTGTTCCA-3′ |
| Inpp5f |
5′-GGAGGCCACTTGTGTAGAT-3′ |
5′-GGAGGCCACTTGTGTAGAT-3′ |
| GAPDH |
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ |
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′ |
Western blot analysis
p-PI3K, p-AKT and p-GSK-3β in rat brain tissue were
detected. The protein concentration was measured using the BCA
method (Thermo Fisher), and the concentration of the protein was
adjusted to 4 µg/µl. The protein was extracted from the tissue with
RIPA lysate, with 50 ng of protein loaded per lane, separated with
12% polyacrylamide gel electrophoresis with an initial voltage of
90 V, moved to the appropriate position of the separation gel
through increasing the voltage to 120 V, transferred to the
membrane [polyvinylidene fluoride (PVDF)] with a constant voltage
of 100 V for 100 min, and sealed at 37°C for 60 min. Then, the
transfer membrane was sealed in 5% skim milk, subjected to an
immune reaction, incubated with primary antibodies (p-PI3K, p-AKT
and p-GSK-3β monoclonal antibodies; Shenyang Wanlei Bio Co., Ltd.;
cat. nos. wl02849, wlp001a and wl03518, respectively; dilution,
1:1,000) overnight at 4°C, washed with PBS over 5 min each time 3
times the next day, incubated with secondary antibody (goat
anti-rat IgG polyclonal antibody; Shanghai Xinyu Bio Co., Ltd.;
cat. no. xyKS016; dilution, 1:1,000) for 1 h at room temperature,
then developed at room temperature for 1 min and fixed for 5 min
with ECL luminescent reagent (Shanghai QiMing Biotechnology Co.,
Ltd., GJ50436). The bands scanned were statistically analyzed using
Quantity One, and the relative expression level of protein = gray
value of the band/gray value of the internal reference. Western
blot detection kit was purchased from Shanghai Youyu Biotechnology
Co., Ltd. with cat. nos. JC-445; p-PI3K, p-AKT and p-GSK-3β
monoclonal antibodies from Shenyang Wanlei Bio Co., Ltd. with cat.
nos. wl02849, wlp001a and wl03518; secondary antibody (goat
anti-rat IgG) from Shanghai Xinyu Bio Co., Ltd., with cat. no.
xyKS016.
Statistical analysis
SPSS19.0 [Asia Analytics (formerly SPSS China)] was
used to analyze the data. Enumeration data were expressed as rate.
Measurement data were expressed as mean ± standard deviation.
Analysis of variance (ANOVA) was used for comparison between groups
as well as for repeated measurements for comparison at different
time-points within the group and LSD test for back testing.
Pearsons correlation analysis was used for the correlation of HDAC2
mRNA with Inpp5f mRNA expression levels. P<0.05 was considered
to indicate a statistically significant difference.
Results
Test results of PWL
There was no statistically significant difference in
PWL between the four groups before modeling (P>0.05), but there
was a statistically significant difference after modeling/before
intervention and three days after intervention (P<0.05). After
modeling/before intervention and three days after intervention, PWL
in the model group and groups A and B was lower than that in the
sham operation group (P<0.05). After modeling/before
intervention, PWL in the model group, groups A and B was not
statistically different (P>0.05). Three days after intervention,
PWL in groups A and B was higher than that in the model group
(P<0.05), which in group A was higher than that in group B
(P<0.05). PWL in the sham operation group was not statistically
different at each time-point (P>0.05), which in the model group
was decreased continuously (P<0.05). In groups A and B, PWL was
lower after modeling/before intervention and three days after
intervention than that before modeling (P<0.05), which was
higher three days after intervention than that after
modeling/before intervention (P<0.05; Table II).
 | Table II.Test results of PWL (sec). |
Table II.
Test results of PWL (sec).
| Variables | Sham operation
group | Model group | Group A | Group B | F value | P-value |
|---|
| Before modeling | 7.07±0.12 | 7.02±0.17 | 7.02±0.21 | 7.02±0.23 |
0.356 | 0.745 |
| After modeling/before
intervention | 6.96±0.22 |
4.59±0.21a,d |
4.61±0.22a,d |
4.59±0.22a,d | 235.647 | <0.001 |
| Three days after
intervention | 6.99±0.21 |
3.89±0.37a,d,e |
6.06±0.18a,b,d,e |
5.03±0.19a–e | 571.433 | <0.001 |
Test results of PWT
There was no statistically significant difference in
PWT between the four groups before modeling (P>0.05), but there
was a statistically significant difference after modeling/before
intervention and three days after intervention (P<0.05). After
modeling/before intervention and three days after intervention, PWT
in the model group, groups A and B was higher than that in the sham
operation group (P<0.05). After modeling/before intervention,
PWT in groups A and B was not different (P>0.05). Three days
after intervention, PWT in groups A and B was lower than that in
the model group (P<0.05), which in group A was lower than that
in group B (P<0.05). PWT in the sham operation group was not
different at each time-point (P>0.05), which in the model group
was increased continuously (P<0.05). In groups A and B, PWT was
higher after modeling/before intervention and three days after
intervention than that before modeling (P<0.05), which was lower
three days after intervention than that after modeling/before
intervention (P<0.05; Table
III).
 | Table III.Test results of PWT (g). |
Table III.
Test results of PWT (g).
| Variables | Sham operation
group | Model group | Group A | Group B | F value | P-value |
|---|
| Before modeling | 46.66±2.57 | 46.45±2.10 | 46.41±2.62 | 46.81±2.16 |
0.124 | 0.946 |
| After modeling/before
intervention | 45.66±2.39 |
66.17±2.48a,d |
65.67±3.03a,d |
65.66±1.47a,d | 350.961 | <0.001 |
| Three days after
intervention | 45.76±2.05 |
72.69±2.13a,d,e |
52.91±1.69a,b,d,e |
58.34±1.83a–e | 696.475 | <0.001 |
Changes in cognitive function
Three days after intervention, there were
statistically significant differences between the four groups in
terms of swimming time, swimming distance, number of errors and
latent time (P<0.05), which in the model group, and groups A and
B were longer than those in the sham operation group (P<0.05),
and which in groups A and B were shorter than those in the model
group (P<0.05), and which in group A were shorter than those in
group B (P<0.05; Table IV).
 | Table IV.Changes in cognitive function. |
Table IV.
Changes in cognitive function.
| Variables | Sham operation
group | Model group | Group A | Group B | F value | P-value |
|---|
| Swimming time
(sec) |
80.42±16.75 |
152.72±28.45a |
103.31±20.72a,b |
124.50±24.90a–c | 35.467 | <0.001 |
| Swimming distance
(cm) | 138.62±31.27 |
361.94±104.71a |
227.52±55.48a,b |
256.74±57.59a–c | 36.981 | <0.001 |
| Number of errors
(times) |
2.34±1.11 |
9.24±3.21a |
4.79±1.87a,b |
6.82±1.75a–c | 38.117 | <0.001 |
| Latent time
(sec) | 13.18±5.42 |
73.41±25.34a |
31.55±18.43a,b |
52.31±17.59a–c | 41.016 | <0.001 |
Test results of HDAC2/Inpp5f
Three days after intervention, there were
statistically significant differences in the expression levels of
HDAC2 mRNA and Inpp5f mRNA between the four groups (P<0.05).
Compared with the sham operation group, the rats in the model group
and groups A and B had higher HDAC2 mRNA expression level
(P<0.05), but lower Inpp5f mRNA expression level (P<0.05).
HDAC2 mRNA expression level was lower in group A than that in the
model group and group B (P<0.05), whereas Inpp5f mRNA expression
level in groups A and B was higher than that in the model group
(P<0.05). There was no statistical difference in HDAC2 mRNA
expression level between the model group and group B (P>0.05),
whereas Inpp5f mRNA expression level in group B was higher than
that in group A (P<0.05; Table
V).
 | Table V.Test results of HDAC2/Inpp5f. |
Table V.
Test results of HDAC2/Inpp5f.
| Variables | Sham operation
group | Model group | Group A | Group B | F value | P-value |
|---|
| HDAC2 mRNA | 1.83±0.34 |
6.23±1.59a |
3.12±0.83a,b |
6.02±0.74a,c | 97.439 | <0.001 |
| Inpp5f mRNA | 1.41±0.28 |
0.59±0.09a |
0.73±0.14a,b |
1.15±0.12a–c | 94.716 | <0.001 |
Test results of PI3K/Akt/GSK-3β signal
pathway related proteins
There were statistically significant differences in
the expression levels of p-PI3K, p-AKT and p-GSK-3β between the
four groups (P<0.05), which in the model group, and groups A and
B were higher than those in the sham operation group (P<0.05),
and which in groups A and B were lower than those in the model
group (P<0.05), and which in group A were lower than those in
group B (P<0.05; Table VI).
 | Table VI.PI3K/Akt/GSK-3β signal pathway
related proteins. |
Table VI.
PI3K/Akt/GSK-3β signal pathway
related proteins.
| Variables | Sham operation
group | Model group | Group A | Group B | F value | P-value |
|---|
| p-PI3K | 0.943±0.024 |
1.463±0.135a |
1.125±0.098a,b |
1.246±0.099a–c | 99.689 | <0.001 |
| p-AKT | 1.399±0.055 |
1.942±0.213a |
1.617±0.123a,b |
1.779±0.163a–c | 47.745 | <0.001 |
| p-GSK-3β | 1.109±0.072 |
1.671±0.154a |
1.285±0.103a,b |
1.375±0.081a–c | 95.838 | <0.001 |
Correlation analysis of HDAC2 mRNA
with Inpp5f mRNA expression levels
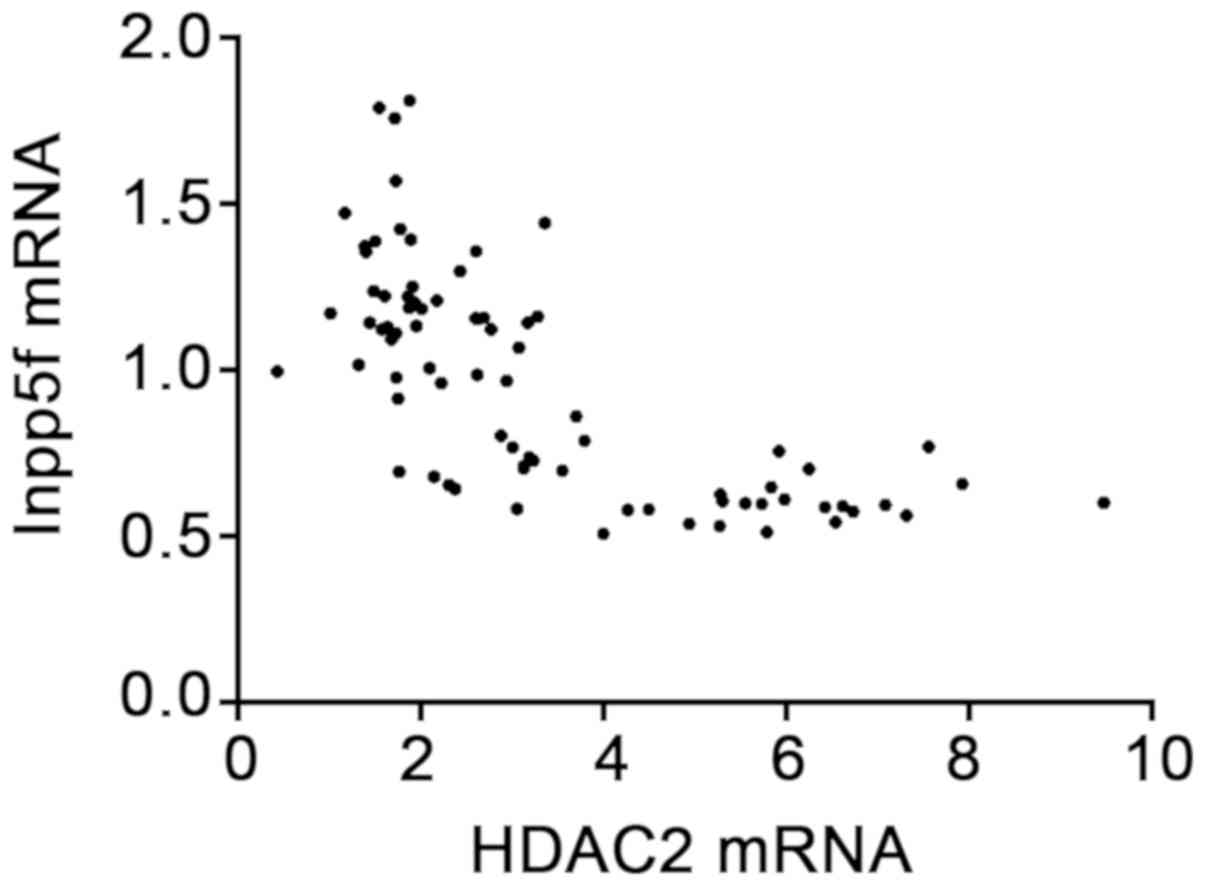
According to Pearsons correlation analysis, the
expression level of HDAC2 mRNA was negatively correlated with that
of Inpp5f mRNA (r=−0.695, P<0.001; Fig. 1).
Discussion
Neuropathic pain is a complex pain syndrome. The
incidence rate is approximately 1.5% in the world, and in China it
is increasing year by year, so finding effective treatments is
urgent (15,16). A rat model of neuropathic pain was
established in this study to explore the antagonism and mechanism
of HDAC2/Inpp5f on neuropathic pain, so as to provide effective
therapeutic targets and experimental bases for the prevention and
treatment of neuropathic pain and cognitive dysfunction.
A rat model of neuropathic pain suffers from
neuropathic pain about 24 h after operation for approximately 10
weeks, which is similar to characteristics of clinical neuropathic
pain (17,18), and meets the requirements of this
experiment. PWL and PWT describe neuropathic pain in rats (19), which are used to describe the rat
model in studies on neuropathic pain. In this study, compared with
the sham operation group, rats in the model group had significantly
lower PWL but higher PWT. Additionally, rats with neuropathic pain
licked and sucked or swung the stimulated hind limbs in the air.
The combination of the two indicates that the model rats had
neuropathic pain, so the rat model of neuropathic pain in this
experiment was successfully established. Three days after
intervention in HDAC2 and Inpp5f expression, PWL was significantly
higher but PWT was significantly lower compared with before
intervention, indicating that inhibition of HDAC2 expression or
promotion of Inpp5f expression has a good antagonistic effect on
neuropathic pain in rats. In this study, inhibition of HDAC2
expression resulted in an increase in Inpp5f expression, whereas
promotion of Inpp5f expression had no obvious effect on HDAC2
expression, and HDAC2 expression level was negatively correlated
with Inpp5f expression level. These findings indicate that there is
one-way regulation between HDAC2 and Inpp5f, and inhibition of
HDAC2 expression can promote Inpp5f expression. Currently, there
are few reports on the roles of HDAC2 and Inpp5f in neuropathic
pain. According to other studies, HDAC2 in LacZ mice resists
cardiomyocyte hypertrophy and pressure-dependent cardiac pachynsis
through increasing Inpp5f expression (11,20). In
addition, HDAC2 promotes tumor development through inhibiting
Inpp5f (21). These studies prove
that HDAC2 has a regulatory relationship with Inpp5f, thus
confirming our conclusions.
In this study, the established rat model of
neuropathic pain activated PI3K/Akt/GSK-3β signal pathway in the
brain tissue, and the expression levels of p-PI3K, p-AKT and
p-GSK-3β were significantly higher than those in the sham operation
group. Besides, after intervention in HDAC2 or Inpp5f expression,
inhibition of HDAC2 expression or promotion of Inpp5f expression
reduced the expression levels of p-PI3K, p-AKT and p-GSK-3β,
suggesting its role as inhibiting PI3K/Akt/GSK-3β signal pathway,
and that inhibition of HDAC2 expression is more effective than
promotion of Inpp5f expression. Based on the previous results,
HDAC2 may promote Inpp5f expression and thus inhibit
PI3K/Akt/GSK-3β signal pathway. However, the regulation of Inpp5f
expression is not the only mechanism of HDAC2. According to a
study, HDAC has a regulatory effect on PI3K/Akt/GSK-3β signal
pathway, and inhibition of HDAC regulates polarization of
microglial cells/macrophages through inhibiting GSK-3β/PTEN/Akt
axis, so as to prevent white matter damage (22). HDAC2 also regulates cardiac
hypertrophy response through inhibiting the activity of GSK-3β
(11).
Neuropathic pain, abnormal pain caused by
neurological deficits, usually leads to cognitive dysfunction
(1–4), so improving the cognitive function of
patients is also an important goal of clinical treatment. According
to this study, inhibition of HDAC2 expression or promotion of
Inpp5f expression improves the cognitive function of rats, but the
former is more effective. In studies on the mouse model of
Alzheimer's disease, knockout of HDAC2 reverses the deacetylation
of histones of learning and memory genes by HDAC2, restores the
structure and synaptic plasticity of neurons, and eliminates
dysmnesia related to neurodegeneration (23,24).
There are few studies on Inpp5f and cognitive function, but
according to a study, inhibition of PI3K/Akt/GSK-3β signal pathway
prevents and treats diabetic cognitive dysfunction (25). The inhibitory effect of Inpp5f on
PI3K/Akt signal pathway is clear (9), so the conclusion that Inpp5f improves
rat cognitive function is credible, which will need to be proved
again in future studies.
In summary, neuropathic pain can cause an increase
in HDAC2 expression level and a decrease in Inpp5f expression
level, and activate the PI3K/Akt/GSK-3β signal pathway. Inhibition
of HDAC2 expression can inhibit the activation of PI3K/Akt/GSK-3β
signal pathway through increasing Inpp5f expression, thus improving
the condition and cognitive disorder of rats with neuropathic
pain.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY wrote the manuscript. CL and YW performed PCR. HY
and TL were responsible for western blot analysis. LY and CL
contributed to observation indexes analysis. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Fifth Hospital in Wuhan (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mu A, Weinberg E, Moulin DE and Clarke H:
Pharmacologic management of chronic neuropathic pain: Review of the
Canadian Pain Society consensus statement. Can Fam Physician.
63:844–852. 2017.PubMed/NCBI
|
|
2
|
Ryan NM, Vertigan AE and Birring SS: An
update and systematic review on drug therapies for the treatment of
refractory chronic cough. Expert Opin Pharmacother. 19:687–711.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Hecke O, Austin SK, Khan RA, Smith BH
and Torrance N: Neuropathic pain in the general population: a
systematic review of epidemiological studies. Pain. 155:654–662.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liem L, Russo M, Huygen FJ, Van Buyten JP,
Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, et al:
One-year outcomes of spinal cord stimulation of the dorsal root
ganglion in the treatment of chronic neuropathic pain.
Neuromodulation. 18:41–48; discussion 48–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu W, Li Z, Xiong L, Yu X, Chen X and Lin
Q: FKBP3 promotes proliferation of non-small cell lung cancer cells
through regulating Sp1/HDAC2/p27. Theranostics. 7:3078–3089. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zimberlin CD, Lancini C, Sno R, Rosekrans
SL, McLean CM, Vlaming H, van den Brink GR, Bots M, Medema JP and
Dannenberg JH: HDAC1 and HDAC2 collectively regulate intestinal
stem cell homeostasis. FASEB J. 29:2070–2080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verrills P, Sinclair C and Barnard A: A
review of spinal cord stimulation systems for chronic pain. J Pain
Res. 9:481–492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kami K, Taguchi S, Tajima F and Senba E:
Histone acetylation in microglia contributes to exercise-induced
hypoalgesia in neuropathic pain model mice. J Pain. 17:588–599.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Genty J, Tetsi Nomigni M, Anton F and
Hanesch U: Maternal separation stress leads to resilience against
neuropathic pain in adulthood. Neurobiol Stress. 8:21–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vakkala M, Järvimäki V, Kautiainen H,
Haanpää M and Alahuhta S: Incidence and predictive factors of
spinal cord stimulation treatment after lumbar spine surgery. J
Pain Res. 10:2405–2411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu W, Trivedi CM, Zhou D, Yuan L, Lu MM
and Epstein JA: Inpp5f is a polyphosphoinositide phosphatase that
regulates cardiac hypertrophic responsiveness. Circ Res.
105:1240–1247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho YK, Eom GH, Kee HJ, Kim HS, Choi WY,
Nam KI, Ma JS and Kook H: Sodium valproate, a histone deacetylase
inhibitor, but not captopril, prevents right ventricular
hypertrophy in rats. Circ J. 74:760–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen XM, Xu J, Song JG, Zheng BJ and Wang
XR: Electroacupuncture inhibits excessive interferon-γ evoked
up-regulation of P2X4 receptor in spinal microglia in a CCI rat
model for neuropathic pain. Br J Anaesth. 114:150–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cho JH, Lee JH, Song KS and Hong JY:
Neuropathic pain after spinal surgery. Asian Spine J. 11:642–652.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gilron I, Baron R and Jensen T:
Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin
Proc. 90:532–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Genda Y, Arai M, Ishikawa M, Tanaka S,
Okabe T and Sakamoto A: microRNA changes in the dorsal horn of the
spinal cord of rats with chronic constriction injury: a
TaqMan® low density array study. Int J Mol Med.
31:129–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen HP, Zhou W, Kang LM, Yan H, Zhang L,
Xu BH and Cai WH: Intrathecal miR-96 inhibits Nav1.3 expression and
alleviates neuropathic pain in rat following chronic construction
injury. Neurochem Res. 39:76–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deer TR, Levy RM, Kramer J, Poree L,
Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J,
et al: Dorsal root ganglion stimulation yielded higher treatment
success rate for complex regional pain syndrome and causalgia at 3
and 12 months: a randomized comparative trial. Pain. 158:669–681.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kee HJ, Eom GH, Joung H, Shin S, Kim JR,
Cho YK, Choe N, Sim BW, Jo D, Jeong MH, et al: Activation of
histone deacetylase 2 by inducible heat shock protein 70 in cardiac
hypertrophy. Circ Res. 103:1259–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu P, Martin E, Mengwasser J, Schlag P,
Janssen KP and Göttlicher M: Induction of HDAC2 expression upon
loss of APC in colorectal tumorigenesis. Cancer Cell. 5:455–463.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang G, Shi Y, Jiang X, Leak RK, Hu X, Wu
Y, Pu H, Li WW, Tang B, Wang Y, et al: HDAC inhibition prevents
white matter injury by modulating microglia/macrophage polarization
through the GSK3β/PTEN/Akt axis. Proc Natl Acad Sci USA.
112:2853–2858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gräff J, Rei D, Guan JS, Wang WY, Seo J,
Hennig KM, Nieland TJ, Fass DM, Kao PF, Kahn M, et al: An
epigenetic blockade of cognitive functions in the neurodegenerating
brain. Nature. 483:222–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmauss C: The roles of class I histone
deacetylases (HDACs) in memory, learning, and executive cognitive
functions: a review. Neurosci Biobehav Rev. 83:63–71. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X and Zhao L: Calycosin ameliorates
diabetes-induced cognitive impairments in rats by reducing
oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem
Biophys Res Commun. 473:428–434. 2016. View Article : Google Scholar : PubMed/NCBI
|