Introduction
Multiple myeloma (MM), a malignant disease of plasma
cells, is one of three major hematological malignancies with the
second highest incidence rate (1).
MM mostly occurs in older patients (2) and is characterized by high monoclonal
immunoglobulin (also called M protein), and is often accompanied by
hypercalcemia, anemia, bone and renal damage (3).
Over the past two decades, there have been new
approved treatment strategies for patients with MM, including
bortezomib, thalidomide or lenalidomide-containing standard
regimens, which have improved the survival of patients with MM
(4). However, most patients relapse
after several lines of therapy, resulting in poor clinical response
and survival outcomes (5).
Therefore, there is an urgent need for more effective therapeutic
strategies for patients with relapsed/refractory multiple myeloma
(RRMM).
Histone deacetylase inhibitors (HDACis) have
demonstrated antitumor activity in other hematological
malignancies. For example, the HDACi vorinostat was initially
approved by the US Food and Drug Administration (FDA) for the
treatment of cutaneous T-cell lymphoma in 2009 (6). MM cells have an abnormal acetylated
spectrum (7), which provides new
insights into the application of HDACis to the treatment of MM.
Basic studies have demonstrated the anti-MM activity of HDACis,
which can induce apoptosis and cell cycle arrest, and degrade
unfolded protein by the aggresome pathway in concert with the
ubiquitin-proteasome system (8–10).
Additionally, panobinostat was approved by the FDA for RRMM therapy
in 2015 following the PANORAMA 2 randomized clinical trial
(11). Currently, there are a number
of HDACis undergoing clinical testing (12). The aim of the present study was to
perform a meta-analysis to evaluate the efficacy and safety of
HDACis in patients with RRMM. Additionally, a subgroup analysis was
performed of patients who were bortezomib-refractory and
lenalidomide-refractory to identify which patients may benefit more
from the application of HDACis.
Materials and methods
Search strategies
Databases, including PubMed, EMBASE, Web of Science
and the Cochrane Library were searched for clinical trials
including phase I, II and III trials published between Jan 2009 and
March 2018, without any language restrictions. Our search criteria
were based on combinations of the following keywords: ‘Relapsed’,
‘refractory’, ‘multiple myeloma’ and ‘histone deacetylase
inhibitor’.
Eligibility criteria
The eligibility criteria included: i) Patients with
RRMM and intent-to-treat individuals in the studies were ≥10; ii)
treatment with an HDACi, including vorinostat, panobinostat,
ricolinostat; iii) the studies provided overall response rate (ORR)
and/or overall survival (OS) and/or progression-free survival
(PFS), as well as adverse event data which allowed statistical
analysis to be performed. Additional relevant references listed in
other reviews and guidelines which met all the criteria of the
present study were also identified. Studies based on animal or cell
line data, case reports, conference abstracts or restricted access
studies were excluded.
Data extraction
Data were independently extracted by two
investigators (XG and XL). Any discrepancies between the two
investigators were solved by a third author (JL). The following
data were extracted from all included publications and exported
manually to Microsoft Excel 2016. Study name, year, therapy
regimens, country, number of included patients, prior treatment
lines and regimens, best response and grade 3 and 4 adverse events
including anemia, neutropenia, thrombocytopenia, fatigue/asthenia,
diarrhea and nausea were recorded. Adverse affects were assessed in
accordance with the Common Terminology Criteria for Adverse Events
version 3.0 (13) and were classed
as: Grade 1, mild; grade 2, moderate; grade 3, severe; grade 4,
life-threatening; grade 5, death. As the literature provided more
information regarding stages 3 and 4, these were selected for
examination in the current study.
Statistical analysis
A random effects model was chosen to account for the
heterogeneity between the selected studies. STATA version 13.0
(StataCorp LP) was used to conduct statistical analysis. P<0.05
was considered to indicate a statistically significant difference.
Heterogeneity was evaluated by I2 value, and significant
heterogeneity was defined as I2>50%.
Results
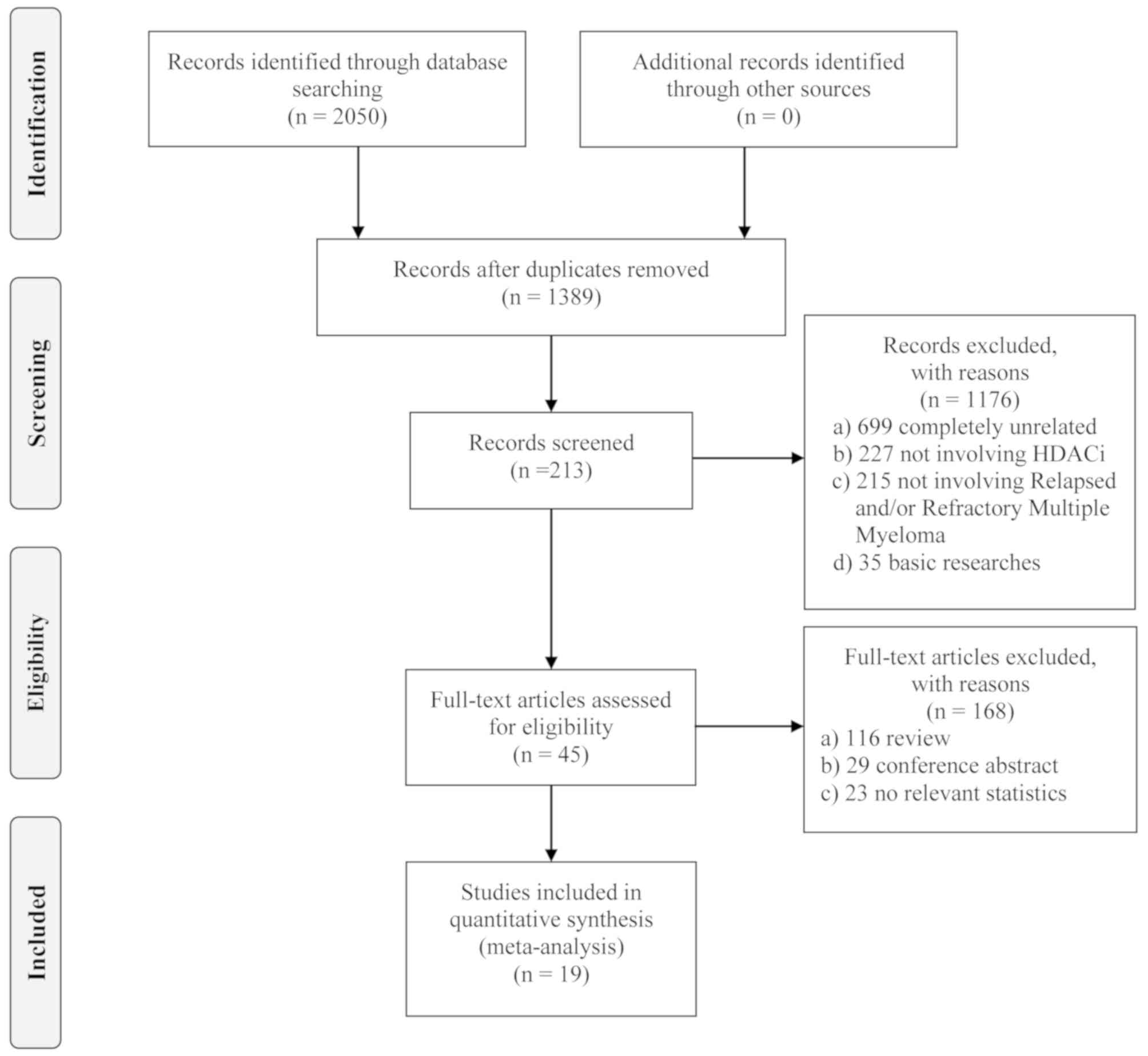
Literature search
In total, 213 potentially relevant studies were
screened after the removal of redundant duplicates and irrelevant
studies. Among the remaining articles, 168 reports were further
excluded as most of them were reviews, conference abstracts or had
no reported data. Following this, 45 full-text articles were
evaluated in detail. Fourteen of these studies did not provide
accessible data, 10 were updates for previously published data, and
one study had a sample size of <10 patients. Ultimately, 19
papers met the selection criteria and were included in the
meta-analysis. The flow diagram of the study design based on PRISMA
2009 (14) is presented in Fig. 1.
Study characteristics
A total of 19 clinical trials with 2,193 evaluable
participants treated with one of three HDACis (panobinostat,
ricolinostat and vorinostat) were included in the present study. In
terms of study design, there was one real-world study (15), 2 phase III studies (11,16), 4
phase II studies (17–20), 5 phase I/II studies (21–25) and
7 phase I studies (26–32). Baseline information of the included
study characteristics and prior therapies are presented in Tables I and II.
 | Table I.Baseline of 19 studies included in the
present meta-analysis. |
Table I.
Baseline of 19 studies included in the
present meta-analysis.
| A, Panobinostat |
|---|
|
|---|
| Study | Year | Country | Phase | Regimen | Patients, n | Dosage of HDACi,
mg | Median age, years
(range) | (Refs.) |
|---|
| Isoda et
al | 2018 | Japan | I | PanVd | 10 | 10/15/20 | 66 (53–77) | (26) |
| Popat et
al | 2016 | UK | I/II | PanVTd | 46 | 10/15/20 | 61 (51–66) | (22) |
| San-Miguel et
al | 2013 | US | Ib | PanV | 62 | 10/20/25/30 | 62 (46–83) | (30) |
| Offidani et
al | 2012 | Italy | II | PanMTd | 12 | 15 | 73 (49–81) | (20) |
|
|
|
|
|
| 19 | 10 | 65 (40–78) |
|
| Berdeja et
al | 2015 | US | I/II | PanK | 44 | 20/30 | 66 (41–82) | (23) |
| Berenson et
al | 2014 | US | I/II | PanM | 40 | 15/20 | 65 (34–88) | (24) |
| Richardson et
al | 2013 | US | I/II | PanVd | 55 | 20 | 61 (41–88) | (25) |
| Wolf et
al | 2012 | US | II | Pan | 38 | 20 | 61 (43–72) | (19) |
| Baertsch et
al | 2018 | Ger | Real-world | PanVd | 24 | 20 | 67 (49–87) | (15) |
| San-Miguel et
al | 2014 | US | III | PanVd | 387 | 20 | 63 (56–69) | (11) |
|
|
|
|
| PboVd | 381 | – | 63 (56–68) |
|
| Chari et
al | 2017 | US | II | PanRd | 27 | 20 | 64 (51–75) | (18) |
|
| B,
Vorinostat |
|
| Study | Year | Country | Phase | Regimen | Patients,
n | Dosage of HDACi,
mg | Median age,
years (range) | (Refs.) |
|
| Sanchez et
al | 2016 | US | IIb | VorRd | 25 | 400 | 65 (48–82) | (17) |
| Vesole et
al | 2015 | US | I | VorKRd | 17 | 300/400 | 61 (48–71) | (29) |
| Voorhees et
al | 2017 | US | I | VorVPLD | 32 | 200/300/400 | 61 (39–75) | (27) |
| Weber et
al | 2012 | US | I | VorV | 34 | 200/300/400 | 61 (45–79) | (31) |
| Dimopoulos et
al | 2013 | US | III | VorV | 317 | 400 | 61 (30–85) | (16) |
|
|
|
|
| PboV | 320 | – | 63 (29–86) |
|
| Badros et
al | 2009 | US | I | VorV | 23 |
100/200/400/500 | 54 (39–78) | (32) |
|
| C,
Ricolinostat |
|
| Vogl et
al | 2017 | US | I/II | Rico | 15 |
40/80/160/240/360 | 70 (51–79) | (21) |
|
|
|
|
| RicoVd | 57 |
40/80/160/240/360 | 65 (47–84) |
|
|
|
|
|
| RicoVd | 20 | ≥160 | 65 (47–83) |
|
|
|
|
|
| RicoVd | 24 | 160 | 67 (48–84) |
|
| Yee et
al | 2016 | US | Ib | RicoRd | 38 | 40/80/160/240 | 63 (57–71) | (28) |
 | Table II.Prior treatments. |
Table II.
Prior treatments.
| A,
Panobinostat |
|---|
|
|---|
|
|
|
| Prior regimens |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Study | Year | Median number of
prior therapies (range) | PIs, n (%) | iMIDs, n (%) | PIs and iMIDs, n
(%) | (Refs.) |
|---|
| Isoda et
al | 2018 | 3.5 (1–5) | – | – | – | (26) |
| Popat et
al | 2016 | 1 (1–4) | 33 (72) | 24 (52) | 8 (17) | (22) |
| San-Miguel et
al | 2013 | 2 (1–10) | 39 (62.9) | T: 28 (45.2) | – | (30) |
|
|
|
|
| R: 28 (45.2) |
|
|
| Offidani et
al | 2012 | – | 8 (67) | T: 7 (58) | – | (20) |
|
|
|
|
| R: 5 (42) |
|
|
| Berdeja et
al | 2015 | 5 (1–10) | 39 (89) | 39 (89) | 35 (80) | (23) |
| Berenson et
al | 2014 | 4 (1–16) | 2 (0–9) | – | – | (24) |
| Richardson et
al | 2013 | 4 (2–11) | 55 (100) | T: 38 (69.1) | – | (25) |
|
|
|
|
| R: 54 (98.2) |
|
|
| Wolf et
al | 2012 | 5 (−) | 2 (5.3) | 3 (7.9) | 24 (63.2) | (19) |
| Baertsch et
al | 2018 | 5 (2–17) | V: 23 (96) | R: 23 (96) |
| (15) |
|
|
|
| K: 7 (29) | Pom: 16 (67) |
|
|
| San-Miguel et
al | 2014 | 1 (1–3) | 169 (44) | R: 72 (19) | 94 (24) | (11) |
| (PANORAMA 1) |
|
|
| T: 205 (53) |
|
|
| Chari et
al | 2017 | 3 (1–10) | V: 27 (100) | R: 27 (100) | – | (18) |
|
|
|
| K: 8 (30) | T: 6 (22) |
|
|
|
|
|
|
| Pom: 10 (37) |
|
|
|
| B,
Vorinostat |
|
|
|
|
| Prior
regimens |
|
|
|
|
|
|
|
|
|
| Study | Year | Median number of
prior therapies (range) | PIs, n
(%) | iMIDs, n
(%) | PIs and iMIDs, n
(%) | (Refs.) |
|
| Sanchez et
al | 2016 | – | 20 (80) | 9 (36) | – | (17) |
| Vesole et
al | 2015 | 4 (1–9) | 17 (100) | 16 (94) | – | (29) |
| Voorhees et
al | 2017 | 2 (1–9) | 25 (78) | 29 (91) | – | (27) |
| Weber et
al | 2012 | 4 (1–14) | – | T: 24 (70) | – | (31) |
|
|
|
|
| R: 19 (56) |
|
|
| Dimopoulos et
al | 2013 | 2 (1–3) | 79 (25) | 192 (61) | – | (16) |
| Badros et
al | 2009 | 7 (3–13) | 19 (82.6) | R: 17 (73.9) | – | (32) |
|
|
|
|
| T: 23 (100) |
|
|
|
| C,
Ricolinostat |
|
|
|
|
| Prior
regimens |
|
|
|
|
|
|
|
|
|
| Study | Year | Median number of
prior therapies (range) | PIs, n
(%) | iMIDs, n
(%) | PIs and iMIDs, n
(%) | (Refs.) |
|
| Vogl et
al | 2017 | 5 (2–13) | V: 36 (63) | T: 12 (21) | – | (21) |
|
|
|
| K: 17 (30) | R: 38 (67) |
|
|
| Yee et
al | 2016 | 2 (1–3) | 11 (29) | R: 12 (32) | – | (28) |
|
|
|
|
| T: 4 (11) |
|
|
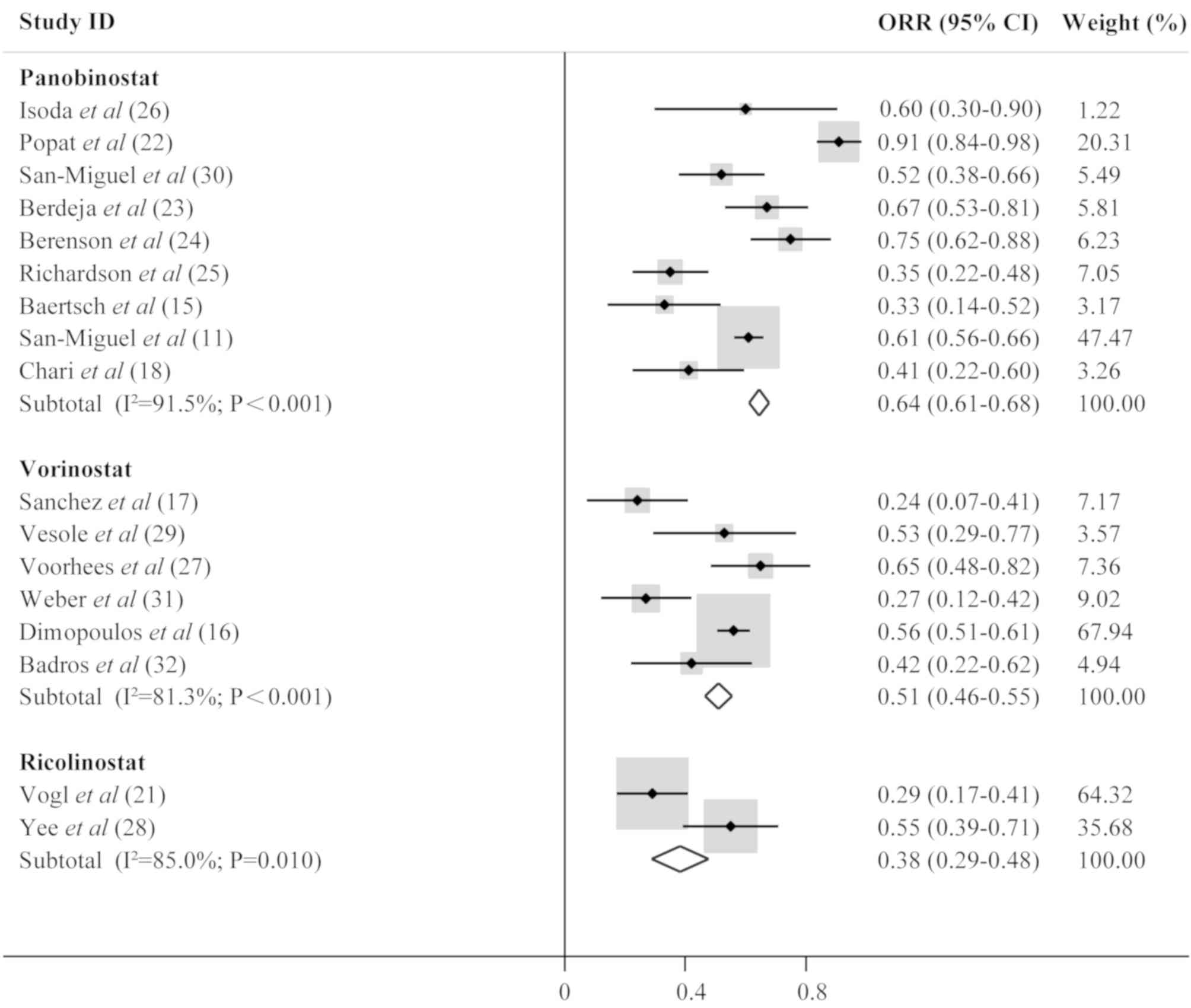
Response to HDACi treatment
The extracted data were categorized into three
groups in order to assess clinical efficacy based on treatment with
panobinostat, ricolinostat and vorinostat. Raw data on the
effectiveness of the treatment are presented in Table III, including ORR, complete
response, very good partial response, partial response, median PFS,
median OS and median follow-up. Analysis of the pooled data
demonstrated that ORR was 0.64 (95% confidence interval [CI],
0.61–0.68; I2, 91.5%; P<0.001) for MM patients
treated with panobinostat, 0.51 (95% CI, 0.46–0.55; I2,
81.3%; P<0.001) for those treated with vorinostat, and 0.38 (95%
CI, 0.29–0.48; I2, 85.0%; P=0.010) for patients treated
with ricolinostat. The results also revealed that the
panobinostat-containing regimen was the most effective treatment
among these three drugs according to the ORR. Forest plots are
presented in Fig. 2.
 | Table III.Response to treatment. |
Table III.
Response to treatment.
| A,
Panobinostat |
|---|
|
|---|
| Study | Year | ORR, n (%) | CR, n (%) | VGPR, n (%) | PR, n (%) | M-PFS, months | (Refs.) |
|---|
| Isoda et
al | 2018 | 6 (60) | 0 (0) | 2 (20) | 4 (40) | 11.5 | (26) |
| Popat et
al | 2016 | 42 (91) | 3 (7) | 21 (46) | 21 (46) | 15.6 | (22) |
| San-Miguel et
al | 2013 | 32 (51.6) | 2 (3.2) | 6 (9.7) | 22 (35.5) | – | (30) |
| Offidani et
al | 2012 | 5 (42.0) | 0 (0) | 1 (8) | 4 (33.5) | 8.1 | (20) |
| Berdeja et
al | 2015 | 28 (67) | – | 14 (33) | 14 (33) | 7.7 | (23) |
| Berenson et
al | 2014 | 3 (7.5) | 0 (0) | 2 (5) | 1 (2.5) | – | (24) |
| Richardson et
al | 2013 | 19 (34.5) | 0 (0) | 3 (5.5) | 18 (32.7) | 5.4 | (25) |
| Wolf et
al | 2012 | – | – | – | 1 (2.6) | – | (19) |
| Baertsch et
al | 2018 | 7 (33) | 0 (0) | 2 (9.5) | 5 (23.8) | 3.5 | (15) |
| San-Miguel et
al | 2014 | 235 (60.7) | 42 (11) | – | 128 (33) | 12.0 | (11) |
| Chari et
al | 2017 | 11 (41) | 2 (7.4) | 4 (14.8) | 5 (18.5) | 7.1 | (18) |
|
| B,
Vorinostat |
|
| Study | Year | ORR (%) | CR (%) | VGPR
(%) | PR (%) | M-PFS,
months | (Refs.) |
|
| Sanchez et
al | 2016 | 6 (24) | 0 (0) | – | 6 (24) | 5.3 | (17) |
| Vesole et
al | 2015 | 9 (53) | – | 2 (12) | 7 (41) | 12 | (29) |
| Voorhees et
al | 2017 | 20 (65) | 2 (6) | 7 (23) | 11 (35) | 13.9 | (27) |
| Weber et
al | 2012 | 9 (27) | 0 (0) | – | 9 (27) | – | (31) |
| Dimopoulos et
al | 2013 | 177 (56.2) | 25 (7.9) | – | 152 (48.3) | 7.6 | (16) |
| Badros et
al | 2009 | 9 (42) | – | 2 (9.5) | 7 (33.3) | – | (32) |
|
| C,
Ricolinostat |
|
| Study | Year | ORR (%) | CR (%) | VGPR
(%) | PR (%) | M-PFS,
months | (Refs.) |
|
| Vogl et
al | 2017 | 29 (29) | – | 7 (7) | 21 (21) | – | (21) |
| Yee et
al | 2016 | 21 (55) | 2 (5) | 7 (18) | 10 (26) | 20.7 | (28) |
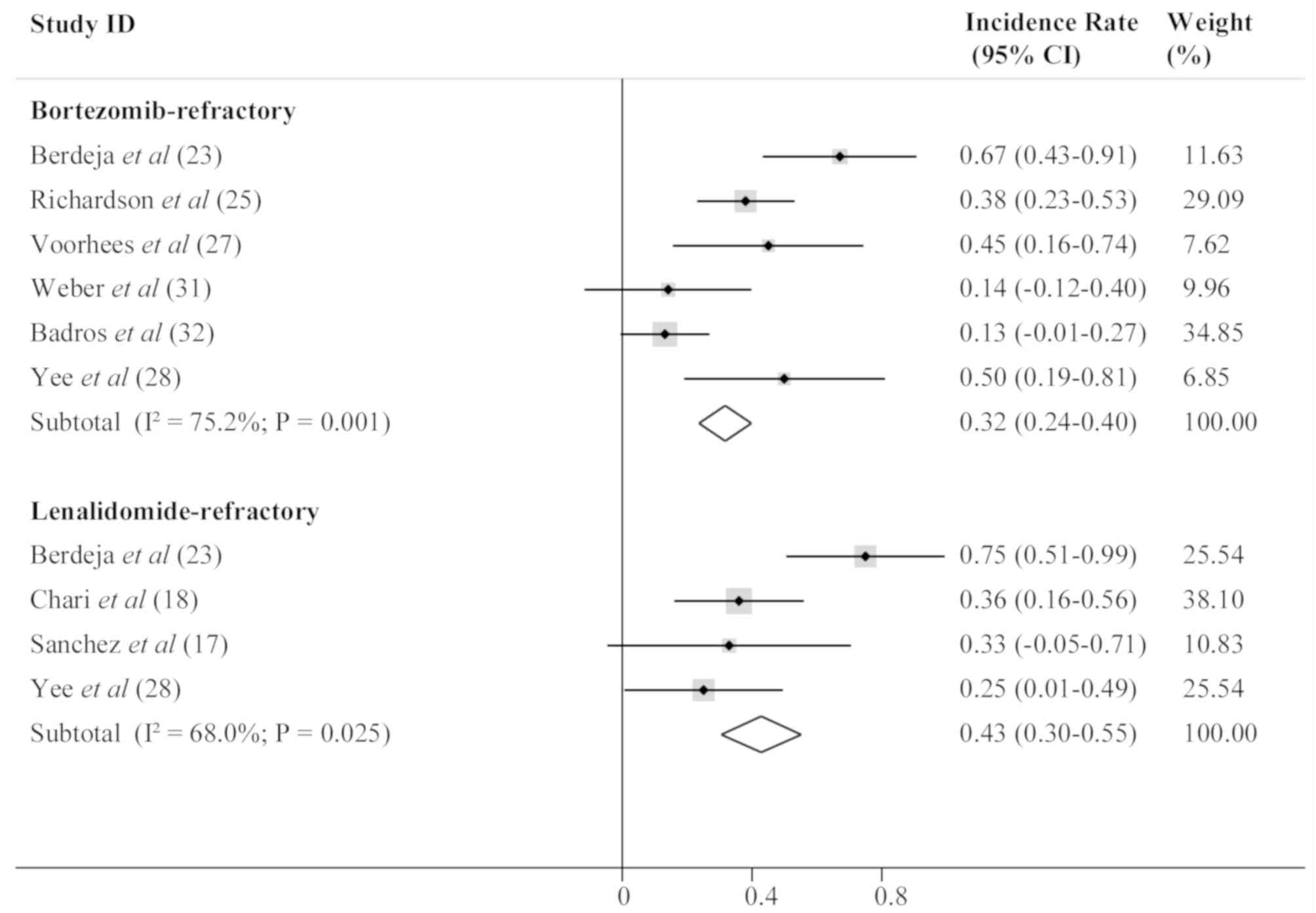
Response of bortezomib and
lenalidomide-refractory patients to HDACi treatment
A subgroup analysis was subsequently conducted to
evaluate the effectiveness of HDACis in bortezomib-refractory and
lenalidomide-refractory patients. ORR was 0.32 (95% CI, 0.24–0.40;
I2, 75.2%; P=0.001) for bortezomib-refractory patients,
and 0.43 (95% CI, 0.30–0.55; I2, 68.0%; P=0.025) for
lenalidomide-refractory patients, suggesting that HDACis were
better for the lenalidomide-refractory MM patients. The results are
presented in Fig. 3.
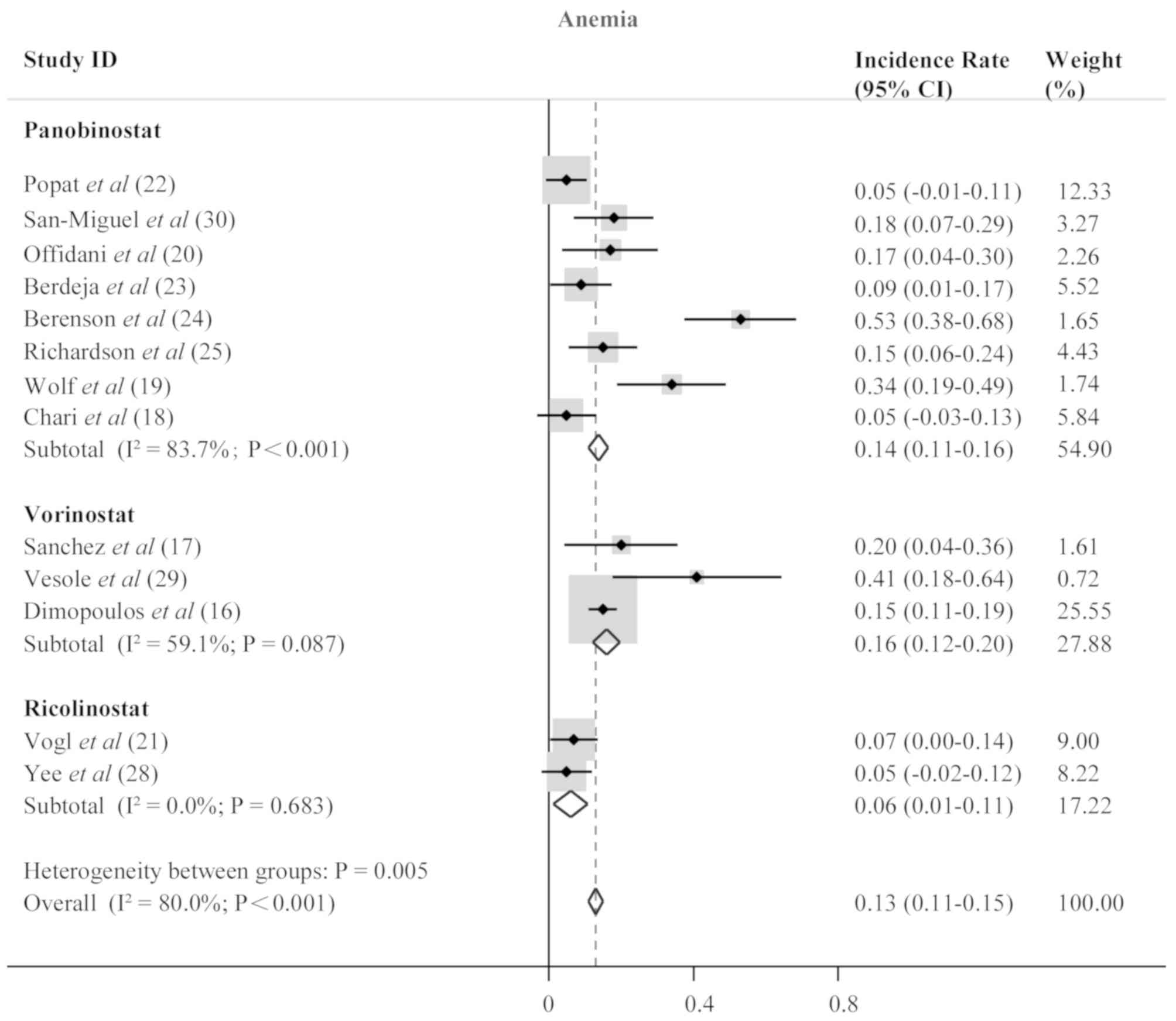
Toxicity
An overview of the adverse events is presented in
Table IV. Analysis of the pooled
data was performed to evaluate the incidence rate of grade 3 and 4
adverse events in all included patients treated with HDACis. The
most common hematological adverse events were anemia with an
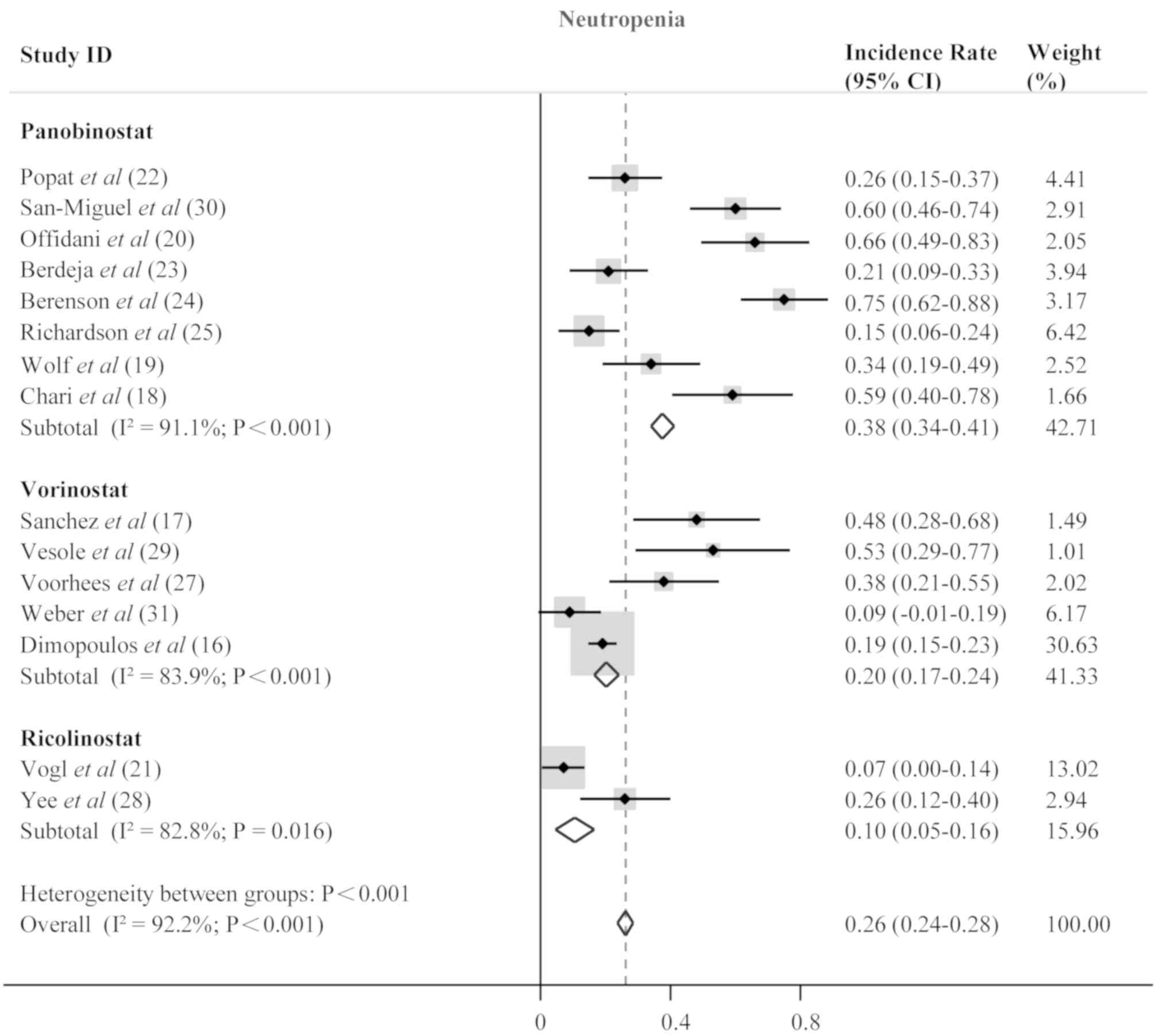
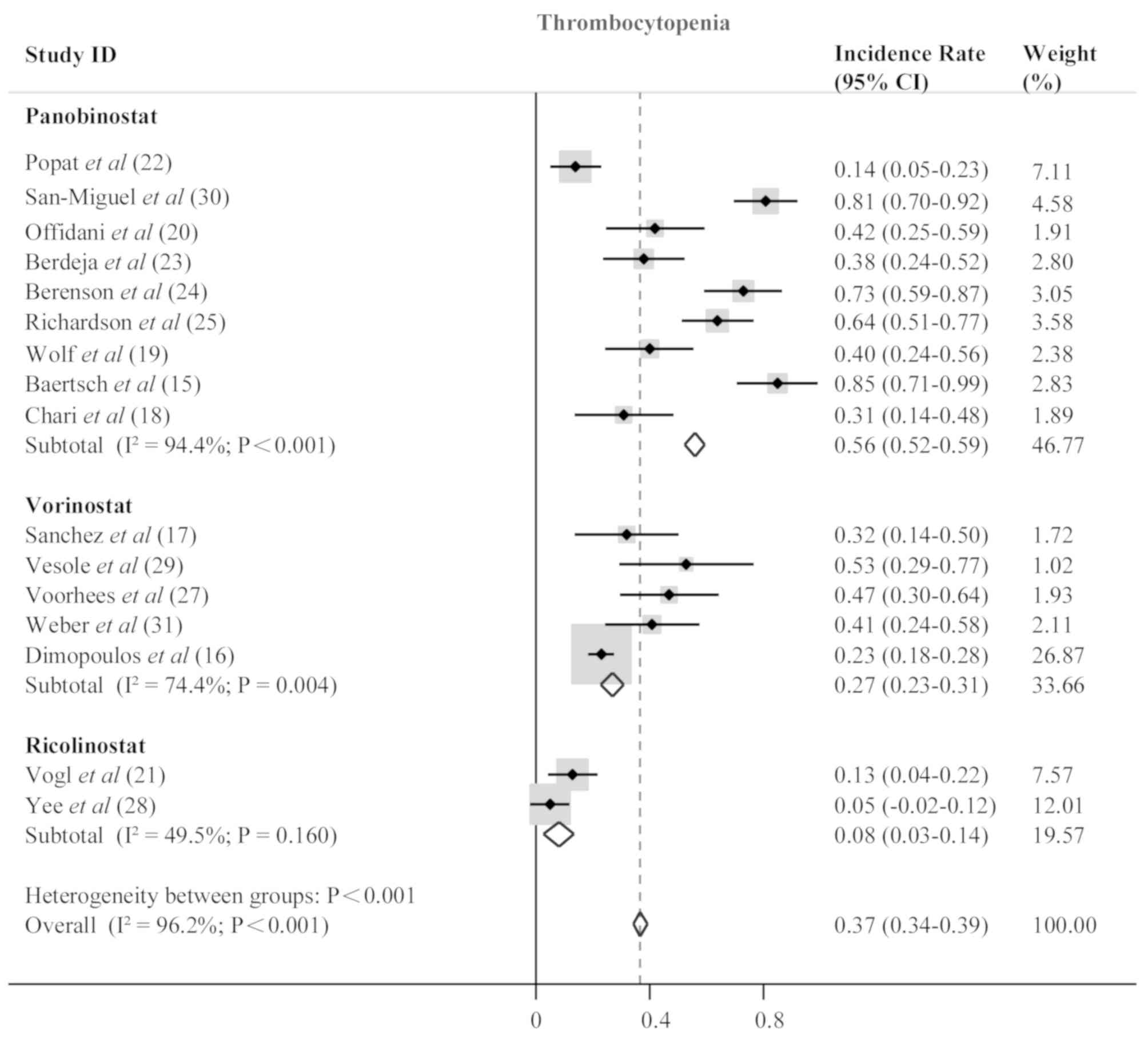
incidence rate of 0.13 (95% CI, 0.11–0.15; P<0.001; Fig. 4), neutropenia (0.26; 95% CI,
0.24–0.28; P<0.001; Fig. 5) and
thrombocytopenia (0.37; 95% CI, 0.34–0.39; P<0.001; Fig. 6). Anemia, neutropenia and
thrombocytopenia were observed at similar frequencies among
panobinostat- and vorinostat-treated patients; however,
ricolinostat-treated patients were affected by neutropenia more
frequently than patients with anemia or thrombocytopenia. The most
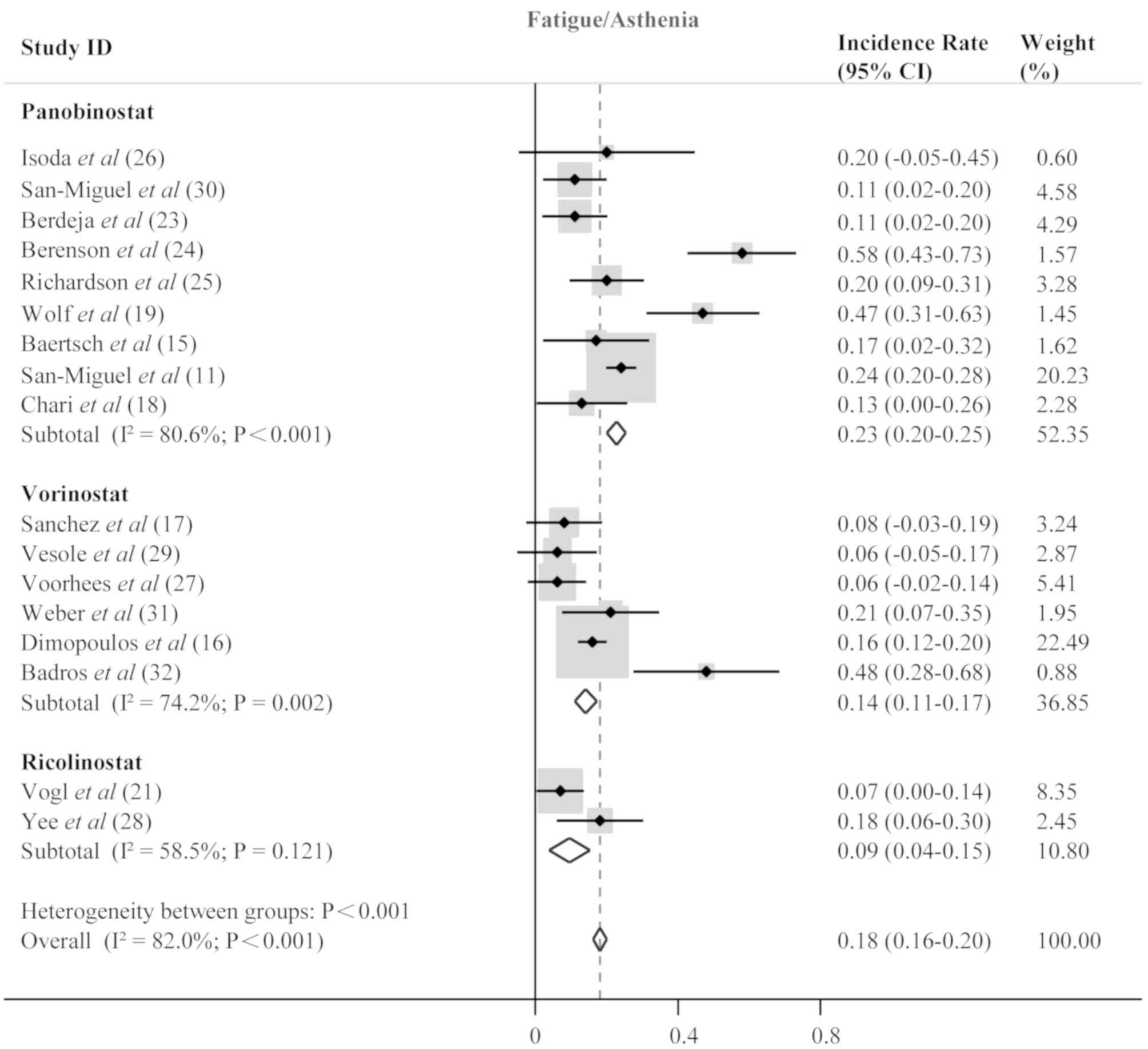
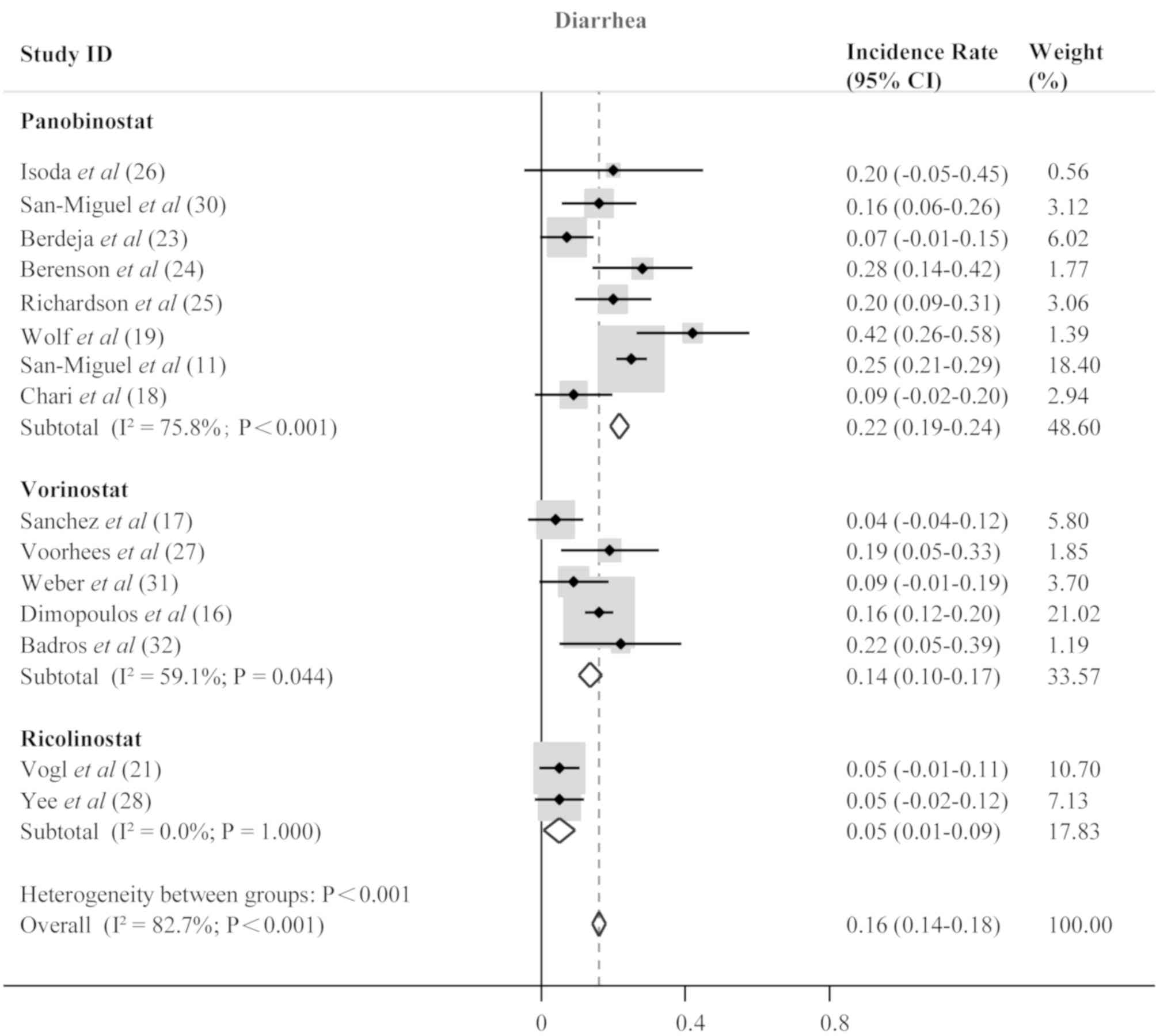
frequent non-hematological adverse events included fatigue/asthenia
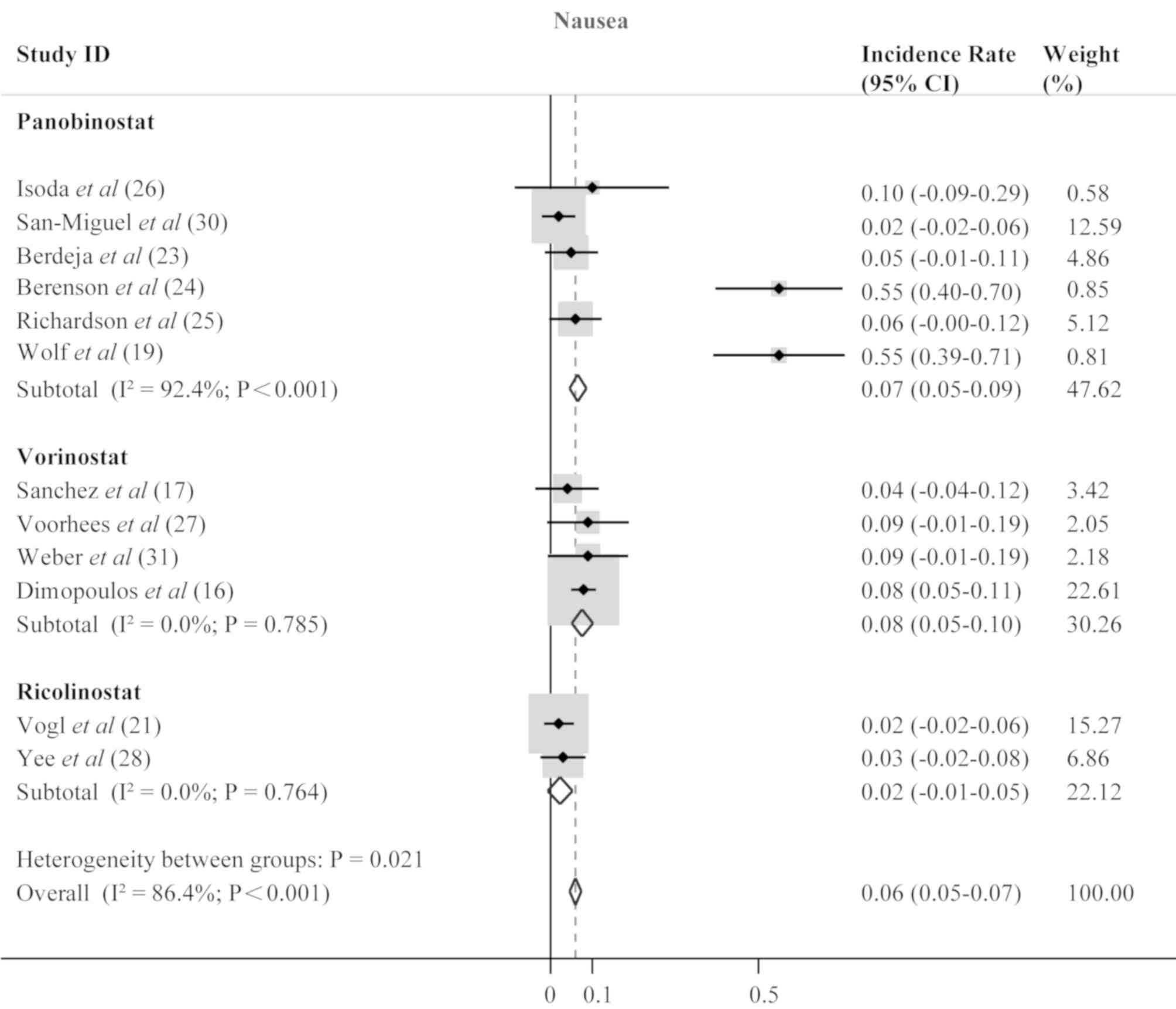
(0.18; 95% CI; 0.16–0.20; P<0.001; Fig. 7), diarrhea (0.16; 95% CI; 0.14–0.18;
P<0.001; Fig. 8), and nausea
(0.06; 95% CI; 0.05–0.07; P<0.001; Fig. 9).
 | Table IV.HDACi treatment related grade 3 and 4
adverse events. |
Table IV.
HDACi treatment related grade 3 and 4
adverse events.
|
|
| Hematological |
Non-hematological |
|
|---|
|
|
|
|
|
|
|---|
| Study | Year | Anemia, n (%) | Neutropenia, n
(%) | Thrombocytopenia, n
(%) | Fatigue/Asthenia, n
(%) | Diarrhea, n
(%) | Nausea, n (%) | (Refs.) |
|---|
| Isoda et
al | 2018 | – | – | – | 2 (20) | 2 (20) | 1 (10) | (26) |
| Popat et
al | 2016 | 3 (5) | 15 (26) | 8 (14) | – | – | 0 (0) | (22) |
| San-Miguel et
al | 2013 | 11 (17.7) | 37 (59.7) | 50 (80.6) | 7 (11.3) | 10 (16.1) | 1 (1.6) | (30) |
| Offidani et
al | 2012 | 2 (17) | 8 (66) | 5 (42) | – | – | – | (20) |
| Berdeja et
al | 2015 | 4 (9) | 9 (21) | 17 (38) | 5 (11) | 3 (7) | 2 (5) | (23) |
| Berenson et
al | 2014 | 21 (52.5) | 30 (75) | 29 (72.5) | 23 (57.5) | 11 (27.5) | 22 (55) | (24) |
| Richardson et
al | 2013 | 8 (14.5) | 8 (14.5) | 35 (63.6) | 11 (20) | 111 (20) | 3 (5.5) | (25) |
| Wolf et
al | 2012 | 13 (34.2) | 13 (34.2) | 15 (39.5) | 18 (47.4) | 16 (42.1) | 21 (55.3) | (19) |
| Baertsch et
al | 2018 | – | – | 17 (85) | 4 (17) | 0 (0) | – | (15) |
| San-Miguel et
al | 2014 | – | – | – | 91 (24) | 97 (25) | 21 (6) | (11) |
| Chari et
al | 2017 | 2 (5) | 19 (59) | 10 (31) | 4 (12.5) | 3 (9.4) | – | (18) |
| Sanchez et
al | 2016 | 5 (20) | 12 (48) | 8 (32) | 2 (8) | 1 (4) | 1 (4) | (17) |
| Vesole et
al | 2015 | 7 (41) | 9 (53) | 9 (53) | 1 (6) | 0 (0) | – | (29) |
| Voorhees et
al | 2017 | 0 (0) | 12 (37.5) | 15 (47) | 5 (16) | 6 (19) | 3 (9) | (27) |
| Weber et
al | 2012 | 0 (0) | 3 (9) | 14 (41) | 7 (21) | 3 (9) | 3 (9) | (31) |
| Dimopoulos et
al | 2013 | 47 (15) | 59 (19) | 74 (23) | 50 (16) | 51 (16) | 24 (8) | (16) |
| Badros et
al | 2009 | – | – | – | 11 (47.8) | 5 (21.7) | – | (32) |
| Vogl et
al | 2017 | 11 (19) | 2 (4) | 21 (37) | 4 (7) | 3 (5) | 1 (2) | (21) |
| Yee et
al | 2016 | 2 (5) | 10 (26) | 2 (5) | 7 (18) | 2 (5) | 1 (3) | (28) |
Discussion
The recommended dose of each of the three drugs was
summarized to provide a reference for future clinical practices. In
the included studies, the dosages of panobinostat ranged from 10 to
30 mg, and the maximum tolerated dose (MTD) was 20 mg, except for
in a phase II clinical trial in which it was combined with
melphalan, prednisone and thalidomide (20). In that study, the MTD was not
determined due to the high rate of dose-limiting toxicities at
doses of 10 and 15 mg (20).
Additionally, the MTD was established as 30 mg when combined with
carfilzomib (23). Vorinostat
inhibited class I, II and IV of HDACs at doses of 100–500 mg in the
selected studies, where the MTD was established at 400 mg. However,
the MTD of vorinostat was not established in studies where it was
combined with carfilzomib, lenalidomide and dexamethasone, or
co-administered with bortezomib (29,31). In
the two studies that included ricolinostat, patients were treated
in cohorts with doses of 40–240 mg. Although the MTD of
ricolinostat was not established, a recommended phase II dose was
defined at 160 mg (28). Overall,
the data suggested that the doses used for the HDAC inhibitors
varied based on different combination regimens.
The meta-analysis results indicated that
panobinostat was more effective and safer than vorinostat and
ricolinostat. In the subgroup analysis, the highest ORR of 0.64 was
observed in RRMM patients treated with panobinostat. This was
followed by ORRs of 0.51 and 0.38 in RRMM patients treated with
vorinostat and ricolinostat, respectively. Panobinostat is a
non-selective HDACi, targeting class I, II and IV HDACs. A study by
Wolf et al (19) found that
only one out of 38 patients achieved a partial response (PR) with
panobinostat monotherapy. The outcome indicated that panobinostat
alone showed little clinical efficacy in the treatment of RRMM.
However, there was a significant improvement in clinical efficacy
when panobinostat was used in combination with proteasome
inhibitors (PIs) or immunomodulatory drugs (iMIDs) for RRMM.
Importantly, a study by Popat et al (22) demonstrated that panobinostat in
combination with bortezomib, thalidomide and dexamethasone
generated an ORR of 0.91 (95% CI, 0.84–0.98), and lower rates of
hematological adverse events, including neutropenia and
thrombocytopenia in comparison to other studies. These results
suggest that multidrug combinations may be more effective treatment
strategies for RRMM. There were only a few studies with small
sample sizes included in the ricolinostat-treated group for the
pooled analysis (21,28). Ricolinostat, a selective inhibitor of
HDAC6, preliminarily showed a weaker anti-MM effect than the other
two drugs in the present study, thereby implying that selective
HDACis are less effective than non selective HDACis
(pan-HDACis).
In the present study, subgroup analysis also
demonstrated that lenalidomide-refractory MM patients had a better
ORR than bortezomib-refractory MM patients after treatment with
HDACi-containing regimens. It was hypothesized that this may be due
to PI-refractory patients not being responsive to HDAC inhibitors
alone, but being responsive to the combined effect of PIs and
HDACis.
In addition, the safety of the three drugs was
evaluated by analyzing the incidence of adverse events.
Panobinostat and vorinostat showed a similar trend of overall
incidence of hematological adverse events of thrombocytopenia,
neutropenia and anemia, and non-hematological adverse events of
fatigue/asthenia, diarrhea and nausea. By contrast, the most common
hematological adverse event for ricolinostat was neutropenia. The
difference in adverse event profiles of HDAC inhibitors should be
carefully considered by clinicians in the clinical management of
patients with RRMM.
There were also several limitations in the present
meta analyses which should be considered. First, in addition to 3
studies from Japan, Italy and Germany, the remaining 16 studies
included in the current analysis were from the United States,
suggesting that the outcome maybe be biased for the American
population. Second, even with using a random effects model in
statistical analysis, the data were still confounded by a high
degree of heterogeneity. This was probably due to the numerous
combination regimens administered in different studies. Third, most
of the studies included in the present analysis were single-arm
clinical trials. There were only two phase III trials (11,16) that
provided OS and PFS data, which are the main indicators used to
evaluate drug efficacy in oncology clinical trials.
In conclusion, panobinostat-containing regimens were
effective in treating patients with RRMM, but ricolinostat and
vorinostat-containing regimens did not yield satisfactory results
for patients with RRMM. Additionally, lenalidomide-refractory
patients may benefit from HDACi treatment more than patients with
bortezomib-refractory. However, a longer follow-up period is
required to investigate crucial study endpoints of PFS and OS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG and LS directed the study and XG wrote the
manuscript. XG and XL extracted data. JL conducted the statistical
analysis of data. LS critically revised the article for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajkumar SV: Multiple myeloma: 2016 update
on diagnosis, risk-stratification, and management. Am J Hematol.
91:719–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV and Kumar S: Multiple myeloma:
Diagnosis and treatment. Mayo Clin Proc. 91:101–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chim CS, Kumar SK, Orlowski RZ, Cook G,
Richardson PG, Gertz MA, Giralt S, Mateos MV, Leleu X and Anderson
KC: Management of relapsed and refractory multiple myeloma: Novel
agents, antibodies, immunotherapies and beyond. Leukemia.
32:252–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mithraprabhu S, Kalff A, Chow A, Khong T
and Spencer A: Dysregulated class I histone deacetylases are
indicators of poor prognosis in multiple myeloma. Epigenetics.
9:1511–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Catley L, Weisberg E, Kiziltepe T, Tai YT,
Hideshima T, Neri P, Tassone P, Atadja P, Chauhan D, Munshi NC and
Anderson KC: Aggresome induction by proteasome inhibitor bortezomib
and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC)
inhibitor LBH589 are synergistic in myeloma cells. Blood.
108:3441–3449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simms-Waldrip T, Rodriguez-Gonzalez A, Lin
T, Ikeda AK, Fu C and Sakamoto KM: The aggresome pathway as a
target for therapy in hematologic malignancies. Mol Genet Metab.
94:283–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hideshima T, Bradner JE, Wong J, Chauhan
D, Richardson P, Schreiber SL and Anderson KC: Small-molecule
inhibition of proteasome and aggresome function induces synergistic
antitumor activity in multiple myeloma. Proc NatI Acad Sci USA.
102:8567–8572. 2005. View Article : Google Scholar
|
|
11
|
San-Miguel JF, Hungria VT, Yoon SS, Beksac
M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn
TN, Siritanaratkul N, et al: Panobinostat plus bortezomib and
dexamethasone versus placebo plus bortezomib and dexamethasone in
patients with relapsed or relapsed and refractory multiple myeloma:
A multicentre, randomised, double-blind phase 3 trial. Lancet
Oncol. 15:1195–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harada T, Hideshima T and Anderson KC:
Histone deacetylase inhibitors in multiple myeloma: From bench to
bedside. Int J Hematol. 104:300–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baertsch MA, Hillengass J, Blocka J,
Schönland S, Hegenbart U, Goldschmidt H and Raab MS: Efficacy and
tolerability of the histone deacetylase inhibitor panobinostat in
clinical practice. Hematol Oncol. 36:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dimopoulos M, Siegel DS, Lonial S, Qi J,
Hajek R, Facon T, Rosinol L, Williams C, Blacklock H, Goldschmidt
H, et al: Vorinostat or placebo in combination with bortezomib in
patients with multiple myeloma (VANTAGE 088): A multicentre,
randomised, double-blind study. Lancet Oncol. 14:1129–1140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanchez L, Vesole DH, Richter JR, Biran N,
Bilotti E, McBride L, Anand P, Ivanovski K and Siegel DS: A phase
IIb trial of vorinostat in combination with lenalidomide and
dexamethasone in patients with multiple myeloma refractory to
previous lenalidomide-containing regimens. Br J Haematol.
176:440–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chari A, Cho HJ, Dhadwal A, Morgan G, La
L, Zarychta K, Catamero D, Florendo E, Stevens N, Verina D, et al:
A phase 2 study of panobinostat with lenalidomide and weekly
dexamethasone in myeloma. Blood Adv. 1:1575–1583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolf JL, Siegel D, Goldschmidt H, Hazell
K, Bourquelot PM, Bengoudifa BR, Matous J, Vij R, de
Magalhaes-Silverman M, Abonour R, et al: Phase II trial of the
pan-deacetylase inhibitor panobinostat as a single agent in
advanced relapsed/refractory multiple myeloma. Leuk Lymphoma.
53:1820–1823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Offidani M, Polloni C, Cavallo F, Liberati
AM, Ballanti S, Pulini S, Catarini M, Alesiani F, Corvatta L,
Gentili S, et al: Phase II study of melphalan, thalidomide and
prednisone combined with oral panobinostat in patients with
relapsed/refractory multiple myeloma. Leuk Lymphoma. 53:1722–1727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogl DT, Raje N, Jagannath S, Richardson
P, Hari P, Orlowski R, Supko JG, Tamang D, Yang M, Jones SS, et al:
Ricolinostat, the first selective histone deacetylase 6 inhibitor,
in combination with bortezomib and dexamethasone for relapsed or
refractory multiple myeloma. Clin Cancer Res. 23:3307–3315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Popat R, Brown SR, Flanagan L, Hall A,
Gregory W, Kishore B, Streetly M, Oakervee H, Yong K, Cook G, et
al: Bortezomib, thalidomide, dexamethasone, and panobinostat for
patients with relapsed multiple myeloma (MUK-six): A multicentre,
open-label, phase 1/2 trial. Lancet Haematol. 3:e572–e580. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Berdeja JG, Hart LL, Mace JR, Arrowsmith
ER, Essell JH, Owera RS, Hainsworth JD and Flinn IW: Phase I/II
study of the combination of panobinostat and carfilzomib in
patients with relapsed/refractory multiple myeloma. Haematologica.
100:670–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berenson JR, Hilger JD, Yellin O, Boccia
RV, Matous J, Dressler K, Ghazal HH, Jamshed S, Kingsley EC, Harb
WA, et al: A phase 1/2 study of oral panobinostat combined with
melphalan for patients with relapsed or refractory multiple
myeloma. Ann Hematol. 93:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richardson PG, Schlossman RL, Alsina M,
Weber DM, Coutre SE, Gasparetto C, Mukhopadhyay S, Ondovik MS, Khan
M, Paley CS and Lonial S: PANORAMA 2: Panobinostat in combination
with bortezomib and dexamethasone in patients with relapsed and
bortezomib-refractory myeloma. Blood. 122:2331–2337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isoda A, Ishikawa T, Miyazawa Y, Mihara M,
Matsumoto M and Sawamura M: Intra-patient dose escalation of
panobinostat in patients with relapsed/refractory multiple myeloma.
Leuk Lymphoma. 59:1277–1278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voorhees PM, Gasparetto C, Moore DT,
Winans D, Orlowski RZ and Hurd DD: Final results of a Phase 1 study
of vorinostat, pegylated liposomal doxorubicin, and bortezomib in
relapsed or refractory multiple myeloma. Clin Lymphoma, Myeloma
Leuk. 17:424–432. 2017. View Article : Google Scholar
|
|
28
|
Yee AJ, Bensinger WI, Supko JG, Voorhees
PM, Berdeja JG, Richardson PG, Libby EN, Wallace EE, Birrer NE,
Burke JN, et al: Ricolinostat plus lenalidomide, and dexamethasone
in relapsed or refractory multiple myeloma: A multicentre phase 1b
trial. Lancet Oncol. 17:1569–1578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vesole DH, Bilotti E, Richter JR, McNeill
A, McBride L, Raucci L, Anand P, Bednarz U, Ivanovski K, Smith J,
et al: Phase I study of carfilzomib, lenalidomide, vorinostat, and
dexamethasone in patients with relapsed and/or refractory multiple
myeloma. Br J Haematol. 171:52–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
San-Miguel JF, Richardson PG, Gunther A,
Sezer O, Siegel D, Bladé J, LeBlanc R, Sutherland H, Sopala M,
Mishra KK, et al: Phase Ib study of panobinostat and bortezomib in
relapsed or relapsed and refractory multiple myeloma. J Clin Oncol.
31:3696–3703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weber DM, Graef T, Hussein M, Sobecks RM,
Schiller GJ, Lupinacci L, Hardwick JS and Jagannath S: Phase I
trial of vorinostat combined with bortezomib for the treatment of
relapsing and/or refractory multiple myeloma. Clin Lymphoma Myeloma
Leuk. 12:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Badros A, Burger AM, Philip S, Niesvizky
R, Kolla SS, Goloubeva O, Harris C, Zwiebel J, Wright JJ,
Espinoza-Delgado I, et al: Phase I study of vorinostat in
combination with bortezomib for relapsed and refractory multiple
myeloma. Clin Cancer Res. 15:5250–5257. 2009. View Article : Google Scholar : PubMed/NCBI
|