Introduction
The development and progression of diabetes results
in a series of complications that affect most major organs in the
human body (1). Diabetic
complications are now considered as one of the leading causes of
mortality in many countries (2,3). Chronic
hyperglycemia in diabetic patients promotes the expression of
pyruvate dehydrogenase kinase-4, which inhibits the activity of
pyruvate decarboxylase, and inhibits the expression of glucose
transporters (4). The metabolic
remodeling damages the myocardium and leads to the development of
DC (5). Diabetic cardiomyopathy (DC)
is ventricular dysfunction developed in diabetic patients that is
not caused by hypertension or coronary artery disease (6). DC affects a considerable portion of
diabetic patients, and its incidence is increasing as the disease
progresses (7). DC is associated
with high mortality rates even with active treatment (8). Therefore, at present, prevention of DC,
rather than treatment, is more critical for the survival of
diabetic patients.
A growing body of literature has shown that
non-coding RNAs (ncRNAs) are key players in vascular complications
associated with diabetes (9). Long
non-coding RNAs (lncRNAs) are a subgroup of ncRNAs that are of
>200 nucleotides in length (10).
At present, several lncRNAs have been proven to be key players in
DC, and their the regulation may contribute to the control of
disease conditions (11,12). Nuclear factor-κB interacting long
non-coding RNA (LncRNA NKILA) is a well-studied tumor suppressor
lncRNA in several types of malignancies (13,14),
while its involvement in other human diseases is unknown. The
present study aimed to explore the role of lncRNA NKILA p in
DC.
Materials and methods
Subjects and specimens
A total of 312 diabetic patients without obvious
complications that were admitted to The People's Hospital of Puyang
(Henan, China) between January 2008 and January 2010 were included
in the present study. The inclusion criteria were as follows: i)
Patients without obvious complications in major organs; ii)
patients with complete medical records; and iii) patients completed
a 8-year-follow-up period. The exclusion criteria were as follows:
i) Patients suffering from complications of other severe diseases,
including heart disease; ii) patients failed to cooperate with
researchers; and iii) patients succumbed during follow-up period.
These patients consisted of 168 males and 144 females, between 31
and 62 years of age (mean, 45.8±5.7). All patients signed informed
consent forms before admission. The present study was approved by
the Ethics Committee of The People's Hospital of Puyang.
Follow-up
All patients were followed up over a period of 8
years to record the occurrence of diabetic complications. A volume
of blood (5 ml) was extracted every 6 months to obtain plasma
samples, which were in tern obtained via centrifugation at room
temperature in EDTA tubes for 14 min at 1,200 × g. Diagnostic
criteria of DC were as follows: i) Diagnosed diabetes; ii) clinical
manifestations of heart failure; iii) heart enlargement with
impaired cardiac systolic function or diastolic dysfunction in
cases of no heart enlargement; and iv) heart failure caused by
other heart diseases including hypertensive heart disease. Patients
with coronary heart disease and rheumatic valvular heart disease
were excluded from the present study.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
To measure the expression of lncRNA NKILA, total RNA
was extracted from the plasma (obtained from patients) or primary
human cardiomyocyte cells using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.), according to manufacturer's protocols. Reverse
transcription was performed using Applied Biosystems™ High-Capacity
cDNA Reverse Transcription Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using the following thermocycling conditions:
25°C for 5 min, 52°C for 15 min and 85°C for 5 min. The subsequent
qPCR reactions were prepared using SYBR® Green
Quantitative RT-qPCR Kit (Sigma-Aldrich; Merck KGaA). The primers
of lncRNA NKILA and β-actin were designed and synthesized by
GenePharma (Shanghai GenePharma Co., Ltd.): NKILA forward,
5′-AACCAAACCTACCCACAACG-3′ and reverse,
5′-ACCACTAAGTCAATCCCAGGTG-3′; β-actin forward,
5′-GCACCACACCTTCTACAAT-3′ and reverse, 5′-TGCTTGCTGATCCACATCTG-3′.
The PCR reactions were carried out using the following
thermocycling conditions: 1 min at 95°C, 15 sec at 95°C and 30 sec
at 55.5°C for a total of 40 cycles. The expression of lncRNA NKILA
was normalized to the endogenous control β-actin using the
2−ΔΔCq method (15).
Cells and cell transfection
Primary human cardiomyocyte cells (T4037; Applied
Biological Materials) were cultured in this study to perform in
vitro cell experiments under conditions as recommend by the
manufacturer. Cells were cultivated in cardiomyocyte growth medium
(ScienCell Research Laboratories, Inc.) at 37°C with 95% humidity
and 5% CO2. Vectors (pcDNA3) expressing lncRNA NKILA and
its corresponding empty vector, as well as the lncRNA NKILA siRNA
(5′-AUCUGGGGUAGGCGCUGGGUAU-3′) with its respective negative
control, were all designed and prepared by Sangon (Sangon Biotech
Co., Ltd.). Lipofectamine® 2000 reagent (11668-019.;
Invitrogen; Thermo Fisher Scientific, Inc.) was used for all cell
transfections of vectors (10 nM) and siRNAs (30 nM). All
experiments were performed in accordance with the manufacturer's
instructions. Transfection with empty vectors or negative control
siRNAs was considered as the negative control (NC) whereas cells
that were treated with only Lipofectamine 2000 reagent without
vectors or siRNAs were considered control (C) cells. Incubation
with the transfection mixture was performed for 5 h at 37°C. The
interval between transfection and subsequent experiments was 24
h.
Cell apoptosis assay
lncRNA NKILA expression reached 200% and knockdown
rate reached 50% at 24 h after the transfection of siRNA and
vectors. Following 24 h transfection, suspensions of primary human
cardiomyocyte cells at a density of 5×104 cells/ml were
prepared using serum-free cell culture medium (as aforementioned)
supplemented with 20 mM D-glucose, before being subsequently seeded
into 6-well plates at 2 ml/well. The cells were then cultured for
48 h, followed by digestion using 0.25% trypsin (Sangon Biotech
Co., Ltd.) before being subjected to Annexin V-Fluorescein
isothiocyanate (FITC; Dojindo Molecular Technologies, Inc.) and
propidium iodide (PI; Dojindo Molecular Technologies, Inc.)
staining. Apoptotic cells were detected using a flow cytometer.
Data were analysed using FCS Express 6 Flow Cytometry Software (De
Novo Software).
Statistical analysis
Experiments were repeated three times and data were
expressed as mean ± standard deviation. All statistical analyses
were performed using the SPSS19.0 software (IBM Corp.). Comparisons
between two groups were performed by Student's t-test, whereas
comparisons between three groups were performed using one-way
analysis of variance followed by Tukey test. Diagnostic values of
plasma lncRNA NKILA for DC were evaluated by applying receiver
operating characteristic (ROC) curve analysis (Graphpad prism 6;
GraphPad, Inc.), with DC patients as true positive cases, and
diabetic patients without obvious complications as true negative
cases. P<0.05 was considered to indicate a statistically
significant difference.
Results
LncRNA NKILA is upregulated in
diabetic patients with DC but not in patients with other
complications
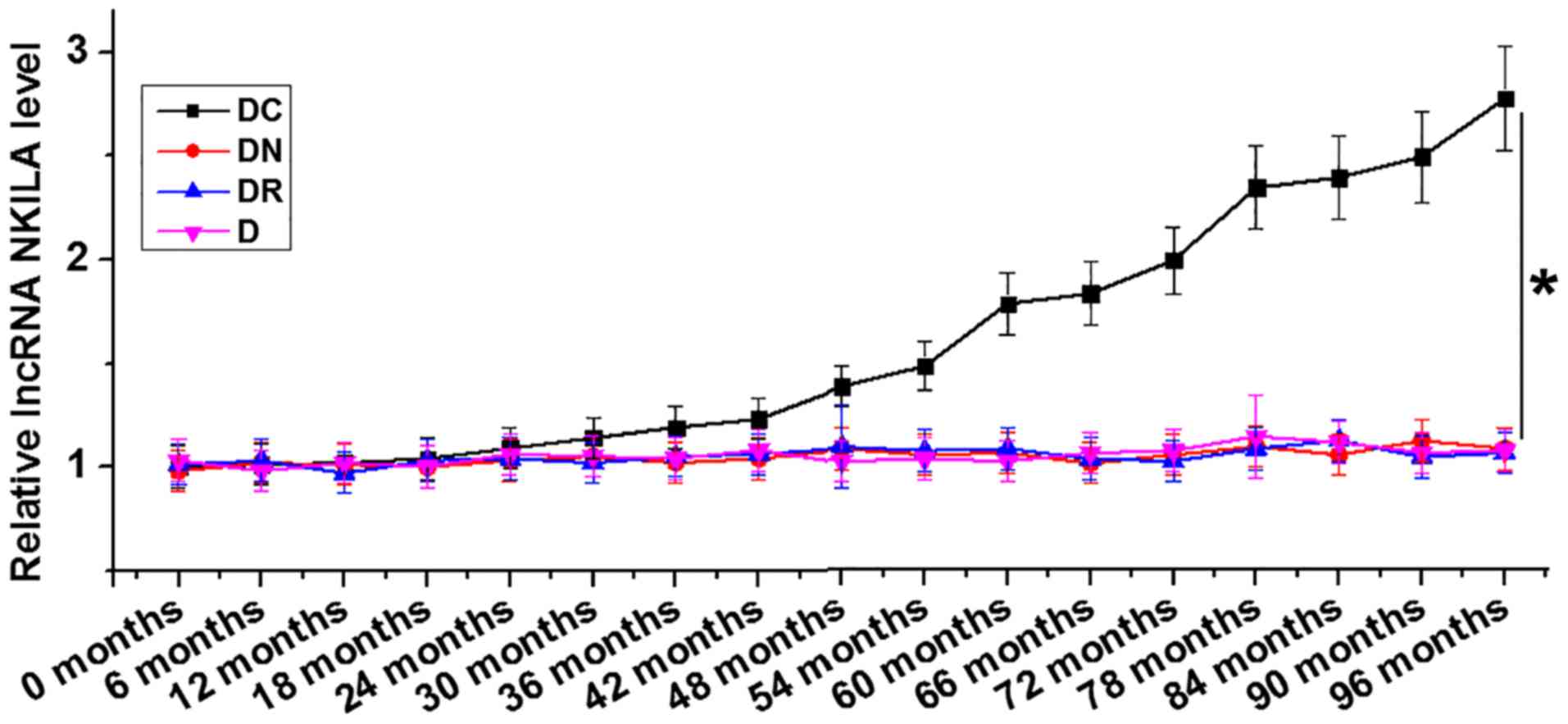
At the end of the 8-year follow-up period, 48
patients were diagnosed with DC only (DC), 42 patients were
diagnosed with diabetic nephropathy only (DN), 34 patients were
diagnosed with diabetic retinopathy only (DR); while no
significantly complications were observed in 44 diabetic patients
(D), and 144 patients were diagnosed with multiple complications.
The group containing 144 patients with multiple complications were
excluded in the following analyses to avoid ambiguity. The
expression of lncRNA NKILA in plasma collected from the different
patient groups at the end of follow-up was analyzed using RT-qPCR.
LncRNA NKILA was demonstrated to be significantly upregulated in
the group of diabetic patients who developed DC compared with all
the remaining patient groups (Fig.
1).
Plasma lncRNA NKILA levels at 6 months
before diagnosis in patients with DC and diabetic patients without
obvious complications
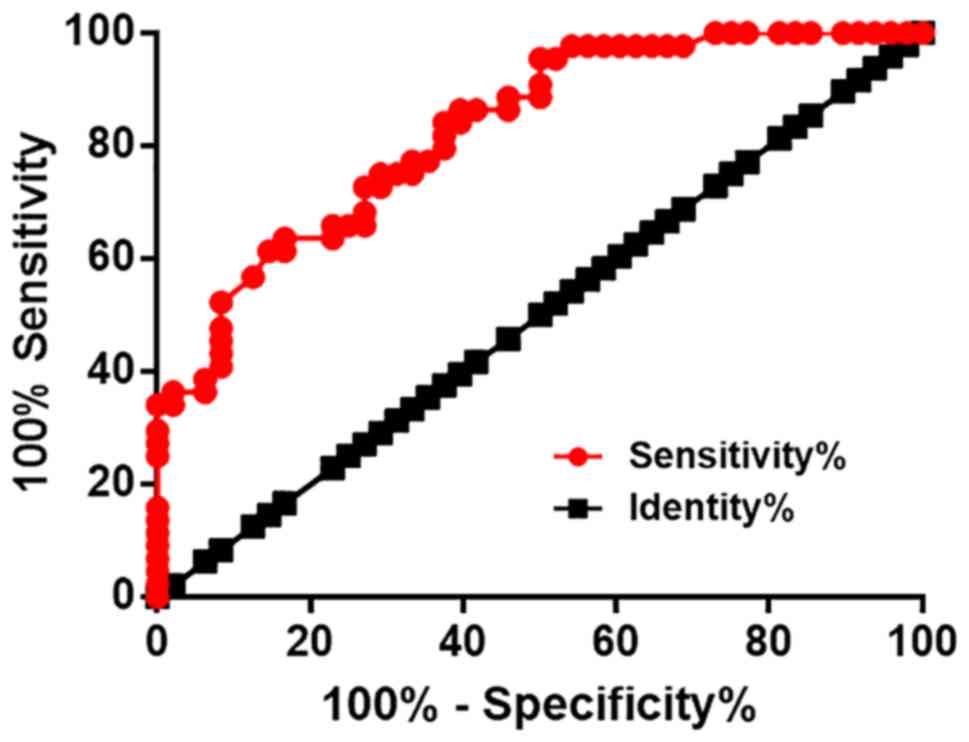
Diagnostic values of plasma lncRNA NKILA were
evaluated using ROC curve analysis, with DC patients as true
positive cases, and diabetic patients without any obvious
complications as true negative cases. For plasma levels of lncRNA
NKILA at 6 months before diagnosis, the area under the curve was
0.83, with a standard error of 0.041 and 95% confidence interval of
0.75–0.91 (Fig. 2). However, plasma
levels of lncRNA NKILA before this time point failed to diagnose DC
(data not shown).
Nevertheless, ROC curve analysis carried out in the
present study illustrated that plasma lncRNA NKILA levels at 6
months before diagnosis is sufficient to distinguish DC patients
from diabetic patients without obvious complications.
Expression of lncRNA NKILA in
cardiomyocytes is not affected by high-glucose treatment
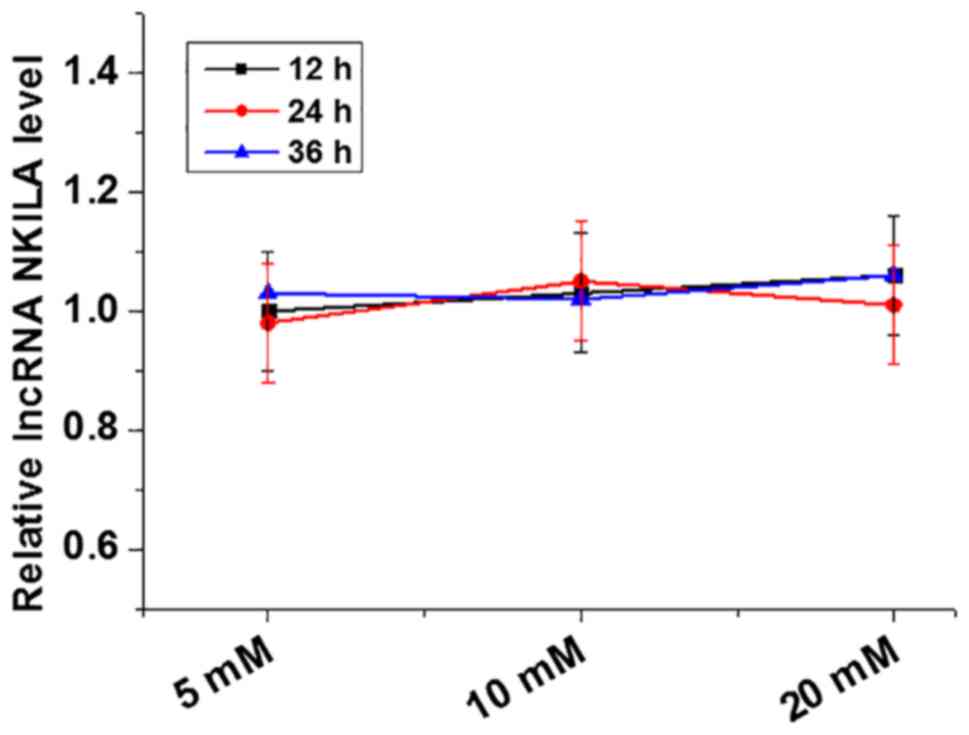
To investigate the effect of glucose on lncRNA NKILA
expression, primary cultured human cardiomyocytes were treated with
D-glucose at concentrations of 5, 10 and 20 mM (5 mM was used as
control as it is within the normal blood glucose range) for 12, 24
and 36 h. Expression of lncRNA NKILA in cardiomyocytes following
D-glucose treatment was measured using RT-qPCR. Treatment with
D-glucose at concentrations of 5 (control), 10 and 20 mM for 12, 24
and 36 h did not lead to any significant effects on lncRNA NKILA
expression in cardiomyocytes (Fig.
3).
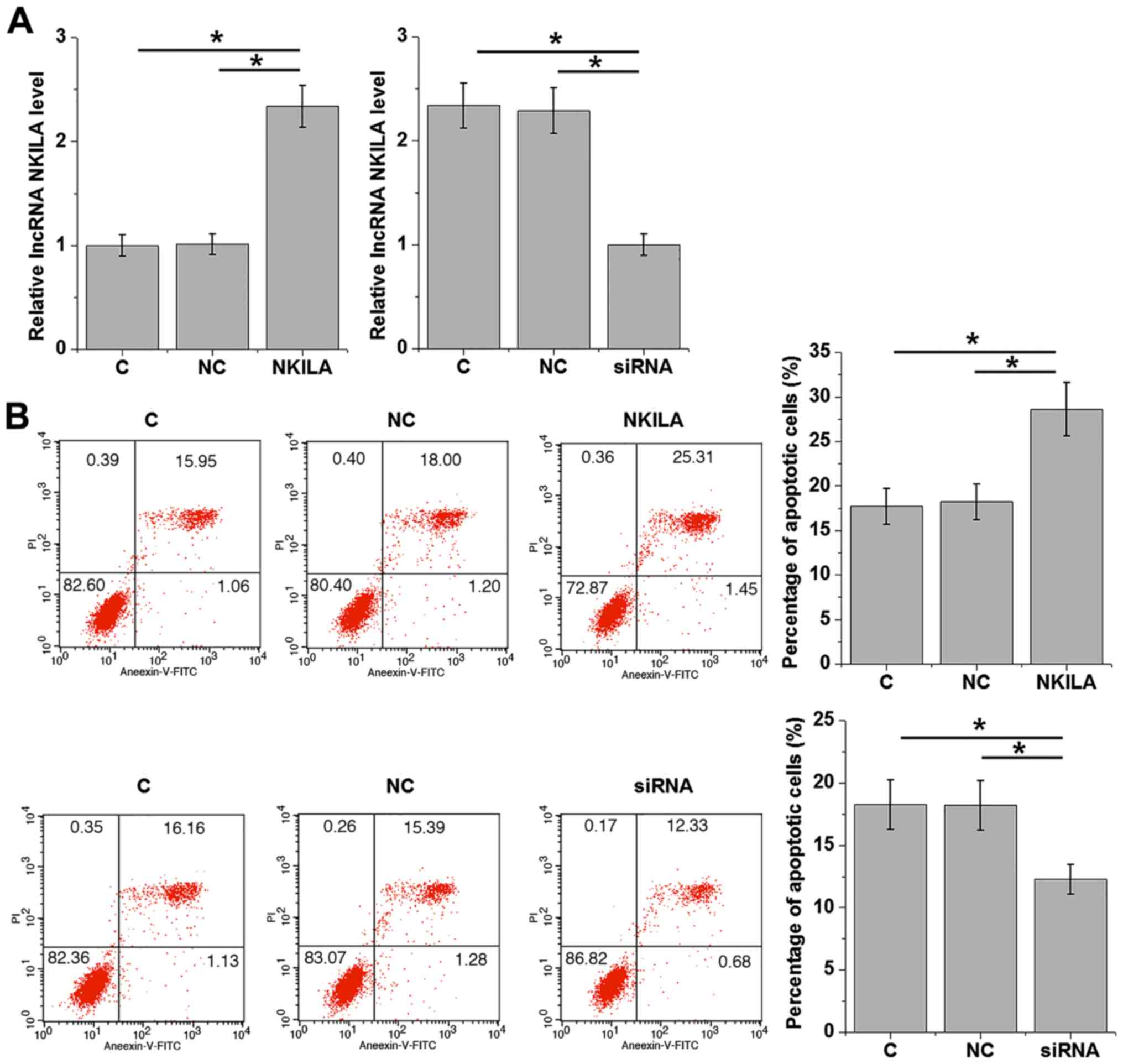
LncRNA NKILA overexpression promotes
apoptosis in cardiomyocytes
Apoptosis of cardiomyocytes contributes to the
pathogenesis of DC (6). Therefore,
the effects of lncRNA NKILA overexpression and depletion by siRNA
knockdown on cardiomyocyte apoptosis was analyzed using Annexin
V-FITC/PI staining by flow cytometry following 20 mM D-glucose
treatment. Following 24 h transfection, ectopic expression of the
lncRNA NKILA vector in cardiomyocytes resulted in a two-fold
increase observed in intracellular lncRNA NKILA mRNA levels
(Fig. 4A). lncRNA NKILA siRNA
transfection induced a 50% reduction in intracellular lncRNA NKILA
mRNA levels compared with controls (Fig.
4A). These efficiencies suggest that the transfection was
successful (Fig. 4A). Compared with
C and NC groups, ectopic lncRNA NKILA expression and lncRNA NKILA
knockdown accelerated and inhibited cardiomyocyte apoptosis,
respectively (Fig. 4B).
Discussion
LncRNA NKILA serves a role as tumor suppressor in
several types of malignancies (13,14). To
the best of our knowledge, the involvement of lncRNA NKILA in
diabetic complications remains poorly understood. The key finding
of the present study is that lncRNA NKILA was upregulated in
diabetic patients who developed DC but not in patients with other
complications. The experimental data presented here demonstrated
that lncRNA NKILA promotes apoptosis in cardiomyocytes, which may
contribute to the progression of DC.
Expression of lncRNA NKILA has been extensively
investigated in many types of cancer in humans (13,14,16,17). As
a tumor suppressor lncRNA, NKILA expression has been illustrated to
be downregulated in various types of cancer malignancies, while its
upregulation inhibits cancer development (16). The 8-year-follow-up study presented
here revealed that lncRNA NKILA expression was specifically
upregulated with the occurrence of DC. In addition, lncRNA NKILA
expression in cardiomyocytes was not significantly affected by
high-glucose treatment, indicating that the involvement of lncRNA
NKILA is specific to DC and not in diabetes per se or other
complications associated with diabetes.
The mortality rate of patients with DC is
unacceptably high even after active treatment (18). Therefore, development of early
prediction markers for DC is critical for developing prevention and
management strategies. In the present study the measurement of
plasma lncRNA NKILA mRNA levels at 6 months before diagnosis was
sufficient to distinguish patients with DC from patients with
diabetes but without significant complications. Therefore, plasma
levels of lncRNA NKILA may act as a potential marker for the
prevention and/or treatment of early DC.
Apoptosis of cardiomyocytes under a high-glucose
environment contributes to the pathogenesis of DC (19), and inhibition of this
pathophysiological process is considered to be a promising
therapeutic target for DC (20). In
the present study, it was illustrated that lncRNA NKILA
overexpression and lncRNA NKILA knockdown accelerated and inhibited
apoptotic cell death in cardiomyocytes under high-glucose
treatment, respectively. Therefore, the suppression of lncRNA NKILA
expression may inhibit the development of DC. Nevertheless, further
clinical trial studies are required to confirmed the results of the
present study. In addition, in the present study lncRNA NKILA
expression did not change in the presence of high-glucose treatment
in vitro. Therefore, lncRNA NKILA expression may be
dysregulated during the formation of heart lesions in diabetic
patients.
Additionally, the present study showed that lncRNA
NKILA levels were upregulated in patients with DC combined with
other complications, including hand and food diseases, and
retinopathy (data not shown). However, diabetic patients with
multiple complications (except cardiomyopathy) exhibited no
significant changes in lncRNA NKILA levels (data not shown),
further implicating the specific involvement of lncRNA NKILA in
DC.
A potential limitation in the data from the current
study is that it did not elucidate the mechanism for the regulation
of cardiomyocyte apoptosis by lncRNA NKILA. Preliminary studies
have indicated that lncRNA NKILA has no binding partners with
apoptosis mediators that are well-characterized in cardiomyocytes,
including miR-133b-5 and miRNA-21 (21,22).
Therefore, the identification of the downstream effectors of lncRNA
NKILA is needed.
In conclusion, the present study demonstrated that
lncRNA NKILA was upregulated specifically in diabetic patients who
developed DC, and lncRNA NKILA overexpression may contribute to the
progression of DC by promoting cardiomyocyte apoptosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL and SN designed the experiments. QL, PL, JS and
SL performed the experiments. XY and YY assisted in performing the
experiments and collected the data. SN drafted the manuscript. All
authors read and approved the final version of the approved this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The People's Hospital of Puyang (Henan, China).
Patient consent for publication
Patients provided consent for the possible
publication of this paper.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forbes JM and Cooper ME: Mechanisms of
diabetic complications. Physiol Rev. 93:137–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang ES, Laiteerapong N, Liu JY, John PM,
Moffet HH and Karter AJ: Rates of complications and mortality in
older patients with diabetes mellitus: The diabetes and aging
study. JAMA Intern Med. 174:251–258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Constantino MI, Molyneaux L,
Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK and
Wong J: Long-term complications and mortality in young-onset
diabetes: Type 2 diabetes is more hazardous and lethal than type 1
diabetes. Diabetes Care. 36:3863–3869. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuentes-Antrás J, Picatoste B, Ramírez E,
Egido J, Tuñón J and Lorenzo Ó: Targeting metabolic disturbance in
the diabetic heart. Cardiovasc Diabetol. 14:172015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Battiprolu PK, Lopez-Crisosto C, Wang ZV,
Nemchenko A, Lavandero S and Hill JA: Diabetic cardiomyopathy and
metabolic remodeling of the heart. Life Sci. 92:609–615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falcão-Pires I and Leite-Moreira AF:
Diabetic cardiomyopathy: Understanding the molecular and cellular
basis to progress in diagnosis and treatment. Heart Fail Rev.
17:325–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huynh K, Bernardo BC, McMullen JR and
Ritchie RH: Diabetic cardiomyopathy: Mechanisms and new treatment
strategies targeting antioxidant signaling pathways. Pharmacol
Ther. 142:375–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia G, DeMarco VG and Sowers JR: Insulin
resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat
Rev Endocrinol. 12:144–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beltrami C, Angelini TG and Emanueli C:
Noncoding RNAs in diabetes vascular complications. J Mol Cell
Cardiol. 89:42–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuo C, Jiang R, Lin X and Shao M: LncRNA
H19 inhibits autophagy by epigenetically silencing of DIRAS3 in
diabetic cardiomyopathy. Oncotarget. 8:1429–1437. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Gu H, Chen J and Zhou X:
Involvement of long noncoding RNA MALAT1 in the pathogenesis of
diabetic cardiomyopathy. Int J Cardiol. 202:753–755. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bian D, Gao C, Bao K and Song G: The long
non-coding RNA NKILA inhibits the invasion-metastasis cascade of
malignant melanoma via the regulation of NF-κB. Am J Cancer Res.
7:28–40. 2017.PubMed/NCBI
|
|
14
|
Bird L: lncRNA NKILA: A killer regulator.
Nat Rev Immunol. 18:666–667. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao F, Xu Y, Yang D, Tian B, Jia Y, Hou J
and Dong M: LncRNA NKILA correlates with the malignant status and
serves as a tumor-suppressive role in rectal cancer. J Cell
Biochem. 119:9809–9816. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ke S, Li RC, Meng FK and Fang MH: NKILA
inhibits NF-κB signaling and suppresses tumor metastasis. Aging
(Albany NY). 10:56–71. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia G, Whaley-Connell A and Sowers JR:
diabetic cardiomyopathy: A hyperglycaemia-and
insulin-resistance-induced heart disease. Diabetologia. 61:21–28.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu W, Liu X and Han L: Apoptosis of
cardiomyocytes in diabetic cardiomyopathy involves overexpression
of glycogen synthase kinase-3β. Biosci Rep. 39:BSR201713072019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diao X and Li G: Down-regulation of
miR-30b reduces cardiomyocyte apoptosis by targeting Bcl-2 in
diabetic cardiomyopathy. Int J Clin Exp Med. 10:5296–5305.
2017.
|
|
21
|
Pan YL, Han ZY, He SF, Yang W, Cheng J,
Zhang Y and Chen ZW: miR-133b-5p contributes to hypoxic
preconditioning-mediated cardioprotection by inhibiting the
activation of caspase-8 and caspase-3 in cardiomyocytes. Mol Med
Rep. 17:7097–7104. 2018.PubMed/NCBI
|
|
22
|
Shan ZX, Lin QX, Deng CY, Zhu JN, Mai LP,
Liu JL, Fu YH, Liu XY, Li YX, Zhang YY, et al: miR-1/miR-206
regulate Hsp60 expression contributing to glucose-mediated
apoptosis in cardiomyocytes. FEBS Lett. 584:3592–3600. 2010.
View Article : Google Scholar : PubMed/NCBI
|