Introduction
Therapeutic hypothermia (TH) is an effective therapy
for comatose patients, improving outcome after return of
spontaneous circulation (ROSC) following cardiac arrest with
ventricular tachycardia or fibrillation as the initial rhythm
(1). All comatose adult patients
after ROSC following cardiac arrest should receive targeted
temperature management (TTM), with a target temperature between
32°C and 36°C (2). However, the
mechanism underlying the therapeutic effect of TH remains elusive.
Following ROSC, the subsequent ischemic reperfusion results in
extensive generation of free radicals, leading to systemic
inflammatory response syndrome (SIRS) which may severely impact
prognosis (3). This type of SIRS has
been termed ‘post-cardiac arrest syndrome’ (PCAS), including
systemic ischemia/reperfusion injury and the subsequent SIRS, as
well as brain injury and myocardial dysfunction (4,5).
However, these studies mainly evaluated the levels of cytokines
(3,6,7). In
contrast, the proteomic approach may provide unbiased data on
alterations in the abundance of proteins (8). Isobaric tag-based quantitative mass
spectrometry has been recently introduced as a high-throughput
technology (9). A previous plasma
proteomics method using the isobaric tag for relative and absolute
quantification (iTRAQ) labeling identified 262 proteins and showed
that 2–3 h of deep hypothermic cardiopulmonary bypass (CPB)
suppressed complement activation compared with normothermic CPB in
aortic surgery (10). Because the
cooling time in TH after cardiac arrest is longer than that
reported in cardiac surgery, it may be easier to detect more
proteins with their expression changes in samples of patients
undergoing TH. However, such studies are rare. For the first time,
we performed gel-free proteomic analysis of plasma samples in
patients undergoing TH after ROSC. The objective of this study was
to assess the effects of cooling/rewarming on the plasma proteome
during TH after ROSC and identify the mechanism underlying its
therapeutic effect.
Patients and methods
Study population
This was a prospective, observational study
including nine patients resuscitated from an in-hospital or
out-of-hospital cardiac arrest and treated with TH (Table I). This prospective, cohort study was
approved by the Ethics Committee of Shimane University Faculty of
Medicine and Shimane Prefectural Central Hospital.
 | Table I.Demographic data. |
Table I.
Demographic data.
| Demographics,
n=9 | Data |
|---|
| Age | 72.6±14.6 |
| Male, n (%) | 4 (44.4%) |
| Bystander-initiated
BLS, n (%) | 5 (55.6%) |
| Time between
collapse to CPR, min |
3.8±4.6 |
| Time between
collapse to ROSC, min | 14.3±4.6 |
| Time between
collapse to start of cooling, min | 154.7±75.0 |
| Time between
initiation of cooling to target temperature 34°C, min |
34.5±25.0 |
| Time between
initiation of rewarming to target temperature 36°C, h | 26.5±7.8 |
| APACHE II
score | 31.2±4.7 |
| pH at initial
examination |
7.13±0.31 |
| HCO3- at
initial examination, mmol/l | 16.8±6.9 |
| Lactate at initial
examination, mmol/l |
20.7±18.6 |
| PaCO2 at
initial examination, mmHg |
52.7±23.8 |
| Patients with good
prognosis, CPC=1-2, n (%) | 3 (33.3%) |
| Patients with poor
prognosis, CPC=3-5, n (%) | 5 (55.6%) |
| Patients who
expired, n (%) | 1 (11.1%) |
Patient management
All patients were admitted to the intensive care
unit (ICU) of Shimane University Hospital and Shimane Prefectural
Central Hospital and treated according to the protocol
predetermined by the study investigators in both hospitals
(Table SI). Written informed
consent was provided by the relatives of the patients. All patients
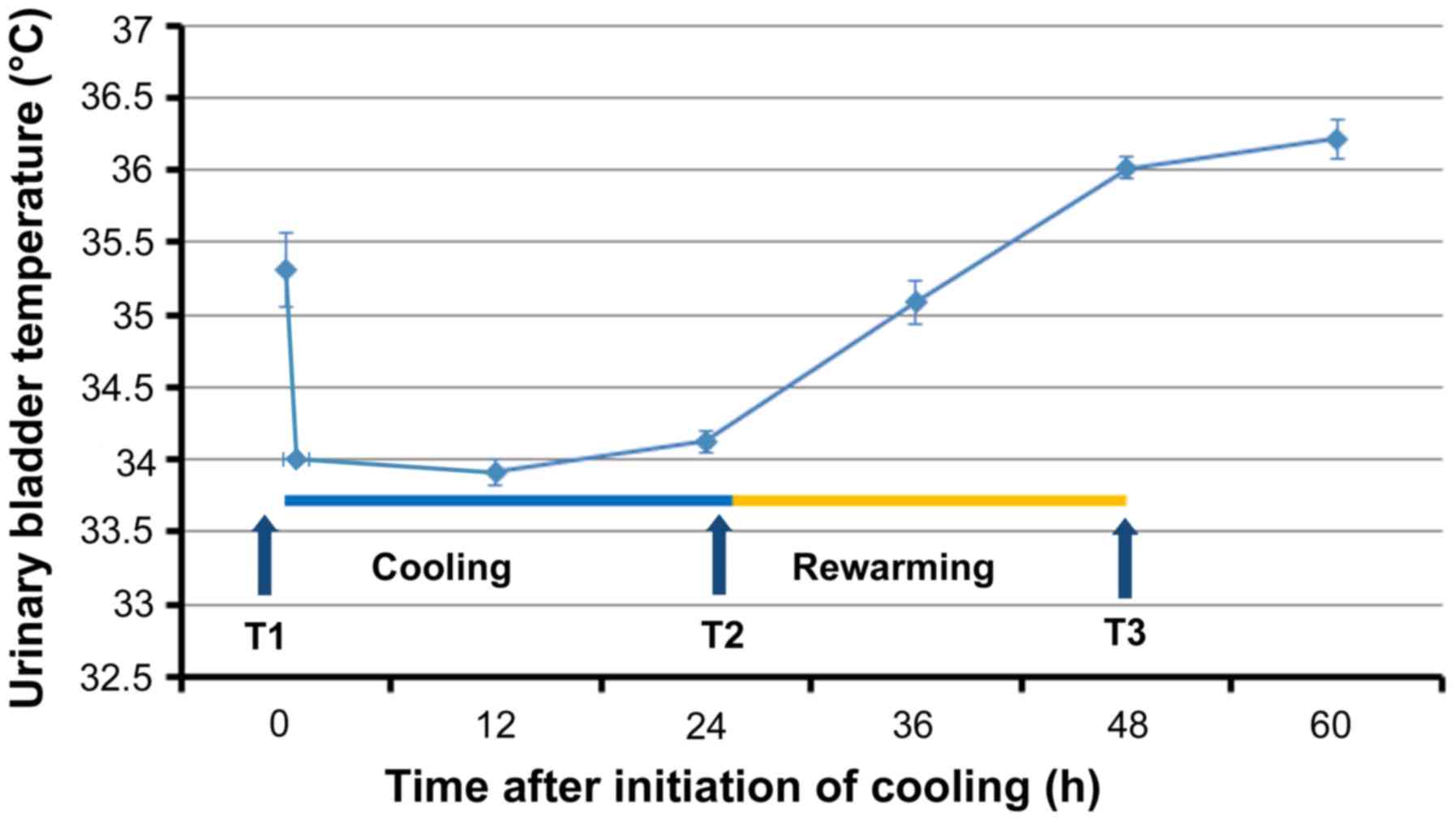
were rapidly cooled to 34°C through surface cooling using the
Arctic Sun® 5000 Temperature Management System
(Medivance, Louisville, CO, USA) and maintained at that temperature
for 24 h. Rewarming was performed slowly at a rate of 0.1°C/hour up
to the target temperature of 36°C (Fig.
1). Patients were sedated using propofol or midazolam plus
fentanyl. Shivering was treated with continuous infusion of
rocuronium. All patients were admitted to the ICU, intubated, and
mechanically ventilated according to the study protocol. The mean
blood pressure was maintained between 60 and 80 mmHg, and diuresis
was controlled at a rate of >1 ml/kg/h. Blood glucose was
controlled between 150 and 200 mg/dl.
Data and sample collection
Demographic and clinical variables were collected
from the electronic files of the patients. Three blood samples were
collected from an arterial line into ethylenediaminetetraacetic
acid (EDTA) tubes as follows: T1 (prior to cooling), T2 (prior to
rewarming, after 24 h of hypothermia), and T3 (immediately after
rewarming at 36°C). Following collection, the blood samples were
immediately centrifuged at 1,400 × g for 5 min, and the
plasma layers were stored at −80°C.
Immunodepletion of abundant
proteins
The two most abundant plasma proteins, albumin and
immunoglobulin (Ig)G, were eliminated using an immunodepletion
column (Albumin and IgG Depletion SpinTrap; GE Healthcare,
Buckinghamshire, UK) according to the manufacturer's instructions
and as previously reported (10).
iTRAQ labeling and strong cation
exchange chromatography
Samples were prepared according to the
manufacturer's instructions (SCIEX, Concord, Canada) and as
previously described (10). Briefly,
equal amounts of immunodepleted T1, T2, and T3 samples from each
patient were denaturated and reduced, and cysteines were alkylated
and digested using trypsin (SCIEX). Each digest was labeled with a
different iTRAQ tag using the iTRAQ Reagent 4-plex kit (SCIEX).
iTRAQ label 114 was chosen for the T1 samples, whereas iTRAQ labels
115, 116, or 117 were randomly selected for the T2 and T3 samples.
Subsequently, the three samples from each patient were combined and
fractionated into six fractions using strong cation exchange (SCX)
chromatography according to the manufacturer's instructions
(SCIEX). Each fraction was desalted according to the manufacturer's
instructions (Waters, Milford, MA, USA).
Nano liquid chromatography (LC) and
matrix-assisted laser desorption/ionization (MALDI)-time-of-flight
(TOF)/TOF mass spectrometry (MS)/MS analysis
One fraction from the SCX chromatography was
fractionated to 171 spots using a DiNa nanoLC system (KYA Tech,
Tokyo, Japan) and collected onto an Opti-TOF LC/MALDI 384 target
plate (SCIEX) in accordance with the manufacturer's instructions
and as previously described (10).
Spotted peptide samples were analyzed using a 5800 MALDI-TOF/TOF
MS/MS Analyzer and the TOF/TOF Series software (version 4.0,
SCIEX). MS/MS data were analyzed using the ProteinPilot™ software
(version 3.0) and the Paragon™ protein database (SCIEX).
Quantitative alterations in protein levels at T2 or T3 were
calculated using the iTRAQ ratios T2:T1 or T3:T1, respectively.
ITRAQ data analysis and bioinformatic
analysis
Identified proteins were tested to determine if they
fulfilled the following two criteria: i) A false discovery rate
(FDR) <5% (FDR was estimated through ‘decoy database searching’
using the ProteinPilot™ software); and ii) protein confidence
>99% (‘unused ProtScore’ >2). Proteins fulfilling these
criteria were defined as statistically significant (10,11). The
DAVID 6.8 software (available at https://david.ncifcrf.gov accessed December 5, 2017)
was used to test for gene enrichment and functional annotation
analysis (12,13) of Gene Ontology terms (GO, available
at http://www.geneontology.org accessed
July 31, 2018) (14). If the number
of identified genes in a particular GO term was significantly
larger than the total number of genes in the entire genome
classified by the same GO term according to the binominal test, the
GO term was termed ‘enriched’. This suggested that a group of genes
in the GO term may play an important role in the pathological
process assessed by this study (13). The correction of multiple testing was
performed using the EASE score, a modified Fisher's exact test, and
Benjamini statistics (12,13). The annotations of identified proteins
were acquired from the Uniprot database (available at: http://www.uniprot.org/ accessed by July 31,
2018).
Western blot analysis and ELISA
Western blotting analysis was performed as
previously described (11). Briefly,
plasma samples were separated through sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
immunoblotted using the mouse monoclonal apolipoprotein A1 (ApoA1)
antibody (Cell Signaling technology, Danvers, MA, USA), rabbit
monoclonal apolipoprotein C3 (ApoC3) antibody (GeneTex, Irvine, CA,
USA), mouse monoclonal Gelsolin (GSN) antibody (Abcam, Tokyo,
Japan), and anti-rabbit IR dye 680-conjugated IgG (LI-COR, Lincoln,
NE, USA). Protein bands were visualized using an Odyssey (LI-COR)
Infrared Imaging System and their intensities were measured through
densitometric analyses of ApoA1, APOC3, and GSN. To confirm equal
levels of proteins per lane, nonspecific proteins stained with
Coomassie Brilliant Blue (CBB) were analyzed. The average value of
three independent experiments was used for statistical analysis.
The levels of free hemoglobin in the plasma were determined using a
solid-phase sandwich ELISA kit (MyBioSource, San Diego, CA, USA)
according to the manufacturer's instructions. The level of the
terminal complement complex (SC5b-9) was determined using a
solid-phase sandwich ELISA kit (Quidel Corp., San Diego, CA,
USA).
Statistical analysis
For the analysis of iTRAQ ratios (i.e., T2:T1 and
T3:T1) of each identified protein, P-values were calculated using a
one-sample t-test of averaged protein ratio against 1 to assess the
validity of the alterations in protein expression (10,11).
Statistical comparisons were performed using the paired t-test or
Wilcoxon signed-rank test followed by a post-hoc Bonferroni test to
estimate significant changes in the iTRAQ ratios of several
important proteins and the corresponding western blotting/ELISA
data.
Results
Proteomic changes during TH after
ROSC
Proteomic analysis identified 189 proteins having an
FDR <5%. Among those, 92 proteins identified in at least five of
the nine samples had significant protein confidence >99% (‘used
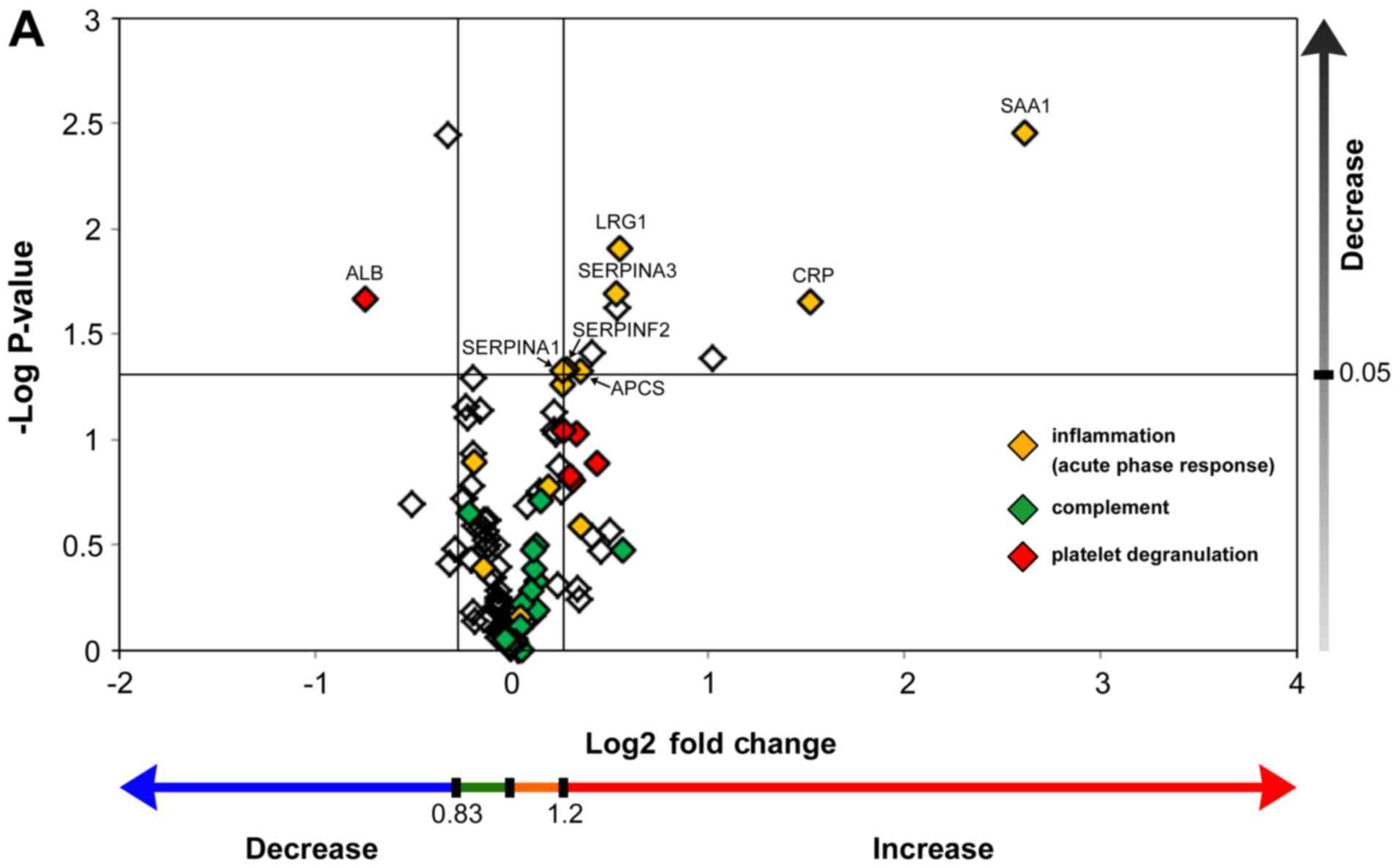
Protscore’ >2). To demonstrate the abundance of proteins using
fold changes and P-values, fold changes (T2/T1, T3/T1) were
combined with their P-values, with the two vertical lines showing a
fold change=1.2 and =0.833, respectively, and the horizontal line
showing P<0.05 (Fig. 2A and B).
Acute phase proteins were significantly upregulated during both
cooling (Fig. 2A) and rewarming
(Fig. 2B). Conversely, complement
proteins were not significantly upregulated during cooling and
rewarming. Fifteen proteins demonstrated statistically significant
alterations, including many upregulated proteins belonging to the
acute-phase response (Table II).
Unexpectedly, both hemoglobin subunit α and β were significantly
downregulated during TH.
 | Table II.Plasma proteins showing significantly
up- (>1.2) or down- (<0.83) regulated T2/T1 and T3/T1 ratio
at the end of cooling or rewarming. |
Table II.
Plasma proteins showing significantly
up- (>1.2) or down- (<0.83) regulated T2/T1 and T3/T1 ratio
at the end of cooling or rewarming.
|
|
|
|
| At end of cooling
(T2/T1) | Just after
rewarming (T3/T1) |
|---|
|
|
|
|
|
|
|
|---|
| Unused
ProtScorea | Gene name | Protein name | Protein
function | Mean ± SD, median
(minimum-maximum) |
P-valueb | Mean ± SD, median
(minimum- maximum) |
P-valueb |
|---|
| 29.32 | SAA1 | Serum amyloid A
protein | Acute-phase
response | 6.13±3.76 | 0.004 | 10.85±8.75 | 0.010 |
| 14 | CRP | C-reactive
protein | Acute-phase
response | 2.88±1.62 | 0.022 | 4.78±3.70 | 0.036 |
| 2.66 | LBP |
Lipopolysaccharide-binding protein | Acute-phase
response, innate immune response | 2.05±0.94 | 0.042 | 2.15±1.37 | 0.095 |
| 19.41 | LRG1 | Leucine-rich
α-2-glycoprotein | TGF β receptor
binding, neutrophil degranulation | 1.47±0.44 | 0.012 | 1.59±0.68 | 0.032 |
| 50.01 | SERPINA3 |
α-1-antichymotrypsin | Acute-phase
response, inhibitor of neutrophil cathepsin G and mast cell
chymase | 1.45±0.47 | 0.020 | 1.94±0.69 | 0.035 |
| 10 | APOC3 | Apolipoprotein
C-III | Lipid
metabolism | 1.34±0.37 | 0.039 | 1.70±0.68 | 0.023 |
| 11 | APCS | Serum amyloid
P-component | Acute-phase
response, the pentraxin family | 1.28±0.33 | 0.047 | 1.68±0.63 | 0.019 |
| 13.43 | SERPINF2 |
α-2-antiplasmin | Acute-phase
response, serine protease inhibitor | 1.22±0.28 | 0.047 | 1.30±0.55 | 0.141 |
| 90.75 | SERPINA1 |
α-1-antitrypsin | Acute-phase
response, blood coagulation, inhibitor of serine proteases
(including elastase) | 1.20±0.26 | 0.047 | 1.43±0.42 | 0.016 |
| 28.06 | ORM1 | α-1-acid
glycoprotein 1 | Acute-phase
response, negative regulation of IL-6 and TNF production | 1.20±0.27 | 0.055 | 1.66±0.68 | 0.019 |
| 101.78 | FGA | Fibrinogen α
chain | Blood coagulation,
adaptive immune response | 1.26±0.41 | 0.094 | 1.44±0.53 | 0.036 |
| 23.5 | HBB | Hemoglobin subunit
β | Oxygen transport,
hydrogen peroxide catabolic process | 0.8 (0.4–3.9) | 1.000 | 0.7 (0.3–1.2) | 0.039 |
| 12.17 | HBA1 | Hemoglobin subunit
α | Oxygen transport,
hydrogen peroxide catabolic process | 0.9 (0.4–3.7) | 1.000 | 0.9 (0.3–1.3) | 0.109c |
| 12.55 | GSN | Gelsolin | Actin filament
capping | 0.8±0.13 | 0.004 | 0.67±0.21 | 0.003 |
| 34.57 | ALB | Serum albumin | Transport | 0.6±0.34 | 0.022 | 0.61±0.30 | 0.013 |
Proteomic profiling using DAVID
Protein levels decreased or increased (<0.833
fold or >1.2 fold, respectively) in at least five of the nine
samples were identified and analyzed for gene enrichment (10,15) and
functional annotation using the DAVID software. Two GO terms,
‘platelet degranulation’ and ‘acute-phase response’, demonstrated
the most statistically significant protein enrichment (Tables III and IV).
 | Table III.Top 10 Gene Ontology terms showing
statistically significant protein enrichment for plasma samples
taken from patients having therapeutic hypothermia during
cooling. |
Table III.
Top 10 Gene Ontology terms showing
statistically significant protein enrichment for plasma samples
taken from patients having therapeutic hypothermia during
cooling.
| Gene Ontology
term | Number of observed
genes | P-value | Benjamini's
correction | Genes |
|---|
| Platelet
degranulation | 11 |
1.6×10−17 |
3.5×10−15 | ALB, FGA, FGB, FGG,
FN1, ITIH3, ORM1, PROS1, SERPINA1, SERPINA3, SERPINF2 |
| Acute-phase
response | 9 |
7.8×10−17 |
1.2×10−14 | CRP, APCS, FN1,
LBP, ORM1, SERPINA1, SERPINA3, SERPINF2, SAA1 |
| Fibrinolysis | 5 |
1.1×10−8 |
7.8×10−7 | FGA, FGB, FGG,
PROS1, SERPINF2 |
| Negative regulation
of endopeptidase activity | 6 |
3.3×10−7 |
1.8×10−5 | C4A, ITIH3, PROS1,
SERPINA1, SERPINA3, SERPINF2 |
| Blood
coagulation | 6 |
2.6×10−6 |
1.2×10−4 | FGA, FGB, FGG, HBB,
PROS1, SERPINA1 |
| Blood coagulation,
fibrin clot formation | 3 |
8.9×10−6 |
3.3×10−4 | FGA, FGB, FGG |
| Innate immune
response | 7 |
1.1×10−5 |
3.3×10−4 | APCS, C4A, FGA,
FGB, LBP, PGLYRP2, SAA1 |
| Platelet
aggregation | 4 |
1.7×10−5 |
4.8×10−4 | FGA, FGB, FGG,
HBB |
| Positive regulation
of peptide hormone secretion | 3 |
4.2×10−5 |
1.0×10−3 | FGA, FGB, FGG |
| Plasminogen
activation | 3 |
5.3×10−5 |
1.2×10−3 | FGA, FGB, FGG |
 | Table IV.Top 10 Gene Ontology terms showing
statistically significant protein enrichment for plasma samples
taken from patients having therapeutic hypothermia after
rewarming. |
Table IV.
Top 10 Gene Ontology terms showing
statistically significant protein enrichment for plasma samples
taken from patients having therapeutic hypothermia after
rewarming.
| Gene Ontology
term | Number of observed
genes | P-value | Benjamini's
correction | Genes |
|---|
| Platelet
degranulation | 10 |
3.7×10−14 |
1.3×10−11 | ALB, FGA, FGB, FGG,
ITIH3, ORM1, PROS1, SERPINA1, SERPINA3, SERPINF2 |
| Acute-phase
response | 8 |
1.8×10−13 |
3.2×10−11 | CRP, APCS, LBP,
ORM1, SERPINA1, SERPINA3, SERPINF2, SAA1 |
| Negative regulation
of endopeptidase activity | 8 |
6.7×10−10 |
8.2×10−8 | AGT, C4A, ITIH3,
PROS1, SERPINA1, SERPINA3, SERPINF2, VTN |
| Fibrinolysis | 5 |
3.1×10−8 |
2.9×10−6 | FGA, FGB, FGG,
PROS1, SERPINF2 |
| Innate immune
response | 9 |
2.5×10−7 |
1.8×10−5 | APCS, APOL1, C1R,
C1S, C4A, FGA, FHB, LBP, SAA1 |
| Lipoprotein
metabolism process | 5 |
3.8×10−7 |
2.3×10−5 | ALB, APOC2, APOC3,
APOE, APOL1 |
| Receptor-mediated
endocytosis | 7 |
4.2×10−7 |
2.2×10−5 | ALB, APOE, APOL1,
HBA1, HBB, SAA1, VTN |
| Protein
polymerization | 4 |
1.0×10−6 |
4.8×10−5 | FGA, FGB, FGG,
VTN |
| Blood
coagulation | 6 |
9.9×10−6 |
4.0×10−4 | FGA, FGB, FGG, HBB,
PROS1, SERPINA1 |
| Regulation of
complement activation | 4 |
1.5×10−5 |
5.4×10−4 | C4A, C9, PROS1,
VTN |
Western blotting and ELISA
analysis
Both units of hemoglobin decreased in seven samples
of patients who did not undergo transfusion (Fig. 3A and B). The difference was
statistically significant. ELISA analysis corroborated this
statistically significant downregulation of free hemoglobin induced
by TH (Fig. 3C). Western blotting
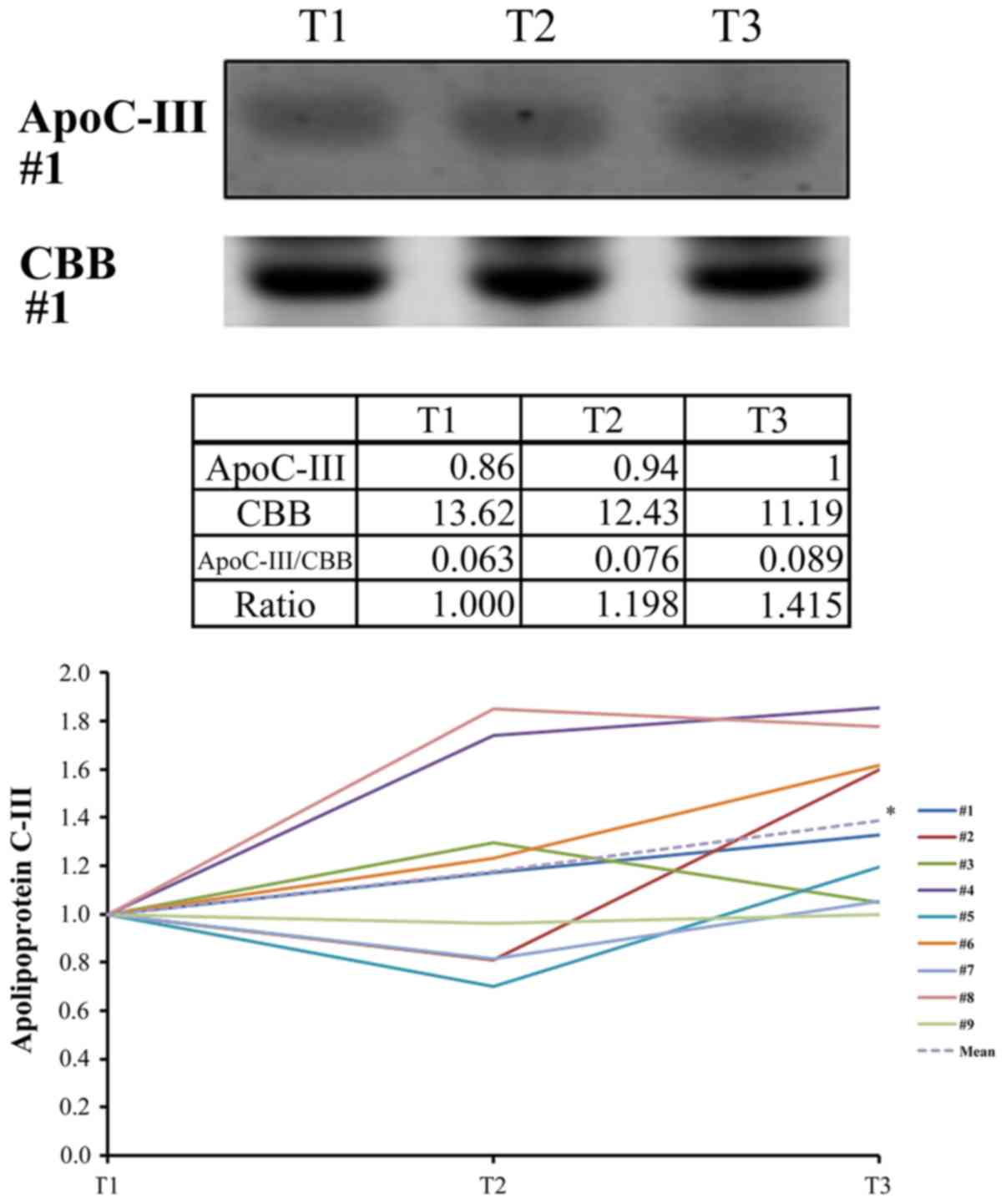
analysis confirmed the statistically significant upregulation of
ApoC3 (Fig. 4) and the statistically
significant downregulation of gelsolin at T3 (T3/T1=0.66±0.27,
P=0.039). Western blotting revealed that the levels of ApoA1 did
not change significantly during TH (T3/T1=0.87±0.16, P=0.091).
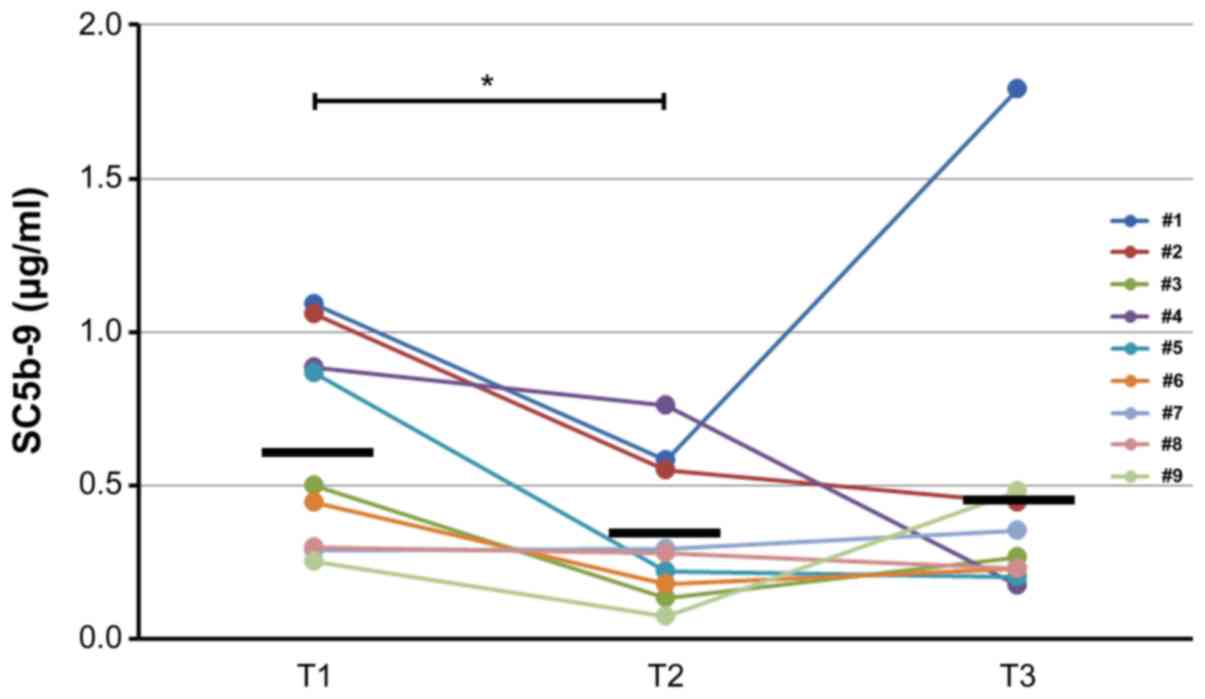
However, the levels of SC5b-9 decreased significantly (P=0.023)
through cooling from T1 to T2 and subsequently increased slightly
through rewarming from T2 to T3 (P=1.00) (Fig. 5).
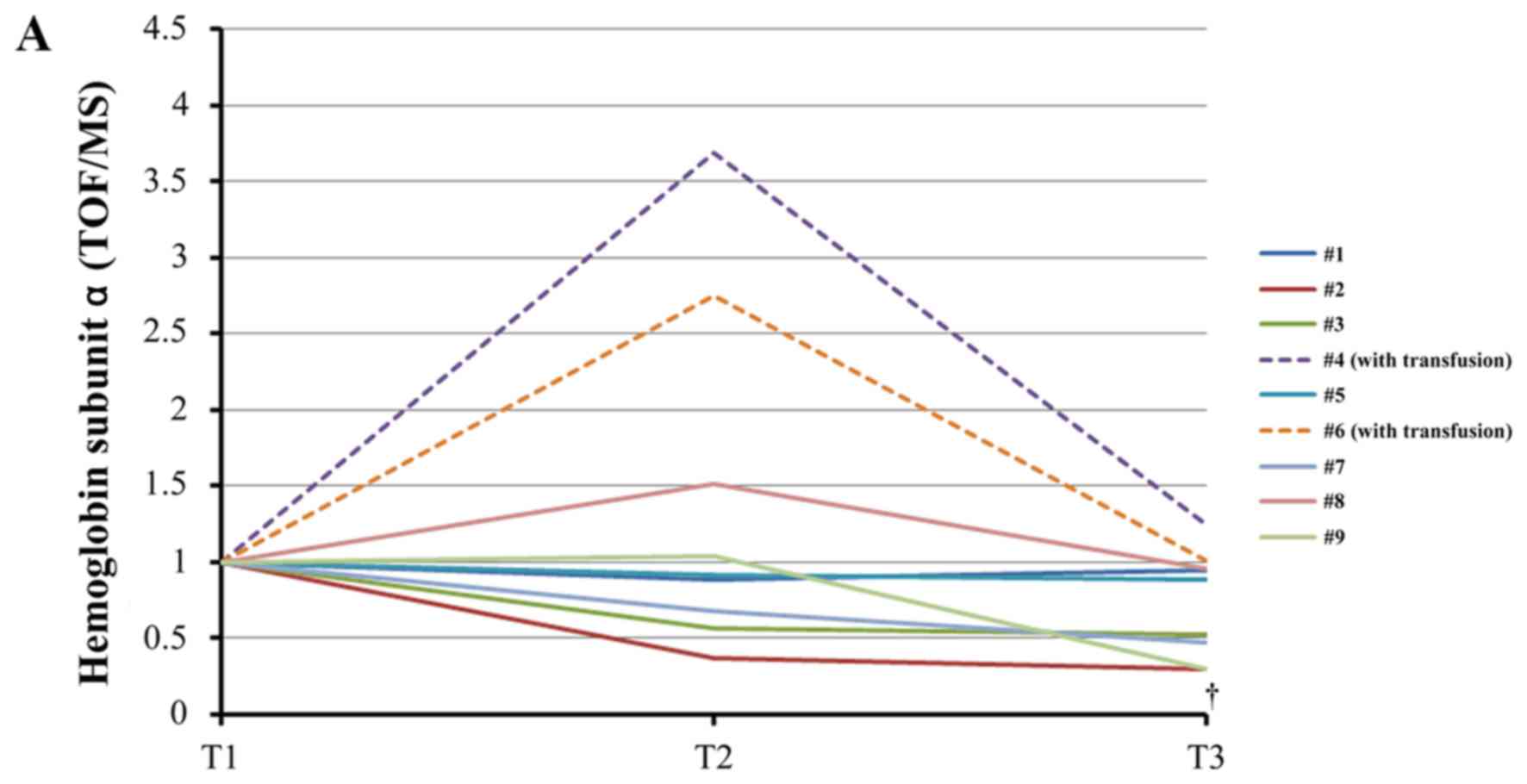 | Figure 3.Changes of plasma free hemoglobin
levels during therapeutic hypothermia. (A) iTRAQ ratio alterations
in the levels of hemoglobin subunit α during TH. †, analysis
excluding patients who received transfusion (#4, #6), P=0.031
between T1 and T3 using the paired t-test with post-hoc analysis
using the Bonferroni method. (B) iTRAQ ratio alterations in the
levels of hemoglobin subunit β levels during TH. *P=0.039 between
T1 and T3 using the Wilcoxon signed-rank test with post-hoc
analysis using the Bonferroni method. †P=0.025 between T1 and T2 in
analysis excluding patients who received transfusion (#4, #6) using
the paired t-test with post-hoc analysis by the Bonferroni method.
‡P=0.003 between T1 and T3 in analysis excluding
patients who received transfusion (#4, #6) using the paired t-test
with post-hoc analysis by the Bonferroni method. (C) Alterations in
the levels of free hemoglobin measured by enzyme-linked
immunosorbent assay (ELISA) during TH. *P=0.042 between T2 and T3
using the paired t-test with post-hoc analysis using the Bonferroni
method. †Analysis excluding patients who received transfusion (#4,
#6), P=0.047 between T1 and T3 using the Wilcoxon signed-rank test
with post-hoc analysis using the Bonferroni method. T1, prior to
cooling; T2, prior to rewarming; T3, immediately after rewarming;
iTRAQ, isobaric tag for relative and absolute quantification; TOF,
time-of-flight; MS, mass spectrometry. |
Discussion
Ethical reasons prohibited the inclusion of a
control group (i.e., ROSC patients after cardiac arrest without TH)
in this study. Therefore, it was not possible to distinguish
between hypothermia-induced changes and those occurring during the
course of SIRS after cardiac arrest (16). To overcome this challenge, we
utilized our serum proteome data previously reported in a study
(17) as ‘control’. The previous and
present studies were conducting using an identical methodology and
analytical approach. The proteomic data obtained in the present
study were compared with the serum proteome data obtained from five
patients (average age: 73 years; four males, one female) pre- and
post-operatively (average of 13.1 days after cardiac surgery).
Cardiac surgery is commonly accompanied by ischemic reperfusion
injury. Thus, SIRS inevitably occurs post-operatively and may lead
to multiple organ dysfunction or death (18–21).
This fact prompted us to use the proteomic data in the post-cardiac
surgery group as a possible surrogate of the control group, namely
ROSC patients after cardiac arrest not undergoing TH.
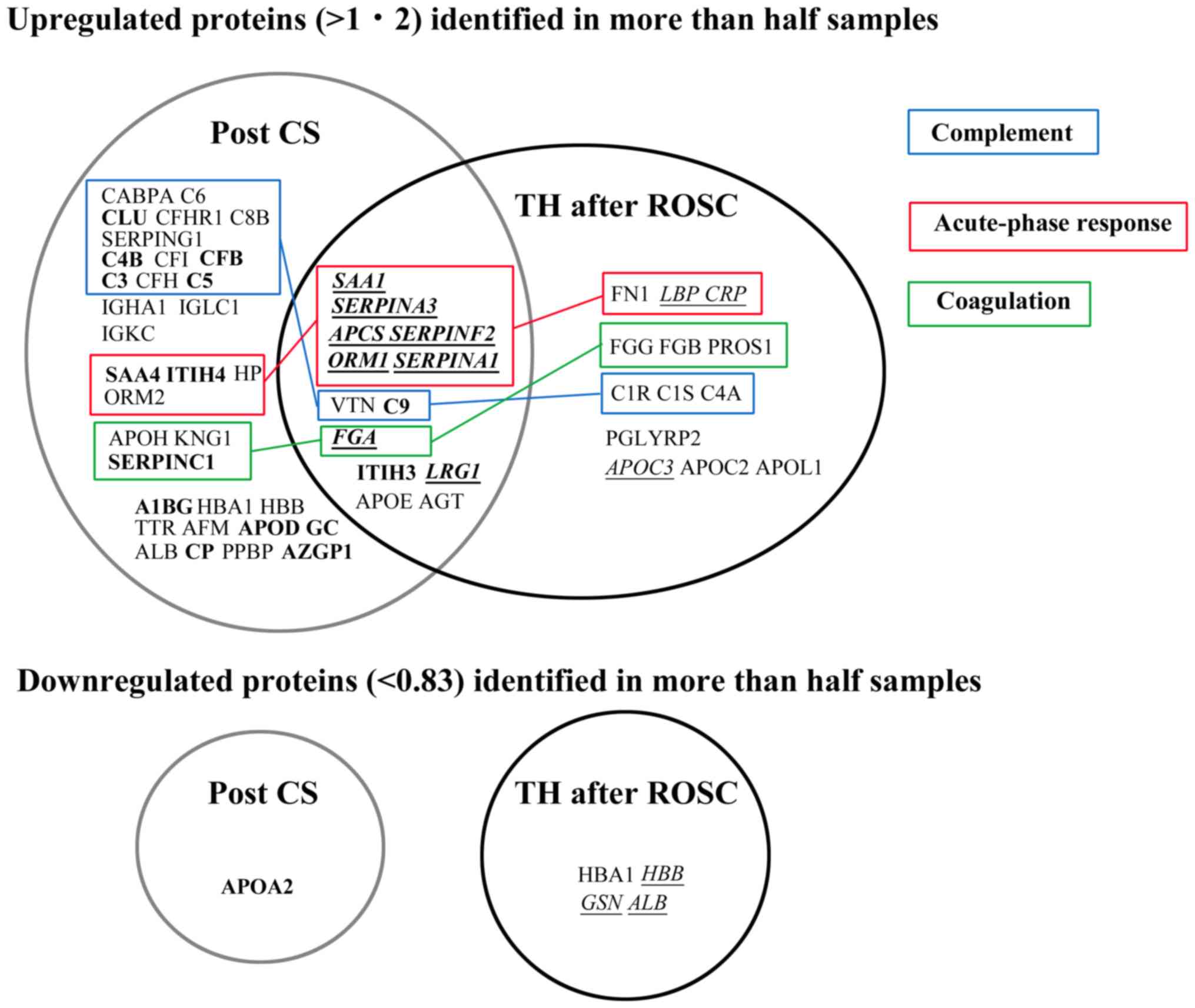
Although many proteins belonging to the acute-phase
response were upregulated in a similar manner in both groups,
proteins related to the complement system were not upregulated in
the TH after ROSC group compared with those in the post-cardiac
surgery group (Fig. 6). For example,
serum amyloid A1 (SAA1) is a major acute-phase protein with
cytokine-like activity during inflammation (22). SAA1 and five other proteins of the
acute-phase response were upregulated in a similar manner in both
the TH after ROSC and post-cardiac surgery groups. In agreement
with recent reports (6,23–25),
these findings imply that TH does not reduce the acute-phase
response after ROSC and that systemic inflammation is not
influenced by TTM at 33°C or 36°C (6). However, there are numerous studies
supporting the hypothermic suppression of inflammation (26). These results suggest that a lower
temperature (i.e., <33°C) may decrease the inflammatory response
(6). The systemic inflammatory
response and apoptosis are thought to continue for several days
after reperfusion or traumatic injuries. Thus, a prolonged duration
of hypothermia (e.g., 72 vs. 24 h) may be more beneficial to
patients (24,26).
According to the analysis performed using the DAVID
software, platelet degranulation was the most enriched GO term in
the TH after ROSC patients. Platelets contain α granule contents,
dense granule contents, and lysosomes (27). These include adhesion molecules,
chemokines, cytokines, coagulation factors, and immunological
molecules (28). Platelet
degranulation is induced by several receptor signaling pathways
including platelet pattern-recognition receptor signaling. The
latter is triggered by pathogen-associated molecular patterns
(PAMPs) or damage-associated molecular patterns (DAMPs) (28). DAMPs were reported to increase at
days 0 and 1 after ROSC (29). The
resultant platelet activation results in inflammation and
thrombosis, leading to the interaction of both processes (30,31).
Although numerous complement proteins were
upregulated in the post-cardiac surgery group, this phenomenon did
not occur in the TH after ROSC group (Fig. 6). Both findings were consistent with
the bioinformatic analysis (Tables
III–V). These suggest that TH
may suppress the complement activation in ROSC patients after
cardiac arrest. In addition, this finding was confirmed by the
decreased levels of soluble C5b-9 (membrane attack complex=MAC)
during TH (Fig. 5), also consistent
with the findings of previous studies (10,24,25,32).
Complement activation is involved in the pathogenesis of ischemic
reperfusion injury and SIRS (33),
and SC5b-9 is a good predictor of prognosis in myocardial/renal
ischemia (34,35). Collectively, these findings indicate
that the therapeutic effect of TH may be the result, at least
partially, of the suppression of complement activation.
 | Table V.Top 10 Gene Ontology terms showing
statistically significant protein enrichment for serum samples
obtained from patients having cardiac surgery. |
Table V.
Top 10 Gene Ontology terms showing
statistically significant protein enrichment for serum samples
obtained from patients having cardiac surgery.
| Gene Ontology
term | Number of observed
genes | P-value | Benjamini's
correction | Genes |
|---|
| Complement
activation, classical pathway | 17 |
2.5×10−24 |
1.0×10−21 | CLU, C3, C4B, C5,
C6, C8B, C9, C4BPA, CFI, IGHA1, IGKC, IGKV3-20, IGKV3D-11, IGKV4-1,
IGLC1, IGLV3-21, SERPING1 |
| Complement
activation | 15 |
2.7×10−21 |
5.6×10−19 | CLU, C3, C4B, C5,
C6, C8B, CFB, CFHR1, CFH, IGKC, IGKV3-20, IGKV3D-11, IGKV4-1,
IGLC1, IGLV3-21 |
| Platelet
degranulation | 15 |
3.3×10−20 |
4.6×10−18 | ALB, A1BG, APOH,
CLU, FGA, ITIH3, ITIH4, KNG1, ORM1, ORM2, PPBP, SERPINA1, SERPINA3,
SERPINF2, SERPING1 |
| Regulation of
complement activation | 11 |
6.0×10−19 |
6.3×10−17 | C3, C4B, C5, C6,
C8B, C9, C4BPA, CFB, CFH, CFI, VTN |
| Receptor-mediated
endocytosis | 15 |
1.6×10−16 |
9.3×10−15 | ALB, APOE, CFI, HP,
HBA1, HBB, IGHA1, IGKC, IGKV3-20, IGKV3D-11, IGKV4-1, IGLC1,
IGKV3-21, SAA1, VTN |
| Negative regulation
of endopeptidase activity | 13 |
1.1×10−15 |
7.8×10−14 | AGT, C3, C4B, C5,
ITIH3, ITIH4, KNG1, SERPINA1, SERPINA3, SERPINC1, SERPINF2,
SERPING1, VTN |
| Acute-phase
response | 12 |
1.7×10−15 |
1.0×10−13 | APCS, HP, ITIH4,
ORM1, ORM2, SERPINA1, SERPINA3, SERPINF2, SAA1, SAA4 |
| Complement
activation, alternative pathway | 6 |
2.4×10−10 |
1.3×10−8 | C3, C5, C8B, C9,
CFB, CFH |
| Immune
response | 12 |
3.3×10−8 |
1.6×10−6 | C3 C8B C9 IGHA1
IGKC IGKV3-20 IGKV3D-11 IGKV4-1 IGLC1 IGLV3-21 PPBP VTN |
| Innate immune
response | 12 |
4.2×10−8 |
1.7×10−6 | APCS CLU C4B C6
C4BPA CFI FGA IGHA1 IGKC1 IGLC1SERPING1 SAA1 |
Free (extracellular) hemoglobin may act as a
peroxidase in the presence of hydrogen peroxide (36,37) and
lead to the consumption of nitric oxide and vasoconstriction
(38), causing acute kidney and lung
injury (39,40). This is the first study to demonstrate
that the levels of free hemoglobin decreased during the cooling and
rewarming phases after ROSC. PCAS shares numerous features with
sepsis, such as elevated levels of cytokines (4). In the early stage of sepsis,
circulating levels of hemoglobin subunit β were found to be
significantly higher in sepsis patients compared with those
reported in the control group (41).
These findings suggest that PCAS may be naturally accompanied by an
increase in the levels of hemoglobin subunit β that may be
suppressed by TH. This reduction of free hemoglobin may be the
consequence of reduced hemolysis and/or augmented scavenging
reaction of hemoglobin. Normal erythrocytes were shown to be lysed
by the formation of the MAC of complement C5b-9 following
activation of the complement system by zymosan (42). This activation has been reported to
decrease at temperatures <37°C (42). Thus, hypothermia may suppress the
activation of the complement system and thereby suppress
hemolysis.
The hemoglobin scavenging system is known as the
haptoglobin-CD163-heme oxygenase-1 pathway and hemopexin-CD91-HO
pathway (43,44). It is well known that increased levels
of free hemoglobin in the plasma accompany the decreased levels of
haptoglobin, as shown in cardiac surgery (45). However, the present plasma proteomic
analysis did not demonstrate any significant changes in the
abundance of haptoglobin (T3/T1=1.19±0.35, P=0.132) and hemopexin
(T3/T1=1.01±0.30, P=0.931) during TH after ROSC. Based on this
evidence, it is inferred that unknown mechanisms may be involved in
the scavenging of free hemoglobin. Although ApoC3 was significantly
upregulated during cooling and rewarming, there was no evidence
regarding the interaction between ApoC3 and cell-free hemoglobin.
However, lipid-free ApoA1 acts as an antioxidant against cell-free
hemoglobin and may facilitate receptor-mediated endocytosis of free
hemoglobin via a scavenger receptor class B type 1 (SR-B1) or an
ApoA1 receptor (46). Furthermore,
in an animal model, small fragments of apolipoprotein E reduced the
levels of free hemoglobin via the ubiquitous heparin sulfate
proteoglycan-associated pathway (47). During the acute-phase response,
marked elevation in the levels of SAA protein in the plasma causes
high-density lipoprotein (HDL) remodeling, with the newly
synthesized SAA1 and SAA2 displacing ApoA1 and becoming an
apolipoprotein of HDL (22,48). This indicates that the increased
levels of SAA1/2 may increase those of free ApoA1 during the
acute-phase response. Although ApoA1 was not upregulated during TH,
it is uncertain whether the levels of free ApoA1 may be altered
during treatment.
The limitations of the present study include the
absence of a control group and the small sample size. Therefore,
further studies involving larger sample sizes and, if possible, a
control group are necessary to confirm the present findings.
In conclusion, plasma proteomic analysis
demonstrated that the abundance of complement proteins did not
change and the levels of SC5b-9 and free hemoglobin decreased in
patients undergoing TH after ROSC. However, proteins belonging to
the acute-phase response were upregulated during TH. These novel
findings may contribute to the current knowledge regarding TH after
ROSC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Yasuko Sonoyama
(Division of Thoracic and Cardiovascular Surgery, Department of
Surgery, Shimane University Faculty of Medicine, Izumo, Japan) for
the sample collection and management.
Funding
The present study was supported by the Japan Society
for the Promotion of Science KAKENHI (grant no. JP23659845).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TO conceived and designed the present study. RI, TN
and KS acquired blood samples and clinical data. AY performed the
proteomic and western blot/ELISA analyses. TO and KM analyzed the
data. TO wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This prospective, cohort study was approved by the
Ethics Committee of Shimane University Faculty of Medicine and
Shimane Prefectural Central Hospital (Izumo, Japan). Written
informed consent was provided by the relatives of the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Geocadin RG, Wijdicks E, Armstrong MJ,
Damian M, Mayer SA, Ornato JP, Rabinstein A, Suarez JI, Torbey MT,
Dubinsky RM and Lazarou J: Practice guideline summary: Reducing
brain injury following cardiopulmonary resuscitation. Report of the
guideline development, dissemination and implementation
subcommittee of the American academy of neurology. Neurology.
88:1–9. 2017. View Article : Google Scholar
|
|
2
|
Callaway CW, Donnino MW, Fink EL, Geocadin
RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM
and Zimmerman JL: Part 8: Post-cardiac arrest care. 2015 American
Heart Association guidelines updates for cardiopulmonary
resuscitation and emergency cardiovascular care. Circulation 132
(18 Suppl 1). S465–S482. 2015. View Article : Google Scholar
|
|
3
|
Bro-Jeppesen J, Kjaergaard J, Wanscher M,
Nielsen N, Friberg H, Mjerre M and Hassager C: Systemic
inflammatory response and potential prognostic implications after
out-of-hospital cardiac arrest: A study of the target temperature
management trial. Crit Care Med. 43:1223–1232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nolan JP, Neumar RW, Adrie C, Aibiki M,
Berg RA, Bottinger BW, Callaway C, Clark RS, Geocadin RG, Jauch EC,
et al: Post-cardiac syndrome: Epidemiology, pathophysiology,
treatment and prognostication. A scientific statement from the
international liaison committee on resuscitation; the American
heart association emergency cardiovascular care committee; the
council on cardiovascular surgery and anesthesia; the council on
cardiopulmonary, perioperative, and critical care; the council on
clinical cardiology; the council on stroke. Resuscitation.
79:350–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakashima R, Hifumi T, Kawakita K, Okazaki
T, Egawa S, Inoue A, Seo R, Inagaki N and Kuroda Y: Critical care
management focused on optimizing brain function after cardiac
arrest. Circ J. 81:427–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bro-Jeppesen J, Kjaergaard J, Wanscher M,
Nielsen N, Friberg H, Bjerre M and Hassager C: The inflammatory
response after out-of-hospital cardiac arrest is not modified by
targeted temperature management at 33°C or 36°C. Resuscitation.
85:1480–1487. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan T, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–1568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skibsted S, Bhasin MK, Aird WC and Shapiro
NI: Bench-to bedside review: Future novel diagnosis for sepsis-a
systems biology approach. Crit Care. 17:2312013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu L, Candille SI, Choi Y, Xie D, Jian L,
Li-Pook-Than J, Tang H and Snyder M: Variation and genetic control
of protein abundance in humans. Nature. 499:79–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oda T, Yamaguchi A, Yokoyama M, Shimizu K,
Toyota K, Nikai T and Matsumoto K: Plasma proteomic changes during
hypothermic and normothermic cardiopulmonary bypass in aortic
surgeries. Int J Mol Med. 34:947–956. 2014.PubMed/NCBI
|
|
11
|
Oda T, Yamaguchi A, Shimizu K, Nikai T and
Matsumoto K: Does the rewarmed heart restore the myocardial
proteome to that of the precooled state? Circ J. 79:2648–2658.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang DW, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Prot. 4:44–57. 2009. View Article : Google Scholar
|
|
14
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: The gene ontology consortium. gene ontology: Tool for the
unification of biology. Nature Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lancaster TS, Jefferson SJ, Hunter JC,
Lopez V, Van Eyk JE, Lakatta EG and Korzick DH: Quantitative
proteomic analysis reveals novel mitochondrial targets of estrogen
deficiency in the aged female rat heart. Physiol Genomics.
44:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beurskens CJ and Jeffermans NP: Changes in
the inflammatory response following cardiac arrest: A matter of
ischemia/reperfusion or induced hypothermia? Crit Care Med.
40:3105–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh H, Yamada K, Maniwa T, Oda T and
Matsumoto K: Monitoring of serial presurgical and postsurgical
changes in the serum proteome in a series of patients with calcific
aortic stenosis. Dis Markers. 2015:694120112015. View Article : Google Scholar
|
|
18
|
Hall R, Smith MS and Rocker G: The
systemic inflammatory response to cardiopulmonary bypass:
Pathophysiological, therapeutic and pharmacological considerations.
Anesth Analg. 85:766–782. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kouchoukos NT, Blackstone EH, Hanley FL
and Kirklin JK: Hypothermia, circulatory arrest and cardiopulmonary
bypass. Cardiac surgery. Fourth. Kouchoukos NT, Blackstone EH,
Hanley FL and Kirklin JK: ELSEVIER; Philadelphia: pp. 67–132.
2012
|
|
20
|
Stoppelkamp S, Veseli K, Stang K,
Schlensak C, Wendel HP and Walker T: Identification of predictive
early biomarkers for sterile-SIRS after cardiovascular surgery.
PLOS One. 10:e01355272015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zakkar M, Taylor K and Hornick PI: Immune
system and inflammatory response to cardiopulmonary bypass.
Cardiopulmonary bypass: Principles and practice. 3rd. Gravlee GP,
Davis RF, Stammers AH and Ungerleider RM: Wolters Kluwer/Lippincott
Williams & Wilkins; Philadelphia, PA: pp. 321–337. 2008
|
|
22
|
Ye RD and Sun L: Emerging functions of
serum amyloid A in inflammation. J Leukoc Biol. 98:923–929. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bisschops LL, Hoedemaekers CW, Mollnes TE
and van der Hoeven JG: Rewarming after hypothermia after cardiac
arrest shifts the inflammatory balance. Crit Care Med.
40:1136–1142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bisschops LL, van der Hoeven JG, Mollnes
TE and Hoedemaekers CW: Seventy-two hours of mild hypothermia after
cardiac arrest is associated with a lowered inflammatory response
during rewarming in a prospective observational study. Crit Care.
18:5462014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beurskens CJ, Horn J, de Boer AM, Schultz
MJ, van Leeuwen EM, Vroom MB and Jeffermans NP: Cardiac arrest
patients have an impaired response, which is not influenced by
induced hypothermia. Crit Care. 18:R1622014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Polderman KH: Mechanisms of action,
physiological effects, and complications of hypothermia. Crit Care
Med 37 (7 Suppl). S186–S202. 2009. View Article : Google Scholar
|
|
27
|
Reed GL: Platelet secretory mechanisms.
Semin Thromb Hemost. 30:441–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Estevez B and Du X: New concepts and
mechanisms of platelet activation signaling. Physiology (Bethesda).
32:162–177. 2017.PubMed/NCBI
|
|
29
|
Timmermans K, Kox M, Gerretsen J, Peters
E, Scheffer GJ, van der Hoeven JG, Pickkers P and Hoedemaekers CW:
The involvement of danger-associated molecular patterns in the
development of immunoparalysis in cardiac arrest patients. Crit
Care Med. 43:2332–2338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esmon CT: Crosstalk between inflammation
and thrombosis. Maturitas. 61:122–131. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Opal SM: Interactions between coagulation
and inflammation. Scand J Infect Dis. 35:545–554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong P, Zhao H, Hua R, Zhang M, Tang Z,
Mei X, Cui J and Li C: Mild hypothermia inhibits systemic and
cerebral complement activation in a swine model of cardiac arrest.
J Cereb Blood Flow Metab. 35:1289–1295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elvington A, Atkinson C, Zhu H, Yu J,
Takahashi K, Stahl GL, Kindy MS and Tomlinson S: The alternative
complement pathway propagates inflammation and injury in murine
ischemic stroke. J Immunol. 189:4640–4647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindberg S, Pedersen SH, Mogelvang R,
Galatius S, Flyvbjerg A, Jensen JS and Bjerre M: Soluble form of
membrane attack complex independently predicts mortality and
cardiovascular events in patients with ST-elevation myocardial
infarction treated with primary percutaneous coronary intervention.
Am Heart J. 164:786–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kotimaa J, Van del Pol P, Leijtens S,
Klar-Mohammad N, Schilders G, Daha MR, Rutjes H and van Kooten C:
Functional assessment of rat complement pathway activities and
quantification of soluble C5b-9 in an experimental model of renal
ischemia/reperfusion injury. J Immunol Methods. 412:14–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gutteridge JM: Iron promoters of the
Fenton reaction and lipid peroxidation can be released from
haemoglobin by peroxides. FEBS Lett. 201:291–295. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kapralov A, Vlasova II, Feng W, Maeda A,
Walson K, Tyurin VA, Huang Z, Aneja RK, Carcillo J, Bayir H, et al:
Peroxidase activity of hemoglobin-haptoglobin complexes: covalent
aggregation and oxidative stress in plasma and macrophages. J Biol
Chem. 284:30395–30407. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meyer C, Heiss C, Drexhage C, Kehmeier ES,
Balzer J, Mühfeld A, Merx MW, Lauer T, Kühl H, Floege J, et al:
Hemodialysis-induced release of hemoglobin limits nitric oxide
bioavailability and impairs vascular function. J Am Coll Cardiol.
55:454–459. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shaver CM, Upchurch CP, Janz DR, Grove BS,
Putz ND, Wickersham NE, Dikalov SI, Ware LB and Bastarache JA:
Cell-free hemoglobin: A novel mediator of acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 310:L532–L541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Neal JB, Shaw AD and Billings FT IV:
Acute kidney injury following cardiac surgery: Current
understanding and future directions. Crit Care. 20:1872016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoo H, Ku SK, Kim SW and Bae J: Early
diagnosis of sepsis using serum hemoglobin subunit beta.
Inflammation. 38:394–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kitamura H, Nagano A and Kitano E:
Hemolysis of normal human erythrocytes by autologous serum
complement. Int Arch Allergy Immunol. 100:209–214. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kristiansen M, Graversen JH, Jacobsen C,
Sonne O, Hoffman HJ, Law SA and Moestrup SK: Identification of the
hemoglobin scavenger receptor. Nature. 409:198–201. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thomsen JH, Etzerodt A, Svendsen P and
Moestrup SK: The haptoglobin-CD163-heme oxygenase-1 pathway for
hemoglobin scavenging. Oxid Med Cell Longev. 2013:5236522013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vermeulen Windsant IC, de Wit NC, Sertorio
JT, van Bijnen AA, Ganushchak YM, Heijmans JH, Tanus-Santos JE,
Jacobs MJ, Maessen JG and Buurman WA: Hemolysis during cardiac
surgery is associated with increased intravascular nitric oxide
consumption and perioperative kidney and intestinal tissue damage.
Front Physiol. 5:3402014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Du R, Winarsih I, Ho B and Ding JL:
Lipid-free apolipoprotein A-I exerts an antioxidant role against
cell-free hemoglobin. Am J Clin Exp Immunol. 1:33–48.
2012.PubMed/NCBI
|
|
47
|
Hanson MS, Xu H, Flewelen TC, Holzhauer
SL, Retherford D, Jones DW, Frei AC, Pritchard KA Jr, Hillery CA,
Hogg N and Wandersee NJ: A novel hemoglobin-binding peptide reduces
cell-free hemoglobin in murine hemolytic anemia. Am J Physiol heart
Circ Physiol. 304:H328–H336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sun L and Ye RD: Serum amyloid A1:
Structure, function and gene polymorphism. Gene. 583:48–57. 2016.
View Article : Google Scholar : PubMed/NCBI
|