Introduction
High-dose chemotherapy along with autologous
hematopoietic stem cell transplantation (HSCT) is a widely used
effective therapeutic option for patients with non-Hodgkin lymphoma
(NHL) and multiple myeloma (MM). HSCT has greatly evolved from its
early days where the only source for the harvesting of
hematopoietic stem cells (HSCs) was the bone marrow. A paradigm
shift in HSC collection has been made possible with the use of
granulocyte-colony stimulating factor (G-CSF) to increase the
number of circulating CD34+ cells and improvements in
collection devices, which allows for HSC collection in fewer
apheresis sessions (1,2).
Successful HSCT is largely dependent on the adequate
collection of CD34+ cells. The American Society for
Blood and Marrow Transplantation recommends 4–5×106
CD34+ cells/kg as the optimal number and
≥2×106 CD34+ cells/kg as the minimum number
of HSCs to support transplantation (3). A large number of cases, however, are
hard-to-mobilize or require multiple mobilization attempts for
successful HSCT. The number of patients who are ‘difficult
mobilizers’ varies considerably, with an incidence ranging from 5
to 10% to approximately 30% (2,4,5). In general, greater mobilization issues
have been found in patients with NHL compared to those suffering
from MM (5).
Plerixafor, a novel bicyclam small-molecule, was
initially developed for managing HIV infections. It reversibly
binds to chemokine receptor CXCR4 and antagonizes the chemokine
stromal cell-derived factor-1α (SDF-1α) interaction, thereby
inducing the mobilization of stem cells into the bloodstream from
the bone marrow (6,7). It has been approved in the US and EU
for the collection of HSCs and autologous transplantation in
patients with NHL and MM, when used in combination with G-CSF
(8). A number of literature reviews
describing the role of plerixafor in HSC mobilization have been
published (2,9,10).
However, to date, and at least to the best of our knowledge,
level-1 evidence in the form of a systematic review and
meta-analysis has been conducted only once (11). The published review analyses data
from the first 2 randomized controlled trials (RCTs) were conducted
by DiPersio et al in 2009 (12,13).
With the addition of new studies (14,15) from
different centers on the efficacy of plerixafor for HSCT, there is
a need for an updated meta-analysis. Therefore, the aim of this
study was to systematically search the published literature and
analyze evidence on the efficacy of additional plerixafor for
successful HSC mobilization in patients with NHL and MM, and to
evaluate the safety of the drug.
Data and methods
This systematic review of the literature was
conducted in line with the Preferred Reporting Items for Systematic
Reviews and Meta-analyses (PRISMA) statement (16) and guidelines of the Cochrane Handbook
for Systematic Reviews of Intervention (17).
Eligibility criteria
We included studies conducted on patients with NHL
or MM, in first or second complete or partial remission, who were
eligible for autologous HSCT and had not undergone any prior failed
mobilization or HSCT. Study intervention was the use of additional
plerixafor for HSC mobilization compared to placebo or no
additional therapy. The outcome of the trial was to report the
number of patients achieving optimal HSC mobilization, the time
required to achieve optimal HSC mobilization and adverse events.
Non-English language studies, studies on healthy volunteers,
uncontrolled and non-randomized studies were excluded.
Search strategy
We searched the PubMed, Scopus, Cochrane Central
Register of Controlled Trials (CENTRAL) and Google scholar
databases (first 100 results) electronically for articles published
up to March, 2019. The key words used in various combinations were
as follows: Lymphoma [MeSH], multiple myeloma [MeSH], non-Hodgkin
lymphoma [MeSH], plerixafor [MeSH], plerixafor hydrochloride
[MeSH], granulocyte-colony stimulating factor [MeSH], filgrastim
[MeSH], placebo effect [MeSH], adult stem cells [MeSH],
hematopoietic stem cell [MeSH], hematopoietic stem cell
mobilization [MeSH] and CD34+ cells [Free text]. Study
designs searched were RCTs. Studies not utilizing a placebo drug in
the control group were also included. Previous meta-analyses and
review articles were analyzed for the identification of any
additional studies.
Data collection and analysis
Potentially eligible studies were evaluated
separately by two reviewers based on the inclusion and exclusion
criteria. Following the removal of duplicates, studies were
scrutinized by their titles and abstracts. The full-texts of
selected articles were then scanned for inclusion in the review.
Any difference in opinion was resolved by discussion. We extracted
the following data from the included trials: Authors, publication
year, inclusion/exclusion criteria, sample size, demographic data,
plerixafor and G-CSF protocol, apheresis protocol, outcomes
assessed and adverse events. The primary objective was to perform
the quantitative analysis of successful optimal HSC mobilization.
The secondary objectives were the following: The analysis of number
of patients achieving minimal HSC mobilization, the quantitative
analysis of the time required to achieve optimal and minimal HSC
mobilization, the number of CD34+ cells collected, the
number of patients subsequently transplanted and adverse
events.
Risk of bias in individual
studies
We assessed the risk of bias in each trial using the
Cochrane Collaboration risk assessment tool for RCTs (18). Seven criteria were evaluated for each
study: Random sequence generation, allocation concealment, blinding
of participants and personnel, blinding of outcome assessment,
incomplete outcome data, selective outcome reporting and other
biases. Studies were scored for each criteria as follows: Low risk
(score of 2), high risk (score of 0), or unclear risk of bias
(score of 1). Based on the scores awarded, individual studies were
grouped as low-(score 0–5), medium-(score 6–10), or high-(score
11–14) quality trials.
Statistical analysis
Meta-analysis was carried out only if at least 3
trials reported data on the same scale. The outcome data extracted
was entered into Review Manager [RevMan, version 5.3; Nordic
Cochrane Centre (Cochrane Collaboration)], Copenhagen, Denmark;
2014) for quantitative analysis. We used Intention to treat data
from the trials for the purpose of analysis. Considering the
heterogeneity amongst studies, a random-effects model was used to
calculate the pooled effect size. Heterogeneity was calculated
using the I2 statistic. I2 values of 25–50%
represented as low, values of 50–75% as medium and >75% were
represented as substantial heterogeneity. For binary outcomes, risk
ratios (RR) with 95% confidence intervals (CI) were calculated. The
mean and standard deviation (SD) scores of the number of
CD34+ cells collected were used for the
meta-analysis.
Results
Search outcome
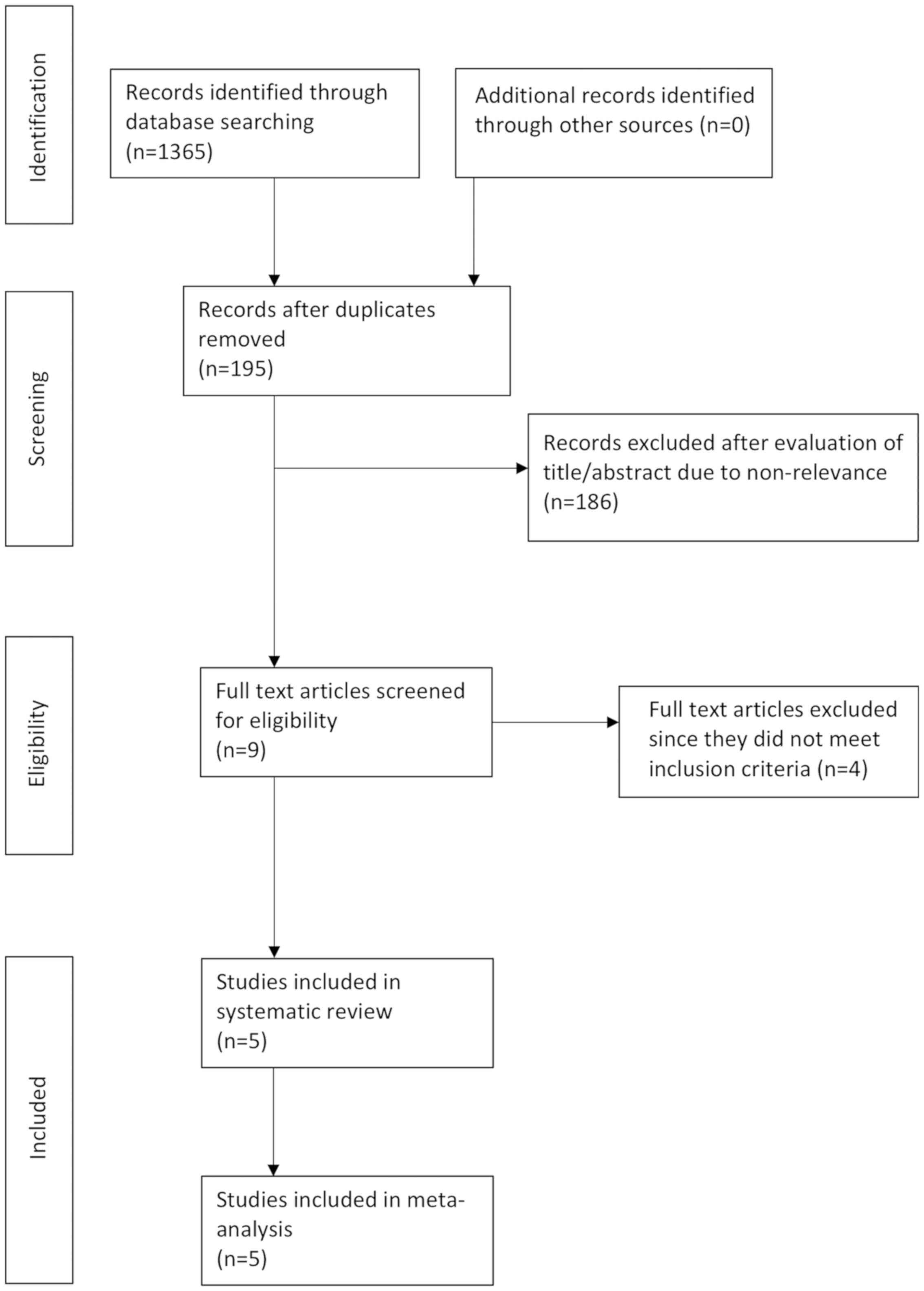
The search outcome of the review is presented in
Fig. 1. A total of 195 articles were
examined by their abstracts. In total, 186 studies were excluded
due to non-relevance, leaving a total of 9 articles (12–15,19–23).
Four studies were excluded from the review, as 2 were
non-randomized (22,23), 1 had no control group (20) and 1 used a retrospective control
group (21). A total of 5 trials
were included in this systematic review and meta-analysis (12–15,19).
Characteristics of included studies
and data analysis
Details of the included studies are presented in
Table I. Three studies (12–14) were
multi-center studies, while 2 were single-center trials (15,19).
Three studies (13–15) were carried out on NHL, while 2 trials
(12,19) were on patients with MM. Two trials [1
on NHL (15) and 1 on MM (19)] did not administer any placebo to the
control group. The G-CSF protocol was standard across the studies,
which consisted of subcutaneous (SC) injections in the morning for
8 days. Similarly, plerixafor was injected by SC injection in the
evening, beginning on day 4 in all studies for up to 4 days and
apheresis was carried out from the morning of day 5 and continued
daily for up to 4 days or till the targeted HSC collection was
reached. In the studies on NHL, the optimal HSC collection was
≥5×106 CD34+ cells/kg, while in the MM
studies, it was ≥6×106 CD34+ cells/kg.
 | Table I.Characteristics of included
studies. |
Table I.
Characteristics of included
studies.
|
|
| No. of
patients | Age (range/mean ±
SD) | Sex: No. of
patients | Remission status
(n) |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Authors/(Refs.),
year | Diagnosis | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | G-CSF protocol | Plerixafor
protocol | Apheresis
protocol |
|---|
| DiPersio et
al (13), | NHL | 150 | 148 | 29–75 | 22–75 | M: 100 | M: 102 | 1st CR: 51 | 1st CR: 44 | 10 µg/kg SC
daily | Beginning on day
4, | Apheresis began
on |
| 2009 |
|
|
|
|
|
|
| 2nd CR: 30 | 2nd CR: 29 | in the morning | patients received
either | the morning of day
5 |
|
|
|
|
|
|
|
|
| 1st PR: 26 | 1st PR: 19 | for up to 8
days | plerixafor 240
µg/kg or | and continued
daily |
|
|
|
|
|
|
|
|
| 2nd PR: 43 | 2nd PR: 54 |
| placebo SC daily
in | for up to 4 days
or |
|
|
|
|
|
|
|
|
|
|
|
| the evening for up
to | until
≥5×106 CD34+ |
|
|
|
|
|
|
|
|
|
|
|
| 4 days or until
≥5×106 | cells/kg were
collected |
|
|
|
|
|
|
|
|
|
|
|
| CD34+
cells/kg were collected |
|
| DiPersio et
al (12), | MM | 148 | 154 | 58.2±8.4 | 58.4±8.6 | M:100 | M:107 | 1st CR: 11 | 1st CR: 18 | 10 µg/kg SC
daily | Beginning on day
4, | Apheresis began
on |
| 2009 |
|
|
|
|
|
|
| 2nd CR: 129 | 2nd CR: 126 | in the morning | patients received
either | the morning of day
5 |
|
|
|
|
|
|
|
|
| 1st PR: 0 | 1st PR: 0 | for up to 8
days | plerixafor 240
µg/kg or | and continued
daily |
|
|
|
|
|
|
|
|
| 2nd PR: 8 | 2nd PR: 10 |
| placebo SC daily
in | for up to 4 days
or |
|
|
|
|
|
|
|
|
|
|
|
| the evening for up
to | until
≥6×106 CD34+ |
|
|
|
|
|
|
|
|
|
|
|
| 4 days or until
≥6×106 | cells/kg were |
|
|
|
|
|
|
|
|
|
|
|
| CD34+
cells/kg were collected | collected |
| Ri et al
(19), | MM | 7 | 7 | 38–71 | 49–67 | M:4 | M:4 | 1st CR: 1 | 1st CR: 0 | 400
μg/m2/day SC | Beginning on day
4, | Apheresis began
on |
| 2017 |
|
|
|
|
|
|
| 2nd CR: - | 2nd CR: - | daily in the
morning | patients in
treatment | the morning of day
5 |
|
|
|
|
|
|
|
|
| 1st PR: 6 | 1st PR: 7 | for up to 8
days | group received
plerixafor | and continued
daily |
|
|
|
|
|
|
|
|
| 2nd PR: - | 2nd PR: - |
| 240 µg/kg SC daily
in | for up to 4 days
or |
|
|
|
|
|
|
|
|
|
|
|
| the evening for up
to | until
≥6×106 CD34+ |
|
|
|
|
|
|
|
|
|
|
|
| 4 days or until
≥6×106 | cells/kg were
collected |
|
|
|
|
|
|
|
|
|
|
|
| CD34+
cells/kg were |
|
| Zhu et al
(14), | NHL | 50 | 50 | 18–66 | 20–60 | M:31 | M:26 | 1st CR: 21 | 1st CR: 22 | 10 µg/kg SC
daily | Beginning on day
4, | Apheresis began
on |
| 2017 |
|
|
|
|
|
|
| 2nd CR: 10 | 2nd CR: 10 | in the morning | patients received
either | the morning of day
5 |
|
|
|
|
|
|
|
|
| 1st PR: 13 | 1st PR: 13 | for up to 8
days | plerixafor 240
µg/kg or | and continued
daily |
|
|
|
|
|
|
|
|
| 2nd PR: 5 | 2nd PR: 5 |
| placebo SC daily
in | for up to 4 days
or |
|
|
|
|
|
|
|
|
|
|
|
| the evening for up
to | until
≥5×106 CD34+ |
|
|
|
|
|
|
|
|
|
|
|
| 4 days or until
≥5×106 | cells/kg were |
|
|
|
|
|
|
|
|
|
|
|
| CD34+
cells/kg were collected | collected |
| Matsue et al
(15), | NHL | 16 | 16 | 39–73 | 27–70 | M:11 | M:12 | 1st CR: 3 | 1st CR: 10 | 400
μg/m2/day SC | Beginning on day
4, | Apheresis began
on |
| 2018 |
|
|
|
|
|
|
| 2nd CR: 7 | 2nd CR: 1 | daily in the
morning | patients in
treatment | the morning of day
5 |
|
|
|
|
|
|
|
|
| 1st PR: 4 | 1st PR: 3 | for up to 8
days | group received
plerixafor | and continued
daily |
|
|
|
|
|
|
|
|
| 2nd PR: 2 | 2nd PR: 2 |
| 240 µg/kg SC daily
in | for up to 4 days
or |
|
|
|
|
|
|
|
|
|
|
|
| the evening for up
to | until ≥5×106
CD34+ |
|
|
|
|
|
|
|
|
|
|
|
| 4 days or until
≥5×106 | cells/kg were |
|
|
|
|
|
|
|
|
|
|
|
| CD34+ cells/kg
were | collected |
|
|
|
|
|
|
|
|
|
|
|
| collected |
|
Data on successful optimal HSC mobilization i.e.,
the collection of ≥5×106 CD34+ cells/kg or
≥6×106 CD34+ cells/kg in ≤4 apheresis days
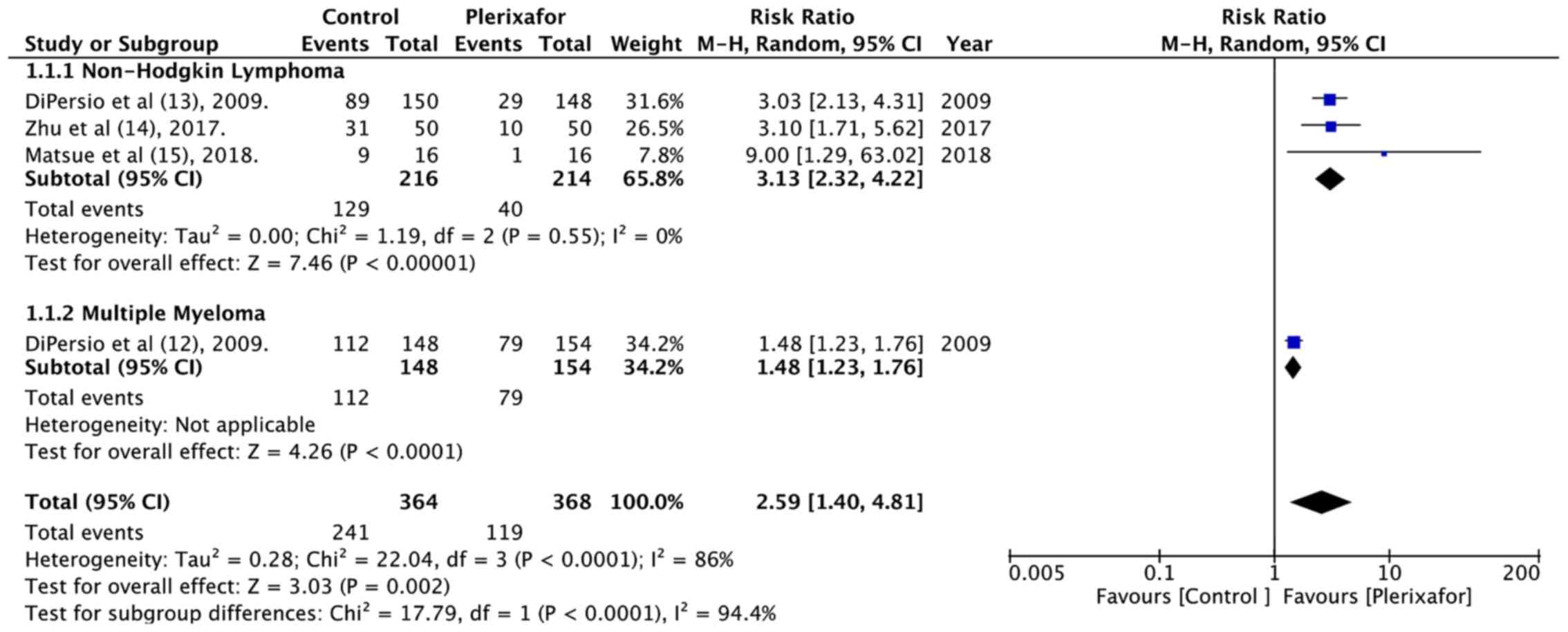
was available from 4 included studies (Table II). From the pooling of the data of
364 patients in the treatment group and 368 patients in the control
group, it was found that patients randomized to plerixafor had
better successful HSC mobilization than those in the control group
(RR=2.59, 95% CI: 1.40 to 4.81; P<0.0001; Fig. 2). There was significant heterogeneity
(I2=86%), which could be attributed to different
underlying conditions or a different targeted stem cell number.
Subgroup analysis based on diagnosis demonstrated a statistically
significant improvement in optimal HSC collection in both the NHL
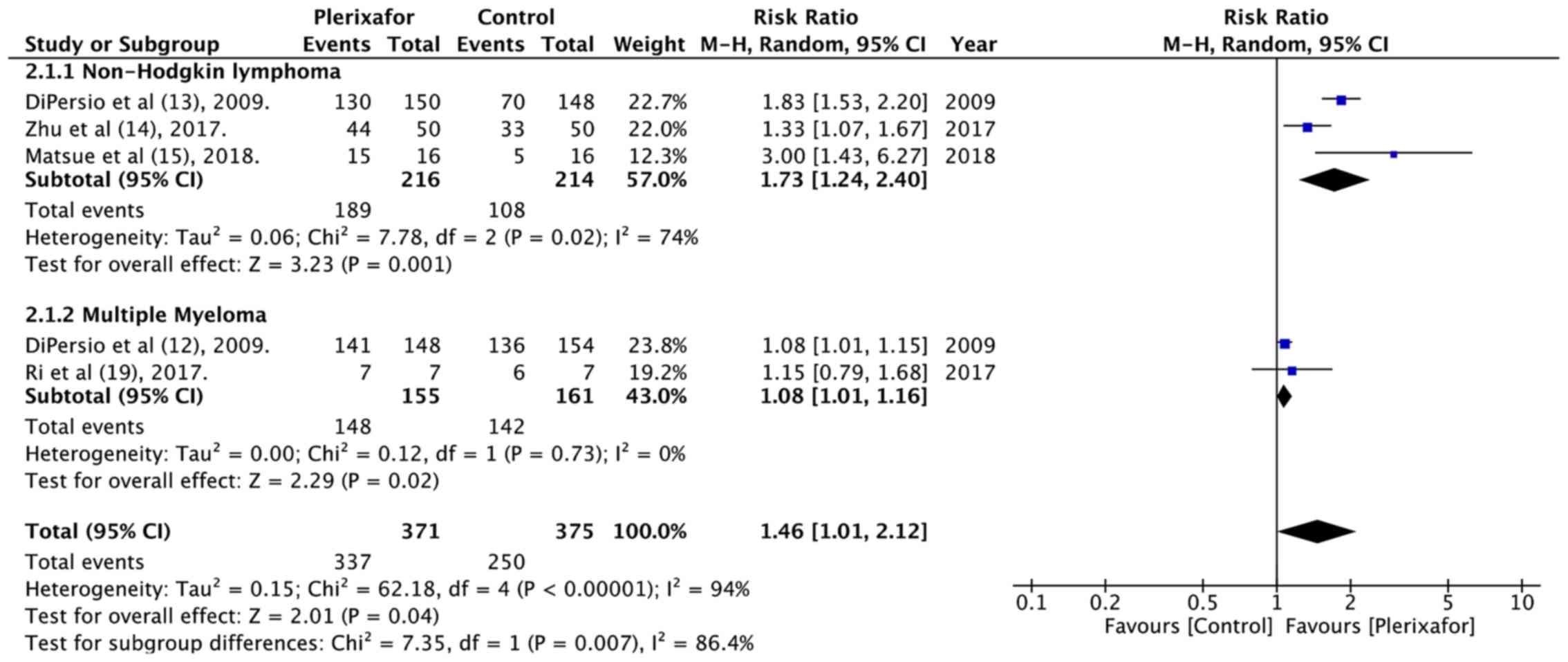
and MM subgroup. Data on successful minimal HSC mobilization i.e.,
the collection of ≥2×106 CD34+ cells/kg in ≤4
apheresis days of 371 patients in the treatment group and 375
patients in the control group was also pooled for analysis. The
results indicated that patients in the plerixafor group had better
minimal HSC mobilization than those in the control group (RR=1.46,
95% CI: 1.01 to 2.12; P=0.04; I2=94%; Fig. 3). Subgroup analysis based on
diagnosis indicated a statistically significant difference in both
the NHL and MM subgroups.
 | Table II.Outcome assessment by included
studies. |
Table II.
Outcome assessment by included
studies.
|
| No. of patients
able to mobilize ≥5/6×106 CD34+ cells/kg in
≤4 apheresis days | No. of patients
able to mobilize ≥2×106 CD34+ cells/kg in ≤4
apheresis days | Median time (days)
required to reach ≥5/6×106 CD34+
cells/kg | Median time (days)
required to reach ≥2×106 CD34+ cells/kg | No. of
CD34+ cells/kg collected in 4 days of apheresis (mean ±
SD) | No. of patients who
underwent auto-HSCT | No. of adverse
events (n/N) |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Authors/(Refs.),year | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control |
|---|
| DiPersio et
al (13), 2009 | 89 | 29 | 130 | 70 | VNR | VNR | VNR | VNR | 5.69 (±NR)
×106 | 1.98 (±NR)
×106 | 105 | 82 | 146/150 | 138/145 |
| DiPersio et
al (12), 2009 | 112 | 79 | 141 | 136 | 1 | 4 | NS | NS | 12.97
(±10.67) ×106 | 7.31
(±5.49) ×106 | 142 | 136 | 140/147 | 140/151 |
| Ri et al
(19), 2017 | 5a | 0a | 7 | 6 | 2 | Not Reached | 1 | 2 | 7.55
(±2.32) ×106 | 3.67
(±1.25) ×106 | NS | NS | 6/7 | 4/7 |
| Zhu et al
(14), 2017 | 31 | 10 | 44 | 33 | 2 | Not Reached | 1 | 2 | NS | NS | 44 | 34 | 32/51 | 31/49 |
| Matsue et al
(15), 2018 | 9 | 1 | 15 | 5 | 3.5 | NR | 1 | VNR | 5.45
(±2.55) ×106 | 2.09
(±1.69) ×106 | NS | NS | 13/16 | 12/16 |
The number of days required to reach optimal
(≥5/6×106 CD34+ cells/kg) or minimal
(≥2×106 CD34+ cells/kg) HSC mobilization
reported by the studies is presented in Table II. Since only median values were
available, data could not be pooled for a quantitative analysis.
The qualitative assessment of results from all 5 studies indicated
that the addition of plerixafor significantly reduced the number of
days required for the collection of optimal HSCs. Similarly, 4
(13,14,15,19) of
the 5 included trials, which studied median time (days) required to
reach ≥2×106 CD34+ cells/kg, concluded that
the number of days required for HSC mobilization to be less with
plerixafor.
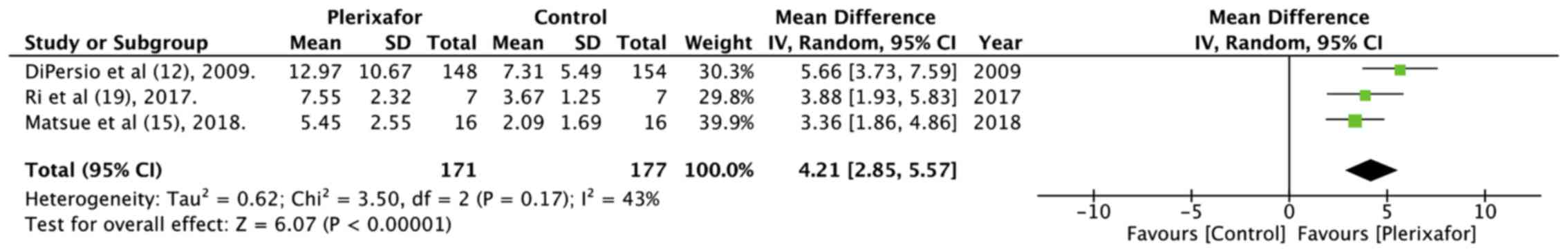
The mean total number of CD34+ cells
collected in up to 4 apheresis days was studied by 4 trials
(12,13,15,19)
(Table II). The data pooled from 3
studies (12,15,19)
indicated that the addition of plerixafor significantly increased
the total collection of CD34+ cells (random: MD=4.21;
95% CI: 2.85 to 5.57; P<0.00001; I2=43%; Fig. 4). In addition, a significantly
greater number of patients randomized to plerixafor eventually
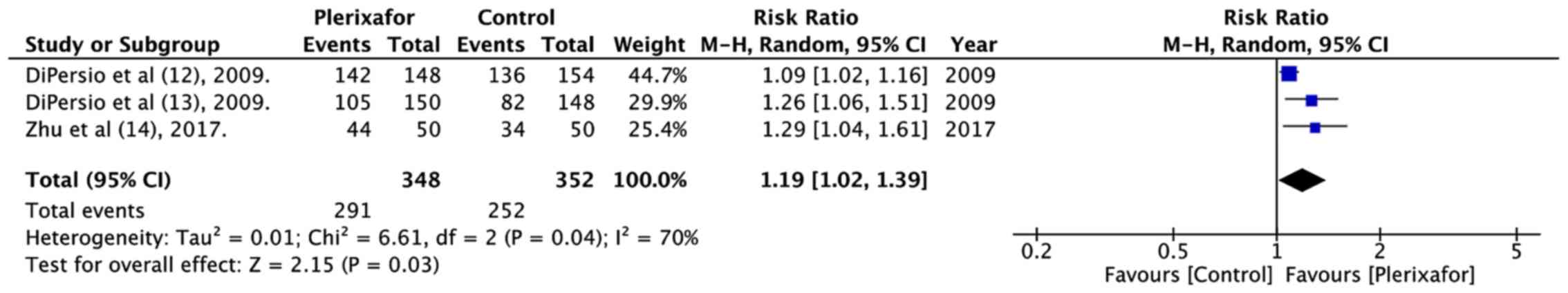
underwent HSCT (RR=1.19, 95% CI: 1.02 to 1.39; P=0.03;
I2=70%; Fig. 5).
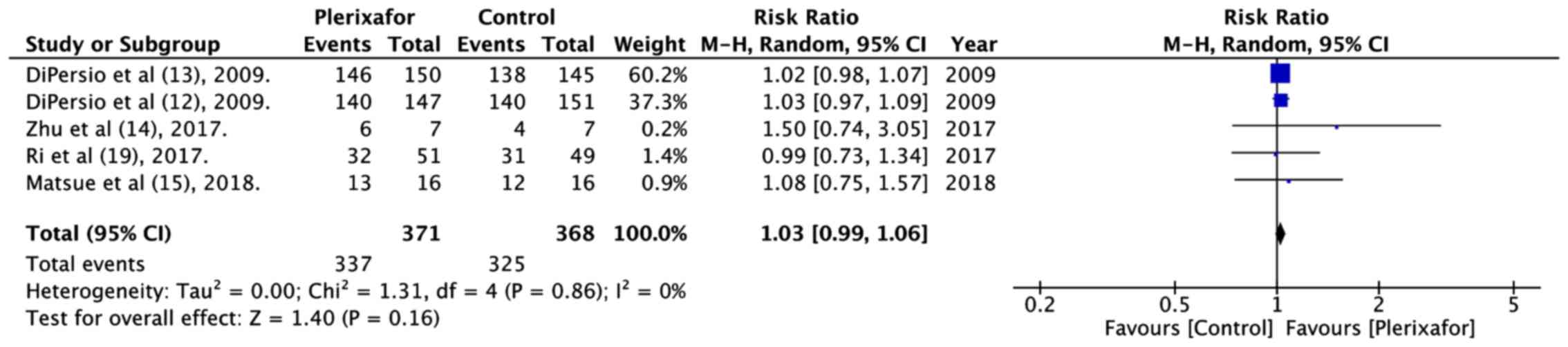
The numbers of patients experiencing at least 1 or
more treatment related adverse events in both groups were pooled.
The meta-analysis of 371 patients in the plerixafor group and 368
patients in the control group indicated no significant increase in
adverse events with the addition of plerixafor (RR=1.03, 95% CI:
0.99 to 1.06; P=0.16; I2=0%; Fig. 6).
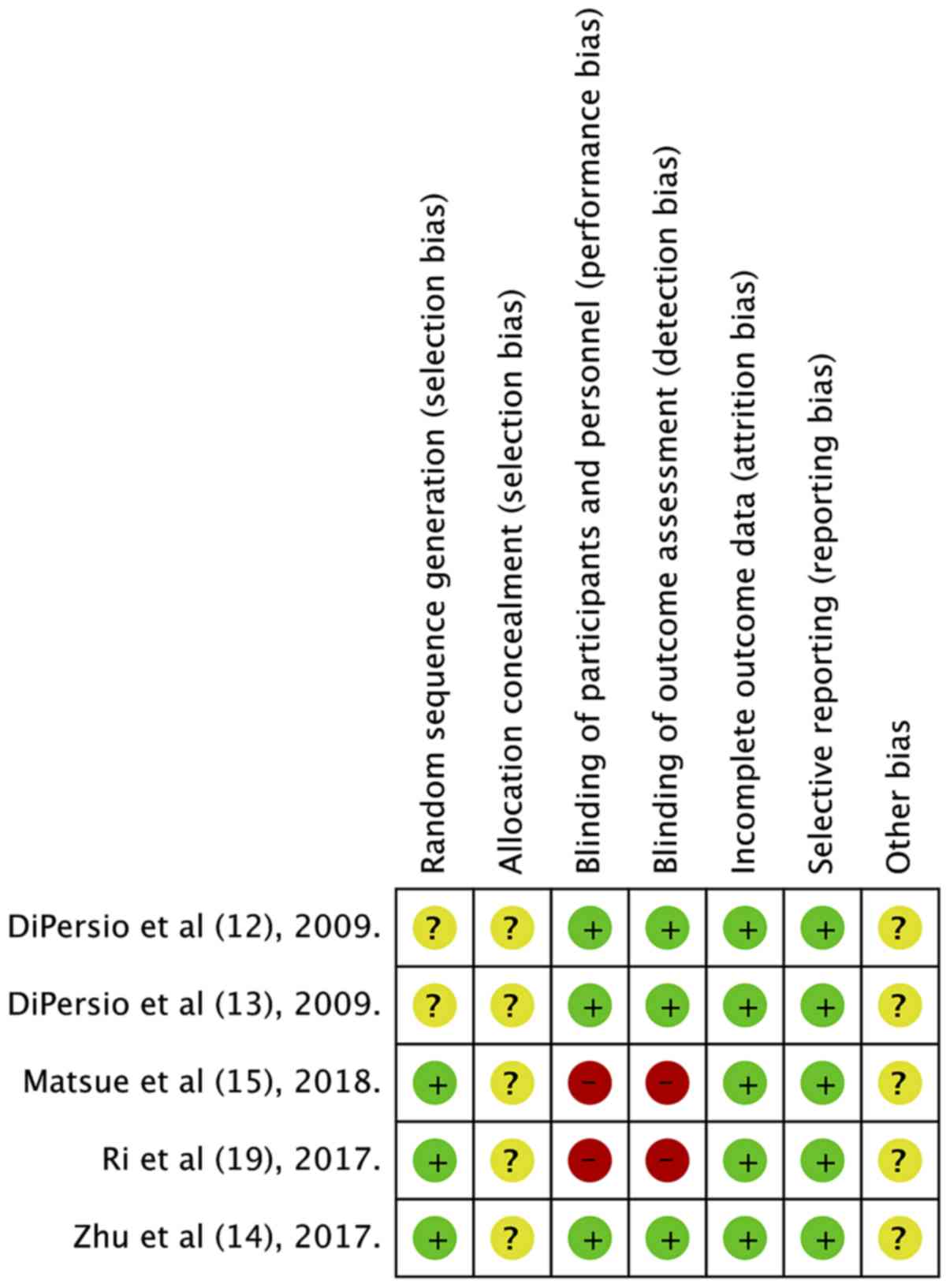
Quality of included studies
The risk of bias summary of the included studies is
presented in Fig. 7. The
randomization method was not described in 2 studies (12,13) and
2 studies were not double blind (15,19).
Since all studies had financial support from pharmaceutical
companies, they were marked with ‘unclear risk’ for other sources
of bias. The overall quality of the studies was rated ‘high’ for 3
trials (12–14) and ‘medium’ for 2 trials (15,19).
Discussion
In 2003, AMD3100, as plerixafor was formerly known,
was studied by Liles et al (24) in healthy volunteers as a potential
HSC mobilizer. The authors reported rapid generalized leukocytosis
and an increase in the number of CD34+ cells in
peripheral blood with a single dose of the drug. Later, in their
study, serial administration of the drug at 80 µg/kg/day for 3 days
resulted in a consistent, reversible increase in the number of
CD34+ cells in peripheral blood with minimal toxicity.
Their findings suggested the potential clinical application of
plerixafor for HSCT. Their results were soon confirmed in patients
with NHL and MM, where a significant increase in the
CD34+ counts was noted (25). Phase 2 trials later established the
superiority of plerixafor + G-CSF as compared to G-CSF alone for
autologous HSC mobilization (26).
Superior evidence in the form of RCTs by DiPersio et al
(12,13) paved the way for the approval of
plerixafor by FDA in December, 2008 (27). The drug was ratified for use in
patients with NHL and MM for the mobilization of HSCs for
autologous transplantation. Since then, a number of studies have
evaluated the role of plerixafor in patients with NHL and MM.
Single arm prospective trials or retrospective studies form the
bulk of the research conducted (28–30). The
results of our systematic review indicated that 5 RCTs evaluating
the role of additional plerixafor to G-CSF have been published to
date. Three of these were conducted on NHL (13–15) and
2 on patients with MM (12,19). The study end-points were more or less
similar across trials which enabled the pooling of the data for the
purposes of our meta-analysis.
The minimum number of CD34+ cells likely
to result in successful engraftment is generally considered to be
≥2×106 cells/kg, whereas the ‘optimal’ number of HSCs
for transplantation is 4–6×106 CD34+ cells/kg
(31). It has been found that the
re-infusion of higher number of CD34+ cells is
associated with earlier engraftment following transplantation, with
better disease-free and overall survival than lower cell doses
(32). As a result, many transplant
centers strive to harvest the optimal number of HSCs rather than
the minimal dose. The primary efficacy end-point in the majority of
the included trials of this review was the collection of optimal
HSCs in 4 or less apheresis days. The results of our meta-analysis
revealed that patients receiving additional plerixafor were
2.59-fold more likely to achieve optimal HSC collection in 4 or
less apheresis days. Similarly, patients in the plerixafor group
were 1.46-fold more likely to mobilize minimal HSCs
(≥2×106 cells/kg in 4 or less apheresis days) as
compared to those receiving G-CSF only. There was slight variation
in the primary efficacy end-point in NHL and MM studies, with NHL
studies reporting the mobilization of ≥5×106 cells/kg,
while MM studies reporting the mobilization of ≥6×106
cells/kg as the optimal number of HSCs for harvest. A higher
primary endpoint is set for the collection of HSCs in patients with
MM as they frequently require a second transplantation. A subgroup
analysis was therefore carried out for NHL and MM, which
demonstrated the effectiveness of plerixafor in both conditions. An
important aspect to note is that only one study (12) was available evaluating the collection
of optimal HSCs in 4 or less apheresis days for MM. The study by Ri
et al (19) reported the
collection of ≥6×106 cells/kg in 2 or less apheresis
days and was not pooled with the other study of DiPersio et
al (12) for this variable.
In addition to the optimal collection of HSCs,
another critical goal of stem cell mobilization is to collect cells
in minimum number of days so as to reduce treatment stress and
financial burden of the patient and also ensure optimal utilization
of medical resources. Despite the fact that pooled analysis was not
possible for the number of days needed for HSC mobilization with or
without plerixafor, all studies included in this review reported
the rapid mobilization with the drug. The median number of days for
both optimal and minimal HSC mobilization was less with the
addition of plerixafor. The more rapid collection of HSCs may lead
to better patient compliance, reduce apheresis-associated risks and
may shorten the interval between mobilization and transplant
(15). The superior efficacy of
plerixafor + G-CSF was also observed in the total number of
CD34+ cells collected and the number of patients who
eventually underwent HSCT.
The safety profile of plerixafor was tested in all
included studies. Based on our analysis and the investigation of
individual trials, plerixafor is generally well-tolerated and there
is no significant increase in treatment-related adverse events with
the addition of the drug. In both the trials of DiPersio et
al (12,13), the majority of adverse events were
gastrointestinal (GI) disorders or injection site reactions. Ri
et al (19) reported
increased headaches, diarrhea and back pain with the addition of
plerixafor. Zhu et al (14)
and Matsue et al (15)
reported GI-related disorders and headaches to be more common in
the plerixafor arm than the control arm. All adverse events
reported were mild to moderate and there were no treatment-related
deaths reported in any of the included studies.
As plerixafor tends to mobilize relatively different
cell populations as compared to other methods of mobilizing
CD34+ cells, post-transplant outcomes, such as
hematopoietic and immune recovery and progression-free survival
(PFS) are important variables of concern to clinicians (2). In total, 2 of the 5 included trials
reported that engraftment and short-term outcomes at 12 months were
similar with plerixafor + G-CSF and plerixafor + placebo.
Importantly, the long-term results of these 2 studies have been
published recently (33). The
results indicated that the probability of overall survival and PFS
evaluated over a period of 5 years did not differ significantly in
patients with NHL or MM treated with plerixafor or the placebo.
There is however, a need for more such long-term studies in order
to validate the safety and efficacy of plerixafor for long-term
PFS.
Some limitations of our review and meta-analysis
need to be elaborated. Firstly, the paucity of RCTs limited the
number of studies for inclusion in our review. Secondly, not all
trials included were high quality studies. In total, 2 of the 5
included studies were sored as ‘medium quality’ based on the
quality assessment tool. Thirdly, data on engraftment, long-term
overall survival and PFS were not available from all included
studies. Fourthly, as with any meta-analysis, heterogeneity as
measured with I2 statistic was significant in all our
investigations. The time period, locations and samples of
individual trials are quite variable across studies, but are
usually balanced between the two arms of the meta-analysis.
Nevertheless, our systematic review and meta-analysis is a
significant update from the last review published on the subject by
Hartmann et al in 2015 (11).
Data from a 3 additional trials were available and added for
quantitative analysis. The results of this study indicate that
addition of plerixafor to G-CSF leads to increased HSC collection
in a shorter period of time with no concomitant increase in adverse
events. Further RCTs with a larger sample size evaluating
short-term efficacy, as well as long-term survival would help to
further strengthen evidence on this subject.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Funding Project (grant no. 8187014).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY conceived and designed the study. MW, FY and ZW
collected the data and performed the literature search. All authors
were involved in writing the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoggatt J, Speth JM and Pelus LM: Concise
review: Sowing the seeds of a fruitful harvest: hematopoietic stem
cell mobilization. Stem Cells. 31:2599–2606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jantunen E, Varmavuo V and Valtola J:
Plerixafor injection: A hematopoietic stem cell mobilizer in
non-Hodgkin lymphoma and multiple myeloma. Expert Rev Hematol.
9:723–732. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duong HK, Savani BN, Copelan E, Devine S,
Costa LJ, Wingard JR, Shaughnessy P, Majhail N, Perales MA, Cutler
CS, et al: Peripheral blood progenitor cell mobilization for
autologous and allogeneic hematopoietic cell transplantation:
Guidelines from the American Society for Blood and Marrow
Transplantation. Biol Blood Marrow Transplant. 20:1262–1273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hosing C, Saliba RM, Ahlawat S, Körbling
M, Kebriaei P, Alousi A, De Lima M, Okoroji JG, McMannis J,
Qazilbash M, et al: Poor hematopoietic stem cell mobilizers: A
single institution study of incidence and risk factors in patients
with recurrent or relapsed lymphoma. Am J Hematol. 84:335–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pusic I, Jiang SY, Landua S, Uy GL, Rettig
MP, Cashen AF, Westervelt P, Vij R, Abboud CN, Stockerl-Goldstein
KE, et al: Impact of mobilization and remobilization strategies on
achieving sufficient stem cell yields for autologous
transplantation. Biol Blood Marrow Transplant. 14:1045–1056. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kessans MR, Gatesman ML and Kockler DR:
Plerixafor: A peripheral blood stem cell mobilizer.
Pharmacotherapy. 30:485–492. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hübel K, Liles WC, Broxmeyer HE, Rodger E,
Wood B, Cooper S, Hangoc G, Macfarland R, Bridger GJ, Henson GW, et
al: Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor
Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther.
1:165–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell N, Douglas K, Ho AD, Mohty M,
Carlson K, Ossenkoppele GJ, Milone G, Pareja MO, Shaheen D,
Willemsen A, et al: Plerixafor and granulocyte colony-stimulating
factor for first-line steady-state autologous peripheral blood stem
cell mobilization in lymphoma and multiple myeloma: Results of the
prospective PREDICT trial. Haematologica. 98:172–178. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilgin YM and de Greef GE: Plerixafor for
stem cell mobilization: The current status. Curr Opin Hematol.
23:67–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Clercq E: Mozobil®
(Plerixafor, AMD3100), 10 years after its approval by the US Food
and Drug Administration. Antivir Chem Chemother.
27:2040206619829382. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hartmann T, Hübel K, Monsef I, Engert A
and Skoetz N: Additional plerixafor to granulocyte
colony-stimulating factors for haematopoietic stem cell
mobilisation for autologous transplantation in people with
malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev.
20:CD0106152015.
|
|
12
|
DiPersio JF, Stadtmauer EA, Nademanee A,
Micallef IN, Stiff PJ, Kaufman JL, Maziarz RT, Hosing C, Früehauf
S, Horwitz M, et al 3102 Investigators, : Plerixafor and G-CSF
versus placebo and G-CSF to mobilize hematopoietic stem cells for
autologous stem cell transplantation in patients with multiple
myeloma. Blood. 113:5720–5726. 2009.PubMed/NCBI
|
|
13
|
DiPersio JF, Micallef IN, Stiff PJ,
Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger
G and Calandra G; 3101 Investigators, : Phase III prospective
randomized double-blind placebo-controlled trial of plerixafor plus
granulocyte colony-stimulating factor compared with placebo plus
granulocyte colony-stimulating factor for autologous stem-cell
mobilization and transplantation for patients with non-Hodgkin's
lymphoma. J Clin Oncol. 27:4767–4773. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu J, Huang H, Chen H, Zhang X, Li Z, Wu
D, Zhou D, Song Y, Hu Y, Liang Y, et al: Plerixafor and
granulocyte-colony-stimulating factor for mobilization of
hematopoietic stem cells for autologous transplantation in Chinese
patients with non-Hodgkin's lymphoma: A randomized Phase 3 study.
Transfusion. 58:81–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsue K, Kumagai K, Sugiura I, Ishikawa
T, Igarashi T, Sato T, Uchiyama M, Miyamoto T, Ono T, Ueda Y, et
al: Plerixafor for mobilization and collection of haematopoietic
stem cells for autologous transplantation in Japanese patients with
non-Hodgkin lymphoma: A randomized phase 2 study. Int J Hematol.
108:524–534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and meta-analyses: The PRISMA statement. PLoS Med.
6:e10000972009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higgins J and Green S: Cochrane Handbook
for Systemic Reviews of Interventions. Version 5. The Cochrane
Collaboration. 2011
|
|
18
|
Higgins J, Altman D and Sterne J; Cochrane
Statistical Methods Group and the Cochrane Bias Methods Group, :
Chapter 8: assessing risk of bias in included studies. Cochrane
Handbook for Systemic Reviews of Interventions, Version 5. The
Cochrane Collaboration. 2011
|
|
19
|
Ri M, Matsue K, Sunami K, Shimazaki C,
Hayashi A, Sunaga Y, Sasaki T and Suzuki K: Efficacy and safety of
plerixafor for the mobilization/collection of peripheral
hematopoietic stem cells for autologous transplantation in Japanese
patients with multiple myeloma. Int J Hematol. 106:562–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dugan MJ, Maziarz RT, Bensinger WI,
Nademanee A, Liesveld J, Badel K, Dehner C, Gibney C, Bridger G and
Calandra G: Safety and preliminary efficacy of plerixafor (Mozobil)
in combination with chemotherapy and G-CSF: An open-label,
multicenter, exploratory trial in patients with multiple myeloma
and non-Hodgkin's lymphoma undergoing stem cell mobilization. Bone
Marrow Transplant. 45:39–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin AP, Richards S, Haycox A, Houten R,
McLeod C, Braithwaite B, Clark JO, Bell J and Clark RE: Evaluating
the use of plerixafor in stem cell mobilisation - an economic
analysis of the PHANTASTIC trial. J Clin Apher. 31:434–442. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Haverkos BM, Huang Y, Elder P, O'Donnell
L, Scholl D, Whittaker B, Vasu S, Penza S, Andritsos LA, Devine SM,
et al: A single center's experience using four different front line
mobilization strategies in lymphoma patients planned to undergo
autologous hematopoietic cell transplantation. Bone Marrow
Transplant. 52:561–566. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Partanen A, Valtola J, Ropponen A, Vasala
K, Penttilä K, Ågren L, Pyörälä M, Nousiainen T, Selander T,
Mäntymaa P, et al: Preemptive plerixafor injection added to
pegfilgrastim after chemotherapy in non-Hodgkin lymphoma patients
mobilizing poorly. Ann Hematol. 96:1897–1906. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liles WC, Broxmeyer HE, Rodger E, Wood B,
Hübel K, Cooper S, Hangoc G, Bridger GJ, Henson GW, Calandra G, et
al: Mobilization of hematopoietic progenitor cells in healthy
volunteers by AMD3100, a CXCR4 antagonist. Blood. 102:2728–2730.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Devine SM, Flomenberg N, Vesole DH,
Liesveld J, Weisdorf D, Badel K, Calandra G and DiPersio JF: Rapid
mobilization of CD34+ cells following administration of the CXCR4
antagonist AMD3100 to patients with multiple myeloma and
non-Hodgkin's lymphoma. J Clin Oncol. 22:1095–1102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flomenberg N, Devine SM, Dipersio JF,
Liesveld JL, McCarty JM, Rowley SD, Vesole DH, Badel K and Calandra
G: The use of AMD3100 plus G-CSF for autologous hematopoietic
progenitor cell mobilization is superior to G-CSF alone. Blood.
106:1867–1874. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brave M, Farrell A, Ching Lin S, Ocheltree
T, Pope Miksinski S, Lee SL, Saber H, Fourie J, Tornoe C, Booth B,
et al: FDA review summary: Mozobil in combination with granulocyte
colony-stimulating factor to mobilize hematopoietic stem cells to
the peripheral blood for collection and subsequent autologous
transplantation. Oncology. 78:282–288. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan S, Palmer JM, Tsai N-C, Dagis A,
Nademanee A and Wang S: Engraftment and outcomes following
autologous stem cell transplantation in Hodgkin lymphoma patients
mobilized with plerixafor. Hematol Oncol. 35:281–287. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milone G, Martino M, Leotta S, Spadaro A,
Zammit V, Cupri A, Avola G, Camuglia MG, Di Marco A, Scalzulli P,
et al: Cost-effectiveness of on-demand plerixafor added to
chemotherapy and granulocyte-colony stimulating factor for
peripheral blood stem cell mobilization in multiple myeloma. Leuk
Lymphoma. 59:42–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohty M, Azar N, Chabannon C, Le Gouill S,
Karlin L, Farina L, Milkovich G, Ostermann H, Glaß B, Noppeney R,
et al: Plerixafor in poor mobilizers with non-Hodgkin's lymphoma: A
multi-center time-motion analysis. Bone Marrow Transplant.
53:246–254. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lemoli RM: New strategies for stem cell
mobilization. Mediterr J Hematol Infect Dis. 4:e20120662012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shpall EJ, Champlin R and Glaspy JA:
Effect of CD34+ peripheral blood progenitor cell dose on
hematopoietic recovery. Biol Blood Marrow Transplant. 4:84–92.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Micallef IN, Stiff PJ, Nademanee AP,
Maziarz RT, Horwitz ME, Stadtmauer EA, Kaufman JL, McCarty JM,
Vargo R, Cheverton PD, et al: Plerixafor Plus Granulocyte
Colony-Stimulating Factor for Patients with Non-Hodgkin Lymphoma
and Multiple Myeloma: Long-Term Follow-Up Report. Biol Blood Marrow
Transplant. 24:1187–1195. 2018. View Article : Google Scholar : PubMed/NCBI
|