Introduction
Breast cancer has developed into the most common and
aggressive cancer among women around the world (1). In 2012, breast cancer accounted for 25%
of all cancer cases and 15% of all cancer deaths amongst females
worldwide (1). Evidence has revealed
that there are a number of risk factors that increase the incidence
of breast cancer, such as obesity, alcohol abuse and genetic
mutations (3). Despite numerous
advances in breast cancer therapy, the mortality remains to
increase (4). Currently, the major
approaches for breast cancer treatment include surgery,
radiotherapy, chemotherapy and hormonotherapy (5). The outcomes of patients with breast
cancer remain poor. Thus, it is crucial to investigate the
potential molecular mechanisms underlying breast cancer and develop
novel therapeutic strategies.
MicroRNAs (miRNAs or miRs) are one group of small
non-coding RNAs that are approximately 22 nucleotides in length
(6). Like long non-coding RNAs,
miRNAs are implicated in the development of multiple cancers, such
as liver cancer (7,8). miRNAs may regulate gene expression
through interacting with the 3′-untranslated region (3′-UTR) of
target mRNAs (9). Evidence has
demonstrated that miRNAs initiate or suppress cancer development by
regulating cell proliferation, metastasis, apoptosis or
differentiation (10). For example,
miR-495 regulates the progression of gastric cancer via the
mammalian target of rapamycin pathway (11). miR-150 suppresses thyroid cancer
development by modulating Ras-related protein Rab-11A/WNT/β-Catenin
signaling (12). miR-1179 inhibits
the proliferation of non-small cell lung cancer cells through the
protein kinase B pathway (13). In
addition, miR-125b-5p has been reported to repress breast cancer
cell growth and metastasis via targeting uncharacterized protein
KIAA1522 (14). Thus, identification
of key miRNAs involved in breast cancer will be important for
understanding its potential underlying molecular mechanism.
miR-876-5p was previously been proven to inhibit
metastasis of head and neck squamous cell carcinoma, liver cancer
and lung cancer (15–17). However, its function in breast cancer
development remains unclear. The aim of the present study was to
analyse miR-876-5p expression in breast cancer and evaluate its
effects on tumor cell malignant behaviors, whilst emphasizing the
essential roles of the miR-876-5p/transcription factor AP-2-α
(TFAP2A) axis in breast cancer.
Materials and methods
Cell culture and transfection
Breast cancer cell lines (MCF-7, MDA-MB-231 and
T47D) and the normal breast epithelial cell line MCF-10A were
obtained from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). These cell lines
were cultured with RPMI 1640 medium containing 10% fetal bovine
serum (both Invitrogen; Thermo Fisher Scientific, Inc.) in a humid
cell incubator at 37°C. miR-876-5p inhibitors
(5′-UGGUGAUUCACAAAGAAAUCCA-3′), miR-876-5p mimics
(5′-UGGAUUUCUUUGUGAAUCACCA-3′) and negative controls
(5′-UUCUCCGAACGUGUCACGUTT-3′) were synthesized by GenePharma
Biotech Corp., and transduced into cell lines at a concentration of
50 nM using Lipofectamine™ 2000 (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For the
rescue assay, the coding sequence of TFAP2A was cloned into pcDNA3
vector (Invitrogen; Thermo Fisher Scientific, Inc.) and transfected
into cells (1 µg per well) using Lipofectamine™ 2000.
Following 48 h, transfection efficiency was confirmed using reverse
transcription-quantitative (RT-qPCR) as described below.
RNA extraction and RT-qPCR
RNAs were isolated from cultured cells using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). cDNAs were reverse transcribed using a PrimeScript RT
reagent kit (Takara Bio, Inc.) according to the manufacturer's
protocol. qPCR analysis were performed using a SYBR Green I Master
Mix kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. miRNA relative expression was normalized
to U6 while mRNA expression was normalized to actin, cytoplasmic 1
(ACTB). Thermocycling conditions were as follows: Denaturation at
95°C for 10 min, followed by 40 cycles of denaturation at 95°C for
15 sec and elongation at 60°C for 1 min. Gene expression was
calculated according to the 2−ΔΔCq method (18). The primers were synthesized by Sangon
Biotech Co., Ltd. The primer sequences were as follows: miR-876-5p:
Forward, 5′-TGGATTTCTTTGTGAATCACCA-3′; reverse,
5′-AACGAGACGACGACAGAC-3′; U6: Forward,
5′-GCAAATTCGTGAAGCGTTCCATA-3′; reverse, 5′-AACGAGACGACGACAGAC-3′;
TFAP2A: Forward, 5′-TGCTACACTGAGACTCCCGT-3′; and reverse,
5′-GAATGCCTGGAAATCGAGCG-3′; and ACTB: Forward
5′-CGGCGCCCTATAAAACCCA-3′ and reverse,
5′-GAGGCGTACAGGGATAGCAC-3′.
Cell proliferation assay
A total of 2,000 cells per well were seeded in the
96-well plate and cultured for 1, 2, 3 or 4 days at 37°C. Then 10
µl Cell Counting Kit (CCK) 8 solution (Dojindo Molecular
Technologies) was added and incubated according the manufacturer's
protocol. Finally, the absorbance at 450 nm was determined using a
multimode microplate reader (Berthold Technologies GmbH & Co.
KG).
Transwell assay
Cell migration and invasion was assessed using
Transwell chambers (8 µm pore size; Corning Inc.). Briefly,
2×104 tumor cells per well were seeded into the upper
chamber in serum-free RPMI 1640 medium (Invitrogen; Thermo Fischer
Scientific, Inc.), and the lower chamber was filled with 600 µl
complete RPMI 1640 medium with 10% FBS. Following incubation for 48
h at 37°C, the cells in the lower chamber were fixed with 4%
paraformaldehyde for 1 h at 25°C and stained with 0.1% crystal
violet for 30 min at 25°C. The cell number was counted using a
light microscope under ×200 magnification. During invasion assay,
Matrigel (Becton, Dickinson and Company) was used to pre-coat the
upper chamber.
Western blotting
Breast cancer cells were lysed in cold
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.), and protein concentration was determined using a
Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific,
Inc.). Protein (40 µg/lane) was separated via SDS-PAGE (10% gel)
and transferred to a polyvinylidene difluoride (PVDF) membrane
(Thermo Fisher Scientific, Inc.). The membrane was blocked using 5%
non-fat milk in PBS (Thermo Fisher Scientific, Inc.) containing
0.1% Tween-20 (Sigma-Aldrich; Merck KGaA) at room temperature for 2
h. Subsequently, the PVDF membrane was incubated with anti-Cyclin
D1 (1:1,000; cat. no. ab16663), anti-TFAP2A (1:1,000; cat. no.
ab52222) and anti-GAPDH (1:1,000; cat. no. ab9485; all from Abcam)
primary antibodies at room temperature for 2 h. Following washing
with PBS for 10 min, the PVDF membrane was incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibodies (1:5,000; cat. no. ab7090; Abcam) at room temperature
for 1 h. Membranes were then washed with PBS for 10 min and the
protein bands were visualized using the Pierce™ ECL
Western Blotting Substrate kit (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Protein densitometry was
performed using ImageJ Software (version 1.41; National Institutes
of Health).
Dual-luciferase reporter assay
The TargetScan7 tool (http://www.targetscan.org/vert_71/) was used to
predict the potential interaction between miR-876-5p and TFAP2A.
The TFAP2A 3′-UTR sequence containing wild-type (WT) or mutant
(MUT) binding site for TFAP2A was constructed into pGL3-reporter
luciferase vector (Promega Corporation). For the luciferase
reporter assay, tumor cells were co-transfected with TFAP2A
reporter vector and miR-876-5p mimics or control using
Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.). Following 48
h, luciferase activity was measured using a dual-luciferase
reporter assay system (Promega Corporation) following the
manufacturer's protocol. Luciferase activities were normalized to
that of Renilla luciferase activity.
Statistical analysis
All experiments were repeated in triplicate and
analyzed using SPSS software (version 22.0; IBM Corp.). The
differences were determined via unpaired Student's t-test or a
one-way analysis of variance followed by a Tukey's post-hoc test.
Results were presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant result.
Results
miR-876-5p suppresses breast cancer
cell proliferation
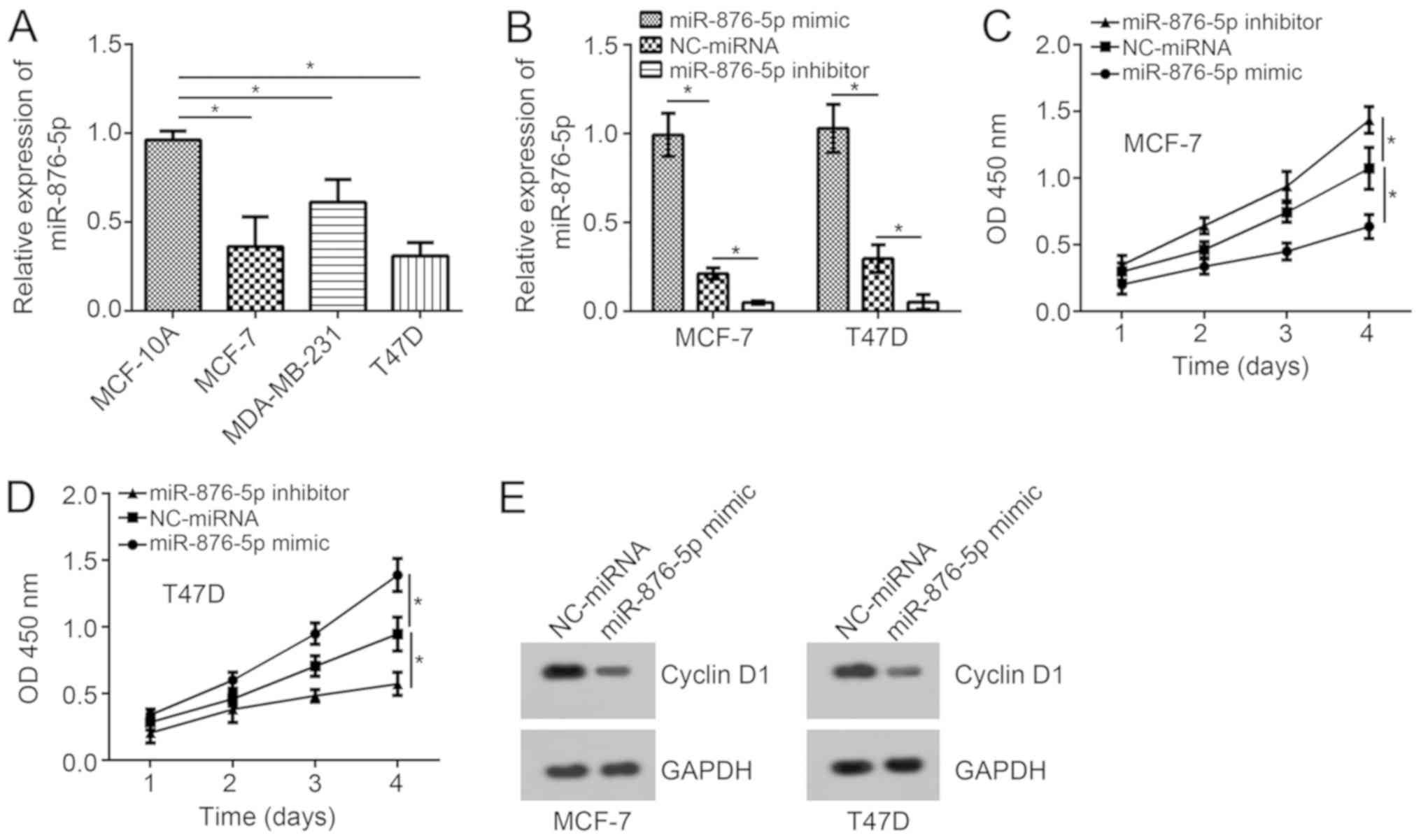
Expression patterns of miR-876-5p in breast cancer
cells were analyzed using RT-qPCR. The results indicated that
miR-876-5p expression levels are significantly downregulated in
tumor cells when compared with MCF-10A cells (Fig. 1A). Among these three breast cancer
cell lines, the expression levels of miR-876-5p were the lowest in
MCF-7 and T47D cells. Thus, these two cell lines were selected for
functional experiments. Using miR-876-5p mimics and inhibitors, the
miR-876-5p expression levels were significantly upregulated and
downregulated, respectively, in MCF-7 and T47D cells compared with
NC-miRNA-transfected cells (Fig.
1B). According to the CCK8 assay, miR-876-5p upregulation
suppressed the proliferation of breast cancer cells on day 4
compared to the NC-miRNA group while inhibition of miR-876-5p
significantly promoted proliferation compared to the control
(Fig. 1C and D). In addition,
miR-876-5p upregulation inhibited the protein levels of Cyclin D1
in MCF-7 and T47D cells (Fig. 1E),
indicating miR-876-5p prevents cell-cycle progression.
miR-876-5p inhibits migration and
invasion of breast cancer cells
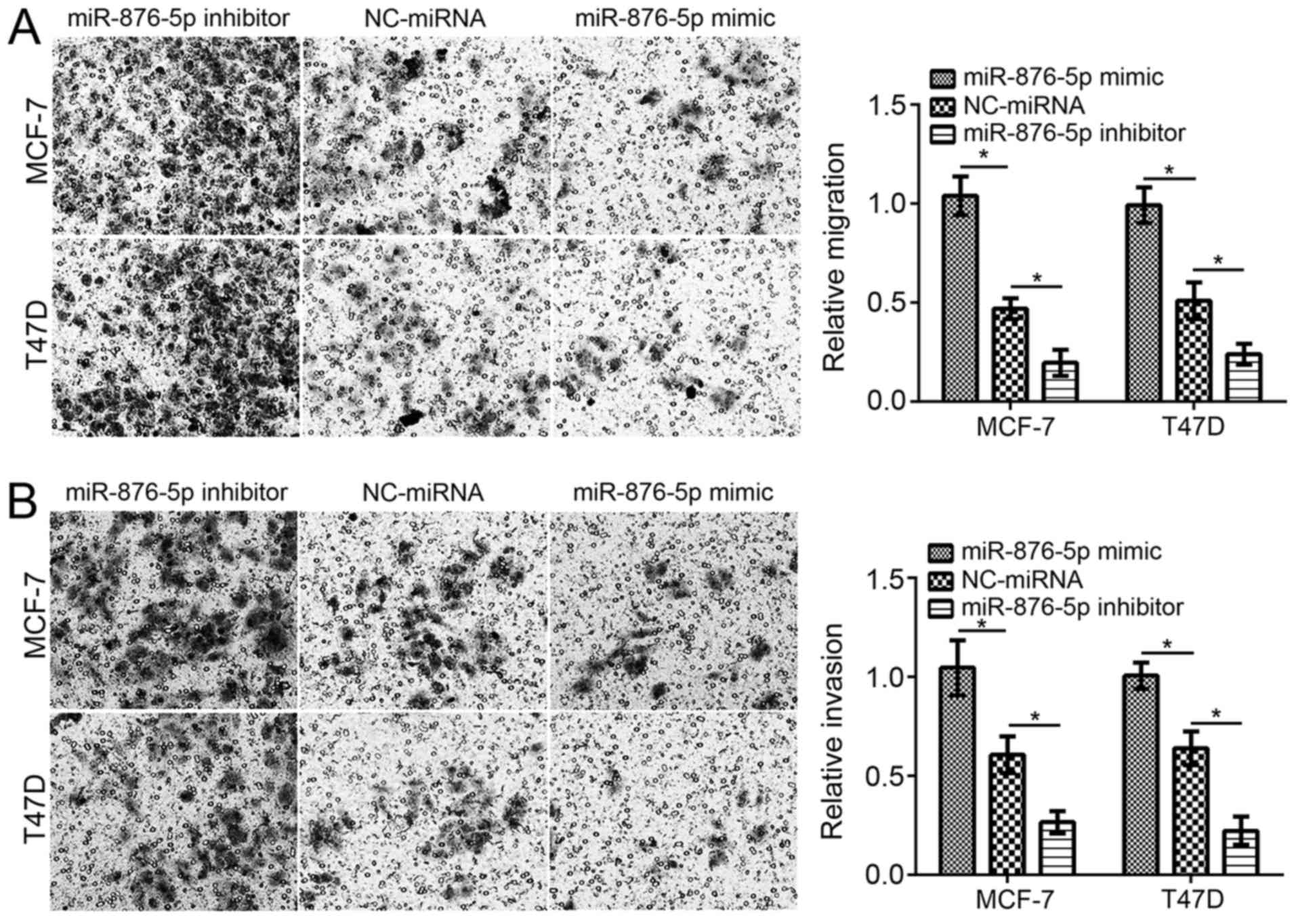
Furthermore, the effect of miR-876-5p on migration
and invasion was evaluated using Transwell assays. Following
miR-876-5p mimic transfection, the cell numbers of migration and
invasion were significantly decreased compared with
NC-miRNA-transfected cells (Fig. 2A and
B). Inhibition of miR-876-5p significantly decreased the
migration and invasion of MCF-7 and T47D cells compared with
NC-miRNA-transfected cells.
miR-876-5p targets TFAP2A in breast
cancer cells
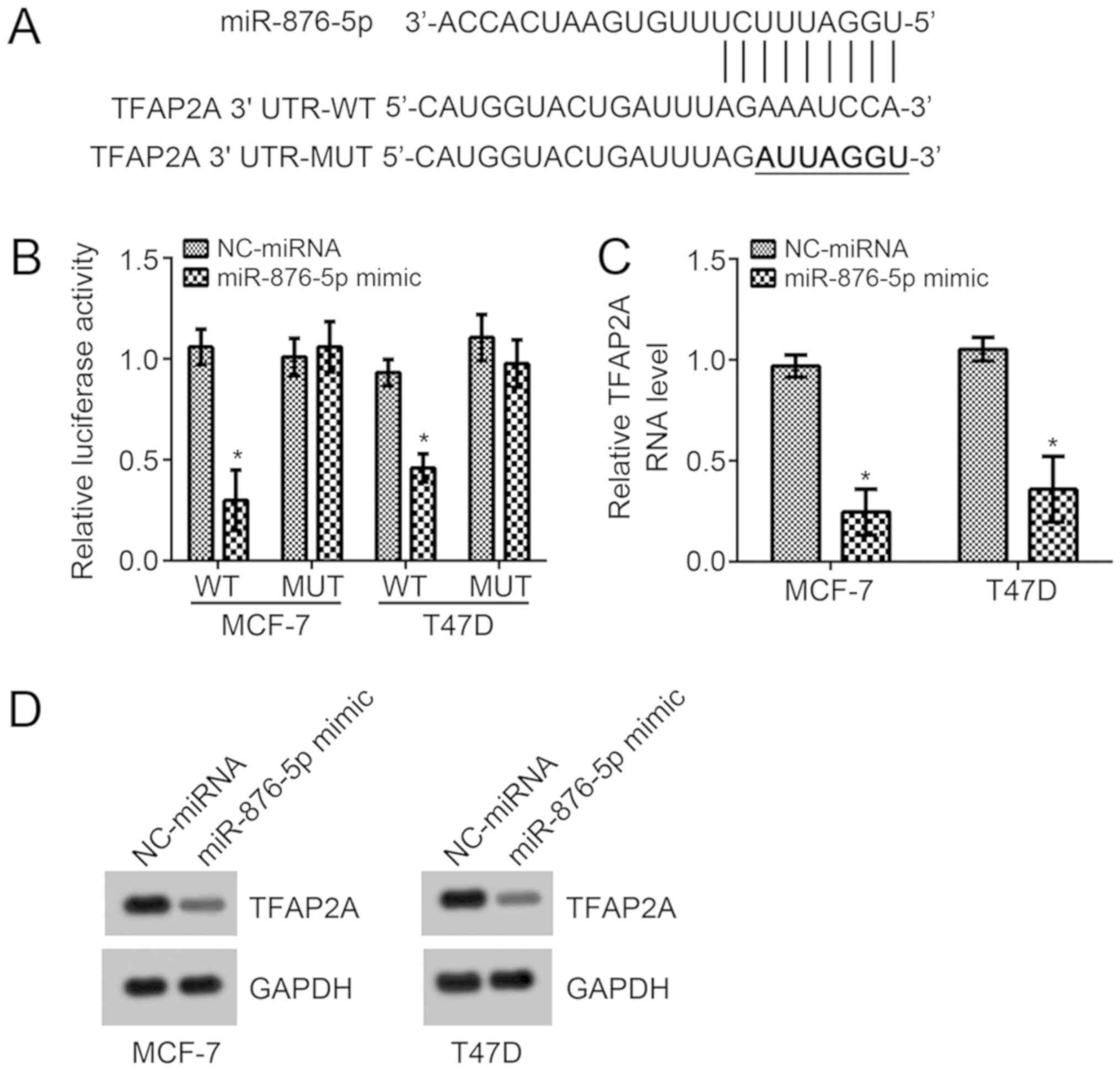
In order to investigate the underlying molecular
mechanism of miR-876-5p, the TargetScan7 tool was used to predict
the potential target gene of miR-876-5p. Among all candidates,
TFAP2A ranked top. The potential binding site for miR-876-5p was
identified in the 3′-UTR region of TFAP2A and it was mutated for
the luciferase reporter assay (Fig.
3A). According to the luciferase reporter assay in MCF-7 and
T47D cells, miR-876-5p mimic transfection significantly suppressed
the activity of WT-reporter plasmid compared with
NC-miRNA-transfected cells (Fig.
3B). Mutation of this site in the reporter plasmid rescinded
the decrease in luciferase activity (Fig. 3B). Furthermore, miR-876-5p mimics
significantly suppressed the mRNA levels compared with
NC-miRNA-transfected cells, and markedly suppressed protein levels
of TFAP2A in MCF-7 and T47D cells (Fig.
3C and D). These results demonstrated that miR-876-5p directly
targets TFAP2A in breast cancer cells.
Restoration of TFAP2A rescinds the
effect of miR-876-5p mimics in MCF-7 cells
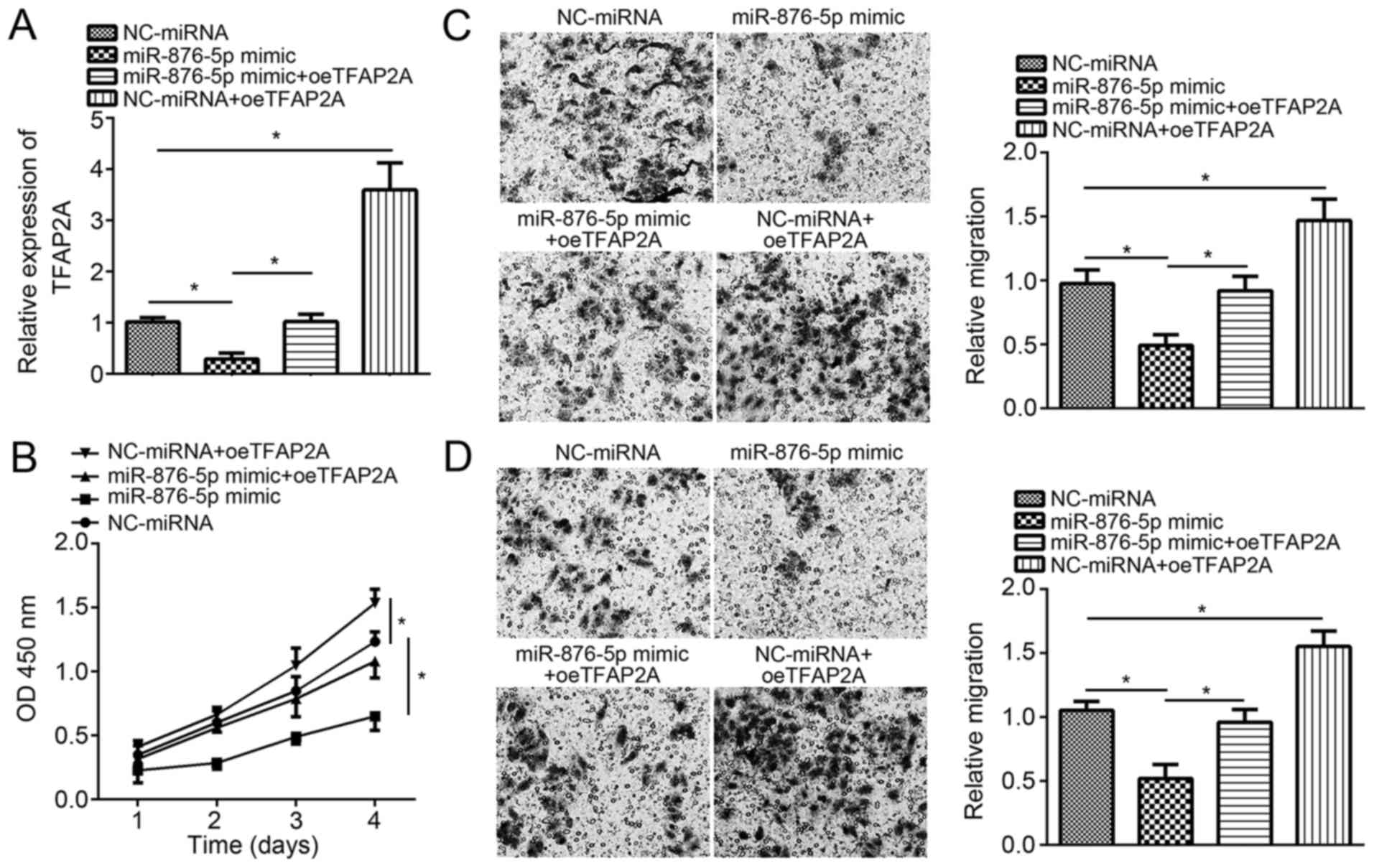
Finally, to validate whether miR-876-5p suppresses
breast cancer progression through targeting TFAP2A, rescue assays
were conducted. RT-qPCR analysis validated that co-transfection
with pcDNA3-TFAP2A vector and miR-876-5p mimics significantly and
successfully restored the expression of TFAP2A in MCF-7 cells
compared with cells transfected with the miR-876-5p mimics alone
(Fig. 4A). The CCK8 assay
demonstrated that TFAP2A restoration markedly rescued the
suppressed proliferation by miR-876-5p in MCF-7 cells (Fig. 4B). Furthermore, individual ectopic
expression of TFAP2A further promoted MCF-7 cell proliferation.
Similarly, TFAP2A restoration following co-transfection
significantly rescued the migration and invasion abilities in MCF-7
cells compared with miR-876-5p mimics-transfected cells (Fig. 4C and D). Overall, these results
demonstrated that miR-876-5p suppresses breast cancer progression
by targeting TFAP2A.
Discussion
Breast cancer remains to be a significant challenge
for the health of women worldwide. However, the outcomes and
survival rate urgently require improvement. A number of studies
have indicated that miRNAs are involved in the progression of
breast cancer (19) and may be good
biomarkers for tumor diagnosis and prognosis (20). Furthermore, uncovering the functional
mechanism of pivotal miRNAs involved in breast cancer will be
helpful for developing novel therapeutic targets against this
disease. The results from the present study indicated that
miR-876-5p expression was decreased in breast cancer cell lines.
miR-876-5p upregulation suppresses the proliferation, migration and
invasion of tumor cells, which, to the best of our knowledge,
indicates its anti-cancer role in breast cancer for the first
time.
Up until now, the roles of miR-876-5p in
tumorigenesis have been poorly understood. Xu et al
(21) reported that elevated levels
of miR-876 promotes apoptosis of endothelial cells. Bao et
al (17) demonstrated that
miR-876-5p inhibits metastasis in lung cancer through targeting
bone morphogenetic protein 4. Sang et al (22) revealed that miR-876-5p targets the
melanoma-associated antigen family to suppress the development of
esophageal squamous cell carcinoma. Furthermore, previous studies
have proved that miR-876-5p also prevents cell invasion in liver
cancer (16,21). In addition, Dong et al
(15) proved that miR-876-5p impeded
the migration of head and neck squamous cancer cells via inhibiting
vimentin. Currently, the way in which miR-876-5p regulates breast
cancer remains unknown. In the present study, it was revealed that
miR-876-5p expression levels were significantly decreased in breast
cancer cell lines. A CCK8 assay demonstrated that miR-876-5p
upregulation attenuates tumor cell growth. Furthermore, a Transwell
assay demonstrated that miR-876-5p mimic transfection impaired the
migration and invasion ability in breast cancer cell lines. The
results from the present study indicate that miR-876-5p suppresses
breast cancer progression. However, the expression levels of
miR-876-5p should be investigated in the future, and analyzing the
association between miR-876-5p and the clinical severity of breast
cancer may further validate whether miR-876-5p could be a
therapeutic target. Furthermore, whether miR-876-5p could serve as
a diagnostic or prognostic marker for patients with breast cancer
needs to be determined, alongside whether miR-876-5p regulates
breast cancer cell apoptosis.
A number of studies have demonstrated that miRNAs
exert roles through targeting the complementary sequence in the
3′UTR region of mRNAs (12,16). For example, miR-363-3p promotes
glioma cell proliferation and metastasis through targeting pyruvate
dehydrogenase B (22). miR-214
contributes to osteosarcoma progression via targeting tumor
necrosis factor receptor-associated factor 3 (23). miR-543 enhances metastasis of gastric
cancer cells by targeting speckle-type POZ protein (24). Furthermore, miR-1258 upregulation
suppresses the growth of colon cancer cells and arrests cell-cycle
progression through targeting transcription factor E2F8 (25). Through bioinformatics analyses, it
was revealed that TFAP2A may be targeted by miR-876-5p. The
luciferase reporter assay demonstrated the direct interaction
between miR-876-5p and TFAP2A in breast cancer cells. Furthermore,
it was also revealed that TFAP2A expression could be suppressed by
miR-876-5p, suggesting that TFAP2A may be a downstream signal of
miR-876-5p.
TFAP2A is a transcription factor involved in many
different types of cancer (26). For
example, Shi et al (27)
revealed that TFAP2A promotes nasopharyngeal carcinoma cell
proliferation and inhibits apoptosis. Notably, studies have
indicated that TFAP2A promotes breast cancer progression (28,29). In
the present study, it was also revealed that TFAP2A overexpression
promotes the proliferation, migration and invasion of breast cancer
cells. Furthermore, restoration of TFAP2A in miR-876-5p-upregulated
breast cancer cells could abolish the suppressive effects of
miR-876-5p mimics. Overall, the results from the present study
demonstrated that miR-876-5p exerts roles by targeting TFAP2A.
To the best of our knowledge, the present study
demonstrates for the first time that miR-876-5p suppresses breast
cancer progression through attenuating proliferation, migration and
invasion by targeting TFAP2A directly. Findings suggested that
miR-876-5p may be a promising therapeutic target for breast cancer
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JX initiated and designed the present study,
analyzed and interpreted the results. JZ, JW and JS performed
various experiments. JX wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao L and Zheng XY: MicroRNA-490 inhibits
tumorigenesis and progression in breast cancer. Onco Targets Ther.
9:4505–4516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudolph A, Chang-Claude J and Schmidt MK:
Gene-environment interaction and risk of breast cancer. Br J
Cancer. 114:125–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu RZ, Garcia E, Glubrecht DD, Poon HY,
Mackey JR and Godbout R: CRABP1 is associated with a poor prognosis
in breast cancer: Adding to the complexity of breast cancer cell
response to retinoic acid. Mol Cancer. 14:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dong Y, Chang C, Liu J and Qiang J:
Targeting of GIT1 by miR-149* in breast cancer suppresses cell
proliferation and metastasis in vitro and tumor growth in vivo.
Onco Targets Ther. 10:5873–5882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu P, Wang Y, Wu J, Huang G, Liu B, Ye B,
Du Y, Gao G, Tian Y, He L and Fan Z: LncBRM initiates YAP1
signalling activation to drive self-renewal of liver cancer stem
cells. Nat Commun. 7:136082016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adams BD, Kasinski AL and Slack FJ:
Aberrant regulation and function of microRNAs in cancer. Curr Biol.
24:R762–R776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu B, Li J and Cairns MJ: Identifying
miRNAs, targets and functions. Brief Bioinform. 15:1–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song B, Lin HX, Dong LL, Ma JJ and Jiang
ZG: MicroRNA-338 inhibits proliferation, migration, and invasion of
gastric cancer cells by the Wnt/β-catenin signaling pathway. Eur
Rev Med Pharmacol Sci. 22:1290–1296. 2018.PubMed/NCBI
|
|
11
|
Li N, Han M, Zhou N, Tang Y and Tang XS:
MicroRNA-495 confers increased sensitivity to chemotherapeutic
agents in gastric cancer via the mammalian target of rapamycin
(mTOR) signaling pathway by interacting with human epidermal growth
factor receptor 2 (ERBB2). Med Sci Monit. 24:5960–5972. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai D, Sun H, Wang X, Lou H, Zhang J, Wang
X and Jiang L: MiR-150 inhibits cell growth in vitro and in vivo by
restraining the RAB11A/WNT/β-catenin pathway in thyroid cancer. Med
Sci Monit. 23:4885–4894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song L, Dai Z, Zhang S, Zhang H, Liu C, Ma
X, Liu D, Zan Y and Yin X: MicroRNA-1179 suppresses cell growth and
invasion by targeting sperm-associated antigen 5-mediated Akt
signaling in human non-small cell lung cancer. Biochem Biophys Res
Commun. 504:164–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Y, Wang Y, Fan H, Zhang Z and Li N:
miR-125b-5p inhibits breast cancer cell proliferation, migration
and invasion by targeting KIAA1522. Biochem Biophys Res Commun.
504:277–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong Y, Zheng Y, Wang C, Ding X, Du Y, Liu
L, Zhang W, Zhang W, Zhong Y, Wu Y and Song X: MiR-876-5p modulates
head and neck squamous cell carcinoma metastasis and invasion by
targeting vimentin. Cancer Cell Int. 18:1212018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Xie Y, Li X, Lin J, Zhang S, Li Z,
Huo L and Gong R: MiR-876-5p acts as an inhibitor in hepatocellular
carcinoma progression by targeting DNMT3A. Pathol Res Pract.
214:1024–1030. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bao L, Lv L, Feng J, Chen Y, Wang X, Han S
and Zhao H: MiR-876-5p suppresses epithelial-mesenchymal transition
of lung cancer by directly down-regulating bone morphogenetic
protein 4. J Biosci. 42:671–681. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagasawa S, Sedukhina AS, Nakagawa Y,
Maeda I, Kubota M, Ohnuma S, Tsugawa K, Ohta T, Roche-Molina M,
Bernal JA, et al: LSD1 overexpression is associated with poor
prognosis in basal-like breast cancer, and sensitivity to PARP
inhibition. PLoS One. 10:e01180022015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Yin W, Lin Y, Yin K, Zhou L, Du Y,
Yan T and Lu J: Downregulated circulating microRNAs after surgery:
Potential noninvasive biomarkers for diagnosis and prognosis of
early breast cancer. Cell Death Discov. 4:212018. View Article : Google Scholar
|
|
21
|
Xu Q, Zhu Q, Zhou Z, Wang Y, Liu X, Yin G,
Tong X and Tu K: MicroRNA-876-5p inhibits epithelial-mesenchymal
transition and metastasis of hepatocellular carcinoma by targeting
BCL6 corepressor like 1. Biomed Pharmacother. 103:645–652. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu DX, Guo JJ, Zhu GY, Wu HJ, Zhang QS and
Cui T: MiR-363-3p modulates cell growth and invasion in glioma by
directly targeting pyruvate dehydrogenase B. Eur Rev Med Pharmacol
Sci. 22:5230–5239. 2018.PubMed/NCBI
|
|
23
|
Rehei AL, Zhang L, Fu YX, Mu WB, Yang DS,
Liu Y, Zhou SJ and Younusi A: MicroRNA-214 functions as an oncogene
in human osteosarcoma by targeting TRAF3. Eur Rev Med Pharmacol
Sci. 22:5156–5164. 2018.PubMed/NCBI
|
|
24
|
Xu J, Wang F, Wang X, He Z and Zhu X:
miRNA-543 promotes cell migration and invasion by targeting SPOP in
gastric cancer. Onco Targets Ther. 11:5075–5082. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Z, Li J, Huang Y, Peng W, Qian W, Gu
J, Wang Q, Hu T, Ji D, Ji B, et al: Upregulated miR-1258 regulates
cell cycle and inhibits cell proliferation by directly targeting
E2F8 in CRC. Cell Prolif. 51:e125052018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan J, Zhang N, Zheng Y, Chen YD, Liu J
and Yang M: LncRNA GAS5 indel genetic polymorphism contributes to
glioma risk through interfering binding of transcriptional factor
TFAP2A. DNA Cell Biol. 37:750–757. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu
L, Wang J, Guo W, Kang T, Huang W and Deng W: TFAP2A regulates
nasopharyngeal carcinoma growth and survival by targeting
HIF-1alpha signaling pathway. Cancer Prev Res (Phila.). 7:266–277.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Allouche A, Nolens G, Tancredi A,
Delacroix L, Mardaga J, Fridman V, Winkler R, Boniver J, Delvenne P
and Begon DY: The combined immunodetection of AP-2alpha and YY1
transcription factors is associated with ERBB2 gene overexpression
in primary breast tumors. Breast Cancer Res. 10:R92008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan F, He Q, Hu X, Li W, Wei K, Li L,
Zhong Y, Ding X, Xiang S and Zhang J: Direct regulation of caspase3
by the transcription factor AP2α is involved in aspirininduced
apoptosis in MDAMB453 breast cancer cells. Mol Med Rep. 7:909–914.
2013. View Article : Google Scholar : PubMed/NCBI
|