Introduction
Satureja hortensis L. is a plant known and
used as remedy for more than 20 centuries. The name was assigned by
the Roman writer Pliny, being derived from the word ‘satyr’ (the
Latin name satureia) which describes a creature from ancient
mythology (half man, half goat) and the legend says that savories
belonged to him (1–3). This perennial plant belongs to an
important family of aromatic and medicinal herbs, Lamiaceae that
includes more than 200 genera widespread in Europe (the southern
and eastern regions), Asia (the western region), South America and
some Spanish islands (3,4). Satureja hortensis L., known as
summer savory, is native of Europe, especially from the Balkan
regions and its leaves and stems are currently utilized as tea,
spice or flavoring agent (2).
The increased interest towards this plant is due to
its chemical composition which affords important biological
activity. The main classes of compounds identified, are: volatile
compounds (carvacrol, thymol, o-cymene, (+)-4-carene,
cis-terpinene, citronellol, geraniol, limonene, linalool,
myrcene, p-cymene, α-pinene and γ-terpinene),
phenolic acids (rosmarinic acid, caffeic acid and gallic acid),
flavonoids and associated compounds (apigenin, quercetin,
naringenin, and their glycosides) and other compounds (e.g.,
enzymes) (3,4). The main structures are presented in
Fig. 1.
Satureja hortensis L. was used in folk
medicine to treat various disorders, such as cramps, muscle pains,
stomach, intestinal, and infectious diseases (3,5). The
modern techniques applied to study the effects of biologically
active substances from its composition revealed a plethora of
beneficial activities, such as antimicrobial, antioxidant,
cytotoxic, insecticidal, fumigant toxicity, insect repellant,
antinociceptive/analgesic, antileishmanial, anti-inflammatory,
antidiarrheal, antispasmodic, matrix metalloproteinase inhibitory
activity, inhibition on blood platelet adhesion, aggregation and
secretion, effect on immune system and on rhinosinusitis (3). Taking into account the great interest
for the treatment and the prophylaxis of different pathologies, the
plant material should be meticulously selected from verified and
certified sources due to the fact that along with the bioactive
compounds, it can also contain toxic compounds (e.g., heavy metals)
(6).
Skin cancers are malignancies most often developed
by people with lighter skin and the incidence is steadily
increasing, especially due to uncontrolled exposure to ultraviolet
radiation, recognized carcinogens (7). Skin cancers are divided into two major
classes: non-melanoma cancers, the most common types diagnosed and
melanoma cancers, the most aggressive with the lowest life
expectancy after diagnosis (8,9).
The present study was aimed to characterize the
biological activity of essential/volatile oil (VO) in comparison
with the total hydro-alcoholic extract (TE) in terms of in
vitro experiments, namely: antioxidant activity (AOA),
antibacterial and antifungal activity, and cytotoxicity on two
normal cell lines (HaCaT, immortalized human keratinocytes; 1BR3,
human skin fibroblasts) and two melanoma cell lines (A375, human
melanoma; B16 melanoma 4A5, mouse melanoma) (Fig. 2).
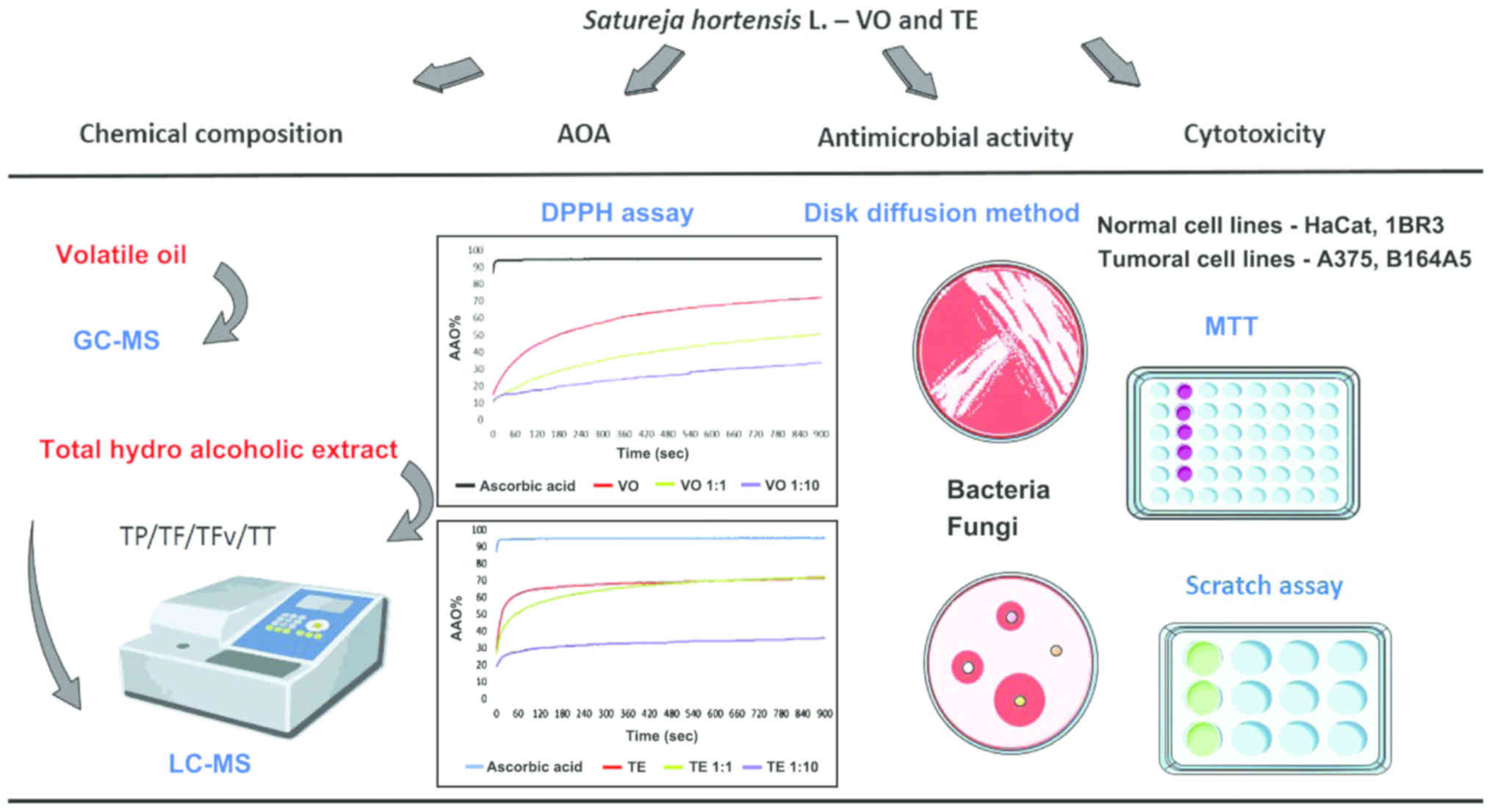 | Figure 2.Schematic overview of the techniques
applied to assess the biological activity of Satureja
hortensis L. essential/VO in comparison with the TE. VO,
volatile oil; TE, total hydro-alcoholic extract; AOA, antioxidant
activity; TP, total polyphenols, TF, total flavonoids, TFv, total
flavonols; TT, total codensed tannins; HaCaT, human immortalized
keratinocytes; 1BR3, skin normal fibroblast; A375, human melanoma;
B164A5, mouse melanoma. |
Materials and methods
Plant material and reagents
Satureja hortensis L. (summer savory) from
spontaneous flora was collected in Timis County (western region of
Romania) during growing season of the year 2017. Botanical
identification of the plant was realized by Professor Diana Antal,
at the Department of Pharmaceutical Botany, Faculty of Pharmacy,
‘Victor Babes’ University of Medicine and Pharmacy (Timisoara,
Romania) and a voucher specimen (no. CD_004) is deposited at the
Herbarium of the Faculty. The plants were harvested at the time
when the volatile oil content was at the maximum percentage
regarding the volatile compounds of interest, namely at full
flowering stage, and were dried in oven at 42°C. Before processing,
the plant material was crushed using an analytical laboratory mill
(A 11 basic Analytical Mill; IKA Werke, Staufen, Germany). All
standard compounds, reagents and solvents used for LC-MS analysis
were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
The reagents used for antioxidant activity (AOA) assessment were
ethanol 96% (v/v) from Chemical Company S.A. (Iasi, Romania),
2,2-diphenyl-1-picrylhydrazyl (DPPH) from Sigma Aldrich; Merck KGaA
and ascorbic acid from Lach-Ner, Ltd. (Neratovice, Czech
Republic).
Extraction procedures
Plant material (aerial parts, 250 g) carefully
selected and dried was subjected to hydro distillation method for
three hours, using an all glass Clevenger-type apparatus according
to the well-known methods described in the literature (10). The final product obtained was dried
and stored at −20°C until further analysis.
In order to obtain summer savory total TE Soxhlet
extraction method was employed. Aerial parts (50 g), crushed and
homogenized, were placed in the Soxhlet apparatus (Solvent
Extractor™, SER 148 Series; Velp Scientifica, Usmate, Italy) and
400 ml of 70% EtOH were used to sequentially extraction for 48 h.
The final extract was filtered through filter paper, the solvent
was removed by a rotary evaporator (Heidolph Hei-VAP Advantage
Rotary Evaporator package) under vacuum, the pellet being
lyophilized and stored in a dark glass tube at −20°C until further
analysis.
Chemical composition of VO and TE
GC-MS analysis
The chemical characterization of essential oil was
realized by using a gas-chromatograph equipment with mass
spectrometer (GS/MS)-QP2010 Plus (Shimadzu, Tokyo, Japan) with a
capillary column with the characteristics: DB-WAX, 30 m length ×
0.32 mm × 1 µm. The carrier gas used was helium with a flow rate of
1 ml/min. The program used for the compounds separation was: start
at 40°C and increased with a rate of 5°C/min to 250°C and hold for
5 min. Injector and ion source temperatures were 250 and 220°C,
respectively. The injection volume was 1 µl at a split ratio of
1:50. The NIST 02 database (webbook.nist.gov), integrated in the device software
was used to identify volatile compounds. The percentages of
individual components were calculated based on GC peak areas
without using correction factors. The linear retention indices
(LRI) were determined under the same operating conditions in
relation to a homologous series of n-alkanes (C8-C24) according to
Van den Dool and Kratz (11):
LRI=100×n+100×(tx-tn)tn+1-tn
where tn and tn+1 are the
retention times of the reference n-alkane hydrocarbons eluting
immediately before and after chemical compound ‘X’ and
tx is the retention time of compound ‘X’.
Total polyphenols, flavonoids,
flavonols, and tannins
The total phenols (TP) evaluation was realized using
Folin-Ciocalteu method as described in the literature (12). Samples (0.5 ml) were treated with
1.25 ml Folin-Ciocalteu reagent (Sigma-Aldrich; Merck KGaA) diluted
1:10 with water and incubated for 5 min at room temperature. After
the addition of 1 ml sodium carbonate 60 g/l the samples were
heated for 30 min at 50°C and then the sample absorbance was
measured at 750 nm using an UV–VIS spectrophotometer (Specord 205;
Analytik Jena AG, Jena, Germany). The calibration curve was
obtained using gallic acid (GA) (Sigma-Aldrich; Merck KGaA) as
positive control, in concentration of 0.03–1 mg/ml. The results
were expressed in mg GAE/g dry material (DM).
Flavonoids/flavonols (TF/TFv) content was evaluated
by using classical test (Al colorimetric analysis) according to
method described in the literature, dilution 1:1, at room
temperature (13). Briefly, for
flavonoid content 500 µl of extract was treated with 500 µl
AlCl3 solution 2% (incubation for 30 min) and absorbance
values measured at 417 nm. For flavonols content, same volume of
extract was mixed with AlCl3 (2%) and
CH3COONa (5%) (incubation for 3 h) and absorbance values
measured at 445 nm. An Secomam UviLine 9400 Spectrophotometer
(Kisker Biotech GmbH and Co., KG, Steinfurt, Germany) was used for
all determinations and rutin was utilized as reference standard
while the data are expressed as rutin equivalents (REs).
The total condensed tannin (TT) was determined by
the vanillin test (14) slightly
modified: Extract (500 µl) was treated for several minutes, in an
ice bath, with 1.5 ml vanillin solution (1% in dilute sulphuric
acid) (incubation for 15 min) and the absorbance was read at 500 nm
on UviLine 9400 Spectrophotometer. The TT was expressed as
milligrams of (+)-catechin equivalents (mg CE/g extract). All
experiments were performed in triplicate.
LC-MS analysis
Quantification of individual phenolic compounds was
performed using a Shimadzu chromatograph equipped with SPD-10A UV
and LC-MS 2010 detectors (Schimadzu, Tokyo, Japan), and EC 150/2
Nucleodur C18 Gravity SB (MACHEREY-NAGEL GmbH & Co., KG, Düren,
Germany), 150 × 2 mm × 5 µm column. Chromatographic conditions were
as follows: mobile phase A, water acidified with formic acid at pH
3.0. Mobile phase B, acetonitrile acidified with formic acid at pH
3.0, gradient program: 0.01–20 min, 5% phase B; 20.01–50 min, 5–40%
phase B; 5–55 min, 40–95% phase B; and 55–60 min, 95% phase B.
Solvent flow rate of 0.2 ml/min, temperature at 20°C. The
monitoring wavelengths were 280 and 320 nm. The calibration curves
were performed in the range of 20–50 µg/ml. The limit of
quantification (LOQ), representing the lowest concentration for
which S/N ≥5, was 0.3 µg/ml. Determinations were performed in
duplicate. All reagents and solvents used were analytical grade
chemicals. Standards were purchased from Sigma-Aldrich; Merck
KGaA.
Antioxidant activity. The DPPH assay was applied to
estimate the radical-scavenging ability of the tested samples.
Briefly, 500 µl of test sample was diluted with 2 ml hydro-alcohol
mixture (ethanol 50%) and 500 µl of DPPH 1 mM was added. The
absorbance was continuously measured at 516 nm with a T70 UV/VIS
Spectrophotometer (PG Instruments Ltd., Leicestershire, UK) for 900
sec to observe the changes in the values of AOA. The antioxidant
activity recorded was compared in each case to that of ascorbic
acid, used as positive control. The percent of AOA activity (%) of
each sample was calculated according to the formula used in our
previous studies (13,15).
Antimicrobial activity
Disc diffusion assay
Extracts of Satureja hortensis L. were tested
for antimicrobial activity against Staphylococcus aureus
(ATCC 25923™), Bacillus cereus (ATCC 8035™), Escherichia
coli (ATCC 25922™), Pseudomonas aeruginosa (ATCC
27853™), Shigella flexneri (ATCC 12022™), Salmonella
typhimurium (ATCC 14028™), Streptococcus pyogenes (ATCC
19615™) and for antifungal activity against Candida albicans
(ATCC 10231™) and Candida parapsilosis (ATCC 22019™) (all
from American Type Culture Collection, Manassas, VA, USA) using the
Disk diffusion method for susceptibility testing, according to the
Standard Rules for Antimicrobial Susceptibility Testing using
Impregnated Disks.
In vitro testing was performed in plates, and
micro-tablets with Gentamicin (for the antimicrobian activity) and
Nystatin (for the antifungal activity) were used as positive
controls. Commercial Gentamicin discs (10 mg, ref. E110712;
BioMaxima, Lublin, Poland) and Nystatin (100 mg - ref. SD 025;
Himedia, Mumbai, Maharashtra, India), alongside filter papers
impregnated with a water:ethanol mixture (as negative controls) and
filter papers impregnated with a known quantity of samples were
assessed. A 10−2 dilution of the fresh fungi cultures
and a 10−3 fresh bacteria cultures were used to perform
the assay, an inoculum equivalent to a 0.5 McFarland standard. The
Petri plates, prepared by a method previously described (16,17) were
seeded and the respective specimens were treated with the VO (10
µl/disk) and TE (250 µg/disk), respectively and were incubated at
30°C for fungi and 37°C in case of bacteria, for 24–48 h. Tests
were performed in triplicate.
In vitro cytotoxicity
Cell lines and specific reagents
The cell lines used in this study: HaCaT,
immortalized human keratinocytes (cat. no. 300493; CLS Cell Lines
Service GmbH, Eppelheim, Germany), 1BR3, human skin fibroblasts
(cat. no. 90011801; European Collection of Authenticated Cell
Cultures, Salisbury, UK), A375, human melanoma cells
(ATCC® CRL-1619™), and B16 melanoma 4A5 cell line from
mouse (ECACC; cat. no. 94042254) were acquired as frozen items and
stored in liquid nitrogen until the experiment began. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
kit was purchased from Roche Diagnostics GmbH (Mannheim, Germany).
The reagents used for cell culture: Dulbecco's modified Eagle's
medium (DMEM) was provided from ATTC and trypsin/EDTA solution,
phosphate-buffered saline (PBS), penicillin/streptomycin mixture,
fetal calf serum (FCS) and Trypan blue solution were supplied by
Sigma-Aldrich; Merck KGaA.
Cell culture
Viability and migration assay. The impact of the
samples on cell viability was tested on all four cell lines: HaCaT,
1BR3, human (A375) and murine (B164A5) melanoma cells. HaCaT, A375
and B164A5 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM), supplemented with 10% FCS and antibiotic mixture
(100 U/ml penicillin and 100 µg/ml streptomycin) and for 1BR3 cells
was used as culture medium the Eagle's Minimum Essential Medium
(EMEM) supplemented with 15% FBS. The culture plates were incubated
at 37°C in a humidified atmosphere with 5% CO2, and
passaged every day. Cell counting was performed with Countess™ II
Automated Cell Counter (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) in Trypan blue presence.
MTT assay is a colorimetric assay which was
performed for cell viability evaluation. The viability percentage
is directly proportional with the mitochondrial reduction of viable
cells which will convert MTT to formazan via succinic dehydrogenase
activity. Briefly, 104 cells/well were plated onto a
96-well plate in 200 µl media and incubated until 90% confluence
was reached. The cells were stimulated with 100 µl media containing
5, 10, 25, 50, and 100 µM (for VO based on the molecular weight of
γ-terpinene and for TE based on the molecular weight of
rosmarinic acid) of test samples. A volume of 10 µl MTT/well was
added at 24 h post stimulation and the mitochondrial reduction of
the tetrazolium salt (MTT) to formazan was determined after a 4 h
contact time. The concentration of formazan was measured at 570 nm
wavelength, via spectrophotometry with a microplate reader (xMark™
Microplate; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The migratory character of the tumor cells used in
this study was examined by applying scratch assay technique. In
brief, 2×105 cells/well were seeded in 12-well plates in
specific culture medium and when the confluence was appropriate
(85–90%) a gap was drawn in the middle of the well with a 10 µl
tip. The capacity of the cells to migrate and fill the gap was
monitored for 24 h by acquiring images at different time-points,
namely 0, 3 and 24 h using an Optika Microscopes Optikam Pro Cool 5
and Optika View (Optika, Ponteranica, Italy).
Statistical analysis
The GraphPad Prism 7 software (GraphPad Software,
San Diego, CA, USA) was employed for the description and
performance of the data. One-way ANOVA followed by Tukey's multiple
comparisons test was used to determine the statistical differences
between the various experimental and control groups (P<0.05,
P<0.01, and P<0.0001). The results were expressed as the mean
± standard deviation (SD).
Results
Chemical composition of VO and TE
The chemical composition of Satureja
hortensis L. VO is presented in Table I. A number of 18 compounds were
identified in total, and the main compounds were:
γ-terpinene (37.862%), o-cymene (15.113%), thymol
(13.491%), carvacrol (13.225%), (+)-4-carene (6.086%),
β-myrcene (3.931%), α-thujene (3.695%),
β-caryophyllene (1.496%), β-pinene (1.374%),
isothymol (0.645%), D-limonene (0.558%), α-thujone (0.546%)
and camphor (0.521%).
 | Table I.Chemical constituents of Satureja
hortensis L. volatile oil (VO). |
Table I.
Chemical constituents of Satureja
hortensis L. volatile oil (VO).
| Compounds | LRI reported in
literature (18) | LRI | Concentration
(%) |
|---|
|
α-Thujene | 1022–1027 | 1024 | 3.695 |
|
β-Pinene | 1105–1108 | 1107 | 1.374 |
| β-Myrcene | 1160 | 1159 | 3.931 |
|
(+)-4-Carene | 1149–1157 | 1148 | 6.086 |
| D-Limonene | 1196–1199 | 1198 | 0.558 |
|
β-Phellandrene | 1195–1212 | 1207 | 0.361 |
|
γ-Terpinene | 1243 | 1245 | 37.862 |
|
o-Cymene | 1268 | 1267 | 15.113 |
|
α-Thujone | 1433–1438 | 1437 | 0.546 |
| Camphor | 1490–1518 | 1511 | 0.521 |
|
β-Caryophyllene | 1597–1618 | 1602 | 1.496 |
|
Bicyclo[5.1.0]octane,
8-(1-methylethylidene) | – | 1698 | 0.274 |
| Anethole | 1817–1819 | 1817 | 0.420 |
| Octanoic acid | 2056–2084 | 2067 | 0.304 |
| Thymol | 2162–2169 | 2168 | 13.491 |
| Isothymol | 2179–2225 | 2183 | 0.645 |
| Carvacrol | 2189 | 2188 | 13.225 |
| Total |
|
| 99.902 |
The resulting hydro alcoholic extract of summer
savory was evaluated for total phenols, flavonoids, flavonols, and
condensed tannins contents with the help of spectrophotometric
methods and the results are presented in the Table II.
 | Table II.Total content of different bioactive
classes from Satureja hortensis L. total hydro alcoholic
extract (TE) determined by spectrophotometric method. |
Table II.
Total content of different bioactive
classes from Satureja hortensis L. total hydro alcoholic
extract (TE) determined by spectrophotometric method.
| Sample | Extraction yield
(%) | TP (mg GAE/g
DM) | TF (mg RE/g
DM) | TFv (mg RE/g
DM) | TT (mg CE/g
DM) |
|---|
| TE | 28.4 | 164.75±2.47 | 24.04±1.26 | 6.65±0.41 | 16.23±0.94 |
Individual quantitative analysis of polyphenols,
revealed the chemical composition found in the total hydro
alcoholic extract of Satureja hortensis L., quercetin and
kaempherol being the major compounds. Moreover, a number of
phenolic acids (caffeic, gallic, rosmarinic, coumaric, ferullic,
and protocatechuic), stilbenoid (resveratrol), flavonoid aglycones
(epicatechin), and glycosides (rutin) were identified in the
extract. The identity of the compounds was certified by LC-MS by
comparison with standards. The polyphenols identified in
composition of summer savory TE are presented in Table III.
 | Table III.The main polyphenols of Satureja
hortensis L. total extract (TE). |
Table III.
The main polyphenols of Satureja
hortensis L. total extract (TE).
| Compounds | Retention time | m/z | Concentration
(µg/g) |
|---|
| Gallic acid |
4.8 | 169 | 28.39 |
| Protocatechuic
acid | 10.8 | 153 | 8.3 |
| Caffeic acid | 21.9 | 179 | 29.12 |
| Epicathechin | 22.7 | 289 | 171.65 |
| Coumaric acid | 24.4 | 163 | 4.15 |
| Ferullic acid | 24.7 | 193 | 29.14 |
| Rutin | 25.7 | 609 | 187.45 |
| Rosmarinic
acid | 28.8 | 359 | 121.05 |
| Resveratrol | 31.9 | 227 | 44.00 |
| Quercetin | 32.1 | 301 | 480.04 |
| Kaempherol | 34.9 | 285 | 3518.99 |
Antioxidant activity
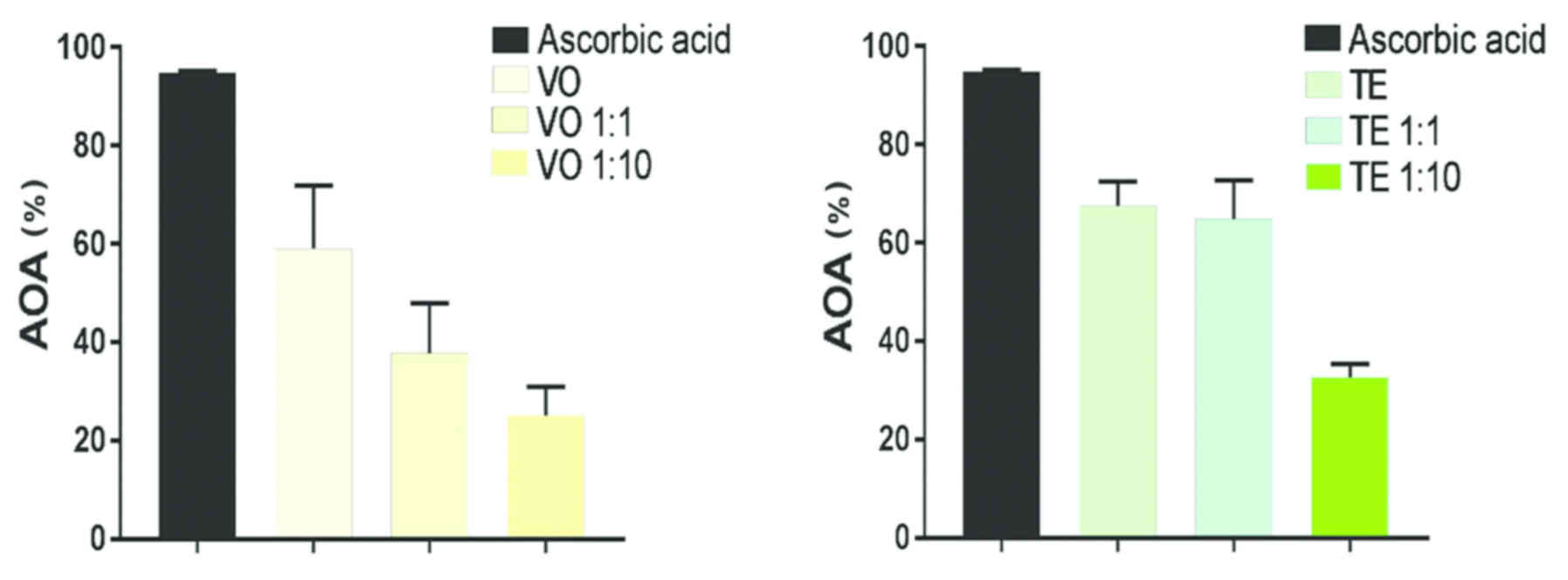
The Fig. 3 presents
the AOA of the summer savory VO and TE which proved to possess a
high activity compared to the one of positive control used,
ascorbic acid. For the evaluation, three samples from each type
were analyzed: VO crude sample, VO 1:1 dilution and VO 1:10
dilution and TE crude sample, TE 1:1 dilution and TE 1:10 dilution,
respectively. VO samples showed a steady increase throughout the
entire time period. Thus, the AOA values after the first 300 sec
were: VO, 57.35%; VO 1:1, 34.98%; and VO 1:10, 22.83% while at the
end of period (900 sec) the values were: VO, 72.12%; VO 1:1,
50.37%; and VO 1:10, 33.73%. In the case of TE samples, data
recorded revealed AOA as follows: after the first 300 sec TE,
67.86%; TE 1:1, 64.56%; and TE 1:10, 32.42% while at the end of
period assessed (900 sec) TE, 71.38%; TE 1:1, 72.19%; and TE 1:10,
35.77%. The graphs prove that TE and TE 1:1 possesses the highest
increase, especially in the first seconds followed by VO.
Antimicrobial activity
In Table IV are
presented the data obtained after the antimicrobial activity
evaluation. The results showed that VO, used in concentration of 10
µl/disk, exhibits an antibacterial effect against both Gram
positive and Gram negative bacteria, respectively and also an
antifungal effect. The most pronounced antibacterial effect was
against Gram positive bacteria S. aureus (16 mm) and the
weakest against Gram negative bacteria S. flexneri and Gram
positive bacteria S. pyogenes (8 mm) while the antifungal
effect was recorded only against C. albicans (10 mm). TE,
used in concentration of 250 µg/disk, exhibited only a slight
effect against S. flexneri, S. typhimurium and S.
pyogenes while no antifungal effect was recorded.
 | Table IV.Antimicrobial and antifungal
activities of the volatile oil and total hydro alcoholic extract
from Satureja hortensis L. by Disk Diffusion method,
expressed as diameter (mm) of inhibition zone (mean ± SD) including
the disc diameter (6 mm). |
Table IV.
Antimicrobial and antifungal
activities of the volatile oil and total hydro alcoholic extract
from Satureja hortensis L. by Disk Diffusion method,
expressed as diameter (mm) of inhibition zone (mean ± SD) including
the disc diameter (6 mm).
| Bacteria and
fungi | VO (mm) | TE (mm) | Positive control
(antibiotic, mm) | Negative control
(solvent) |
|---|
| Bacteria |
| S.
aureus, ATCC 25923(+) | 16±0.58 | – | 15 | – |
| E.
coli, ATCC 25922(−) |
9±0.50 | – | 12 | – |
| P.
aeruginosa, ATCC 27853(−) | 10±0.61 | – | 15 | – |
| B.
cereus, ATCC 8035(+) | 12±0.71 | – | 15 | – |
| S.
flexneri, ATCC 12022(−) |
8±0.36 | 8±0.21 | 13 | – |
| S.
typhimurium, ATCC 14028(−) | 11±0.41 | 8±0.26 | 12 | – |
| S.
pyogenes, ATCC 19615(+) |
8±0.35 | 7±0.25 | 21 | – |
| Fungi |
| C.
albicans, ATCC 10231 | 10±0.54 | – | 12 | – |
| C.
parapsilosis, ATCC 22019 | – | – | 13 | – |
Cell viability
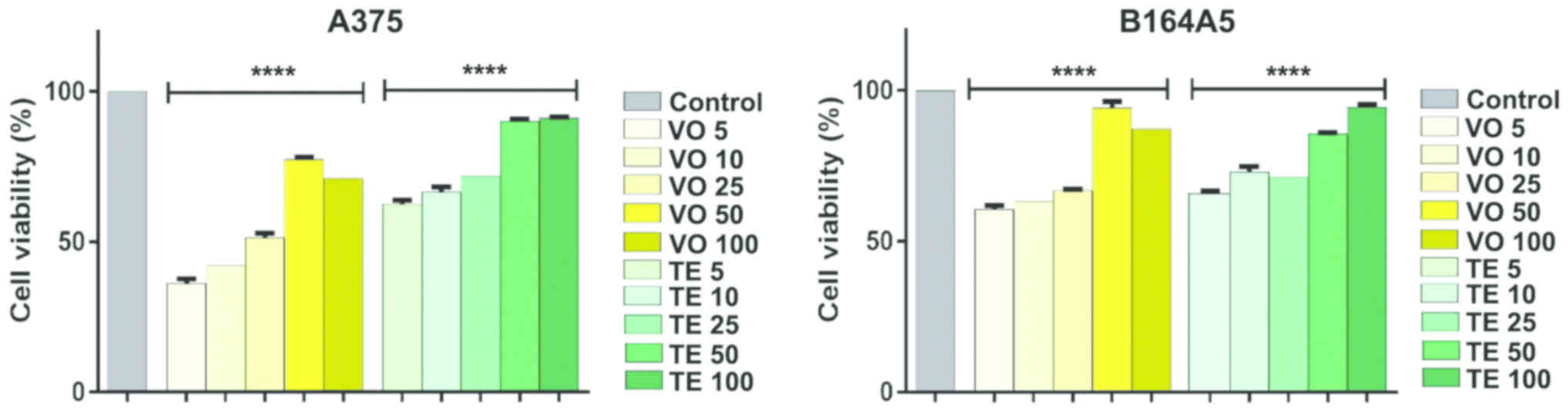
In order to assess the effects induced by VO and TE
on A375 and B16 melanoma 4A5 cell viability, different
concentrations (5, 10, 25, 50 and 100 µM) were tested in a 24 h
exposure. The results revealed that: i) VO induced a significant
decrease of A375 living cells percentage at low concentrations, ii)
the effect was dose-dependent, and iii) the concentrations lower
than 25 µM were more toxic for the cells (Fig. 4). The IC50 value was 22.27
µM, which proves the potent inhibitory effect of VO on A375 cells
viability. The evaluation of TE revealed a reduced viability
percentage of A375 cells, but much lower compared to VO
(IC50 value for TE was 40.38 µM). The viability rate (%)
of A375 cells decreased at concentrations lower than 25 µM, whereas
the highest concentration tested of 100 µM did not affect the cells
(91.22±0.27% viable cells) (Fig.
4).
The B164A5 cells also seemed to be sensitive to VO
effect (IC50, 34.16 µM). The media percentages of viable
cells at low concentrations of 5 and 10 µM have been reduced, but
not in the same manner as in the case of A375 cells (5 µM:
60.55±1.28 vs. 36.18±1.51 and 10 µM: 63.26±1.83 vs. 42.14±2.06,
respectively). TE induced a lower decrease of living cell
percentage in the case of B164A5 cells as compared to the one
determined for A375 cells, the calculated IC50 value
being 204.4 µM with a non-significantly reduced percentage of
viable cells at 100 µM (94.46±0.87) (Fig. 4).
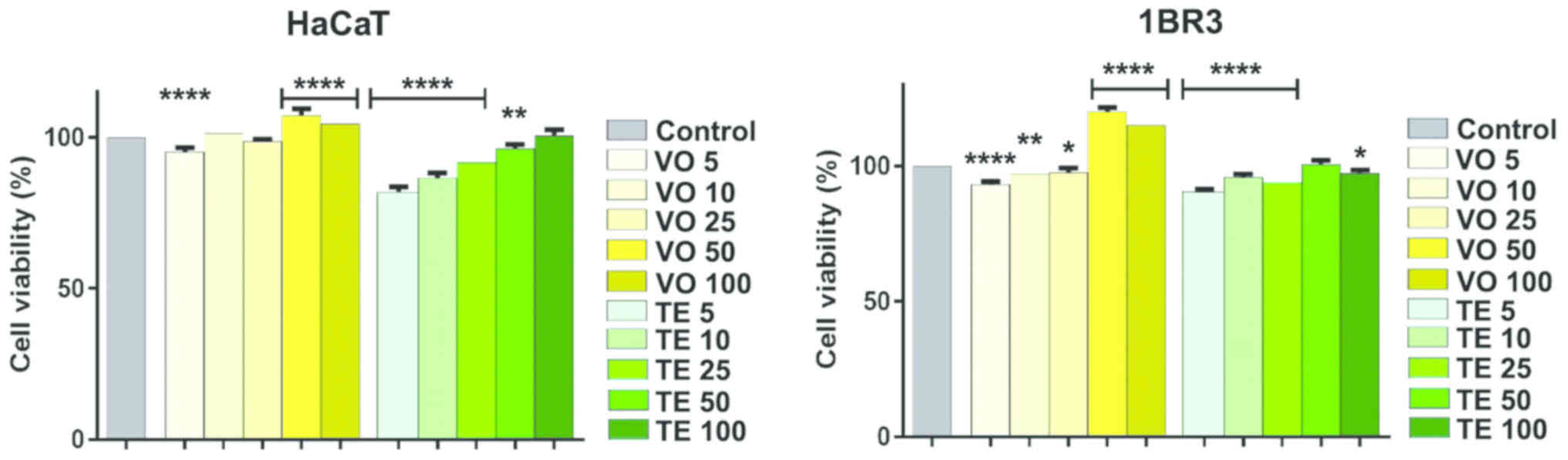
On normal human keratinocyte and fibroblasts, the
concentrations of VO and TE used did not show a significant
toxicity, whereas a potent dose-dependent stimulatory effect was
observed (Fig. 5). In the case of
keratinocytes, TE had a low cytotoxic effect on cell viability at
the lowest concentrations (~82% at 5 µM and ~86% at 10 µM, viable
cells), while volatile oil exerted a weak stimulating effect at the
highest concentrations used. Human fibroblasts were affected in
terms of viability, only by small sample concentrations, for both
VO (~93% at 5 µM) and TE (~90% at 5 µM), respectively whereas
highest concentrations of VO utilized presented a strong
stimulatory effect (~120% at 50 µM and ~116% at 100 µM).
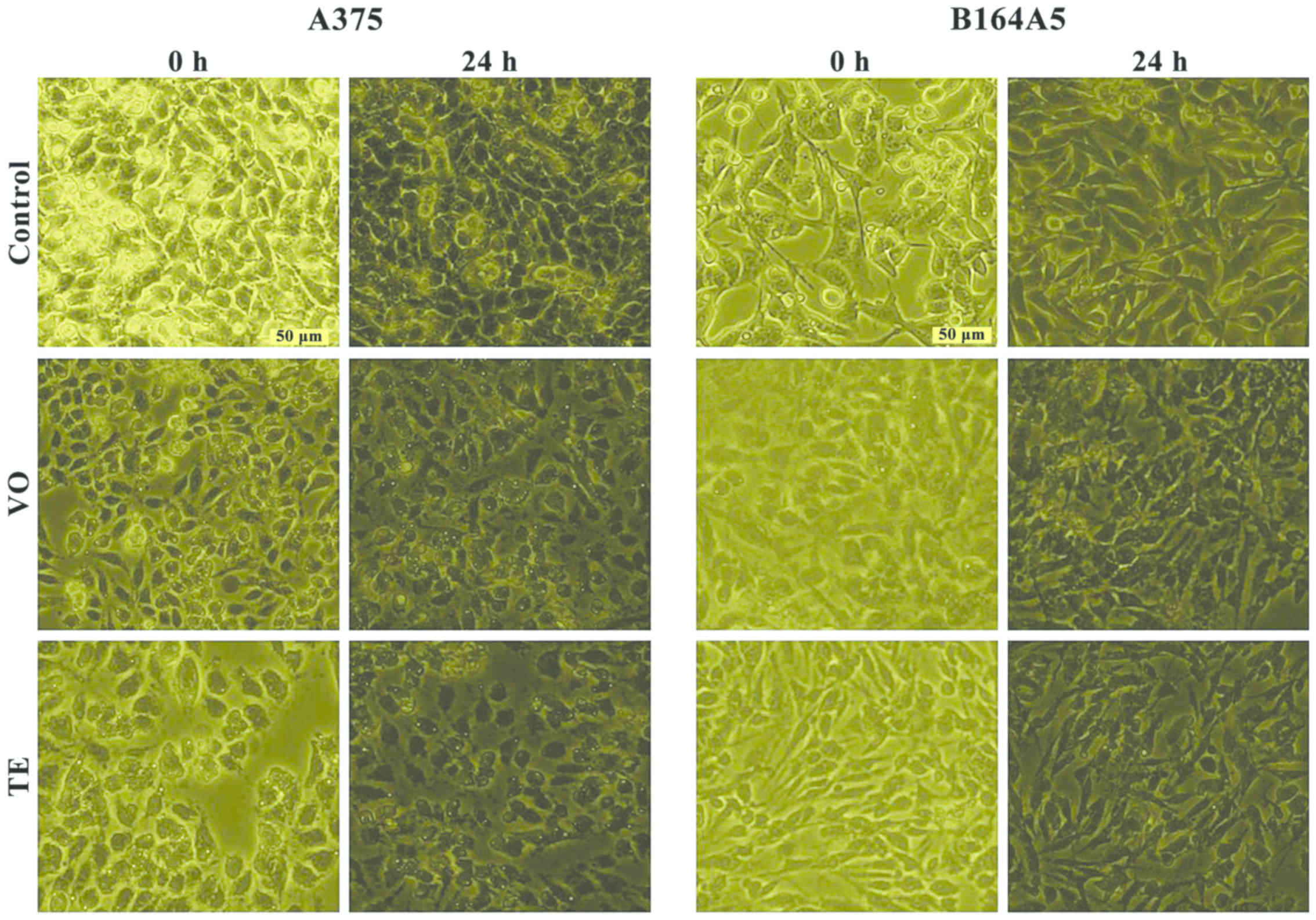
Cell morphology
Since the test compounds (VO and TE), exhibited a
significant cytotoxic effect against tumor cells A375 and B164A5,
the impact of these compounds on cell morphology was monitored by
light microscopy (Optikam Pro Cool 5; Optika Microscopes,
Ponteranica, Italy). In the case of A375, the control cells
(unstimulated) displayed a normal epithelial morphology, with
spindle and cobblestone shapes, strongly bound and adherent to
culture plate, and highly confluent after 24 h. The A375 cells
stimulated with VO and TE for 24 h did not present differences in
terms of morphology, still a reduced confluence and loose bonds was
detected between the cells as compared to control cells, and
several cells were floating in the culture medium (Fig. 6). B164A5 control cells presented a
healthy fibroblastic-like morphology, with polygonal shape,
increased adherence to culture plate and confluence at 24 h. The VO
and TE stimulation led to several changes in cell shape, becoming
shrunken with a reduced confluence tendency and started to detach
from the culture plate (Fig. 6),
results that confirm the data recorded for cell viability
tests.
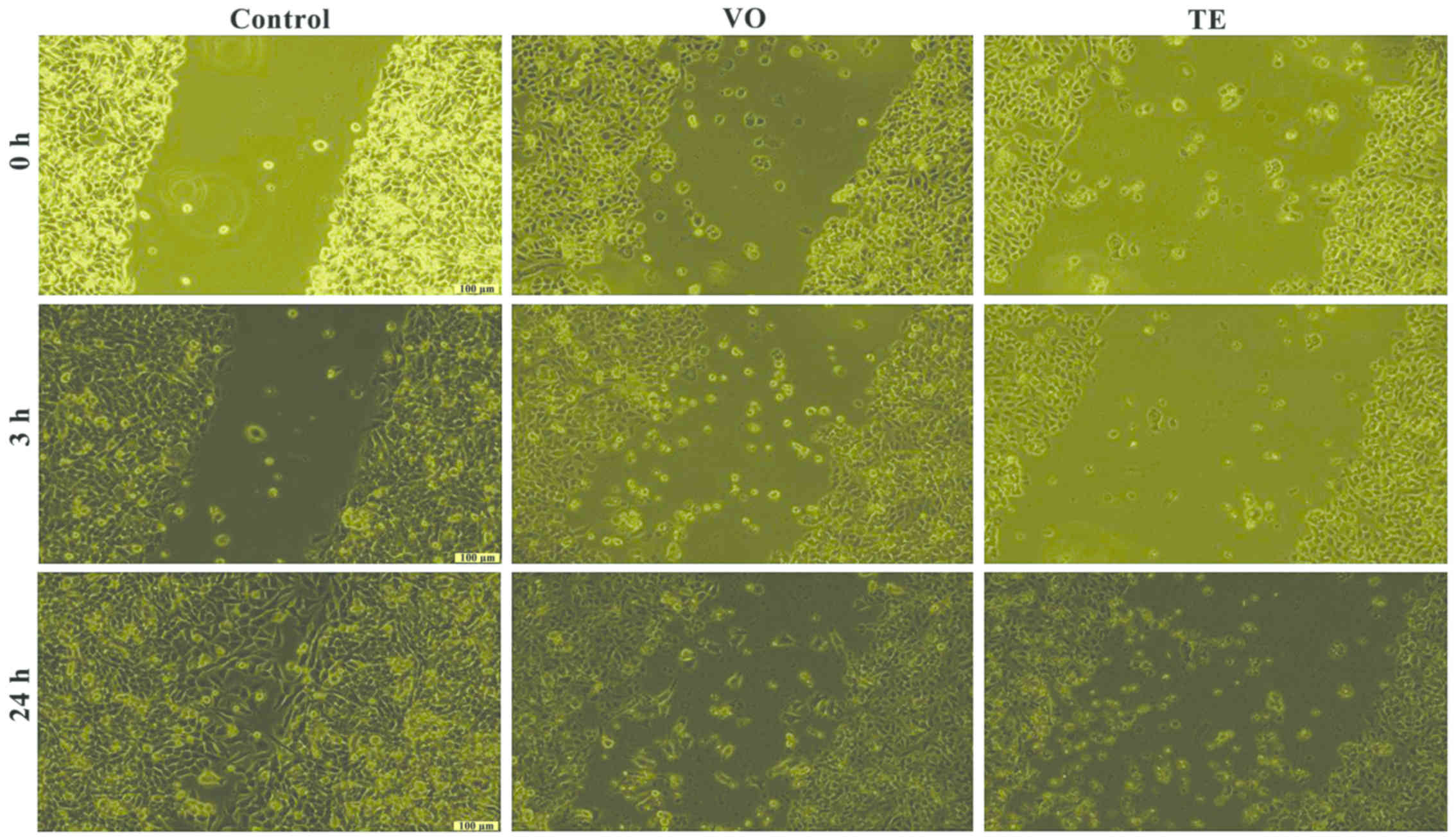
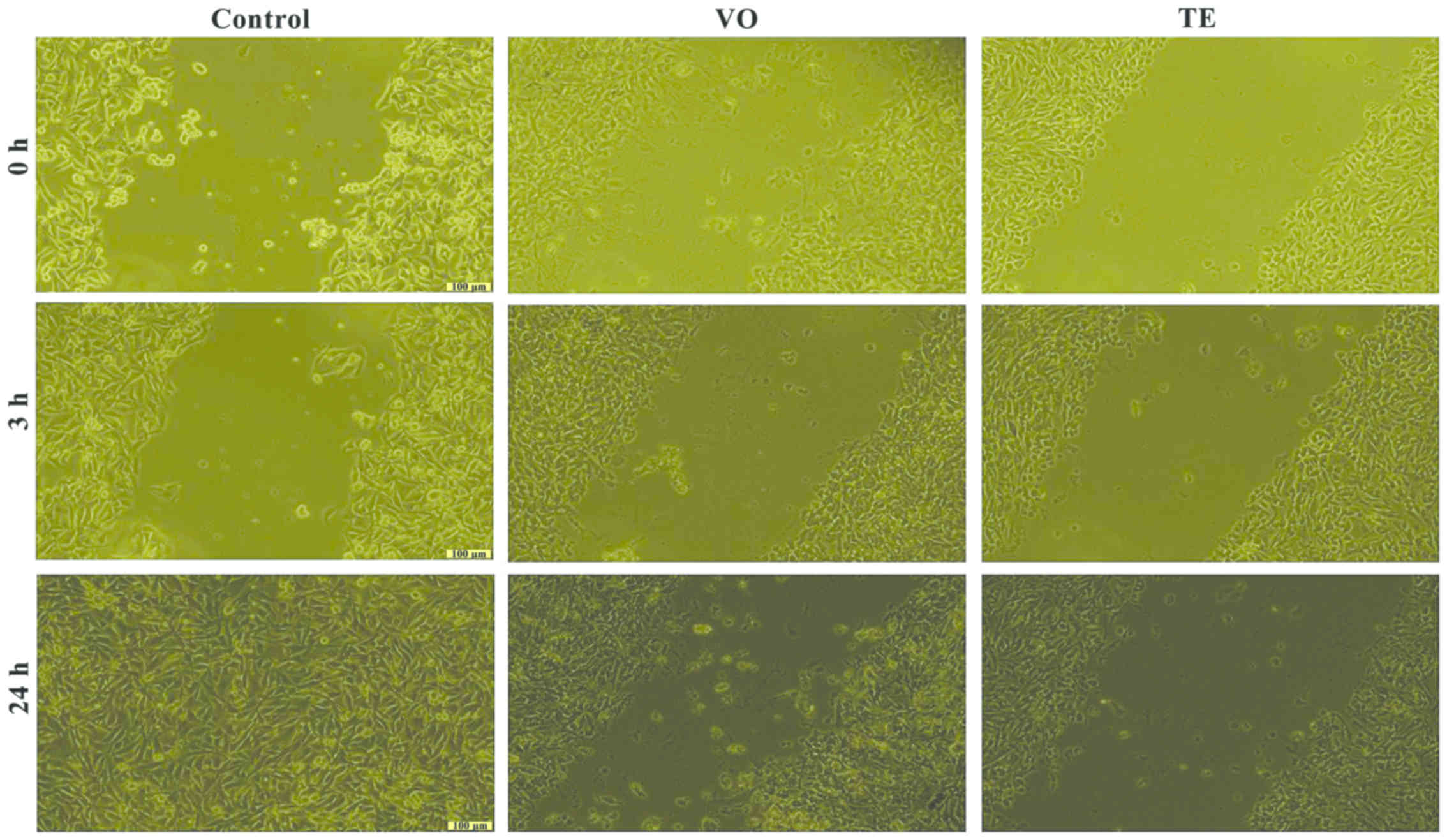
Cell migration and proliferation
Based on the results obtained for the cell viability
test, the effects of VO and TE on cell migration were assessed by
using only the concentrations that did not induce a significant
cell death of the cells (25 µM). The effect of the VO and TE on
cell migration and proliferation was evaluated by the means of
scratch assay, a wound healing type technique. After the scratches
were drawn (when the confluence of the cells was higher than 85%),
the cells were stimulated for 24 h. Images were taken at three
different time-points, namely 0, 3, and 24 h in order to study the
impact of the VO and TE on cell migration and proliferation.
The A375 control cells presented migratory capacity
by covering the wound area after 24 h but in the case of cells
treated with VO and TE an inhibition of the cell migration process
was observed, more pronounced after 24 h (Fig. 7). Similar effects were detected in
the case of B164A5 cells, but the effect exerted by VO was more
pronounced (Fig. 8).
The VO did not affect the normal keratinocyte and
fibroblast migration after 3 h, neither after 24 h, moreover, the
sample had a stimulatory effect on cell proliferation. The cells
were abundant on the plate and well attached. The TE induced a
similar effect on HaCaT cell migration and proliferation as that
described for VO: a stimulatory effect, results that were in
agreement with the data recorded for the cytotoxicity test.
Discussion
A variety of factors can influence the chemical
composition of Satureja hortensis L. essential oils
including environmental factors, extraction and isolation
procedures, and storage conditions (19,20). To
obtain the essential oil, the hydro distillation method was chosen
due to the reduced time of obtaining, the facile way of preparation
and the increased content of volatile compounds of interest.
Sefdikon et al (19) noted
that drying the aerial parts in the oven at 45°C and application of
the above method represents the proper alternative for obtaining
the desired yields (19). Studies
regarding the chemical composition of essential oils obtained from
the aerial parts of the plant harvested from different regions of
the world showed that the main compounds are carvacrol,
γ-terpinene, p-cymene, α-terpinene and myrcene
(19,21,22).
However, it should be mentioned that there is a lack of data in the
literature regarding the chemical composition in terms of the
region, cultivation conditions and environmental factors. Some
authors mentioned that the percentages of carvacrol and terpinene
are highly influenced by the climate, especially by ultraviolet
radiation so they can lead to an increase in carvacrol content and
a decrease in terpinene (21,23). The
data obtained in the present study is partly consistent with the
literature, specifying that the percentages of the mentioned
compounds decreased in the following order γ-terpinene
(~38%) > o-cymene (~15%) > thymol (~13.5%) >
carvacrol (~13%) as detailed in Table
I.
As in the case of essential oil, the chemical
composition of the extract varies depending on a number of factors,
such as plant origin, harvest period, geographic and climatic
factors, and extraction method. Chkhikvishvili et al
(24) identified in the ethanolic
extract of Satureja hortensis L. by HPLC analysis, a series
of compounds: rosmarinic and ferulic acids were the major
compounds, and caffeic, p-coumaric acids, catechin,
epicatechin, luteolin, apigenin, rutin, hesperidin, and
apigenin-7-glucoside were also detected (24). By applying Soxhlet extraction method
and using EtOH, 96% as solvent, an extract concentrated in
rosmarinic acid and quercetin was obtained, with lower
concentrations of other polyphenols such as apigenin, kaempherol,
luteolin, rutin, p-coumaric acid and chlorogenic acid
(25).
A number of causes, including a disorganized
lifestyle, an inadequate diet or particular conditions developed by
foreign stimuli lead to the formation of reactive oxygen species.
Thus, peroxide (ROO•), hydroxyl (HO•), nitric oxide (NO•),
superoxide anion (O2•−), hydrogen peroxide
(H2O2), and other free radicals are produced
in vivo by partial reduction of O2 through
mitochondrial respiration/oxidative phosphorylation as a protection
mechanism. Increased production of these species most often lead to
alterations in the DNA, proteins and lipids, with serious
consequences on the body in terms of cellular aging, mutagenicity,
carcinogenicity, and other (26).
Antioxidant compounds possess the ability to react
in different conditions transferring electrons, binding metal ions,
activating enzymes, reducing radicals and inhibiting oxidases, all
of these leading to the neutralization of the reactive oxygen
species (27). Two types of
antioxidant compounds have been described, endogenous antioxidants
naturally produced in the body and exogenous antioxidants which are
found in different sources of natural origin (e.g., fruits,
vegetables and plants). Antioxidant properties of VO and TE, were
found to be significant; therefore, due to the rich content in
compounds with antioxidant properties, which possess an increased
number of OH groups capable of participating in the reactions
mentioned above, their properties are explored in the study of
certain pathologies.
Considering the antimicrobial activity, volatile
oils exert some actions on cell membranes, such as interference and
destabilization, with repercussions on the phospholipid bilayer and
alteration of enzyme activity (28).
The inhibitory effect against bacteria and fungi of summer savory
VO can be attributed to the increased content of biologically
active compounds of the monoterpenes class, especially terpinene,
thymol and carvacrol (5). Some
studies reported that thymol and carvacrol possess an increased
activity against bacterial and fungal strains, while
γ-terpinene and p-cymene are active against fungal
strains (29,30). Other data mention that thymol has an
important inhibitory activity against S. aureus, carvacrol
and p-cymene against E. coli and γ-terpinene
against S. aureus and C. albicans (5). The volatile oil of Satureja
hortensis L. possesses a wide antimicrobial spectrum, against
both bacteria and fungi (25 bacteria, 8 fungi, and 1 yeast species)
(31). Mihajilov et al
(32) proved the activity against
E. coli, S. typhimurium, S. aureus, L. monocytogenes, P.
putida strain isolated from meat (32). In the present study both VO and TE
were tested; VO showed activity against tested bacteria and fungi,
with the indication that S. aureus was the most sensitive
while TE exerts only a slight effect against three bacteria. These
aspects are in agreement with the hypothesis that only the volatile
oil has in its composition more antimicrobial compounds than the
extracts (31).
Data related to the activity of different natural
compounds, volatile oil or extract obtained from different
medicinal plants have captured the attention in recent years and
are extensively studied and tested in various cancer pathologies
(33,34). Extracts from Satureja
hortensis L. on melanoma cells are scarce and also briefly
described. In one study, Stanojković et al (35) tested the methanol extract of
Satureja hortensis L. and observed a strong antitumor
activity against Fem-x human malignant melanoma cells with an
IC50 of 39.66±2.71 µg/ml (35). It was also proved that the extract
highly inhibited the K562 cell viability at low concentrations of
10 µg/ml (IC50, 52 µg/ml), and in case of Jurkat cell
line the IC50 value was 66.7 µg/ml (36). Another study showed that Satureja
hortensis L. and its rosmarinic acid-rich fraction are able to
protect Jurkat cells against oxidative stress caused by
H2O2 (24). In
the case of Hep2c, human cervix carcinoma, RD, human
rhabdomyosarcoma and L2OB, murine fibroblast cell lines, the
calculated inhibitory concentrations were in the ranges of
13.23–35.29, 18.43–31.03 and 20.51–34.09 µg/ml, respectively for
ethanolic summer savory extracts (25).
Multiple studies were focused on the chemical
compounds found in the composition of volatile oil and extract.
Common active principles of S. hortensis L. such as
α-pinene, γ-terpenene, caryophyllene and others, presented
antiproliferative activity against K562 cells (IC50
value between 98–329 µM/ml) (37).
Consequently, carvacrol exerted cytotoxic activity against breast
cancer (MCF-7), skin cancer (SK-ML-2), colon cancer (HCT-15) and
pancreatic cancer (MIAPaCa-2) (38);
kaempherol presented antiproliferative activity (IC50,
20 µM) against human melanoma (A375) after 48 h incubation
(39) and showed various suppressive
effects on melanoma A375SM cells, at a concentration of 20 µM
(40); quercetin slightly decreased
the survival of human melanoma cells (A375 and A2058) in a time-
and dose-dependent manner with IC50 values of 99.6 and
118.1 µM after 48 h treatment and the viability of murine melanoma
cells (B16F10) decreased in a dose-dependent manner (41,42);
quercetin and other compounds from its class, such as
epigallocatechin, kaempherol, myricetin and luteolin showed
inhibitory actions on HGF-stimulated melanoma cell migration and
invasion. These findings denoted that these compounds shared
similar activities and may be taken into account in melanoma
treatment and prevention (42).
The novelty of this study consists in the biological
assessment of essential oil and total hydro-alcoholic of
Satureja hortensis L. both as individuals and in comparative
terms. VO and TE of summer savory possessed different antimicrobial
activity against tested bacteria and fungi. VO proved to be active
against all tested bacteria, especially against S. aureus
while TE showed poor activity against three of the tested bacteria
(S. flexneri, S. typhimurium and S. pyogenes) with no
activity on fungi. The antitumor activity against melanoma cells
was present, and no toxic effects on healthy cell lines tested
(immortalized human keratinocytes and human skin fibroblast) was
recorded. VO showed a greater efficacy as compared to TE on human
melanoma cells (A375) while in the case of murine melanoma cells
(B164A5) the impact on cell viability was similar. The activity of
the compounds tested was higher at low concentrations whereas at
high concentrations the percentages of viability were increased.
Further in vitro and in vivo studies are necessary in
order to elucidate the anticancer mechanisms of activity, and for a
better understanding of the role played by the biological active
compounds.
Acknowledgements
Part of the in vitro experiments was
conducted within the Center of Genomic Medicine from the ‘Victor
Babes’ University of Medicine and Pharmacy (POSCCE Project ID:
1854, SMIS code: 48749, ‘Center of Genomic Medicine v2’, contract
no. 677/09.04.2015; Timisoara, Romania). The GC-MS and LC-MS
analysis of VO and TE were performed in the Interdisciplinary
Research Platform (PCI), belonging to Banat's University of
Agricultural Sciences and Veterinary Medicine ‘King Michael I of
Romania’ (Timisoara, Romania).
Funding
The present study was financially supported by an
internal grant funded by ‘Victor Babes’ University of Medicine and
Pharmacy (project no. P III-C4-PCFI-2016/2017-04; Timisoara,
Romania).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RAP, IPi, CAD, HTS and DV made a major contribution
to the conceptualization, validation, writing, reviewing and
editing of the manuscript. IPo and EA obtained, analyzed and
interpreted the data regarding volatile oil and extracts. CGF, DC
and CD performed in vitro assays (analysis and
interpretation). DV and VL contributed to the writing of the
manuscript, methodology and project resources. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing of
interests.
References
|
1
|
Zavatti M, Zanoli P, Benelli A, Rivasi M,
Baraldi C and Baraldi M: Experimental study on Satureja
montana as a treatment for premature ejaculation. J
Ethnopharmacol. 133:629–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charles DJ: Savory. In: Antioxidant
Properties of Spices, Herbs and Other Sources. Springer; New York,
NY: pp. 531–536. 2012
|
|
3
|
Tepe B and Cilkiz M: A pharmacological and
phytochemical overview on Satureja. Pharm Biol. 54:375–412.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yesiloglu Y, Sit L and Kilic I: In vitro
antioxidant activity and total phenolic content of various extracts
of Satureja hortensis L. collected from Turkey. Asian J
Chem. 25:8311–8316. 2013. View Article : Google Scholar
|
|
5
|
Hamidpour R, Hamidpour S, Hamidpour M,
Shahlari M and Sohraby M: Summer Savory: From the selection of
traditional applications to the novel effect in relief, prevention,
and treatment of a number of serious illnesses such as diabetes,
cardiovascular disease, Alzheimer's disease, and cancer. J Tradit
Complement Med. 4:140–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Antal DS, Coricovac D, Soica CM, Ardelean
F, Panzaru I, Danciu C, Vlaia V and Toma C: High cadmium content in
wild-growing medicinal plants from South-Western Romania unexpected
results of a survey on 29 species. Rev Chim. 65:1122–1125.
2014.
|
|
7
|
Dehelean CA, Soica C, Pinzaru I, Coricovac
D, Danciu C, Pavel I, Borcan F, Spandidos DA, Tsatsakis AM and
Baderca F: Sex differences and pathology status correlated to the
toxicity of some common carcinogens in experimental skin carcinoma.
Food Chem Toxicol. 95:149–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lupu M, Caruntu A, Caruntu C, Papagheorghe
LML, Ilie MA, Voiculescu V, Boda D, Constantin C, Tanase C, Sifaki
M, et al: Neuroendocrine factors: The missing link in non-melanoma
skin cancer (Review). Oncol Rep. 38:1327–1340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Neagu M, Caruntu C, Constantin C, Boda D,
Zurac S, Spandidos DA and Tsatsakis AM: Chemically induced skin
carcinogenesis: Updates in experimental models (Review). Oncol Rep.
35:2516–2528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
European Pharmacopoeia 7.0, . https://www.scribd.com/doc/38363641/European-Pharmacopoeia-7-0-2011February
5–2018
|
|
11
|
Van den Dool H and Kratz PD: A
generalization of the retention index system including linear
temperature programmed gas - liquid partition chromatography. J
Chromatogr A. 11:463–471. 1963. View Article : Google Scholar
|
|
12
|
Zălaru C, Crişan CC, Călinescu I, Moldovan
Z, Ţârcomnicu I, Litescu SC, Tatia R, Moldovan L, Boda D and Iovu
M: Polyphenols in Coreopsis tinctoria Nutt. fruits and the
plant extracts antioxidant capacity evaluation. Open Chem.
12:858–867. 2014.
|
|
13
|
Pinzaru I, Heghes A, Marti D, Dehelean C,
Coricovac D, Tăculescu (Moacă) EA, Moatar M and Camen D:
Therapeutically potential of Medicago sativa extracts
chemical and in vitro assessments. Rev Chim. 69:121–124. 2018.
|
|
14
|
Zengin G, Locatelli M, Carradori S, Mocan
AM and Aktumsek A: Total phenolics, flavonoids, condensed tannins
content of eight centaurea species and their broad inhibitory
activities against cholinesterase, tyrosinase, α-amylase and
α-glucosidase. Not Bot Horti Agrobot Cluj-Napoca. 44:195–200. 2016.
View Article : Google Scholar
|
|
15
|
Coricovac DE, Moacă EA, Pinzaru I, Cîtu C,
Soica C, Mihali CV, Păcurariu C, Tutelyan VA, Tsatsakis A and
Dehelean CA: Biocompatible colloidal suspensions based on magnetic
iron oxide nanoparticles: Synthesis, characterization and
toxicological profile. Front Pharmacol. 8:1542017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cocan I, Alexa E, Danciu C, Radulov I,
Galuscan A, Obistioiu D, Morvay AA, Sumalan RM, Poiana MA, Pop G,
et al: Phytochemical screening and biological activity of Lamiaceae
family plant extracts. Exp Ther Med. 15:1863–1870. 2018.PubMed/NCBI
|
|
17
|
Călina D, Docea AO, Rosu L, Zlatian O,
Rosu AF, Anghelina F, Rogoveanu O, Arsene AL, Nicolae AC, Drăgoi
CM, et al: Antimicrobial resistance development following surgical
site infections. Mol Med Rep. 15:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Institute of Standards and
Technology, . NIST Chemistry WebBook. https://webbook.nist.govAugust 21–2017
|
|
19
|
Sefidkon F, Abbasi K and Khaniki GB:
Influence of drying and extraction methods on yield and chemical
composition of the essential oil of Satureja hortensis. Food Chem.
99:19–23. 2006. View Article : Google Scholar
|
|
20
|
Mohtashami S, Rowshan V, Tabrizi L,
Babalar M and Ghani A: Summer savory (Satureja hortensis L.)
essential oil constituent oscillation at different storage
conditions. Ind Crops Prod. 111:226–231. 2018. View Article : Google Scholar
|
|
21
|
Katar D, Kacar O, Kara N, Aytac Z, Göksu
E, Kara S, Katar N, Erbaş S, Telci I and Elmastaş M: Ecological
variation of yield and aroma components of summer savory
(Satureja hortensis L.). J Appl Res Med Aromat Plants.
7:131–135. 2017.
|
|
22
|
Alizadeh A, Khoshkhui M, Javidnia K,
Firuzi O, Tafazoli E and Khalighi A: Effects of fertilizer on
yield, essential oil composition, total phenolic content and
antioxidant activity in Satureja hortensis L. (Lamiaceae)
cultivated in Iran. J Med Plants Res. 4:33–40. 2010.
|
|
23
|
Tozlu E, Cakir A, Kordali S, Tozlu G, Ozer
H and Aytas Akcin T: Chemical compositions and insecticidal effects
of essential oils isolated from Achillea gypsicola, Satureja
hortensis, Origanum acutidens and Hypericum scabrum
against broadbean weevil (Bruchus dentipes). Sci Hortic
(Amsterdam). 130:9–17. 2011. View Article : Google Scholar
|
|
24
|
Chkhikvishvili I, Sanikidze T, Gogia N,
Mchedlishvili T, Enukidze M, Machavariani M, Vinokur Y and Rodov V:
Rosmarinic acid-rich extracts of summer savory (Satureja
hortensis L.) protect Jurkat T cells against oxidative stress.
Oxid Med Cell Longev. 2013:4562532013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mašković P, Veličković V, Mitić M, Đurović
S, Zeković Z, Radojković M, Cvetanović A, Švarc-Gajić J and Vujić
J: Summer savory extracts prepared by novel extraction methods
resulted in enhanced biological activity. Ind Crops Prod.
109:875–881. 2017. View Article : Google Scholar
|
|
26
|
Coricovac DE and Dehelean CA: Pathological
aspects with global impact induced by toxicants at cellular level.
Toxicology Studies - Cells. Drugs and Environment. Andreazza AC and
Scola G: InTech; Rijeka: pp. 3–21. 2015
|
|
27
|
Corina D, Florina B, Iulia P, Cristina D,
Rita A, Alexandra P, Virgil P, Hancianu M, Daliana M and Codruta S:
Rutin and its cyclodextrin inclusion complexes: Physico-chemical
evaluation and in vitro activity on B164A5 murine melanoma cell
line. Curr Pharm Biotechnol. 18:1067–1077. 2018. View Article : Google Scholar
|
|
28
|
Rezvanpanah S, Rezaei K, Golmakani MT and
Razavi SH: Antibacterial properties and chemical characterization
of the essential oils from summer savory extracted by
microwave-assisted hydrodistillation. Braz J Microbiol.
42:1453–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Farzaneh M, Kianib H, Sharicfi R, Raeisi M
and Hadian J: Chemical composition and antifungal effects of three
species of Satureja (S. hortensis, S. spicigera, and S.
khuzistanica) essential oils on the main pathogens of
strawberry fruit. Postharvest Biol Technol. 109:145–151. 2015.
View Article : Google Scholar
|
|
30
|
Adiguzel A, Ozer H, Kilic H and Cetin B:
Screening of antimicrobial activity of essential oil and methanol
extract of Satureja hortensis on food borne bacteria and
fungi. Czech J Food Sci. 25:81–89. 2007. View Article : Google Scholar
|
|
31
|
Mahboubi M and Kazempour N: Chemical
composition and antimicrobial activity of Satureja hortensis
and Trachyspermum copticum essential oil. Iran J Microbiol.
3:194–200. 2011.PubMed/NCBI
|
|
32
|
Mihajilov-Krstev T, Radnović D, Kitić D,
Zlatković B, Ristić M and Branković S: Chemical composition and
antimicrobial activity of Satureja hortensis L. essential
oil. Cent Eur J Biol. 4:411–416. 2009.
|
|
33
|
Sani TA, Mohammadpour E, Mohammadi A,
Memariani T, Yazdi VM, Rezaee R, Calina D, Oana DA, Goumenou M,
Etemad L, et al: Cytotoxic and apoptogenic properties of
Dracocephalum kotschyi aerial part different fractions on
calu-6 and mehr-80 lung cancer cell lines. Farmacia. 65:189–199.
2017.
|
|
34
|
Caruntu C, Boda D, Constantin C, Caruntu A
and Neagu M: Catecholamines increase in vitro proliferation of
murine B16F10 melanoma cells. Acta Endocrinol (Copenh). 10:545–558.
2014.
|
|
35
|
Stanojković T, Kolundžija B, Ćirić A,
Soković M, Nikolić D and Kundaković T: Cytotoxicity and
antimicrobial activity of Satureja kitaibelii wierzb. Ex
heuff (Lamiaceae). Dig J Nanomater Biostruct. 8:845–854. 2013.
|
|
36
|
Esmaeilbeig M, Kouhpayeh SA and
Amirghofran Z: An investigation of the growth inhibitory capacity
of several medicinal plants from Iran on tumor cell lines. Iran J
Cancer Prev. 8:e40322015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lampronti I, Saab AM and Gambari R:
Antiproliferative activity of essential oils derived from plants
belonging to the Magnoliophyta division. Int J Oncol.
29:989–995. 2006.PubMed/NCBI
|
|
38
|
Rajput JD, Bagul SD, Tadavi S and Bendre
RS: Comparative anti-proliferative studies of natural phenolic
monoterpenoids on human malignant tumour cells. Med Aromat Plants
(Los Angel). 5:2792016.
|
|
39
|
Yang J, Xiao P, Sun J and Guo L:
Anticancer effects of kaempferol in A375 human malignant melanoma
cells are mediated via induction of apoptosis, cell cycle arrest,
inhibition of cell migration and downregulation of m-TOR/PI3K/AKT
pathway. J BUON. 23:218–223. 2018.PubMed/NCBI
|
|
40
|
Heo JR, Lee GA, Kim GS, Hwang KA and Choi
KC: Phytochemical-induced reactive oxygen species and endoplasmic
reticulum stress-mediated apoptosis and differentiation in
malignant melanoma cells. Phytomedicine. 39:100–110. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cao HH, Tse AK, Kwan HY, Yu H, Cheng CY,
Su T, Fong WF and Yu ZL: Quercetin exerts anti-melanoma activities
and inhibits STAT3 signaling. Biochem Pharmacol. 87:424–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li
T, Tse AK, Kwan HY, Yu H and Yu ZL: Quercetin inhibits HGF/c-Met
signaling and HGF-stimulated melanoma cell migration and invasion.
Mol Cancer. 14:1032015. View Article : Google Scholar : PubMed/NCBI
|