Introduction
Gliomas comprise ~30% of brain and central nervous
system tumors and 80% of all malignant brain tumors (1). The prognosis for patients with
high-grade gliomas is generally poor, particularly in older
patients. Notably, the median overall survival for grade IV
glioblastoma is ~15 months (2).
Catenins are a family of proteins found in complexes with the cell
adhesion molecule, cadherin, in animal cells (3). The first two catenins that were
identified were α-catenin and β-catenin. α-catenin can bind to
β-catenin and actin. β-catenin binds the cytoplasmic domain of
numerous cadherins (4). β-catenin is
a dual function protein as it is involved in the coordination and
regulation of cell-cell adhesion and gene transcription (5). The β-catenin gene is a proto-oncogene
and mutations in the gene are commonly found in a variety of
cancers, including primary hepatocellular carcinoma, colorectal
cancer, skin cancer, prostate cancer and glioblastoma (6–10).
miR-24 is conserved in various species, and is
clustered with miR-23 and miR-27 on human chromosome 9 and 19
(11). miR-24 was reported to
suppress the expression of genes that are crucial for cell cycle
control in hematopoietic differentiation, including E2F2 and myc
(12). miR-24 also promoted the
differentiation of keratinocytes by repressing actin-cytoskeleton
regulators, including PAK4, Tsk5 and Rho GTPase-activating protein
19 (13). miR-24 was revealed to
reduce the mRNA and protein levels of human activin receptor
type-1B by targeting the 3′-untranslated region of the mRNA
(14). Tripartate motif-containing
protein 11, a direct target of miR-24-3p, was reported to promote
cell proliferation and inhibit apoptosis in colon cancer (15). Additionally, overexpression of
miR-24-3p in the small cell lung cancer cell line H446/EP led to a
reduction of the autophagy related 4a cysteine peptidase (ATG4A)
protein level, allowing small cell lung cancer cells to
re-sensitize to the combination of chemotherapeutic etoposide
(VP16) and cisplatin (DDP) (16).
Therefore, to examine the direct function of miR-24, the mRNA
expression of ATG4A, and protein expression of Beclin1 and
microtubule-associated proteins 1A/1B light chain 3B (LC3B) were
measured in the current study. Beclin1 and LC3B are essential
proteins associated with autophagy (17). The effects of miR-24 on cell
viability and autophagy of glioma cells, and how these biological
processes are regulated by β-catenin remain unclear. Therefore, the
role of β-catenin in regulating the effects of miR-24 on cell
viability and autophagy of glioma cells was also examined.
Materials and methods
Cells, animals and reagents
Rat glioma C6 cells were purchased from iCell
Bioscience, Inc. (Shanghai, China). Three male Sprague Dawley rats
(weight range, 180 to 220 g; 6 weeks old) were purchased from JSJ
Laboratory, Inc. (Shanghai, China). Three Sprague Dawley rats
bearing C6 glioma were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). The animals were housed
altogether in cages at ambient temperature under a 12-h light/dark
cycle with free access to standard pelleted food and water. The
project and number of animals were approved by the Animal Ethics
Committee of Shanghai Changhai Hospital (Shanghai, China).
Dulbecco's modified Eagle medium (DMEM), fetal
bovine serum, trypsin, penicillin and streptomycin antibiotics were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). MTT cell proliferation assay kit was purchased from Aladdin
Shanghai Biochemical Technology Co., Ltd. (Shanghai, China).
Primary antibodies against GAPDH (ab181602) and LC3B (ab48394) were
from Abcam (Cambridge, MA, USA); primary antibodies against Beclin1
(3495) and β-catenin (8480) were from Cell Signaling Technology,
Inc. (Danvers, MA, USA); goat anti-rabbit antibody (65–6120) for
western blotting and TRIzol™ reagent were from
Invitrogen (Thermo Fisher Scientific, Inc.). TUNEL apoptosis
detection kit was from Shanghai Yeasen Biotechnology Co., Ltd.
(Shanghai, China). QuantScript RT kit and miRcute miRNA qPCR
Detection kit were from Tiangen Biotech Co., Ltd. (Beijing, China).
Specific primers for miR-24 and U6 were purchased from Sangon
Biotech Co., Ltd. (Shanghai, China).
The equipment used was as follows: Cell incubator
(Thermo Fisher Scientific, Inc.); microplate reader (Shanghai Kehua
Bio-engineering Co., Ltd., Shanghai, China); light microscope
(Olympus Corporation, Tokyo, Japan); table-type refrigerated
centrifuge (USTC Zonkia Inc., Hefei, China); electric thermostatic
drying oven (Huyue Inc., Shangyu, China); electrophoresis system
(Beijing Liuyi Biotechnology Co., Ltd., Beijing, China); T-100 PCR
machine (Bio-Rad Laboratories, Inc., Hercules, CA, USA); and
StepOnePlus™ Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.).
Transfection of miRNA
Following the culturing of rat glioma C6 cells at
37°C for 24 h, the complete DMEM medium was replaced with DMEM
medium without serum and antibiotics. A mixture of Lipofectamine
2000 (Thermo Fisher Scientific, Inc.) and miRNAs was prepared,
including negative control miRNA (5-UUC UCC GAA CGU GUC ACG UTT-3),
miR-24-3p mimics (5-UGG CUC AGU UCA GCA GGA ACA G-3) and miR-24-3p
inhibitors (5-CUG UUC CUG CUG AAC UGA GCC A-3). Cells that were
transfected with miRNA negative control served as controls. A total
of 125 µl OPTI-MEM (Thermo Fisher Scientific, Inc.) was utilized to
dilute RNA. A total 125 µl OPTI-MEM was also used to dilute 10 µl
Lipofectamine 2000 and incubated at room temperature for 5 min. The
prepared miRNA mixture and Lipofectamine 2000 mixture were then
mixed, incubated at room temperature for 20 min, and added into
antibiotic-free DMEM culture medium with glioma C6 cells. The final
concentration of miR-24 inhibitors used was 100 nM and the final
concentration of miR-24 mimics was 50 nM. The culture medium was
changed to the complete DMEM medium after transfection of 4–8 h.
The cells were typically cultured at 37°C for 48 h until harvested
for subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Glioma C6 cells were treated with miR-24-3p mimics,
XAV-939 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) or a
combination of XAV-939 and miR-24-3p mimics for 48 h. XAV-939 is a
well-established Tankyrase inhibitor of the Wnt/β-catenin signaling
cascade (18). For the combination
group, the C6 cells were first treated with 50 nM miR-24-3p mimics
then with 1 µM XAV-939 until harvested for the next procedure.
Total RNA of C6 cells was extracted and purified by TRIzol,
according to the manufacturer's protocol. A QuantScript RT kit
(Tiangen Biotech Co., Ltd.) was utilized for reverse transcription
at 37°C for 60 min. Each reaction contained 1 µl random hexamer
primers (0.2 µg/µl) and 40 U M-MuLV Reverse Transcriptase (20
U/µl). The specific primer for the detection of miR-24 was 5-GGC
TCA GTT CAG CAG GAA CA-3, whereas the patented reverse primer was
supplied by the kit. he primer for ATG4A mRNA was forward (F):
5-AAC TGT GAC TGA GCC GAT TG-3′; reverse (R): 5-GTC TTT CAG GGA TGA
CTT GGT G-3′. The primer for U6 was F: 5-CTC GCT TCG GCA GCA CA-3;
R: 5-AAC GCT TCA CGA ATT TGC GT-3. miRcute miRNA qPCR Detection kit
(Tiangen Biotech Co., Ltd.) was used for qPCR analysis. PCR
conditions were as follows: Pre-denaturing at 94°C for 2 min, 40
cycles of denaturing at 94°C for 20 sec, and annealing and
polymerization at 60°C for 34 sec, according to the supplied
manual. PCR was performed in a StepOnePlus™ Real-Time PCR system.
The relative expression of miR-24 and ATG4A was determined with the
2−ΔΔCq method (19),
using U6 as the reference gene.
Immunohistochemistry (IHC)
The rats were euthanized by intraperitoneal
injection with 40 mg/body weight kg sodium pentobarbital, following
the guidelines by the Animal Ethics Committee of Shanghai Changhai
Hospital. Normal brain tissue and glioma tissue from C6
glioma-bearing rats were surgically isolated and fixed in 10%
neutral buffered formalin (Thermo Fisher Scientific, Inc.) at room
temperature for 2 h, embedded in paraffin, and cut into 5 µm
sections. The slides were then dewaxed and rehydrated. Following
antigen retrieval, slides were blocked with bovine serum albumin,
and incubated at 4°C overnight with anti-LC3B (1:400) and
anti-Beclin1 (1:130) antibodies (Abcam). Next the slides were
washed with PBS and incubated in the dark at 37°C with horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies
(1:50; OriGene Technologies, Inc., Beijing, China) for 30 min.
Slides were observed under light microscope.
Western blotting analysis
The expression levels of LC3B and Beclin1 proteins
were detected by western blotting. Cellular proteins of glioma C6
cells in different treatment groups were extracted with
self-prepared radioimmunoprecipitation assay buffer (25 mM Tris,
150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100).
Total protein concentration was determined by the BCA assay. The
total proteins (~30 µg per lane) were separated by electrophoresis
(120 V) on a 10% SDS-PAGE. The separated proteins were then
electrophoretically (100 V for 120 min) transferred to
polyvinylidene fluoride membranes. Following the blocking of the
membranes with 5% non-fat milk powder for 1 h at room temperature,
the membranes were incubated with anti-LC3B (1:1,000), anti-Beclin1
(1:1,000) and anti-GAPDH (1:5,000) antibodies at 4°C overnight.
Following incubation, the membranes were washed three times with a
solution of Tris-buffered saline with Tween-20. The membranes were
then incubated for 1 h at room temperature with goat anti-rabbit
secondary antibody labeled with HRP (1:3,000). They were washed and
incubated for a short time period in enhanced chemiluminescence
(ECL) solution (Chemiluminescent Western Blot Detection Kit, Thermo
Fisher Scientific, Inc.). The films were exposed in a dark room.
The densitometry was determined suing ImageJ bundled with 64-bit
Java 1.6.0_24 (National Institutes of Health).
MTT assay
The cell viability of glioma C6 cells in different
treatment groups was detected by MTT assay. Following culturing, 20
µl MTT solution (5 mg/ml) was added to each well in the 96 well
plates and the cells were cultured at 37°C for 4 h. Cell
supernatants were removed and discarded, and 150 µl dimethyl
sulfoxide was added to each well. The plates were then agitated for
15 min to dissolve the purple formazan crystals and the absorbance
of each sample was detected at 570 nm using an ELISA microplate
reader. Relative cell viability was calculated by dividing the
absorbance of the experimental groups by the absorbance of the
control group.
Statistical analysis
Statistical analysis was performed and figures were
created using GraphPad Prism 5.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA). Data are presented as the mean ± standard error
of the mean and each experiment was performed in triplicate.
Comparison between two groups was performed using Student's t tests
and nonparametric tests. Differences between ≥3 groups were
compared by one-way analysis of variance followed by the Bonferroni
post hoc test. Differences between two groups were compared by
unpaired t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
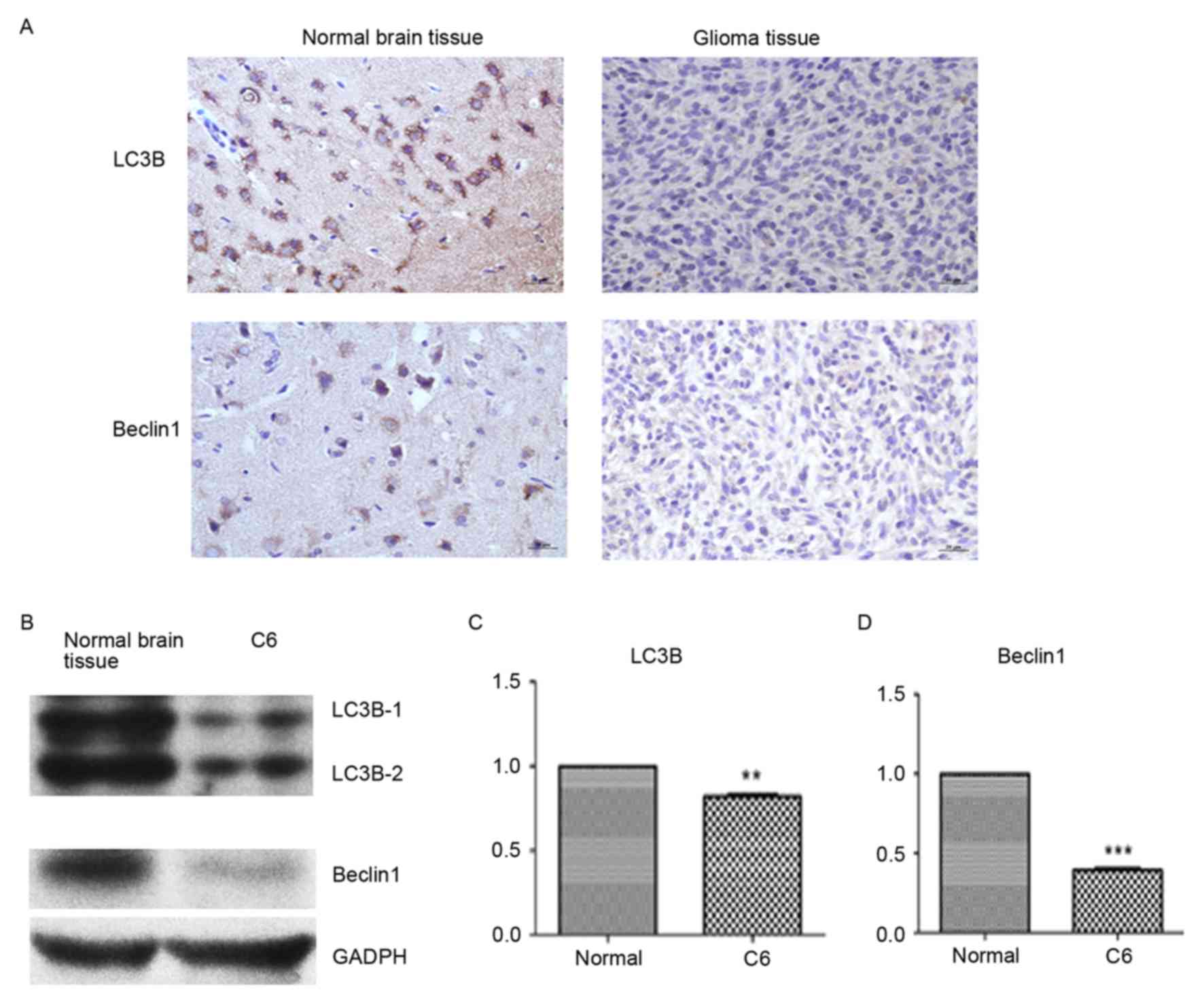
LC3B and Beclin1 protein expression
decreases in glioma tissue and glioma C6 cells
The expression of LC3B and Beclin1 was detected by
colorimetric IHC and western blotting. Compared with normal brain
tissue, the expression of LC3B and Beclin1 was visually markedly
decreased in glioma tissue (Fig.
1A). Similarly, the protein expression of LC3B and Beclin1 was
detected by western blotting and determined by densitometry to be
markedly decreased compared with normal brain tissue (Fig. 1B). LC3B (P<0.01) and Beclin1
(P<0.001) protein expression levels were significantly lower in
glioma tissue compared to normal brain tissue (Fig. 1C and D).
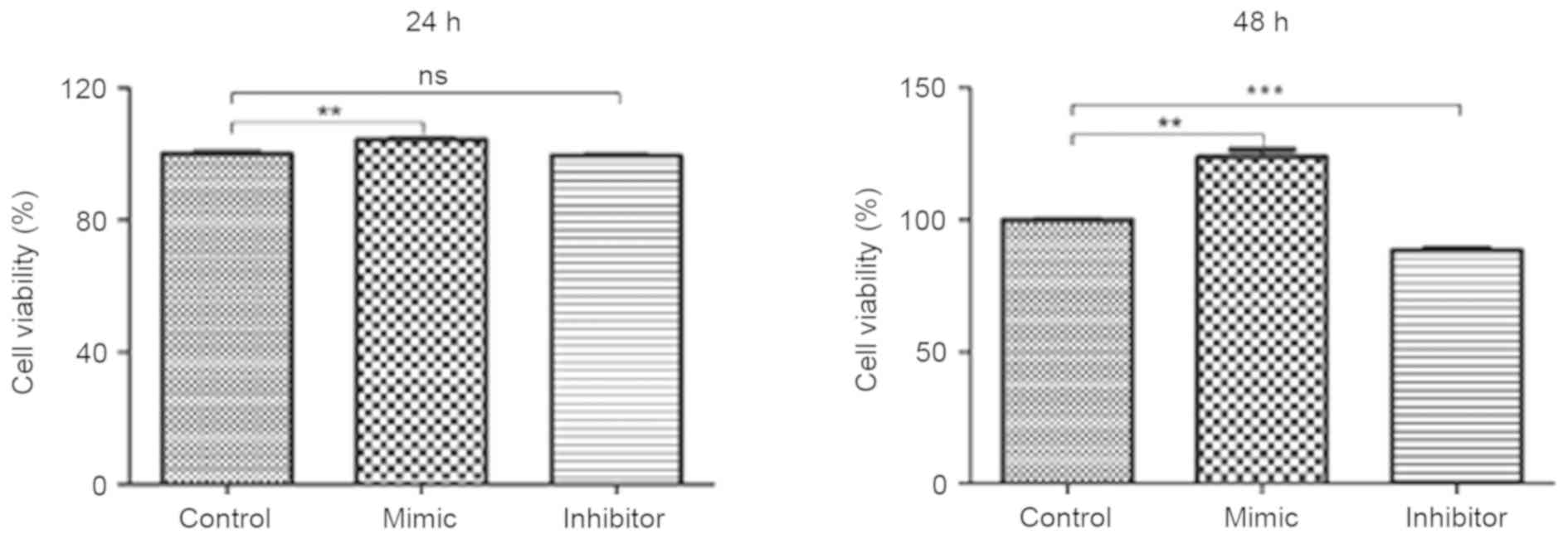
Glioma C6 cells transfected with
miR-24 mimics exhibit greater cell viability
Glioma C6 cells were transfected with miR-24 mimic,
miR-24 inhibitor and the negative control. An MTT assay was
utilized to evaluate the viability of C6 cells. Compared with the
negative control group, C6 cells transfected with miR-24 mimics
exhibited significantly greater cell viability at 24 and 48 h (both
P<0.01; Fig. 2). C6 cells
transfected with miR-24 inhibitor exhibited significantly decreased
cell viability at 48 h compared with the negative control group
(P<0.001). No significant differences were identified in cell
viability between the miR-24 inhibitor and negative control groups
at 24 h.
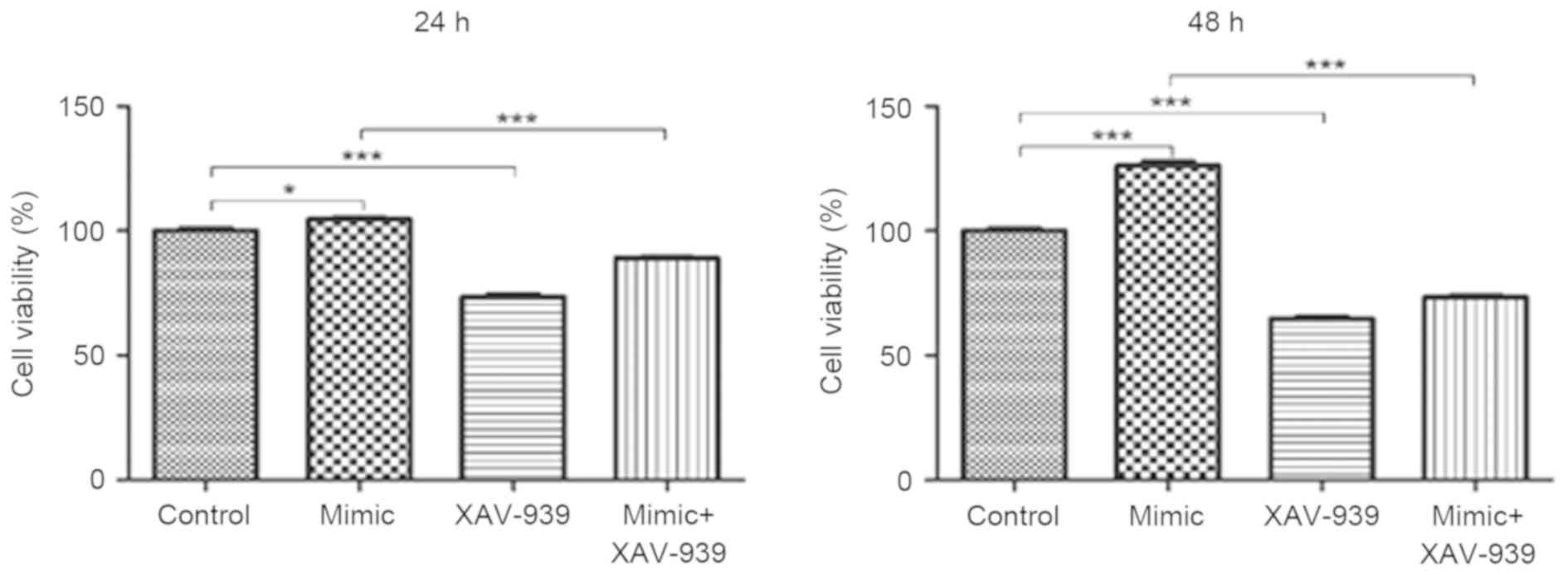
XAV-939 attenuates the miR-24
mimic-induced increase on the viability of glioma C6 cells
Glioma C6 cells transfected with miR-24 mimics or
the negative control miRNA were treated with the β-catenin
inhibitor, XAV-939. MTT assays were utilized to evaluate the
viability of C6 cells. The viability of C6 cells was significantly
decreased following the treatment with β-catenin inhibitor XAV-939
at 24 and 48 h compared with the negative control group
(P<0.001, Fig. 3). In addition,
the viability of C6 cells transfected with miR-24 mimics and
treated with XAV-939 was significantly decreased at 24 and 48 h
compared with C6 cells that were transfected with miR-24 mimics and
untreated (P<0.001).
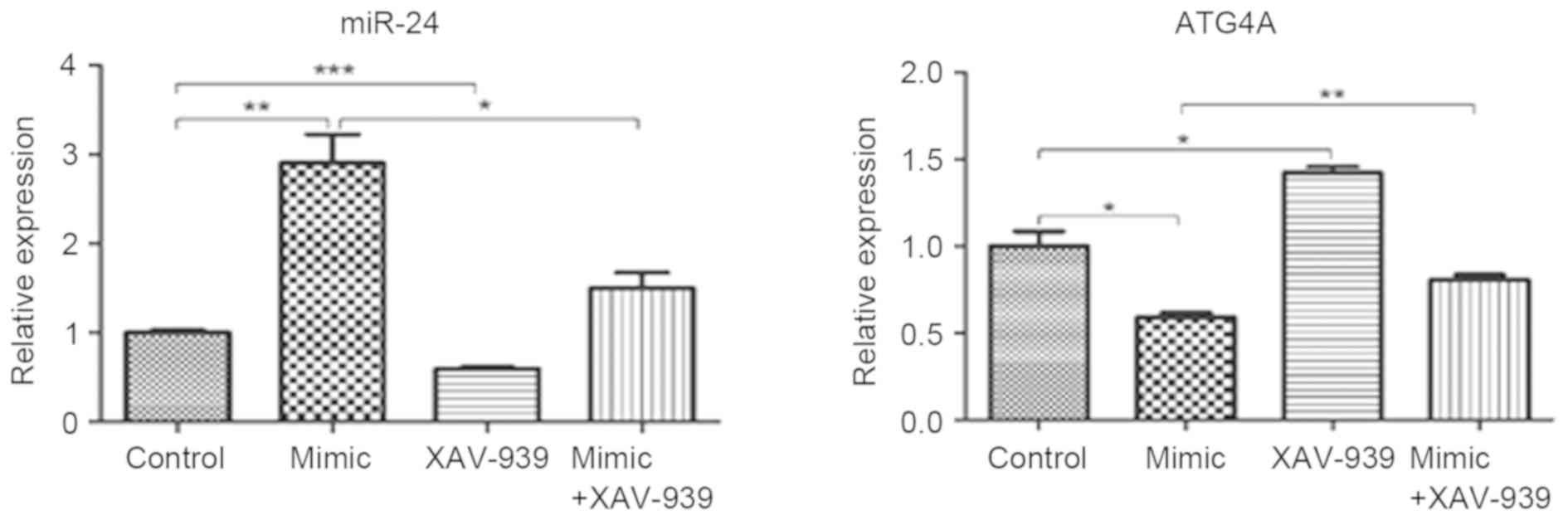
Expression of miR-24 decreases and
ATG4A increases following treatment with XAV-939 in glioma C6 cells
transfected with miR-24
Glioma C6 cells transfected with miR-24 mimics or
negative control miRNA were treated with β-catenin inhibitor
XAV-939. The mRNA expression levels of miR-24 and ATG4A were
detected by RT-qPCR analysis. The expression of miR-24
significantly decreased in C6 cells transfected with miR-24 mimics
and treated with XAV-939 compared with C6 cells transfected with
miR-24 mimics (P<0.05; Fig. 4).
Similarly, in C6 cells transfected with the negative control miRNA,
miR-24 expression significantly decreased following XAV-939
treatment (P<0.001). In addition, ATG4A expression significantly
increased following XAV-939 treatment either in C6 cells
transfected with miR-24 mimics (P<0.01) or the negative control
miRNA (P<0.05).
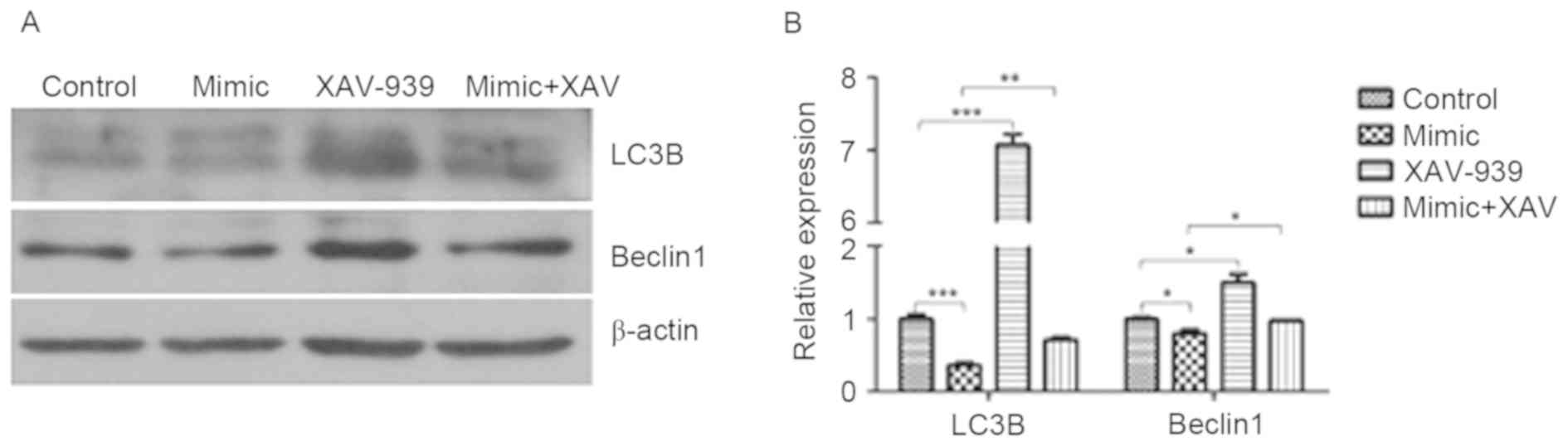
XAV-939 attenuates the miR-24-induced
decrease in the protein expression of LC3B and Beclin1
The protein expression of LC3B and Beclin1 in glioma
C6 cells was detected by western blotting. miR-24 induced a
significant decrease in the protein expression of LC3B (P<0.001)
and Beclin1 (P<0.05) compared with the cells transfected with
negative control miRNA (Fig. 5). The
protein expression of LC3B and Beclin1 in negative control miRNA
and XAV-939-treated C6 cells was significantly increased compared
with those treated with the negative control miRNA (P<0.001 and
P<0.05, respectively). The protein expression of LC3B and
Beclin1 in miR-24 mimic and XAV-939-treated C6 cells was
significantly increased compared with those treated with miR-24
mimics alone (P<0.01 and P<0.05, respectively).
Discussion
It was demonstrated in the present study that the
β-catenin inhibitor, XAV-939, attenuates the miR-24 mimic-induced
increase on the viability of glioma C6 cells. XAV-939 attenuated
the miR-24-induced decrease in autophagy markers by decreasing
miR-24 expression and increasing ATG4A expression in glioma C6
cells. This indicates that β-catenin promotes an increase in
miR-24-induced increase cell viability in glioma C6 cells. It is
likely that there is a β-catenin binding site at the promoter
region of miR-24 or miR-24 stimulates cell proliferation via the
β-catenin signaling pathway. The β-catenin gene is a proto-oncogene
and its mutations are commonly found in a variety of cancers,
including primary hepatocellular carcinoma, colorectal cancer, skin
cancer, prostate cancer and glioblastoma (6–8,20,21).
miR-96 was previously demonstrated to contribute to glioma tumor
progression by activating the Wnt/β-catenin signaling pathway.
Additionally, the miR-96/HMG box-containing protein 1/Wnt/β-catenin
regulatory circuitry promoted the proliferation of glioma cells
(22). It was also revealed that
miR-603 promoted glioma cell growth via the Wnt/β-catenin signaling
pathway by inhibiting Wnt inhibitory factor 1 and
β-catenin-interacting protein 1 (23).
The ATG4A gene is the target gene of miR-24
(16). The results of the current
study revealed that β-catenin increased miR-24 expression, thereby
inhibiting autophagy in gliomas. β-catenin may bind to the promoter
region of miR-24, and thus increase the expression and function of
miR-24. In addition, it is possible that the β-catenin signaling
pathway is involved in the downstream effects of miR-24 on
autophagy. TGF-β1-induced autophagy was demonstrated to link
β-catenin and Smad signaling to promote epithelial-mesenchymal
transition in mouse tubular epithelial cells through the
pY654-β-catenin/p-Smad2/integrin-linked protein kinase signaling
pathway (20). Tissue kallikrein was
reported to promote cell survival rate and β-catenin degradation in
serum-starved SH-SY5Y cells via increasing autophagy (24). Additionally, the overexpression of
progranulin inhibited TNF-α-induced inflammation in keratinocytes
by positively mediating autophagy through the Wnt/β-catenin
signaling pathway (25). More
studies are required to elucidate the detailed role of β-catenin in
the miR-24-mediated inhibition of autophagy. To the best of our
knowledge, this is the first to demonstrate that β-catenin
regulates the intracellular effects of miR-24 on the viability and
autophagy of glioma cells. The present results also provide a
direct mechanistic basis to support the growing pharmaceutical
interest in targeting WNT signaling in high grade brain tumors
(26).
Acknowledgements
Not applicable.
Funding
The present study received financial support from
the National Science Foundation of China (grant no. ISIS:
81502163).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZY and DD conceived the idea and designed the study.
HC and QL conducted the experiments, analysed the data and drafted
the manuscript. CC, YD, YL and WM participated in the data
acquisition and analysis. All authors read and approved the final
version of this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Shanghai Changhai Hospital (Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bleeker FE, Molenaar RJ and Leenstra S:
Recent advances in the molecular understanding of glioblastoma. J
Neurooncol. 108:11–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weis WI and Nelson WJ: Re-solving the
cadherin-catenin-actin conundrum. J Biol Chem. 281:35593–35597.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saldanha G, Ghura V, Potter L and Fletcher
A: Nuclear beta-catenin in basal cell carcinoma correlates with
increased proliferation. Br J Dermatol. 151:157–164. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tao H, Guo L, Chen L, Qiao G, Meng X, Xu B
and Ye W: MSX1 inhibits cell migration and invasion through
regulating the Wnt/β-catenin pathway in glioblastoma. Tumour Biol.
37:1097–1104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh T and Katiyar SK: Green tea
polyphenol, (−)-epigallocatechin-3-gallate, induces toxicity in
human skin cancer cells by targeting β-catenin signaling. Toxicol
Appl Pharmacol. 273:418–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong HJ, Jang GB, Lee HY, Park SR, Kim JY,
Nam JS and Hong IS: The Wnt/β-catenin signaling/Id2 cascade
mediates the effects of hypoxia on the hierarchy of
colorectal-cancer stem cells. Sci Rep. 6:229662016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei A, Fan B, Zhao Y, Zhang H, Wang L, Yu
X, Yuan Q, Yang D and Wang S: ST6Gal-I overexpression facilitates
prostate cancer progression via the PI3K/Akt/GSK-3β/β-catenin
signaling pathway. Oncotarget. 7:65374–65388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen J, Rajasekaran M, Xia H, Zhang X,
Kong SN, Sekar K, Seshachalam VP, Deivasigamani A, Goh BK, Ooi LL,
et al: The microtubule-associated protein PRC1 promotes early
recurrence of hepatocellular carcinoma in association with the
Wnt/β-catenin signalling pathway. Gut. 65:1522–1534. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lal A, Kim HH, Abdelmohsen K, Kuwano Y,
Pullmann R Jr, Srikantan S, Subrahmanyam R, Martindale JL, Yang X,
Ahmed F, et al: p16(INK4a) translation suppressed by miR-24. PLoS
One. 3:e18642008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lal A, Navarro F, Maher CA, Maliszewski
LE, Yan N, O'Day E, Chowdhury D, Dykxhoorn DM, Tsai P, Hofmann O,
et al: miR-24 inhibits cell proliferation by targeting E2F2, MYC,
and other cell-cycle genes via binding to ‘seedless’ 3′UTR microRNA
recognition elements. Mol Cell. 35:610–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amelio I, Lena AM, Viticchiè G,
Shalom-Feuerstein R, Terrinoni A, Dinsdale D, Russo G, Fortunato C,
Bonanno E, Spagnoli LG, et al: miR-24 triggers epidermal
differentiation by controlling actin adhesion and cell migration. J
Cell Biol. 199:347–363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Q, Huang Z, Xue H, Jin C, Ju XL, Han
JD and Chen YG: MicroRNA miR-24 inhibits erythropoiesis by
targeting activin type I receptor ALK4. Blood. 111:588–595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin Y, Zhong J, Li SW, Li JZ, Zhou M, Chen
Y, Sang Y and Liu L: TRIM11, a direct target of miR-24-3p, promotes
cell proliferation and inhibits apoptosis in colon cancer.
Oncotarget. 7:86755–86765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan B, Chen Y, Song H, Xu Y, Wang R and
Chen L: Mir-24-3p downregulation contributes to VP16-DDP resistance
in small-cell lung cancer by targeting ATG4A. Oncotarget.
6:317–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cherra SJ III, Kulich SM, Uechi G,
Balasubramani M, Mountzouris J, Day BW and Chu CT: Regulation of
the autophagy protein LC3 by phosphorylation. J Cell Biol.
190:533–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Y, Ma W, Lei F, Li Q, Su Y, Lin X, Lin
C, Zhang X, Ye L, Wu S, et al: Prostate tumour overexpressed-1
promotes tumourigenicity in human breast cancer via activation of
Wnt/β-catenin signalling. J Pathol. 239:297–308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu S, Wang S, Zheng S, Verhaak R, Koul D
and Yung WK: MSK1-mediated β-catenin phosphorylation confers
resistance to PI3K/mTOR inhibitors in glioblastoma. Mol Cancer
Ther. 15:1656–1668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan Z, Wang J, Wang C, Jiao Y, Qi W and
Che S: miR-96/HBP1/Wnt/β-catenin regulatory circuitry promotes
glioma growth. FEBS Lett. 588:3038–3046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo M, Zhang X, Wang G, Sun J, Jiang Z,
Khadarian K, Yu S, Zhao Y, Xie C, Zhang K, et al: miR-603 promotes
glioma cell growth via Wnt/β-catenin pathway by inhibiting WIF1 and
CTNNBIP1. Cancer Lett. 360:76–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Cui M, Lu Z, Yang Q and Dong Q:
Tissue kallikrein promotes survival and β-catenin degradation in
SH-SY5Y cells under nutrient stress conditions via autophagy. Mol
Med Rep. 13:1389–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tian R, Li Y and Yao X: PGRN suppresses
inflammation and promotes autophagy in keratinocytes through the
Wnt/β-catenin signaling pathway. Inflammation. 39:1387–1394. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McCord M, Mukouyama YS, Gilbert MR and
Jackson S: Targeting WNT signaling for multifaceted glioblastoma
therapy. Front Cell Neurosci. 11:3182017. View Article : Google Scholar : PubMed/NCBI
|