Introduction
Psoriasis is a complex erythema scaly skin disease
(1), and basically cellular immune
disorders, genetic, environmental and infectious factors determine
its pathogenesis (2,3). Psoriasis is an autoimmune disease,
triggered with periods of remission or aggravation by certain
infectious factors such as streptococcal infections (4,5).
Although there are many studies on psoriasis, an effective
treatment without many side effects has not been discovered
yet.
Western medicine mainly focusses on symptomatic
treatment and physical therapy (6,7),
including inhibition of cell proliferation, immunosuppressive and
anti-inflammatory effects (8).
Recently, many biological agents have been designed with limited
effects, but their usage is not efficient because of their unknown
long-term effects (9–11). Currently, alternative medicine gives
hope to patients with psoriasis disease by using various
traditional herbal medicines.
Traditional chinese medicine (TCM) is a part of
alternative medicine and extensively used in China. Recent studies
proved that the application of TCM in psoriasis treatment shows
acceptable results with less side effects than modern western
treatment (12–14). Acitretin is a common drug used in the
treatment of psoriasis (15). It can
also regulate the epidermal keratinocytes (KCs) of the body by
inhibiting the excessive proliferation of epithelial cells. In
addition, the drug has significant immunomodulatory effects on
abnormal epidermal cells, which can effectively lead to the
reduction of inflammatory response in the skin tissue of the
patients (16). Xiaoyin granule is a
pure Chinese medicine agent (17).
The main components of Xiaoyin granule are: Angelica, raw land,
Radix Sophora flavescens, honeysuckle, Sophora
flavescens and peony skin. TCM claims that psoriasis is mainly
caused by blood stasis, blood heat and blood deficiency. Xiaoyin
granule has remarkable effects on cooling and nourishing blood,
dispelling cold, moistening dryness and alleviating itching. Each
part of Xiaoyin granule has unique effects. The Radix Sophora
flavescens activate blood circulation and they have also
nourishing and cooling effects on blood (18). Radix Sophorae, honeysuckle,
white fresh skin and windproof have detoxifying, antipruritic
effects and also reducing effect on blood heat stress (19). Red flower and Angelica can promote
blood circulation and dissipate blood stasis (20).
The excessive proliferation of KCs is an important
feature of psoriasis, and proliferating cell nuclear antigen (PCNA)
is the most reliable index to evaluate cell proliferation (21). PCNA is a nucleoprotein involved in
DNA synthesis, which is may be related to the pathogenesis of
psoriasis (22). Granulocyte colony
stimulating factor (GM-CSF) is now considered to be an important
natural immune activator, which activates mature white blood and
immune cells and is involved in the chronic stage of inflammatory
and autoimmune diseases. The expression of GM-CSF is abnormal in
psoriasis and correlated with the severity of psoriasis (23,24).
Based on history and unique green plant cover of
China, TCM has great potential as treatment in chronic disease. The
present study was designed to evaluate the protective effect of
white mange mixture and Xiaoyin granules in comparison with regular
treatment (acitretin) on the mouse vaginal psoriasis model.
Epithelial KC cell PCNA, T lymphocyte apoptosis and GM-CSF were
evaluated for the first time.
Materials and methods
Main reagents
Phosphate-buffered saline (PBS) (Changde Beekman
Biotechnology Co., Ltd.); RPMI-1640 (Xibao Biotech Co., Ltd.);
hematoxylin and eosin (H&E) stain solution (Shanghai Xinfan
Biotechnology Co., Ltd.); mercuric oxide, aluminium potassium
sulfate, 5% glacial acetic acid and 1% Hydrochloric acid solution
(Hubei Xinkang Pharmaceutical Chemical Co., Ltd.); PCNA (Beijing
Zhongshan Jinqiao Biotechnology Co., Ltd.); chromogenic agent (DAB)
(code WK294; Beijing Baiaolaibo Technology Co., Ltd.); SP
Immunohistochemical commercial assay kit (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.); and GM-CSF enzyme-linked immunosorbent
assay (ELISA) kit (Shenzhen Dako Biotechnology Co., Ltd.).
Main experimental equipment
Micropipettes, electronic balances and surgical
instruments (Tianjin Celiss Automation Technology Co., Ltd.);
JEM-100CX II transmission electron microscope (JEOL Ltd.);
microplate reader (Shanghai Flash Spectrum Biotechnology Co.,
Ltd.); BY-160C type medical centrifuge (Beijing Baiyang Medical
Instruments Co., Ltd.).
Experimental animals
Seventy female BALB/c mice were purchased from
Beijing Longmidas Science and Technology Development Co., Ltd. The
animals were 6 to 8 weeks old, weighing 18 and 22 g at the
beginning of the study. The animals were acclimatized for 2 weeks
at standard conditions of 24±2°C temperature, humidity between 40
and 60% and 12 h light/dark cycle. The animals had free access to
tap water and food. The animal experiment has been approved by the
Ethics Committee of the Guiyang University of Chinese Medicine
(Guiyang, China).
Experimental groups and treatment of
mice
Seventy mice were randomly divided into 7 groups
(n=10) as follows: negative control group, positive control group,
acitretin group, Xiaoying granule groups, high-dose white mange
mixture group, medium-dose white mange mixture group and low-dose
white mange mixture group. The doses of white mange mixture,
Xiaoying granules and acitretin were settled based on the
equivalent received by humans (25).
For example, a person with the weight of 70 kg receives a dose of
1.610 mg white mange mixture per day. According to the proportion
of body surface area of the human and the mice we conclude that
mice of 10 g should receive a dose of 0.23 mg white mange mixture
per day that is equivalent to 23 mg/kg/day. The drugs were
dissolved in drinking water. The stock solution (10 ml) (acitretin
group: 11.5 mg/ml; Xiaoying granule group: 2.438 mg/ml; high-,
medium-, and low-dose of white mange mixture groups: 46, 23 and
11.5 mg/ml, respectively, was prepared. A mouse weighing 20 g
received a dose of 0.2 ml from stock solution. Negative control
group received every day the same amount of drinking water by
gavage. Acitretin group received a dose of 5.75 mg/kg/day of and
Xiaoying granule group 1.219 mg/kg/day, respectively. High-,
medium- and low-dose of white mange mixture groups received 23,
11.5 and 5.75 mg/kg/day, respectively. The treatment was for 28
consecutive days. Experimental drugs used in the protocol are
presented in Table I.
 | Table I.Experimental drugs. |
Table I.
Experimental drugs.
| Drugsa | Components |
|---|
| White mange
mixture | 10 g Fructus
amomi, 20 g Figwort, 20 g Chinese Angelica, 20 g Scutellaria
baicalensis, 20 g Madder, 15 g Radix Arnebiae, 25 g Rhizoma
Imperatae, 15 g Honeysuckle, 20 g Cortex Moutan, 15 g Licorice |
| Acitretin | Acitretin |
| Xiaoyin
granules | 10 g Chinese
Angelica, 10 g Rhizoma Chuanxiong, 10 g Mirabilite, 10 g
Polygonum multiflorum, 15 g Dried Rehmannia Root, 15 g Radix
Paeoniae Rubra, 15 g Madder, 15 g Cortex Dictam, 15 g Tribulus
terrestris, 15 g Flos sophorae, 9 g Flos
carthami, 6 g Licorice, 30 g Lignum millettiae |
White mange mixture is a Chinese medicine which is
effective in the treatment of psoriasis. It contains: 10 g of
Fructus amomi, 20 g Figwort, 20 g Chinese Angelica, 20 g
Scutellaria baicalensis, 20 g Madder, 15 g Radix Arnebiae,
25 g Rhizoma imperatae, 15 g Honeysuckle, 20 g Cortex
Moutan, and 15 g Licorice (these drugs are made into powder and
dissolved in water when they are used).
Xiaoyin Granules is a Chinese medicine for treating
psoriasis, macular and itchy skin diseases. It has obvious
anti-histamine, anti-inflammation, anti-bacterial and antivirus
effects, and improves microcirculation. It contains: 10 g Chinese
Angelica, 10 g Rhizoma chuanxiong, 10 g Mirabilite, 10 g
Polygonum multiflorum, 15 g Dried Rehmannia Root, 15 g Radix
Paeoniae Rubra, 15 g Madder, 15 g Cortex Dictam, 15 g Tribulus
terrestris, 15 g Flos sophorae, 9 g Flos
carthami, 6 g Licorice and 30 g Lignum millettiae (these
drugs are made into powder and dissolved in water when they are
used).
Murine model of vaginal psoriasis
The mice from experimental groups received estradiol
(Guangzhou Baiyun Mountain Mingxing Pharmaceutical Co., Ltd.),
intraperitoneally in doses of 5 mg/kg/day, for 3 consecutive days,
while the mice from negative control group received saline
solution. On the fourth day, each animal was weighed and treated
for 28 consecutive days as mentioned above.
Blood sample collection
At the end of the treatment the blood samples were
collected directly from heart of each mouse. The samples were
centrifuged (BY-160C type medical centrifuge; Beijing Baiyang
Medical instruments Co., Ltd.) at 671 × g for 10 min and the sera
were stored at −80°C.
Histopathological lesions
The vaginal epithelial tissue was removed
immediately, fixed in 10% neutral formalin (Zhongshan Kang Naixin
Biomedical Science and Technology Co., Ltd.) for 24 to 48 h, and
then processed to obtain paraffin blocks. Paraffin-embedded blocks
were routinely processed; 5 µm thick sections were prepared
(26).
PCNA evaluation
PCNA level was determined by using SP
Immunohistochemical commercial assay kit (Beijing Zhongshan Jinqiao
Biotechnology Co., Ltd.). The staining procedure was performed
according to the protocol of the manufacturer. Briefly, after
deparaffinization, the slides were immersed in antigen retrieval
solution (pH 6.0) and heated in microwave for 15 min so as to
unmask antigens. The sections were then incubated in 3%
H2O2 for 10 min to block the activity of
endogenous peroxidase. Each reagent (A, mouse PCNA antibody, B and
C) was added consecutively and each step contained incubation of
the samples at room temperature for 15 min and then washed by PBS
(each step was repeated 3 times).
Finally, DAB (code WK294; Beijing Baiaolaibo
Technology Co., Ltd.) was exposed to samples and then gray value
tones were measured by Leica Q550CW image acquisition and analysis
system (both from Leica Microsystem Trading Co., Ltd.). The gray
mean value evaluation was measured by positive cell count/unit
area. The system set the white gray value to 255 and black gray
value to 0. The higher the gray value, the weaker the
expression.
T lymphocyte isolation and
evaluation
Under deep anesthesia (0.6% sodium pentobarbital;
Shanghai Xinya Pharmaceutical Co., Ltd.) the spleen tissue was
extracted at aseptic conditions and placed in a Petri dish. The
tissue was rinsed off using PBS (0.1 mol/l, pH 7.4) (Changde
Beekman Biotechnology Co., Ltd.) and then was centrifuged twice at
377 × g for 5 min. The red blood cells were suspended in RPMI-1640,
10% FBS and 1% antibiotics (Xibao Biotech Co., Ltd.) cultivating at
37°C in 5% CO2 incubator for 24 h. Then, the
non-adherent cell suspension (spleen lymphocytes) was collected
(27).
Apoptosis of T lymphocytes was determined by
JEM-100CX II transmission electron microscope (JEOL Ltd.) (28). For this, 100 µl of cell suspension
was added to each well of the culture plates (n=5). Then, 20 µl
freshly prepared 5%
3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
(Sigma-Aldrich; Merck KGaA) solution was added into each well
followed by incubation for more than 4 h. Glutaraldehyde 2.5% was
added to collect T lymphocytes for 2 h. After rinsing off the cells
using PBS, they were fixed for 1 h using 1% osmium acid. The
samples underwent dehydration of gradient ethanol and acetone
(Changsha Zhenxiang Biotechnology Co., Ltd.) and then were soaked
overnight in epoxy resin EMbed 812 (Electron Microscopy Sciences).
Polymerization was performed at 40 and 60°C for 24 h. Finally, LKBV
ultra-thin slicing machine (Guangzhou Jiuying Machinery Equipment
Co., Ltd.), was used to prepare the samples at 500–600A thickness.
After uranium acetate (Suzhou Weiboyi Chemical Co., Ltd.) and lead
citrate double staining, the samples were observed using
transmission electron microscope. The number of apoptotic cells was
counted. There were 5 fields in each pore under high-power lens,
and the average value was calculated and analyzed.
Granulocyte macrophage-colony
stimulating factor evaluation
The level of GM-CSF was determined by commercially
available ELISA kit (GM-CSF ELISA kit; Shenzhen Dako Biotechnology
Co., Ltd.). The procedure was performed according to manufacturer
protocol. Briefly, the absorbance (OD) value of samples was
determined at 450 nm wavelength (n=3). The regression equation
resulted from drawing standard curve using standard absorbance (OD)
and its corresponding concentration. The absorbance value of the
sample and the content of GM-CSF that was calculated according to
the regression equation, were used for the statistical
analysis.
Statistical methods
Statistical analysis was carried out by using SPSS
statistical software (version 20.0; IBM SPSS, Armonk, NY, USA).
Data are presented in mean ± standard error (SE). Differences in
measured parameters among the seven groups were analyzed with one
way ANOVA test followed by Least Significant Difference post hoc.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of white mange mixture on
PCNA
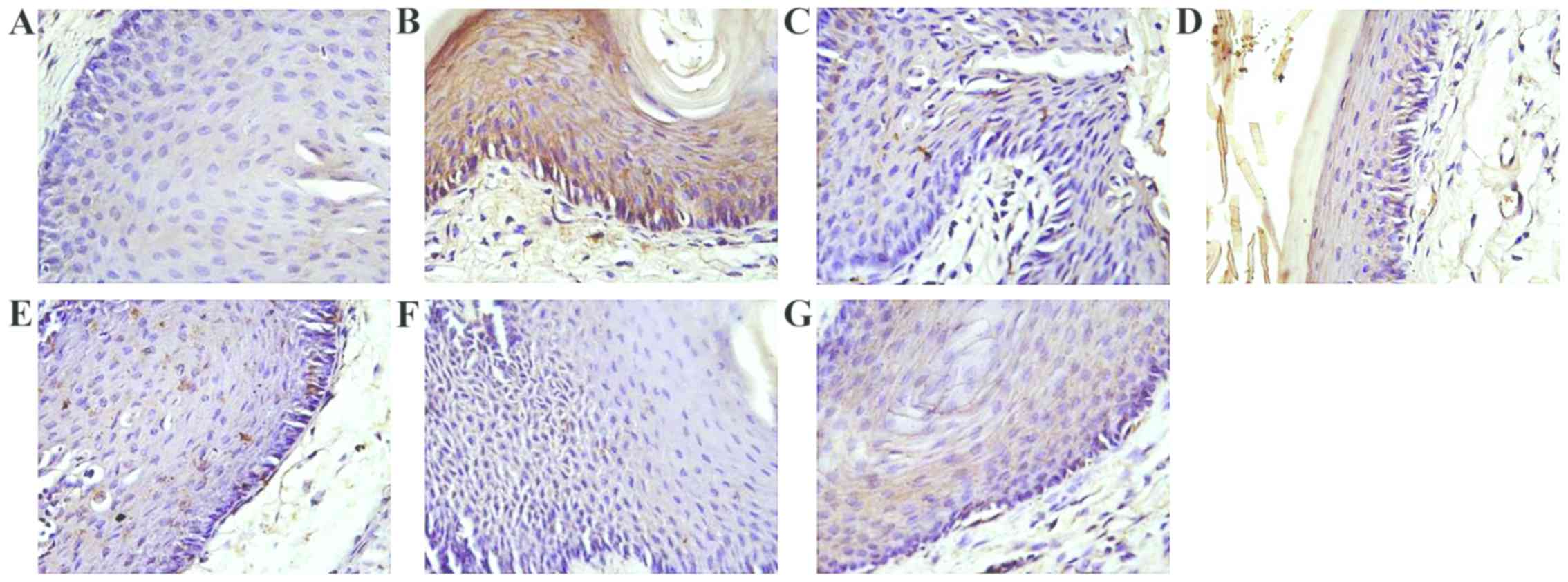
The PCNA protein expression is shown in Table II and Fig. 1. PCNA in negative control group
showed the highest grey value with regard to the experimental
groups. The mice in the positive control group (vaginal psoriasis
model groups) showed lowest color level around 98.17. It was also
observed that the grey value of the white mange mixture group was
significantly increased by increasing the dose to 23 mg. In
addition, the grey values of high- and medium-dose groups were very
close. Also, average grey value in low white mange mixture,
acitretin and Xiaoying granule groups were similar. However, the
inhibitory effect of TCM in high- and-medium dose groups was more
obvious than acitretin group and Xiaoying granules group.
 | Table II.The expression of PCNA in the vaginal
tissue of mice. |
Table II.
The expression of PCNA in the vaginal
tissue of mice.
| Groups | Dose
(mg/kg/day) | Average grey
value |
|---|
| Negative control
group | – |
180.03±11.24b,c,e |
| Positive control
group | ′ |
98.17±5.91a,c,e |
| Acitretin
group | 5.75 |
119.31±5.23a,b |
| Xiaoying granules
group | 1.219 |
116.33±7.75a,b |
| High-dose white
mange mixture group | 23 |
140.83±4.13a–c,e |
| Medium-dose white
mange mixture group | 11.5 |
137.33±3.46a–d |
| Low-dose white
mange mixture group | 5.75 |
115.28±2.97a,b |
The results suggested that white mange mixture is
effective on vaginal psoriasis compared with acitretin and Xiaoying
granules. Low-dose of white mange mixture had similar effect to
acitretin and Xiaoying granules on inhibiting the cell
proliferation of mouse vaginal epithelium KC PCNA.
T lymphocyte cell apoptosis
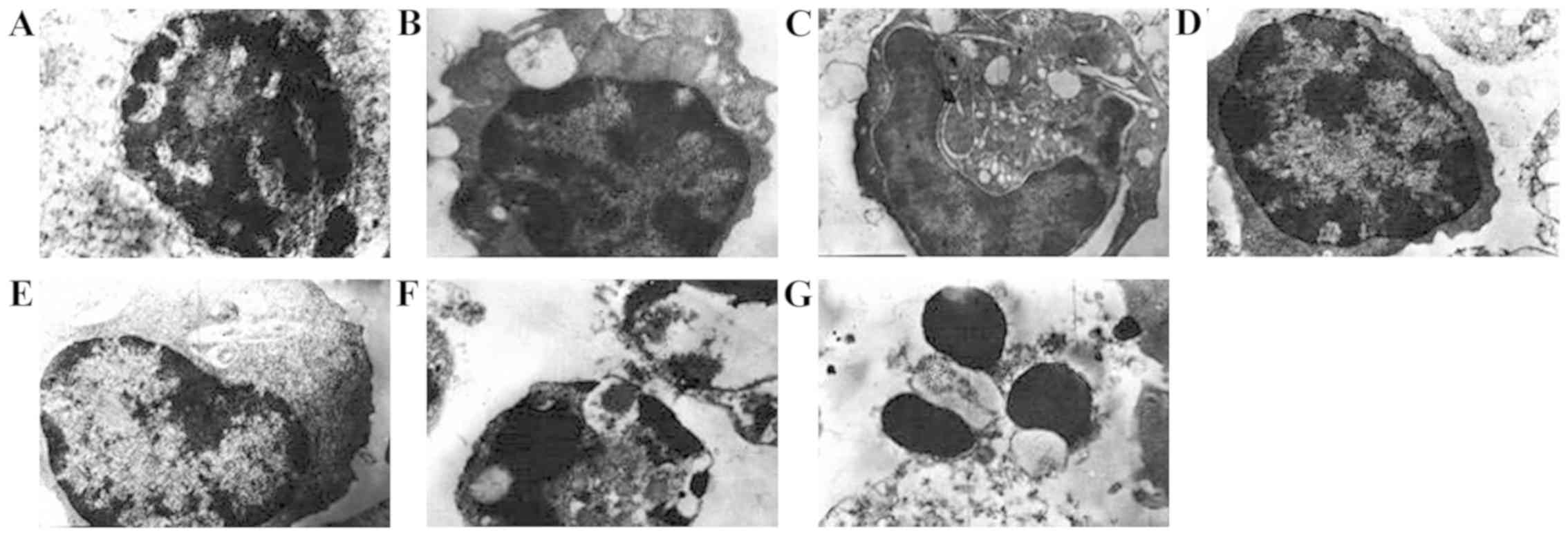
The T lymphocyte cell apoptosis is shown in Fig. 2. T lymphocytes in the negative
control group had normal appearance; the nucleus did not show any
segmentation. T lymphocyte cells in positive control group showed
normal but large nucleus compared to negative control group. In all
the white mange mixture groups cell apoptosis was significantly
observed but apoptosis ratio in high-dose group (4.20±3.0 cells)
and medium-dose group (4.00±2.0 cells) (nucleus was big and
circular and heterochromatin was distributed in the periphery) were
increased compared to low-dose group (4.0±2.0 cells). Apoptosis was
also observed in Xiaoying granules (2.00±1.0 cells) and acitretin
groups (2.20±1.0 cells) but apoptotic trend was decreased compared
with white mange mixture groups (Fig.
2).
Serum GM-CSF levels
Serum GM-CSF levels are shown in Table III. Serum GM-CSF levels in negative
control and positive groups were 10.41 and 21.83 pg/ml,
respectively. Compared with the negative control group, the level
of GM-CSF in the serum of the psoriasis model increased
significantly (P<0.01). In our results, acitretin group showed
decrease in serum GM-CSF levels to minimum compared to all
treatment groups. GM-CSF serum levels were decreased in Xiaoyin
granules and white mange mixture group, at high- and medium-dose,
but there was no significant difference between these groups
(P>0.05). The results indicated that the effect of white mange
mixture, acitretin and Xiaoyin granules was similar on inhibiting
the inflammatory cytokine GM-CSF serum levels.
 | Table III.GM-CSF levels in serum of each group
of mice. |
Table III.
GM-CSF levels in serum of each group
of mice.
| Groups | GM-CSF (pg/ml) |
|---|
| Negative control
group |
10.41±0.36b,d,f |
| Positive control
group |
21.83±0.47a,d,f |
| Acitretin
group |
12.02±0.41a,b |
| Xiaoying granules
group |
14.29±0.36a,b |
| High-dose white
mange mixture group |
14.41±0.81a,b |
| Medium-dose white
mange mixture dose |
14.92±0.42a–c |
| Low-dose white
mange mixture group |
16.87±0.36a,b,d,e |
Discussion
Basic clinical signs of psoriasis are erythema,
papule, differentiation and proliferation of KCs (29–31). The
vaginal epithelial hyperplasia can be used as a murine model of
vaginal psoriasis (32). In this
model, estrogen (which can simulate the rapid growth of epidermal
hyperplasia) ejection significantly increases mitosis and cell
proliferation (33,34).
SP immunohistochemistry results showed that white
mange mixture in different doses had inhibitory effect on mouse
vaginal epithelium PCNA protein expression and the effect was
better than that of Xiaoying granules and acitretin. This finding
leads to the conclusion that white mange mixture had inhibitory
effect on mouse vaginal epithelium PCNA expression. Zhang et
al (35) detected the effect of
white mange mixture on mitosis and PCNA expression in vaginal
epithelium of mice with psoriasis in estrogen stage by using
immunohistochemistry methods. They confirmed that white mange
mixture can promote the formation of granular layer in the tail
scale model of mice, and reduce the expression of PCNA in the
vaginal epithelium of the female mice. In our study white mange
mixture reduced also the expression level of PCNA in the vaginal
epithelium.
Liu et al (36) studied the effect of Xiaoyin granules
on histopathology of skin lesions and PCNA content in guinea pigs
with psoriasis. The results of studies showed that Xiaoyin granules
can play an important role in the treatment of psoriasis by
improving the histopathological score and inhibiting the expression
of PCNA. Results of Liu et al were similar to our Xiaoyin
granule results on serum PCNA expression.
Zhou and Yu (37)
observed the clinical effect of Xiaoyin capsule on psoriasis
vulgaris blood heat syndrome and explored its effect on Ki-67 and
PCNA expression in skin lesions, showing that the expression of
PCNA in the skin lesions of the patients before treatment was
strongly positive, and the expression intensity was significantly
higher than in normal skin (P<0.05). After treatment, the
expression intensity was significantly decreased, showing a weak
positive expression or near normal skin expression. Therefore,
Xiaoyin capsule can achieve therapeutic effect by inhibiting the
proliferation of epidermal cells.
T lymphocytes have a key role in the pathogenesis of
psoriasis (38). Many studies have
shown that dermal infiltration of T lymphocytes is an important
pathological feature of psoriasis (39,40). The
relation between cytokines and psoriatic lesions is unclear and
requires further research (41,42).
According to our electron microscope findings, apoptotic appearance
was observed in the high and middle white mange mixture groups. The
cause of psoriasis lies mainly in the abnormality of T lymphocyte
activation. Yuan and Li (43)
studied the activation and regulation of T cells in the model of
guinea pig with psoriasis and proved that the white mange mixture
can improve the excessive proliferation of skin in guinea pigs with
psoriasis. Their data show correlation with our findings. According
to our results, white mange mixture induced apoptosis of T cells,
as pictured by the atomic force microscope. Chen et al
(44) investigated the effects of
white mange mixture on the expression level of IL-6 and CXCR2
protein ratio in HaCa T cells. Their results showed that white
mange mixture inhibits the secretion of IL-6 and reduce the
expression of IL-6 mRNA and CXCR2 protein during psoriasis
treatment. Xu et al (45)
detected T lymphocyte subsets in peripheral blood before and after
treatment with white mange mixture and the results showed that
white mange mixture has few side effects and low relapse rate in
treatment of psoriasis.
GM-CSF is now considered to be involved in the
chronic phase of inflammatory and autoimmune diseases (46,47).
GM-CSF has a role in the regulation of neutrophils (48,49). In
addition, GM-CSF promotes the secretion of IL-1 cytokines and is
involved in the pathogenesis process of psoriasis (50). This research adopted the ELISA method
to detect mouse serum GM-CSF concentrations. The results showed
that GM-CSF concentration in mouse serum of the psoriasis model
controls were higher than the negative control group showing that
GM-CSF have a key role in the pathogenesis of psoriasis. GM-CSF
levels of Chinese medicine group with different doses, were
significantly lower than the positive control group. The data
showed that white mange mixture inhibited the expression of GM-CSF
serum levels mainly in high- and medium-doses. Yang et al
(51) detected the level of cytokine
IL-2, −6 and −8 in the skin lesion of the guinea pig psoriasis
model by radioimmunoassay and the effect of white mange mixture on
the cytokine in the skin lesion was observed. The results showed
that white mange mixture may inhibit the proliferation of KCs and
regulate cellular inflammatory factors. Studies showed that the
levels of GM-CSF in psoriatic lesions and serum are increased
(52,53). Cai et al (54) studied the effect of Xiaoyin granules
on the expression of monocyte chemoattractant protein-1 (MCP-1),
macrophage colony-stimulating factor (M-CSF) and macrophage
inflammatory protein-1α (MIP-1α) in patients with psoriasis and its
results showed that Xiaoyin granules can improve the expression of
MCP-1, M-CSF and MIP-1α in patients with psoriasis, by improving
the state of oxidative stress and inhibiting the local inflammatory
responses.
In conclusion, our study showed that the white mange
mixture has some unique effects: i) inhibition of proliferation of
mouse vaginal epithelium; ii) KC cell PCNA protein expression; iii)
regulation and induction of apoptosis to T lymphocytes; and iv)
decrease in GM-CSF serum levels. However, the mechanism of action
is not yet completely revealed, and further research is needed to
unmask intracellular pathways.
Acknowledgements
Not applicable.
Funding
This study was supported by Cooperative Innovation
Center for Medical Research of Miao Medicine [2015]05; Key
Laboratory of Miao Medicine in Guizhou Province: Qianmiao Medicine
K word [2017]103; Key Laboratory of TCM of Colleges and
Universities in Guizhou Province: Qian Teaching Combined KY word
[2016]005; National Medicine of traditional Chinese Medicine in
Guizhou Province (Miao Medicine) New Dosage Form and New
Preparation Engineering Research Center: Qian Teaching Combined KY
word [2014]22; New Dosage and New Technology Innovation Team of
Drug in Guizhou Province: Qian Department Combined Platform Talent
word [2017]5655.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JG contributed to the conception and design of the
study. JG and JL were responsible for the collection and assembly
of the data, and wrote the manuscript. JL was involved in the data
analysis and interpretation. Both authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Guiyang University of Chinese Medicine (Guiyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nickoloff BJ and Nestle FO: Recent
insights into the immunopathogenesis of psoriasis provide new
therapeutic opportunities. J Clin Invest. 113:1664–1675. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leonardi CL, Powers JL, Matheson RT, Goffe
BS, Zitnik R, Wang A and Gottlieb AB; Etanercept Psoriasis Study
Group, : Etanercept as monotherapy in patients with psoriasis. N
Engl J Med. 349:2014–2022. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Menter A, Korman NJ, Elmets CA, Feldman
SR, Gelfand JM, Gordon KB, Gottlieb AB, Koo JY, Lebwohl M, Lim HW,
et al: Guidelines of care for the management of psoriasis and
psoriatic arthritis: Section 4. Guidelines of care for the
management and treatment of psoriasis with traditional systemic
agents. J Am Acad Dermatol. 61:451–485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gudjonsson JE, Johnston A, Dyson M,
Valdimarsson H and Elder JT: Mouse models of psoriasis. J Invest
Dermatol. 127:1292–1308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ungureanu A, Zlatian O, Mitroi G, Drocaş
A, Ţîrcă T, Călina D, Dehelean C, Docea AO, Izotov BN, Rakitskii
VN, et al: Staphylococcus aureus colonisation in patients
from a primary regional hospital. Mol Med Rep. 16:8771–8780. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cargill M, Schrodi SJ, Chang M, Garcia VE,
Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese
JJ, et al: A large-scale genetic association study confirms IL12B
and leads to the identification of IL23R as psoriasis-risk genes.
Am J Hum Genet. 80:273–290. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krueger JG: The immunologic basis for the
treatment of psoriasis with new biologic agents. J Am Acad
Dermatol. 46:1–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reich K, Nestle FO, Papp K, Ortonne JP,
Evans R, Guzzo C, Li S, Dooley LT and Griffiths CE; EXPRESS study
investigators, : Infliximab induction and maintenance therapy for
moderate-to-severe psoriasis: A phase III, multicentre,
double-blind trial. Lancet. 366:1367–1374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nomura I, Goleva E, Howell MD, Hamid QA,
Ong PY, Hall CF, Darst MA, Gao B, Boguniewicz M, Travers JB, et al:
Cytokine milieu of atopic dermatitis, as compared to psoriasis,
skin prevents induction of innate immune response genes. J Immunol.
171:3262–3269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stern RS, Nijsten T, Feldman SR, Margolis
DJ and Rolstad T: Psoriasis is common, carries a substantial burden
even when not extensive, and is associated with widespread
treatment dissatisfaction. J Investig Dermatol Symp Proc.
9:136–139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gottlieb AB, Evans R, Li S, Dooley LT,
Guzzo CA, Baker D, Bala M, Marano CW and Menter A: Infliximab
induction therapy for patients with severe plaque-type psoriasis: A
randomized, double-blind, placebo-controlled trial. J Am Acad
Dermatol. 51:534–542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sani TA, Mohammadpour E, Mohammadi A,
Memariani T, Yazdi MV, Rezaee R, Calina D, Docea AO, Goumenou M,
Etemad L, et al: Cytotoxic and apoptogenic properties of
Dracocephalum kotschyi aerial part different fractions on
calu-6 and mehr-80 lung cancer cell lines. Farmacia. 65:189–199.
2017.
|
|
13
|
Ho SG, Yeung CK and Chan HH: Methotrexate
versus traditional Chinese medicine in psoriasis: A randomized,
placebo-controlled trial to determine efficacy, safety and quality
of life. Clin Exp Dermatol. 35:717–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu C, Deng J, Li L, Wang D and Li G:
Application of metabolomics on diagnosis and treatment of patients
with psoriasis in traditional Chinese medicine. Biochim Biophys
Acta. 1844:280–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geiger JM and Walker M: Is there a
reproductive safety risk in male patients treated with acitretin
(neotigason/soriatane?). Dermatology. 205:105–107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dogra S and Yadav S: Acitretin in
psoriasis: An evolving scenario. Int J Dermatol. 53:525–538. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang L, Wu X and Ma J: Traditional Chinese
medicine for the treatment of psoriasis vulgaris: A systematic
review. Afr J Microbiol Res. 6:7040–7047. 2012.
|
|
18
|
Liu C, Bi Z, Zhu Y and Li P: Simultaneous
determination of four kinds of bioactive components in radix
scrophulariae by HPLC. Chung Kuo Yao Hsueh Tsa Chih. 42:1614–1616.
2007.(In Chinese).
|
|
19
|
Allan BF, Dutra HP, Goessling LS, Barnett
K, Chase JM, Marquis RJ, Pang G, Storch GA, Thach RE and Orrock JL:
Invasive honeysuckle eradication reduces tick-borne disease risk by
altering host dynamics. Proc Natl Acad Sci USA. 107:18523–18527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stpiczyńska M, Nepi M and Zych M:
Nectaries and male-biased nectar production in protandrous flowers
of a perennial umbellifer Angelica sylvestris L. (Apiaceae).
Plant Syst Evol. 301:1099–1113. 2015. View Article : Google Scholar
|
|
21
|
Zhang Y, Tu C, Zhang D, Zheng Y, Peng Z,
Feng Y, Xiao S and Li Z: Wnt/β-catenin and Wnt5a/Ca pathways
regulate proliferation and apoptosis of keratinocytes in psoriasis
lesions. Cell Physiol Biochem. 36:1890–1902. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kawahira K: Immunohistochemical staining
of proliferating cell nuclear antigen (PCNA) in malignant and
nonmalignant skin diseases. Arch Dermatol Res. 291:413–418. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yonei T, Watanabe K, Sato T and Yamadori
I: Induction of psoriasis by human recombinant granulocyte colony
stimulating factor in a patient with small cell lung cancer. Nihon
Kokyuki Gakkai Zasshi. 39:438–441. 2001.(In Japanese). PubMed/NCBI
|
|
24
|
Mössner R, Beckmann I, Hallermann C,
Neumann C and Reich K: Granulocyte
colony-stimulating-factor-induced psoriasiform dermatitis resembles
psoriasis with regard to abnormal cytokine expression and epidermal
activation. Exp Dermatol. 13:340–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stoddart CA, Joshi P, Sloan B, Bare JC,
Smith PC, Allaway GP, Wild CT and Martin DE: Potent activity of the
HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice. PLoS
One. 2:e12512007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshiji H, Kuriyama S, Yoshii J, Ikenaka
Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M,
et al: Vascular endothelial growth factor and receptor interaction
is a prerequisite for murine hepatic fibrogenesis. Gut.
52:1347–1354. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song XY, Sun LN, Zheng NN and Zhang HP:
Effect of hyperbaric oxygen preconditioning on spleen lymphocytes
and cell adhesion molecules after skin transplantation in mice.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 18:1275–1277. 2010.(In
Chinese). PubMed/NCBI
|
|
28
|
Koynova R, Tarahovsky YS, Wang L and
MacDonald RC: Lipoplex formulation of superior efficacy exhibits
high surface activity and fusogenicity, and readily releases DNA.
Biochim Biophys Acta. 1768:375–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng H and Li X: Effect of white mange
medicine mixture on mice models of psoriasis. Chin J Derm Venerol
Integr Trad W Med. 11:149–151. 2012.
|
|
30
|
Kryczek I, Bruce AT, Gudjonsson JE,
Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling
TH, et al: Induction of IL-17+ T cell trafficking and
development by IFN-γ: Mechanism and pathological relevance in
psoriasis. J Immunol. 181:4733–4741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XJ, Huang W, Yang S, Sun LD, Zhang
FY, Zhu QX, Zhang FR, Zhang C, Du WH, Pu XM, et al: Psoriasis
genome-wide association study identifies susceptibility variants
within LCE gene cluster at 1q21. Nat Genet. 41:205–210. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tyring S, Gottlieb A, Papp K, Gordon K,
Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, et al:
Etanercept and clinical outcomes, fatigue, and depression in
psoriasis: Double-blind placebo-controlled randomised phase III
trial. Lancet. 367:29–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiricozzi A, Guttman-Yassky E,
Suárez-Fariñas M, Nograles KE, Tian S, Cardinale I, Chimenti S and
Krueger JG: Integrative responses to IL-17 and TNF-α in human
keratinocytes account for key inflammatory pathogenic circuits in
psoriasis. J Invest Dermatol. 131:677–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park JS, Rhyu JW, Kim CJ, Kim HS, Lee SY,
Kwon YI, Namkoong SE, Sin HS and Um SJ: Neoplastic change of
squamo-columnar junction in uterine cervix and vaginal epithelium
by exogenous estrogen in hpv-18 URR E6/E7 transgenic mice. Gynecol
Oncol. 89:360–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Peng T and Shi H: Effects of
BAIBI capsules on psoriatic mice and its mechanism of action. J
Pract Dermatol. 10:234–239. 2017.(In Chinese).
|
|
36
|
Liu H, Li Q, Wen X and Ye J: Xiaoyin no.
2′ effect of content on the histopathology and the content of PCNA
in psoriatic lesions of Guinea pig. J Yunnan Univ Tradit Chin Med.
38:13–16. 2015.(In Chinese).
|
|
37
|
Zhou Y and Yu R: Observation of combined
acitretin capsule and Xiaoyin granule therapy for 60 patients with
psoriasis vulgaris. Jilin Med J. 33:6504–6505. 2012.(In
Chinese).
|
|
38
|
Nanjappa MK, Medrano TI, March AG and
Cooke PS: Neonatal uterine and vaginal cell proliferation and
adenogenesis are independent of estrogen receptor 1 (ESR1) in the
mouse. Biol Reprod. 92:782015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou X, Krueger JG, Kao MC, Lee E, Du F,
Menter A, Wong WH and Bowcock AM: Novel mechanisms of T-cell and
dendritic cell activation revealed by profiling of psoriasis on the
63,100-element oligonucleotide array. Physiol Genomics. 13:69–78.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lew W, Bowcock AM and Krueger JG:
Psoriasis vulgaris: Cutaneous lymphoid tissue supports T-cell
activation and ‘Type 1’ inflammatory gene expression. Trends
Immunol. 25:295–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Laggner U, Di Meglio P, Perera GK,
Hundhausen C, Lacy KE, Ali N, Smith CH, Hayday AC, Nickoloff BJ and
Nestle FO: Identification of a novel proinflammatory human
skin-homing Vγ9Vδ2 T cell subset with a potential role in
psoriasis. J Immunol. 187:2783–2793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fujita H, Shemer A, Suárez-Fariñas M,
Johnson-Huang LM, Tintle S, Cardinale I, Fuentes-Duculan J,
Novitskaya I, Carucci JA, Krueger JG, et al: Lesional dendritic
cells in patients with chronic atopic dermatitis and psoriasis
exhibit parallel ability to activate T-cell subsets. J Allergy Clin
Immunol. 128:574–582. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan Q and Li X: Experimental study on
regulating action of psoriasis model of guinea pig T cell
activation by TCM psoriasis mixture. Clin J Chin Med. 6:46–49.
2014.(In Chinese).
|
|
44
|
Chen X, Lu Y and Li X: Effects of white
mange mixture on Expression of CXCR2 protein of HaCaT cells and
content of IL-6. Zhonghua Zhongyiyao Xuekan. 33:2710–2712. 2015.(In
Chinese).
|
|
45
|
Xu W, Mao C, Feng R and Wang J:
Correlation analysis of blood heat syndrome with psoriasis vulgaris
and TH17 related cytokines. Guiding J Tradit Chin Med Pharm.
22:5–28. 2016.(In Chinese).
|
|
46
|
Kruglikov IL and Wollina U: The role of
subcutaneous adipose tissue in psoriasis. J Biol Regul Homeost
Agents. 32:159–161. 2018.PubMed/NCBI
|
|
47
|
Jobanputra P: Polyarteritis nodosa.
Diagnostic challenges in a patient with cutaneous vasculitis,
psoriasis, psoriatic arthritis and pancytopenia: Fatal progression
after treatment with G-CSF. Oxf Med Case Rep. 2016:86–90. 2016.
View Article : Google Scholar
|
|
48
|
Scholz T, Weigert A, Brüne B, Sadik CD,
Böhm B and Burkhardt H: GM-CSF in murine psoriasiform dermatitis:
Redundant and pathogenic roles uncovered by antibody-induced
neutralization and genetic deficiency. PLoS One. 12:e01826462017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Witte E, Kokolakis G, Witte K, Philipp S,
Doecke WD, Babel N, Wittig BM, Warszawska K, Kurek A,
Erdmann-Keding M, et al: IL-19 is a component of the pathogenetic
IL-23/IL-17 cascade in psoriasis. J Invest Dermatol. 134:2757–2767.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martin G, Guérard S, Fortin MM, Rusu D,
Soucy J, Poubelle PE and Pouliot R: Pathological crosstalk in vitro
between T lymphocytes and lesional keratinocytes in psoriasis:
Necessity of direct cell-to-cell contact. Lab Invest. 92:1058–1070.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang C, Chen M and Tao C: Effect of baibi
ointment to psoriasis guinea pig model. J Chengdu Univ TCM.
34:55–57. 2011.(In Chinese).
|
|
52
|
Shi XL, Pan YM, Ma HY and Yang XF:
Treatment of psoriasis vulgaris by NB-UVB combined with traditional
Chinese materia medica bath: A clinical observation. Chin J Laser
Med Surg. 20:314–317. 2011.(In Chinese).
|
|
53
|
Buquicchio R, Foti C, Loconsole F,
Polimeno L and Ventura MT: Clusterin serum level: How does it
affect psoriatic patients? J Biol Regul Homeost Agents. 31:785–789.
2017.PubMed/NCBI
|
|
54
|
Cai Y, Zhang J, Yan Z, Zhang L, Yin J, Ren
Y, Zhang G, Yin Z and Li H: Effect of Caishi Xiaoyin on serum
levels of MCP-1, M-CSF and MIP-1 alpha in patients with psoriasis
vulgaris. Chin J Clin Ration Drug Use. 8:107–108. 2015.(In
Chinese).
|