Introduction
Craniofacial defects can be caused by inflammation,
trauma, tumor and congenital malformations amongst other things.
For reconstruction of defects or deformities in oral and
maxillofacial regions, distraction osteogenesis (DO) techniques
have been broadly applied to regenerate bone tissues and are
considered to be the gold-standard treatment. However, the longer
period required for bone consolidation may lead to clinical
complications such as fracture of the distraction device or
infections (1). Therefore,
acceleration of bone formation is urgently required.
The nervous system has been demonstrated to have
pivotal regulatory effects on the metabolism and repair of bones
(2,3). Damage to peripheral nerves is often
followed by osteoporosis (4). Our
previous study reported that a locally applied nerve growth factor
(NGF) promotes the rehabilitation of the inferior alveolar nerve
(IAN) (5). In addition, delivery of
human NGFβ in gels accelerates bone regeneration in DO rabbit
models (6). Skeletal sensory
terminals can secrete various neurotransmitters including substance
P, calcitonin gene-related peptide (CGRP), which regulates the
metabolism of osteoblasts and osteoclasts (7,8). In a
bilateral periodontal defect model, the osteoprotegerin expression
levels decrease along with CGRP when rats undergo transection of
the left inferior alveolar nerve (9). Furthermore, the secretion of CGRP from
sensory peptidergic fibers in the periosteum and bone is functional
during load-induced bone regeneration (10). However, it remains unclear whether
CGRP accelerates bone regeneration in DO by targeting bone marrow
mesenchymal stem cells (BMMSCs). CGRP8-37 is the most widely used
selective pharmacological antagonist of CGRP (11). In the present study, the effects of
CGRP and CGRP-37 on BMMSCs were investigated in vitro and
in vivo in a rat model of mandibular DO.
Materials and methods
Rat BMMSC culture and
identification
A total of 4 male Sprague-Dawley rats (6 weeks old;
60–80 g) from the animal care center of the Fourth Military Medical
University were housed under specific pathogen-free conditions
(22°C; 12-h light/dark cycle; 50% humidity) with free access to
food pellets and tap water. Briefly, rats were sacrificed and
mandibles were resected aseptically (12,13). The
cell suspension was prepared by repeated aspiration through a
20-gauge needle. The collected cells were then seeded into 6 well
plates at a density of 1×106 cells per well and
incubated under standard conditions (5% CO2, 37°C). The culture
medium consisted of α-minimum essential medium (αMEM) supplemented
with 10% (v/v) fetal bovine serum (FBS), 0.25 µg/ml Fungizone, and
1% (v/v) penicillin and streptomycin (all purchased from
Sigma-Aldrich; Merck KGaA).
To characterize rat BMMSCs, the morphology of
adherent BMMSCs was observed using a phase contrast microscope
(Nikon TE 2000-U; Nikon Corporation). Immunofluorescence staining
of MSC markers thy-1 cell surface antigen (CD90) and integrin
subunit β1 (CD29) was performed using a standard procedure. Cells
were fixed for 30 min at room temperature in 4% paraformaldehyde,
washed twice with PBS then treated with 0.1% Triton X-100 at room
temperature for 1–2 min to permeate the cell membrane. In brief,
cells were blocked with 5% bovine serum albumin (BSA)/PBS (1 h,
25°C), then incubated in 1% BSA/PBS with either isotype controls,
anti-CD29 (cat. no. 610468; 1:1,000; BD Biosciences) or anti-CD90
antibodies (cat. no. 554893; 1:1,000; BD Biosciences) for 12 h at
4°C. The cells were then washed three times with 0.5% BSA/PBS.
Samples were then incubated in TRITC goat anti-rabbit fluorescent
secondary antibody (cat. no. 150077; 1:200; Abcam). Stained cells
were observed using a fluorescence microscope (magnification, ×400;
DXM 1200F; Nikon Corporation). In addition, the
multidifferentiation potential of rat BMMSCs was determined using
adipogenic or osteogenic media followed by Oil Red (Sigma-Aldrich,
Merck KGaA) or Alizarin Red staining (ARS) (Sigma-Aldrich; Merck
KGaA), respectively.
Mineralization assay
ARS is used to quantify calcium within the deposited
mineral matrix. Following 3 weeks of culture, cells were stained
with 2% Alizarin red for 10 min. When bound to the calcium salts in
cell matrix, Alizarin red was eluted with 10% cetylpyridinium
chloride. The absorbance of supernatant aliquots (~150 µl) were
measured at 540 nm using a spectrophotometer then standardized with
the absorbance of crystal violet staining at 590 nm.
Cell proliferation assessment
BMMSCs at passage 3 were cultured with α-MEM
containing 10−7 mol/l CGRP, α-MEM medium containing 10-7
mol/l CGRP8-37 or α-MEM medium only. 5-bromo-2′-deoxyuridine (BrdU;
3.1 µg/ml) was added at 72 h post-seeding then incubated for 4 h.
After washing in PBS, cultures were fixed in ice-cold 70% ethanol
for 10 min and denatured in 4 mmol/l HCl for 20 min. Samples were
then incubated with mouse anti-BrdU primary antibody (cat. no.
5292S; 1:1,000; Cell Signaling Technology, Inc.) for 1 h at 37°C
and then with HRP-conjugated second antibodies (cat. no. 7076S;
1:200; Cell Signaling Technology, Inc.) for 1 h at RT. The samples
were washed then stained with diamino-benzidine (DAB) and
hematoxylin for nuclei staining. Cell proliferation was measured as
a percentage of BrdU+ cells relative to the total number
of nuclei. Images were captured in 5 randomly selected fields per
slide (magnification, ×200). The BrdU+ cells were
counted manually by an experienced pathologist. All the experiments
were conducted in triplicate.
Alkaline phosphatase (ALP)
activity
Cultured BMMSCs were fixed in fresh 10% neutral
buffered formalin at week 1, 2, and 3. Then ALP activity was
determined by the commercial ALP activity kit. After all protocols
were performed according to the manufacturer's instructions, the
absorbance was measured at 410 nm with a spectrophotometer and the
cell number was counted using crystal violet staining as previously
reported (14,15,16).
Animal study
A total of 30 male Sprague-Dawley rats (10 weeks
old; 100–120 g) from the animal care center of the Fourth Military
Medical University were randomly divided into three groups:
control, CGRP and CGRP8-37. The rats were housed under specific
pathogen-free conditions (22°C; 12-h light/dark cycle; 50%
humidity) with free access to food pellets and tap water. This
study was specifically approved by the Institutional Ethics
Committee at the Fourth Military Medical University (approval
number ky-034). All animals were cared for according to the
guidelines published by the Animal Center for Medical Experiment at
Fourth Military Medical University.
Surgical procedures
Animals were anaesthetized with 1% pentobarbital
sodium (intraperitoneal injection 30 mg/kg). A vertical osteotomy
was made in the right mandible between the molar region and the
mandibular ramus. Surgeries were performed cautiously to avoid
damage in the IAN. A custom-made distraction device was fixed along
a plane perpendicular to the osteotomy cut. The device was
activated following a latency period of 5 days, and the distraction
was carried out at the rate of 0.2 mm, twice per day for 10 days.
During the distraction period, the CGRP group received a daily CGRP
injection (Sigma-Aldrich; Merck KGaA; 10−8 mmol/l, in
normal saline, total volume of 0.2 ml), the CGRP8-37 group was
given a daily injection of CGRP8-37 (Sigma-Aldrich; Merck KGaA;
10−6 mol/l, in normal saline, total volume of 0.2 ml),
and the control group received normal saline (17). All the drugs were injected into the
local callus at the distraction side percutaneously. Following 14
days of treatment, animals were sacrificed and mandibular tissues
were collected for histological and micro-computed tomography
(micro-CT) analysis. All the analyses were conducted blinded.
Micro-CT evaluation
All mandibular samples were examined with a Micro-CT
system (Inveon CT; Siemens AG) at the standard resolution
(1888×2048 pixels). Each mandibular sample included ~1,000 images
with an isotropic voxel size of 15 µm. The scanning was set at 500
mA, 80 kV, and the integration time was 800 msec. The bone and
accurate 3-dimensional data sets were isolated using the same
optimal thresholds from all segmented images. The region of
interest was defined as a rectangle of 2.5×10 mm covering the
distraction gap. The micro-CT measurements contained both bone
mineral density (BMD) and bone volume/total volume (BV/TV), which
indicates the portion of the mineralized tissue in bony defects.
The newly generated BV in the distraction gap was quantified and
compared to the total distraction area. Following micro-CT
evaluation, all samples underwent histological analysis.
BMD analysis and
immunohistochemistry
The mandibular samples were fixed in 4%
paraformaldehyde for 24 h and decalcified in 30% buffered formic
acid for 7 days at room temperature. Following dehydration and
cleaning, samples were stained with hematoxylin and eosin
(H&E). Analysis of the histological sections was performed
using the Image-Pro Plus analysis software (v11.0; Media
Cybernetics, Inc.) by a single, unbiased examiner who was blinded
in this study.
For immunohistochemistry, endogenous peroxidase
activity was eradicated by 3% hydrogen peroxide for 10 min at room
temperature. Then the tissue slices were permeabilized with 0.25%
Triton X-100 in PBS for 30 min, and blocked in 5% fetal bovine
serum in PBS, 2% glycine and 2% BSA for 1 h at room temperature.
The slices were then incubated with anti-nestin primary antibodies
(cat. no. 4760S; 1:1,000; Cell Signaling Technology, Inc.)
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibodies (1:200; cat. no. 150077;
Abcam) for 1 h at room temperature. Samples were washed and then
stained with diamino-benzidine (DAB). To quantify the proportions
of nestin+ cells in bone generating areas, these areas,
consisting of woven bones and fibrosis, were characterized by
presence of bone trabeculae and osteoid rimmed osteoblasts within a
bridging callus. Nestin+ cells were manually counted
under a fluorescent microscope (DMLRB; Leica Microsystems, Inc.) in
5 random high-power fields (magnification, ×400) per slide. Each
assay was performed in triplicate.
ELISA
Rat BMMSC supernatant was analyzed for stromal
cell-derived factor 1 (SDF-1) using SDF-1 ELISA kit (cat. no.
E-EL-R0922; Civic Bioscience Inc.) following the manufacturer's
protocols.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BMMSCs using the RNeasy
Mini Kit (Qiagen, Inc.), then the purity and concentration were
determined spectrophotometrically. For each sample, 1 µg of total
RNA was reverse transcribed to cDNA using the PrimeScript™ RT
reagent kit (Takara Bio, Inc.). The normalized cDNA was then
amplified with SYBR Premix ExTM Taq II RT-PCR kit in Applied
Biosystems 7,500 RT-PCR System. Thermocycling conditions were as
follows: 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec
and 60°C for 30 sec. mRNA expression was calculated as previously
reported (18), with GAPDH used as
the housekeeping gene. The primer sequences were as follows: SDF-1
forward, 5′-GAGAGCCACATCGCCAGAGC-3′ and reverse,
5′-GGATCCACTTTAATTTCGGGTCAA-3′; runt-related transcription factor 2
(Runx2) forward, 5′-CACTGGCGCTGCAACAAGA-3′ and reverse,
5′-CATTCCGGAGCTCAGCAGAATAA-3′; ALP forward,
5′-GCTCCCTTGTCTGGTCTTT-3′ and reverse, 5′-GGACGCCGTGAAGCAGGTGA-3′;
and GAPDH forward, 5′-AGCCGCATCTTCTTTTGCGTC-3′ and reverse,
5′-TCATATTTGGCAGGTTTTTCT-3′.
Western blot analysis
Rat BMMSCs from cell culture were collected for
western blot analysis. Proteins were extracted from the cells using
Membrane protein isolation mammalian protein extraction reagent
(Thermo Fisher Scientific, Inc.). Bicinchoninic acid assay was used
to quantify protein concentration. A total of 25% volume of protein
sample buffer was added, boiled for 5 min then proteins (50 µg
loaded per lane) were separated via SDS-PAGE on an 8% gel. The
separated proteins were then transferred to polyvinylidene
difluoride membranes. Following blocking with 7% fat-free dry milk
for 2.5 h at 4°C, the membranes were incubated with primary
antibodies against ALP (cat. no. 194297; 1:1,000; Abcam) and Runx2
(cat. no. 12556S; 1:1,000; Cell Signaling Technology, Inc.) at 4°C
overnight. Following primary incubation, membranes were incubated
with horseradish peroxidase-conjugated IgG secondary antibody (cat.
no. 150077; 1:200; Abcam) for 1 h. Membranes were then treated with
enhanced chemiluminescent substrate (Thermo Fisher Scientific,
Inc.) to visualize the bands. β-actin was used as an endogenous
reference. Each experiment was performed three times.
Statistical analysis
The statistical analysis was performed by SPSS
software (v.19.0; IBM Corp.) and the data were presented as mean ±
standard deviation. One-way analysis of variance followed by the
Neuman-Keuls post hoc test was used for analysis. P<0.05 was
considered to indicate statistical significance.
Results
Effects of CGRP on osteogenic
differentiation and proliferation in vitro
Using a well-established method, rat BMMSCs were
isolated. It was determined that morphology was typically
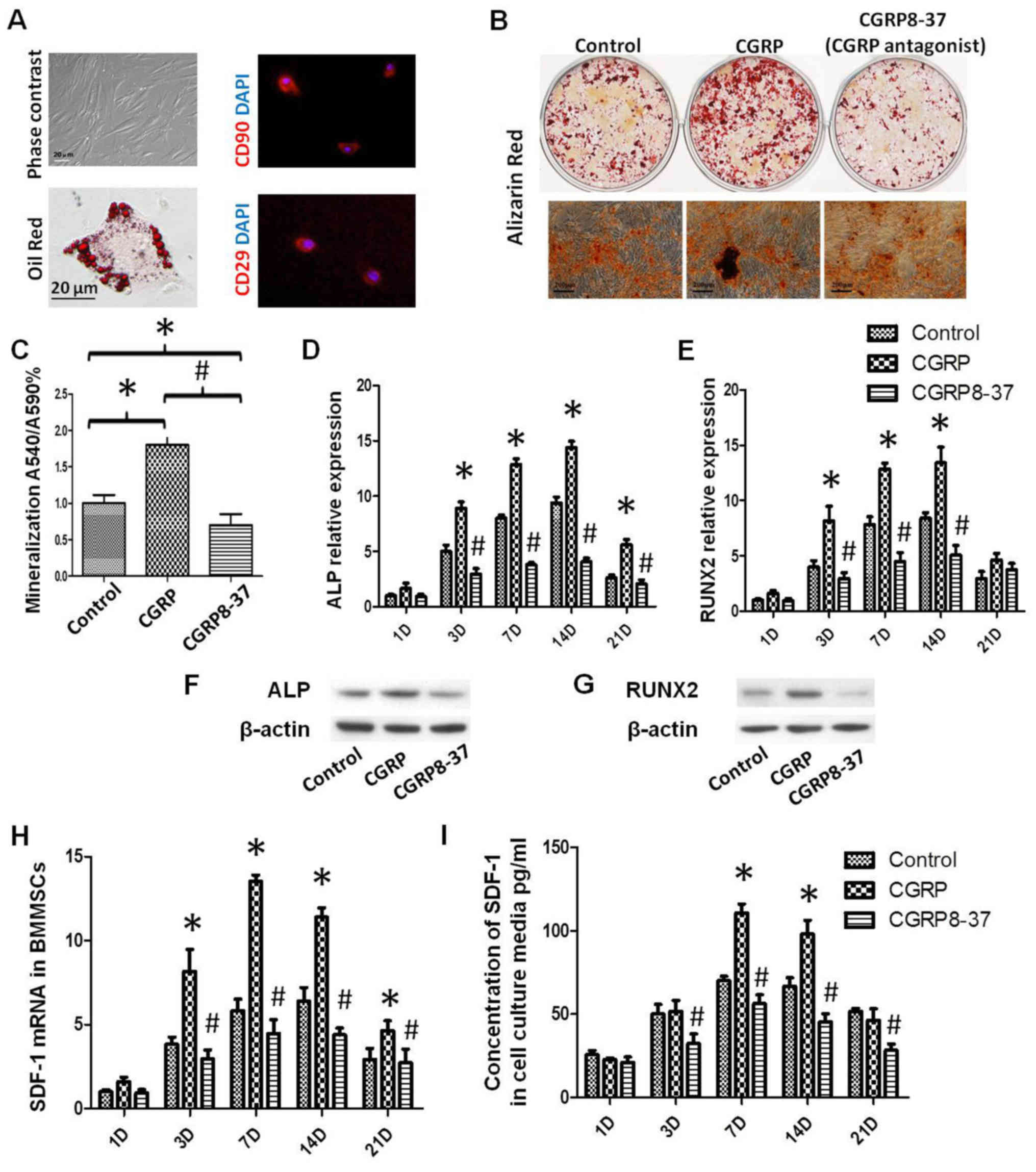
fibroblastic at P0 and spindle-like at P3 (Fig. 1A). Immunofluorescence staining
demonstrated that rat BMMSCs were positive for MSC markers CD90 and
CD29 (Fig. 1A). In addition, rat
BMMSCs differentiated into adipocytes using adipogenic medium or
osteoblasts using osteogenic medium, as indicated by Oil Red or
ARS, respectively (Fig. 1A and B).
The osteoblast phenotype of the control, CGRP and CGRP8-37
treatment groups was confirmed by the presence of matrix
mineralization. After 21 days, osteogenic differentiation of BMMSCs
was significantly enhanced by CGRP treatment and attenuated by
CGRP8-37 (Fig. 1B and C). Runx2,
SDF-1 and ALP mRNA expression levels were evaluated at 1, 3, 7, 14
and 21 days. mRNA expression began to rise from day 3 and peaked at
day 14. At day 14, ALP expression in the CGRP group increased
4.2-fold (P<0.05; Fig. 1D), SDF-1
increased 3.8-fold (P<0.05; Fig.
1H) and Runx2 increased 3.2-fold (P<0.05; Fig. 1E), compared to day 1. ALP and Runx2
expression of cells was further validated using western blot
analysis (Fig. 1F and G). ELISA
analysis further confirmed that concentrations of SDF-1
significantly increased in the CGRP group at day 7 and 14
(P<0.05; Fig. 1I). The effect of
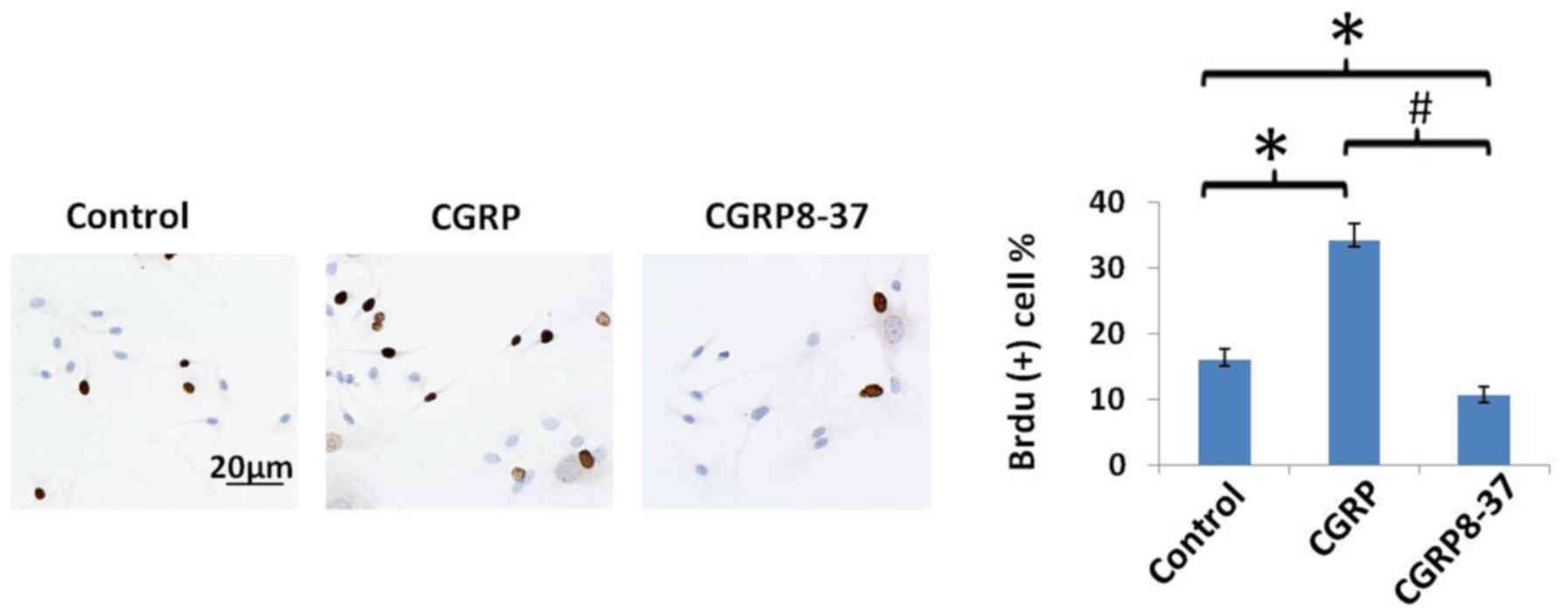
CGRP on cell proliferation was assessed by the BrdU incorporation
assay. CGRP significantly increased the percentage of
BrdU+ BMMSCs over the total number of cells, whilst
CGRP8-37 significantly decreased the percentage of BrdU+
BMMSCs (P<0.05; Fig. 2).
Effects of CGRP on bone regeneration
in vivo
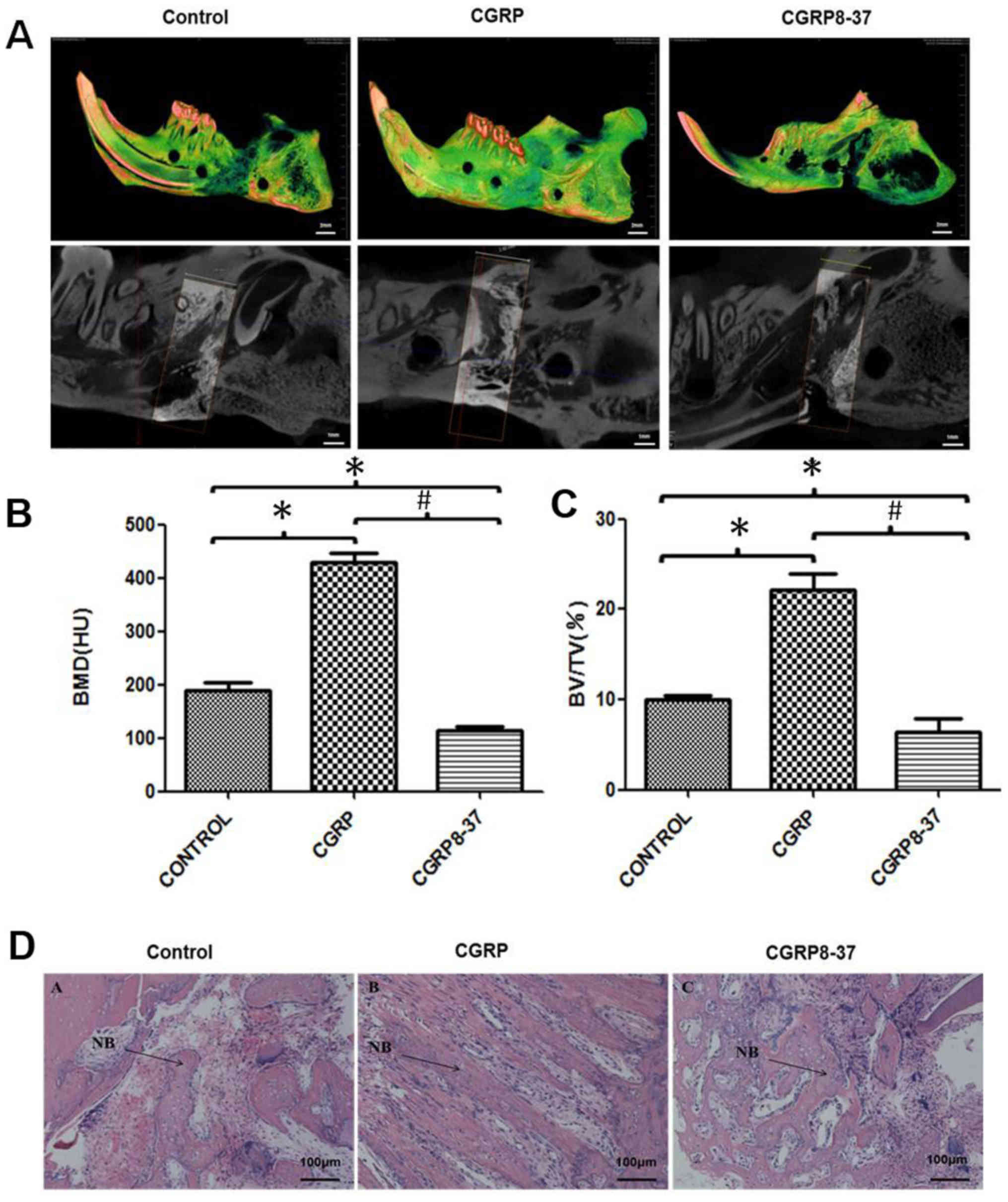
All three groups demonstrated successful mandible
lengthening. Micro-CT imaging determined that the distraction gaps
were filled with newly formed bone in the control and CGRP groups,
while only scattered bone trabeculae were present in the CGRP8-37
group (Fig. 3A). The BMD and BV/TV
ratio in the CGRP group were significantly higher than other two
groups, which indicated increased new bone generation (P<0.05;
Fig. 3B and C). The morphology of
new bone in the CGRP group was tighter and more continuous compared
with the control group (Fig.
3D).
HE staining demonstrated that there was no
inflammation in any group. The CGRP group had a large amount of
woven bone in the distraction axial direction, which is associated
with osteoblasts and new blood vessels. In addition, the trabeculae
were orderly arranged. The CGRP8-37 group exhibited increased
fibrous connective tissues and fewer bone masses and trabeculae.
The trabeculae were disorderly arranged in the CGRP8-37 group
(Fig. 3B).
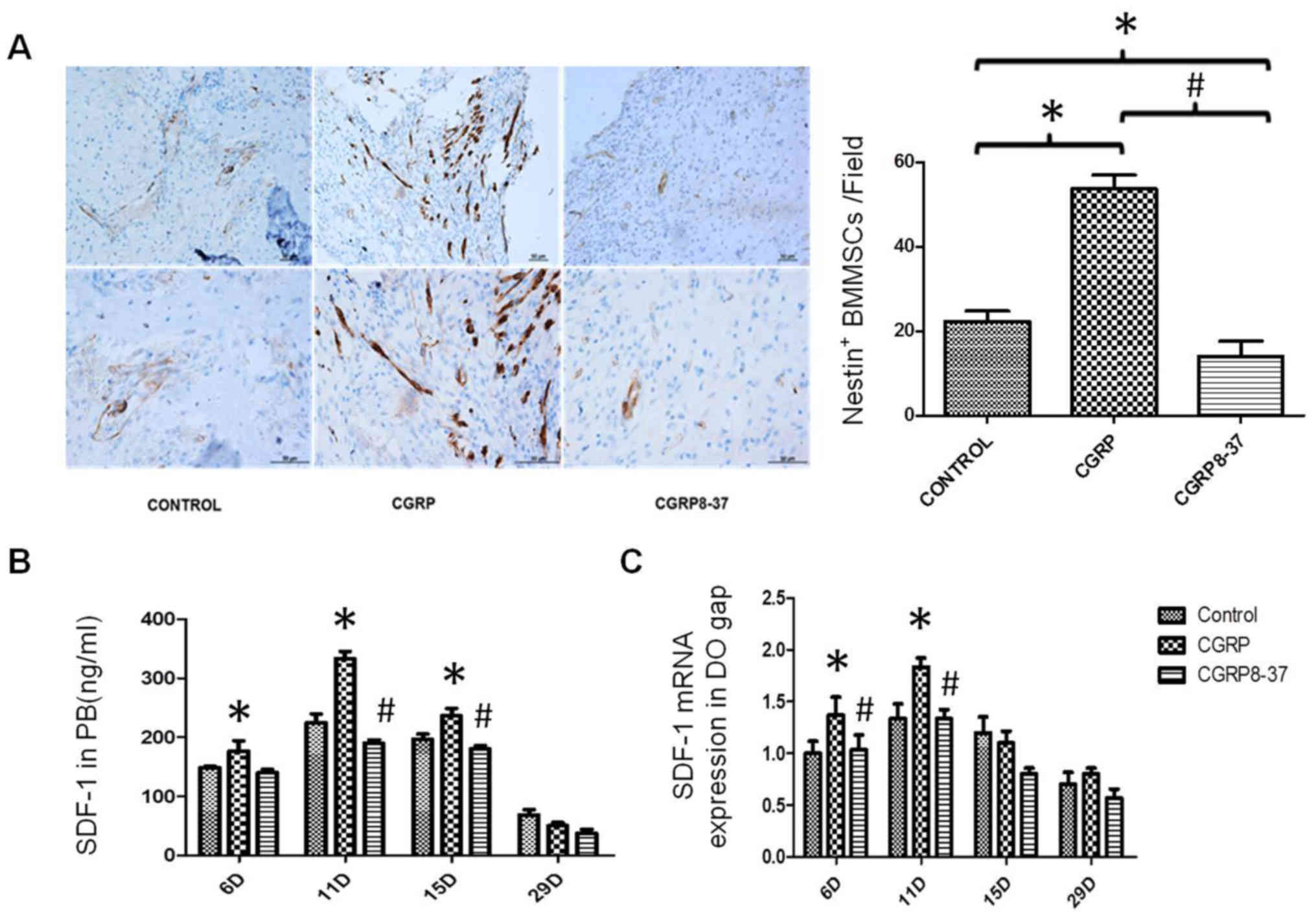
To further investigate the relationship between new
bone and Nestin+ BMMSCs, immunohistochemistry was used
to evaluate the migration of Nestin+ BMMSCs from the
perivascular area to the DO gap. In the CGRP group, the
Nestin+ BMMSCs were extensively distributed in bone
generating areas of the callus whilst they were mainly distributed
in the perivascular areas, rather than bone generating areas, in
the other two groups (Fig. 4A). The
number of Nestin+ cells in bone generating areas were
notably higher in the CGRP compared with the control (P<0.05;
Fig. 4A). In addition, SDF-1
expression in both the peripheral blood and regenerating region
were significantly higher in the CGRP group compared with the other
groups (P<0.05; Fig. 4B and C).
These findings suggested that CGRP treatment improved the migration
of BMMSCs from the perivascular area to bone generating areas.
Discussion
The present study investigated the effects of CGRP
throughout DO in vivo, as well as proliferation,
differentiation and migration of BMMSCs in vitro. A modified
titanium distractor device, similar to previously described devices
(19,20) was utilized to successfully establish
the DO model. Micro-CT and histomorphometric analysis demonstrated
that local injection of CGRP effectively increased and accelerated
osteogenesis, whilst CGRP8-37 significantly impaired bone
formation. In addition, Nestin+ cells in the DO gap and
SDF-1 expression was significantly increased following CGRP
administration, which suggested that CGRP promoted the recruitment
of BMMSCs to the new bone generating area. To support the in
vivo study, in vitro results demonstrated that CGRP
treatment modulated bone metabolism in osteoprogenitor
differentiation and maturation.
Neurotransmitters (e.g., substance P) have been
proven to stimulate proliferation and osteogenic differentiation of
MSCs in numerous animal models (29). Our previous study demonstrated that
local injection of substance P accelerates the generation of bone
during mandibular distraction in rats (21). Hong et al (8) demonstrated that systemic administration
of substance P promotes the mobilization of BMMSC to the bone
generating regions. Therefore, the present study hypothesized that
CGRP may have an effect on sensory nerves serving a similar role in
promoting the mobilization of MSCs akin to substance P.
CGRP belongs to the calcitonin superfamily of
peptides consisting of calcitonin, amylin, adrenomedullin,
adrenomedullin 2 (intermedin) and calcitonin-receptor-stimulating
peptide. Calcitonin is mainly produced by thyroid C cells whilst
CGRP is secreted and stored in the nervous system. Calcitonin can
inhibit osteoclast motility and cause bone resorption disorders;
however, there is limited evidence of the direct effect of
calcitonin on osteoblasts. Notably, neuropeptides, such as CGRPs,
are pivotal in suppressing bone resorption via the receptor
activator of nuclear factor-κB/osteoprotegerin pathway (22). CGRPs are less potent than calcitonin
in inducing hypocalcemia by several orders of magnitude (23,24) but
it has been suggested that CGRP stimulates osteoblasts and bone
formation. Activation of osteoblastic CGRP receptors enhances the
proliferation of osteoblasts in vitro (25) and treatment of the osteocalcin
promoter on transgenic mice resulted in an enhanced bone mass level
(26). It has also been reported
that exogenous CGRP can accelerate the proliferation of MSCs in the
logarithmic growth phase (27).
Furthermore, CGRP modulates the differentiation of SCs towards
osteoblasts in vivo (28) and
also regulates cell differentiation in the induced pluripotent and
embryoid body stages (29).
Therefore, CGRP has an important role in mediating osteoblasts in
cellular and autocrine activities. The CGRP-containing fibers
distribute abundantly in bone marrow and periosteum, which suggests
a mechanistic connection between osteoblasts and CGRP-containing
nerve fibers. In addition, CGRP-containing fibers in the epiphyseal
trabeculae are rarely covered by the Schwann cell sheaths which may
promote the interaction of BMMSCs with CGRP released from the
fibers (29). Another characteristic
of CGRP-containing fibers is their persistent contact with the bone
surface. The links between CGRP-containing fibers and osteoblasts
by the uncovered varicosities are maintained by bone generation.
After bone injury or fracture, the CGRP-containing fibers may
trigger bone regeneration around the blood vessels. However, CGRP
mRNA and protein expression in osteoblasts has also been observed
(28). Therefore, it is highly
reasonable that CGRP may influence osteoblastogenesis and the
metabolism of bone formation by regulating BMMSC activity. Our
future work will determine other osteogenic markers and further
investigate the molecular mechanisms underlying the role of CGRP in
osteogenic differentiation and BMMSC recruitment.
In conclusion, the present study determined that
CGRP could accelerate bone formation during DO. These findings may
provide an insight into the mechanisms underlying bone regeneration
promoted by sensory nerves, and also provides evidence for the
efficiency and safety of DO treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 81771046 and 81270015),
Shanghai Talent Development (grant no. 2018042) and Shanghai Summit
and Plateau Disciplines. The funders had no role in study design,
data collection and analysis, or preparation of the manuscript.
Availability of data and materials
The datasets generated and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
LW and WY designed and supervised the project. SJ,
SZ, XW, ZY and YS performed the experiments. AG, RH, DL and KH
analyzed the data. SJ, XW, AG and LW wrote the article. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol for animal experimentation was approved
by the Institutional Ethics Committee at the Fourth Military
Medical University (approval no. ky-034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Strijen PJ, Breuning KH, Becking AG,
Perdijk FB and Tuinzing DB: Complications in bilateral mandibular
distraction osteogenesis using internal devices. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 96:392–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garcia-Castellano JM, Diaz-Herrera P and
Morcuende JA: Is bone a target-tissue for the nervous system? New
advances on the understanding of their interactions. Iowa Orthop J.
20:49–58. 2000.PubMed/NCBI
|
|
3
|
Suzuki A, Uemura T and Nakamura H: Control
of bone remodeling by nervous system. Neural involvement in
fracture healing and bone regeneration. Clin Calcium. 20:1820–1827.
2010.(In Japanese). PubMed/NCBI
|
|
4
|
Anderson JJ, Woelffer KE, Holtzman JJ and
Jacobs AM: Bisphosphonates for the treatment of Charcot
neuroarthropathy J Foot Ankle Surg. 43:285–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Du ZJ, Wang L, Lei DL, Liu BL, Cao J,
Zhang P and Ma Q: Nerve growth factor injected systemically
improves the recovery of the inferior alveolar nerve in a rabbit
model of mandibular distraction osteogenesis. Br J Oral Maxillofac
Surg. 49:557–561. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang L, Cao J, Lei DL, Cheng XB, Zhou HZ,
Hou R, Zhao YH and Cui FZ: Application of nerve growth factor by
gel increases formation of bone in mandibular distraction
osteogenesis in rabbits. Br J Oral Maxillofac Surg. 48:515–519.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Shi X, Zhao R, Halloran BP, Clark
DJ, Jacobs CR and Kingery WS: Calcitonin-gene-related peptide
stimulates stromal cell osteogenic differentiation and inhibits
RANKL induced NF-kappaB activation, osteoclastogenesis and bone
resorption. Bone. 46:1369–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong HS, Lee J, Lee E, Kwon YS, Lee E, Ahn
W, Jiang MH, Kim JC and Son Y: A new role of substance P as an
injury-inducible messenger for mobilization of CD29(+) stromal-like
cells. Nat Med. 15:425–435. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu X, Lv L, Zhang J, Zhang T, Xiao C and
Li S: Expression of neuropeptides and bone remodeling-related
factors during periodontal tissue regeneration in denervated rats.
J Mol Histol. 46:195–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sample SJ, Heaton CM, Behan M, Bleedorn
JA, Racette MA, Hao Z and Muir P: Role of calcitonin gene-related
peptide in functional adaptation of the skeleton. PLoS One.
9:e1139592014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wimalawansa SJ: Calcitonin gene-related
peptide, and its receptors: Molecular genetics, physiology,
pathophysiology and therapeutic potentials. Endocr Rev. 17:533–585.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Z, Wu B, Jia S, Zhao Y, Hou R, Liu X,
Wang X, Chen L, Yang X, Lei D and Wang L: The mechanically
activated p38/MMP-2 signaling pathway promotes bone marrow
mesenchymal stem cell migration in rats. Arch Oral Biol. 76:55–60.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang ZH, Wu BL, Ye C, Jia S, Yang XJ, Hou
R, Lei DL and Wang L: Targeting P38 pathway regulates bony
formation via MSC recruitment during mandibular distraction
osteogenesis in rats. Int J Med Sci. 13:783–789. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Zhao Y, Liu Y, Akiyama K, Chen C,
Qu C, Jin Y and Shi S: IFN-γ and TNF-α synergistically induce
mesenchymal stem cell impairment and tumorigenesis via NFκB
signaling. Stem Cells. 31:1383–1395. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Liu S, Zhao Y, Liu D, Liu Y, Chen
C, Karray S, Shi S and Jin Y: Osteoblast-induced osteoclast
apoptosis by fas ligand/FAS pathway is required for maintenance of
bone mass. Cell Death Differ. 22:1654–1664. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu B, Wang L, Yang X, Mao M, Ye C, Liu P,
Yang Z, Yang X, Lei D and Zhang C: Norepinephrine inhibits
mesenchymal stem cell chemotaxis migration by increasing stromal
cell-derived factor-1 secretion by vascular endothelial cells via
NE/abrd3/JNK pathway. Exp Cell Res. 349:214–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Villa I, Melzi R, Pagani F, Ravasi F,
Rubinacci A and Guidobono F: Effects of calcitonin gene-related
peptide and amylin on human osteoblast-like cells proliferation.
Eur J Pharmacol. 409:273–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Cao J, Du ZJ, Zhang YB, Liu YP,
Wang L and Lei DL: Effects of sympathetic innervation loss on
mandibular distraction osteogenesis. J Craniofac Surg.
23:1524–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YB, Wang L, Jia S, Du ZJ, Zhao YH,
Liu YP and Lei DL: Local injection of substance P increases bony
formation during mandibular distraction osteogenesis in rats. Br J
Oral Maxillofac Surg. 52:697–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imai S, Tokunaga Y, Maeda T, Kikkawa M and
Hukuda S: Calcitonin gene-related peptide, substance P and tyrosine
hydroxylase-immunoreactive innervation of rat bone marrows: An
immunohistochemical and ultrastructural investigation on possible
efferent and afferent mechanisms. J Orthop Res. 15:133–140. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo YM, Kwag JH, Kim KH and Kim CH:
Effects of neuropeptides and mechanical loading on bone cell
resorption in vitro. Int J Mol Sci. 15:5874–5883. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
MacIntyre I: Amylinamide, bone
conservation and pancreatic beta cells. Lancet. 2:1026–1027. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tippins JR, Morris HR, Panico M, Etienne
T, Bevis P, Girgis S, MacIntyre I, Azria M and Attinger M: The
myotropic and plasma-calcium modulating effects of calcitonin
gene-related peptide (CGRP). Neuropeptides. 4:425–434. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cornish J, Callon KE, Lin CQ, Xiao CL,
Gamble GD, Cooper GJ and Reid IR: Comparison of the effects of
calcitonin gene-related peptide and amylin on osteoblasts. J Bone
Miner Res. 14:1302–1309. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ballica R, Valentijn K, Khachatryan A,
Guerder S, Kapadia S, Gundberg C, Gilligan J, Flavell RA and
Vignery A: Targeted expression of calcitonin gene-related peptide
to osteoblasts increases bone density in mice. J Bone Miner Res.
14:1067–1074. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu G and Jiang D: The role and mechanism
of exogenous calcitonin gene-related peptide on mesenchymal stem
cell proliferation and osteogenetic formation. Cell Biochem
Biophys. 69:369–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang Z, Yang Q, Xiong W, Li GH, Liao H,
Xiao J and Li F: Effect of CGRP-adenoviral vector transduction on
the osteoblastic differentiation of rat adipose-derived stem cells.
PLoS One. 8:e727382013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagao S, Goto T, Kataoka S, Toyono T,
Joujima T, Egusa H, Yatani H, Kobayashi S and Maki K: Expression of
neuropeptide receptor mRNA during osteoblastic differentiation of
mouse iPS cells. Neuropeptides. 48:399–406. 2014. View Article : Google Scholar : PubMed/NCBI
|