|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rajkumar SV: Multiple myeloma: 2016 update
on diagnosis, risk-stratification, and management. Am J Hematol.
91:719–734. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV and Kumar S: Multiple myeloma:
Diagnosis and treatment. Mayo Clin Proc. 91:101–119. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chim CS, Kumar SK, Orlowski RZ, Cook G,
Richardson PG, Gertz MA, Giralt S, Mateos MV, Leleu X and Anderson
KC: Management of relapsed and refractory multiple myeloma: Novel
agents, antibodies, immunotherapies and beyond. Leukemia.
32:252–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mithraprabhu S, Kalff A, Chow A, Khong T
and Spencer A: Dysregulated class I histone deacetylases are
indicators of poor prognosis in multiple myeloma. Epigenetics.
9:1511–1520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Catley L, Weisberg E, Kiziltepe T, Tai YT,
Hideshima T, Neri P, Tassone P, Atadja P, Chauhan D, Munshi NC and
Anderson KC: Aggresome induction by proteasome inhibitor bortezomib
and alpha-tubulin hyperacetylation by tubulin deacetylase (TDAC)
inhibitor LBH589 are synergistic in myeloma cells. Blood.
108:3441–3449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simms-Waldrip T, Rodriguez-Gonzalez A, Lin
T, Ikeda AK, Fu C and Sakamoto KM: The aggresome pathway as a
target for therapy in hematologic malignancies. Mol Genet Metab.
94:283–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hideshima T, Bradner JE, Wong J, Chauhan
D, Richardson P, Schreiber SL and Anderson KC: Small-molecule
inhibition of proteasome and aggresome function induces synergistic
antitumor activity in multiple myeloma. Proc NatI Acad Sci USA.
102:8567–8572. 2005. View Article : Google Scholar
|
|
11
|
San-Miguel JF, Hungria VT, Yoon SS, Beksac
M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn
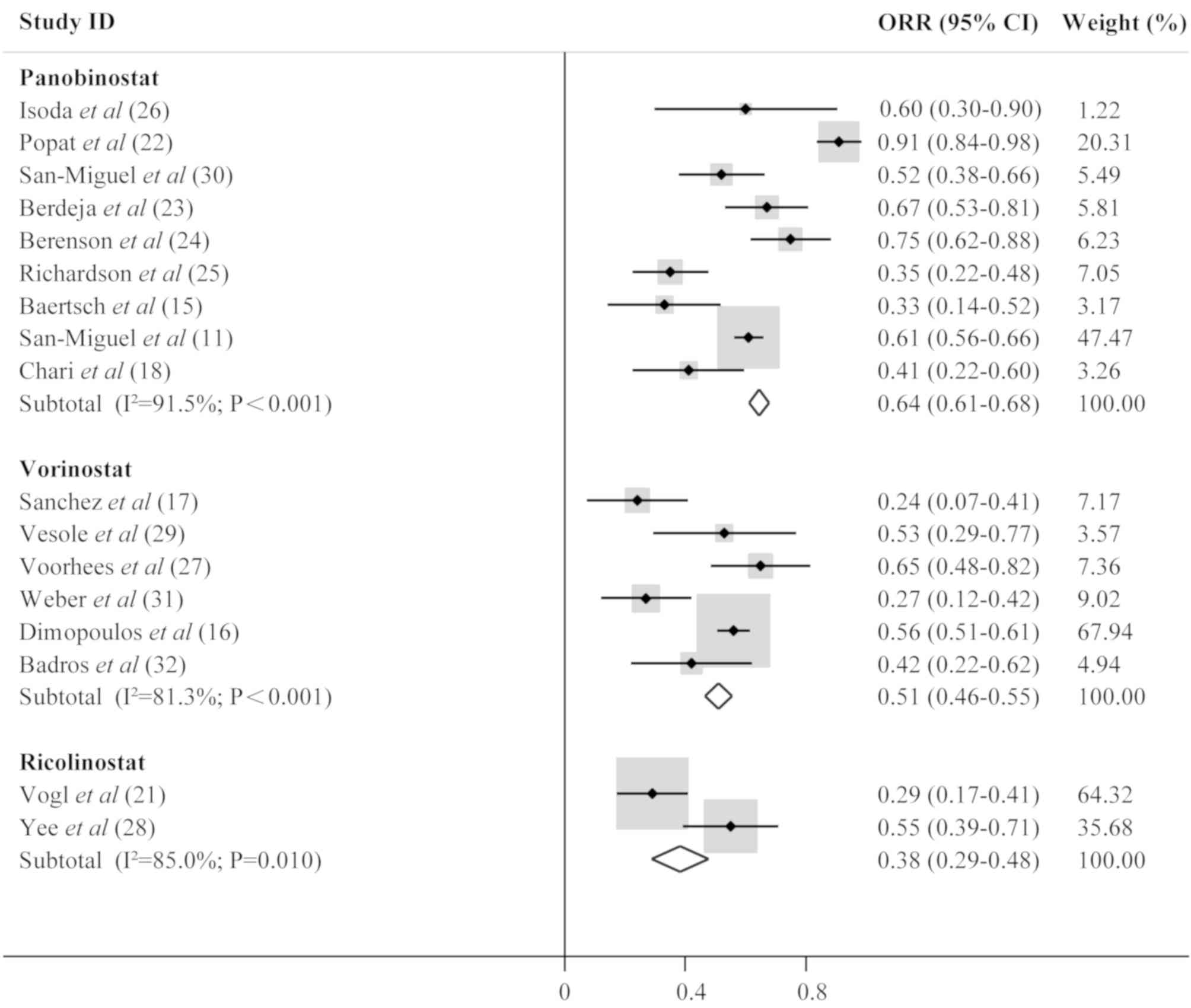
TN, Siritanaratkul N, et al: Panobinostat plus bortezomib and
dexamethasone versus placebo plus bortezomib and dexamethasone in
patients with relapsed or relapsed and refractory multiple myeloma:
A multicentre, randomised, double-blind phase 3 trial. Lancet
Oncol. 15:1195–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harada T, Hideshima T and Anderson KC:
Histone deacetylase inhibitors in multiple myeloma: From bench to
bedside. Int J Hematol. 104:300–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Preferred reporting items for systematic reviews and
meta-analyses: The PRISMA statement. PLoS Med. 6:e10000972009.
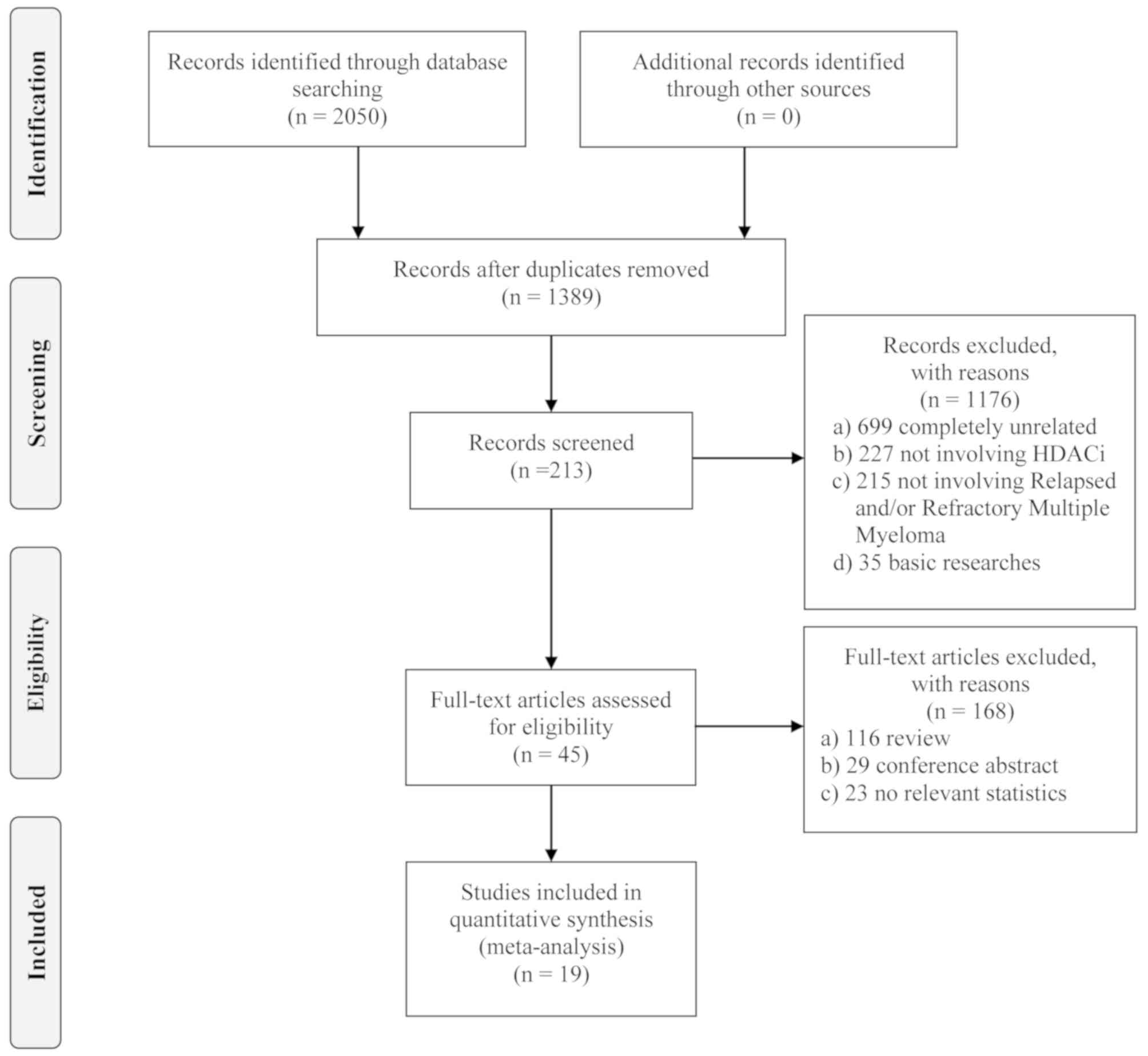
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baertsch MA, Hillengass J, Blocka J,
Schönland S, Hegenbart U, Goldschmidt H and Raab MS: Efficacy and
tolerability of the histone deacetylase inhibitor panobinostat in
clinical practice. Hematol Oncol. 36:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
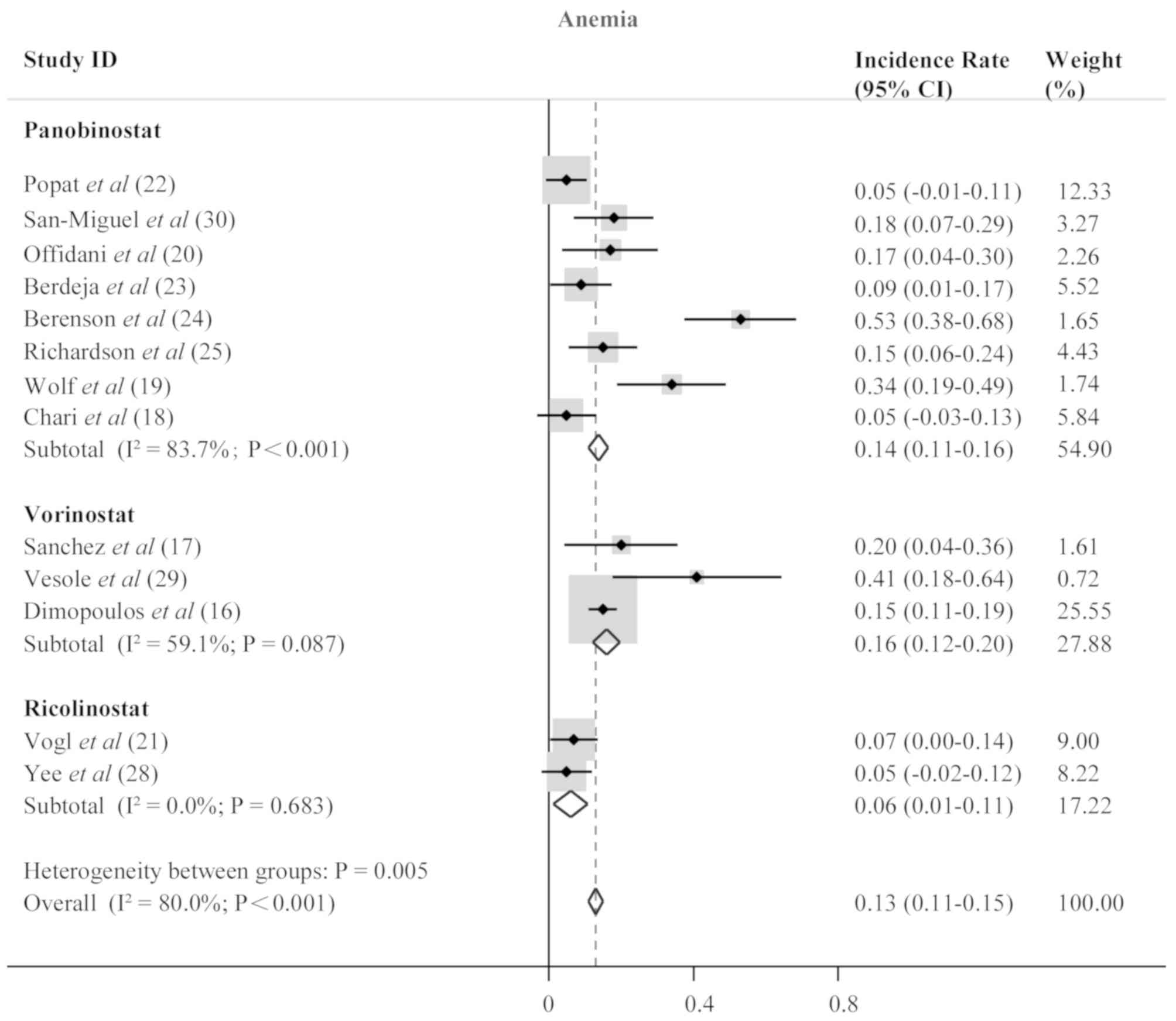
Dimopoulos M, Siegel DS, Lonial S, Qi J,
Hajek R, Facon T, Rosinol L, Williams C, Blacklock H, Goldschmidt
H, et al: Vorinostat or placebo in combination with bortezomib in
patients with multiple myeloma (VANTAGE 088): A multicentre,
randomised, double-blind study. Lancet Oncol. 14:1129–1140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanchez L, Vesole DH, Richter JR, Biran N,
Bilotti E, McBride L, Anand P, Ivanovski K and Siegel DS: A phase
IIb trial of vorinostat in combination with lenalidomide and
dexamethasone in patients with multiple myeloma refractory to
previous lenalidomide-containing regimens. Br J Haematol.
176:440–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chari A, Cho HJ, Dhadwal A, Morgan G, La
L, Zarychta K, Catamero D, Florendo E, Stevens N, Verina D, et al:
A phase 2 study of panobinostat with lenalidomide and weekly
dexamethasone in myeloma. Blood Adv. 1:1575–1583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wolf JL, Siegel D, Goldschmidt H, Hazell
K, Bourquelot PM, Bengoudifa BR, Matous J, Vij R, de
Magalhaes-Silverman M, Abonour R, et al: Phase II trial of the
pan-deacetylase inhibitor panobinostat as a single agent in
advanced relapsed/refractory multiple myeloma. Leuk Lymphoma.
53:1820–1823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Offidani M, Polloni C, Cavallo F, Liberati
AM, Ballanti S, Pulini S, Catarini M, Alesiani F, Corvatta L,
Gentili S, et al: Phase II study of melphalan, thalidomide and
prednisone combined with oral panobinostat in patients with
relapsed/refractory multiple myeloma. Leuk Lymphoma. 53:1722–1727.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogl DT, Raje N, Jagannath S, Richardson
P, Hari P, Orlowski R, Supko JG, Tamang D, Yang M, Jones SS, et al:
Ricolinostat, the first selective histone deacetylase 6 inhibitor,
in combination with bortezomib and dexamethasone for relapsed or
refractory multiple myeloma. Clin Cancer Res. 23:3307–3315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Popat R, Brown SR, Flanagan L, Hall A,
Gregory W, Kishore B, Streetly M, Oakervee H, Yong K, Cook G, et
al: Bortezomib, thalidomide, dexamethasone, and panobinostat for
patients with relapsed multiple myeloma (MUK-six): A multicentre,
open-label, phase 1/2 trial. Lancet Haematol. 3:e572–e580. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
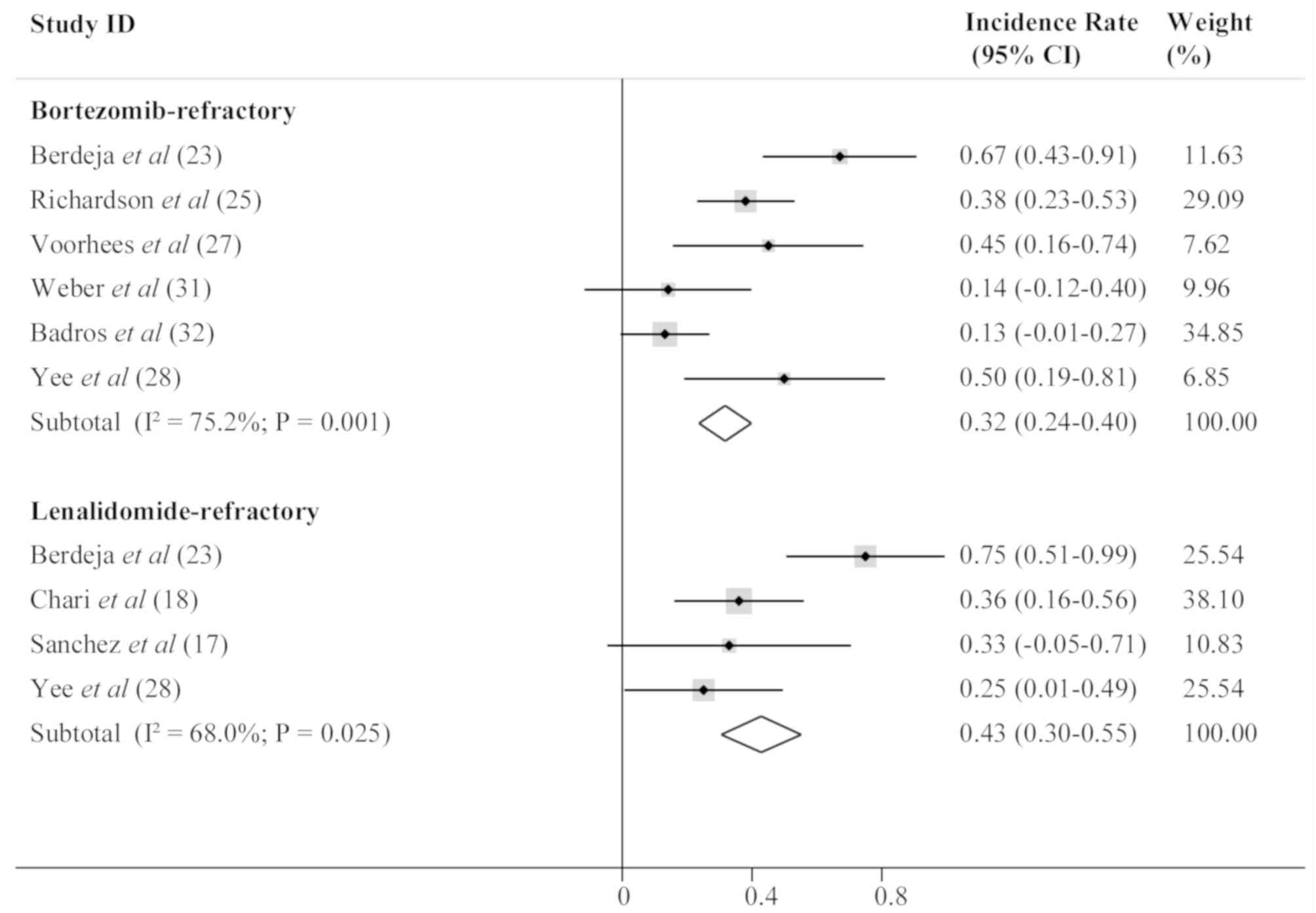
Berdeja JG, Hart LL, Mace JR, Arrowsmith
ER, Essell JH, Owera RS, Hainsworth JD and Flinn IW: Phase I/II
study of the combination of panobinostat and carfilzomib in
patients with relapsed/refractory multiple myeloma. Haematologica.
100:670–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berenson JR, Hilger JD, Yellin O, Boccia
RV, Matous J, Dressler K, Ghazal HH, Jamshed S, Kingsley EC, Harb
WA, et al: A phase 1/2 study of oral panobinostat combined with
melphalan for patients with relapsed or refractory multiple
myeloma. Ann Hematol. 93:89–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richardson PG, Schlossman RL, Alsina M,
Weber DM, Coutre SE, Gasparetto C, Mukhopadhyay S, Ondovik MS, Khan
M, Paley CS and Lonial S: PANORAMA 2: Panobinostat in combination
with bortezomib and dexamethasone in patients with relapsed and
bortezomib-refractory myeloma. Blood. 122:2331–2337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isoda A, Ishikawa T, Miyazawa Y, Mihara M,
Matsumoto M and Sawamura M: Intra-patient dose escalation of
panobinostat in patients with relapsed/refractory multiple myeloma.
Leuk Lymphoma. 59:1277–1278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voorhees PM, Gasparetto C, Moore DT,
Winans D, Orlowski RZ and Hurd DD: Final results of a Phase 1 study
of vorinostat, pegylated liposomal doxorubicin, and bortezomib in
relapsed or refractory multiple myeloma. Clin Lymphoma, Myeloma
Leuk. 17:424–432. 2017. View Article : Google Scholar
|
|
28
|
Yee AJ, Bensinger WI, Supko JG, Voorhees
PM, Berdeja JG, Richardson PG, Libby EN, Wallace EE, Birrer NE,
Burke JN, et al: Ricolinostat plus lenalidomide, and dexamethasone
in relapsed or refractory multiple myeloma: A multicentre phase 1b
trial. Lancet Oncol. 17:1569–1578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vesole DH, Bilotti E, Richter JR, McNeill
A, McBride L, Raucci L, Anand P, Bednarz U, Ivanovski K, Smith J,
et al: Phase I study of carfilzomib, lenalidomide, vorinostat, and
dexamethasone in patients with relapsed and/or refractory multiple
myeloma. Br J Haematol. 171:52–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
San-Miguel JF, Richardson PG, Gunther A,
Sezer O, Siegel D, Bladé J, LeBlanc R, Sutherland H, Sopala M,
Mishra KK, et al: Phase Ib study of panobinostat and bortezomib in
relapsed or relapsed and refractory multiple myeloma. J Clin Oncol.
31:3696–3703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weber DM, Graef T, Hussein M, Sobecks RM,
Schiller GJ, Lupinacci L, Hardwick JS and Jagannath S: Phase I
trial of vorinostat combined with bortezomib for the treatment of
relapsing and/or refractory multiple myeloma. Clin Lymphoma Myeloma
Leuk. 12:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Badros A, Burger AM, Philip S, Niesvizky
R, Kolla SS, Goloubeva O, Harris C, Zwiebel J, Wright JJ,
Espinoza-Delgado I, et al: Phase I study of vorinostat in
combination with bortezomib for relapsed and refractory multiple
myeloma. Clin Cancer Res. 15:5250–5257. 2009. View Article : Google Scholar : PubMed/NCBI
|