Introduction
As a type of human malignancy originating from the
glial cells of the spine or the brain, glioma accounts for >30%
of cases of central nervous system and brain tumors and >80% of
all malignant brain tumors (1,2).
Patients with brain glioma usually show headaches, seizures,
cranial nerve disorders and vomiting, which are caused by the
increased intracranial pressure. By contrast, weakness, pain and
numbness in the extremities are the main symptoms of optic nerve
glioma (3). Hereditary genetic
disorders and activation of certain oncogenes have been associated
with the development of gliomas (4,5). At
present, the molecular mechanism of the pathogenesis of glioma
remains unclear, leading to poor treatment outcomes for patients
with glioma.
Survivin, also known as baculoviral inhibitor of
apoptosis repeat-containing 5 (BIRC5), is a member of the inhibitor
of apoptosis family that inhibits programmed cell death or
apoptosis through the inhibition of caspase activation (6). A growing body of literature has shown
that survivin is frequently overexpressed in human cancers, and the
overexpression of survivin inhibits cancer cell apoptosis (7,8). At
present, inhibition of survivin is reported to be a promising
therapeutic target for cancer treatment (9,10). It
has been reported that the expression and degradation of survivin
in cancer development can be regulated by long non-coding RNAs
(lncRNAs) (11,12). Long intergenic non-coding RNA for
kinase activation (LINK-A) lncRNA has been characterized as an
oncogenic lncRNA in triple negative breast cancer (13). In breast cancer, LINK-A lncRNA
promotes cancer development by activating normoxic
hypoxia-inducible factor 1-α (HIF1α) signaling (13). Based on current knowledge, the
involvement of LINK-A lncRNA in other human diseases is unknown. In
addition, the interaction between LINK-A and survivin is still
unknown. In the present study, the role of LINK-A lncRNA in the
regulation of survivin expression and glioma cell apoptosis was
investigated.
Materials and methods
Cell lines and human specimens
Blood was extracted from 52 patients with glioma and
38 healthy volunteers to prepare serum. The participants were
admitted to the General Hospital of Ningxia Medical University
(Yinchuan, China) between May 2015 and May 2018. The inclusion
criteria for patients were: i) Diagnosis by histopathological
examination; ii) no other severe diseases diagnosed; and iii)
patients who understood the experimental protocol and signed
informed consent. The exclusion criteria were as follows: i)
Diagnosis with multiple diseases; and ii) patients who were treated
within the 3 months before blood extraction. The 52 patients with
glioma included 30 males and 22 females, with a mean age of
45.8±5.6 years (range, 32–64 years). There were 12 cases of grade
I, 13 cases of grade II, 10 cases of grade III and 17 cases of
grade IV. The 38 healthy volunteers included 21 males and 17
females with a mean age of 47.1±5.4 years (range, 33–67 years).
Patient and control groups showed similar age and sex
distributions. The present study was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University.
All patients and healthy volunteers signed written informed
consent.
Human glioma cell lines CCD-25Lu and Hs 683 were
provided by American Type Culture Collection (ATCC).
ATCC-formulated Eagle's Minimum Essential Medium (cat. no. 30-2003)
containing 10% fetal bovine serum (ATCC) was used to culture cells
of both cell lines at 37°C with 5% CO2 and 95%
humidity.
ELISA
Human Survivin Quantikine ELISA kit (DSV00; R&D
Systems China Co., Ltd.) was used to measure levels of survivin in
serum from patients with glioma and healthy controls. The assay was
performed according to the manufacturer's protocol. Serum levels of
survivin were expressed as pg/ml.
Reverse transcription quantitative PCR
(RT-qPCR)
RNA extractions were performed using GenElute™
Plasma/Serum RNA Purification Mini kit (RNB500-50RXN;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Next, cDNA was synthesized using RevertAid RT Reverse
Transcription kit (Thermo Fisher Scientific, Inc.). Samples were
prepared for PCR using SYBR™ Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions for qPCR were as follows: 1 min at 95°C, followed by 40
cycles of 10 sec at 95°C and 40 sec at 58°C. Primers were as
follows: LINK-A forward, 5′-TTCCCCCATTTTTCCTTTTC-3′ and reverse,
5′-CTCTGGTTGGGTGACTGGTT-3′; β-actin forward,
5′-GACCTCTATGCCAACACAGT-3′ and reverse, 5′-AGTACTTGCGCTCAGGAGGA-3′;
and survivin forward, 5′-AAGAACTGGCCCTTCTTGGA-3′ and reverse,
5′-CAACCGGACGAATGCTTTT-3. The 2−ΔΔCq method (14) was used to quantify relative RNA
levels and β-actin was used as the reference gene.
Vectors, siRNAs and cell
transfection
SiRNA (5′-UCCACACACCGCCUCCCACCU-3′) targeting LINK-A
lncRNA, scrambled negative control siRNA
(5′-UUCUCCGAACGUGUCACGUdTdT-3′), LINK-A lncRNA expression vectors
and survivin expression vectors were designed and synthesized by
Shanghai GenePharma Co., Ltd. using pcDNA3.1 vector. Cell
transfections were performed using Lipofectamine™ 2000 transfection
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
concentration of vector used for transfection was 15 nM and the
concentration of siRNAs was 45 nM. Cells treated with transfection
reagent only, without siRNA or vectors, were control cells. Cells
transfected with scrambled negative control siRNA or empty vectors
were negative control cells. At 24 after transfection, expression
of LINK-A lncRNA and survivin was detected by RT-qPCR to confirm
that expression was increased to >200% or decreased to <50%
prior to subsequent experiments (data not shown).
Cell apoptosis assay
Cells were harvested at 24 h after transfection.
Cells with different treatments (LINK-A and survivin expression
vector transfection groups, LINK-A siRNA transfection group, as
well as corresponding control and negative control groups)
aforementioned were harvested to make single cell suspensions
(6×104 cells/ml) using serum-free cell culture medium.
Each well of a 6-well plate was filled with 10 ml cell suspension.
Cells were cultivated under normal conditions for 48 h. The cells
were then subjected to trypsin (0.25%) digestion. After staining
with Annexin V-fluorescein isothiocyanate (Dojindo Molecular
Technologies, Inc.) and propidium iodide (PI; Dojindo Molecular
Technologies, Inc.), apoptotic cells were detected by flow
cytometry. Cyflogic free flow cytometry software version 1.2.1
(http://www.cyflogic.com/) was used to analyze
data.
Western blot analysis
Total Protein Extraction (TPE™) kit (VWR
International Co.) was used for the extraction of total protein
from samples. A BCA kit (Sangon Biotech Co., Ltd.) was used to
measure protein concentrations. After denaturing, protein samples
were separated by SDS-PAGE using a 10% gel with 20 µg per lane.
Following transfer to PVDF membranes, blocking was performed in PBS
containing 5% fat-free milk at room temperature for 2 h. The
membranes were then incubated with primary antibodies of Rabbit
anti-human survivin (1:1,400; ab76424; Abcam) and rabbit anti-human
GAPDH (1:1,400; ab9485; Abcam) overnight at 4°C. Following this,
membranes were then incubated with goat anti-rabbit IgG-horseradish
peroxidase secondary antibody (1:1,300; MBS435036; MyBioSource,
Inc.) at 37°C for 2 h. Pierce ECL western blotting substrate
(Thermo Fisher Scientific, Inc.) was used to develop signals.
MYECL™ Imager (Thermo Fisher Scientific, Inc.) was used to scan
signals and ImageJ version 1.46 software (National Institutes of
Health) was used to quantify band intensity.
Statistical analysis
Experiments were repeated three times. Data are
presented as mean ± standard deviation and Graphpad Prism 6
software (GraphPad Software, Inc.) was used for all statistical
analyses. Diagnostic analysis was performed by receiver operating
characteristic (ROC) curve analysis with glioma patients as true
positive cases and healthy controls as true negative cases.
Correlation between serum levels of LINK-A lncRNA and survivin was
analyzed using Pearson's correlation coefficient. Comparisons were
performed using Student's t-test when comparing two groups and
one-way analysis of variance followed by Tukey test when comparing
more than two groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Serum levels of LINK-A lncRNA and
survivin are significantly higher in patients with glioma compared
with healthy controls
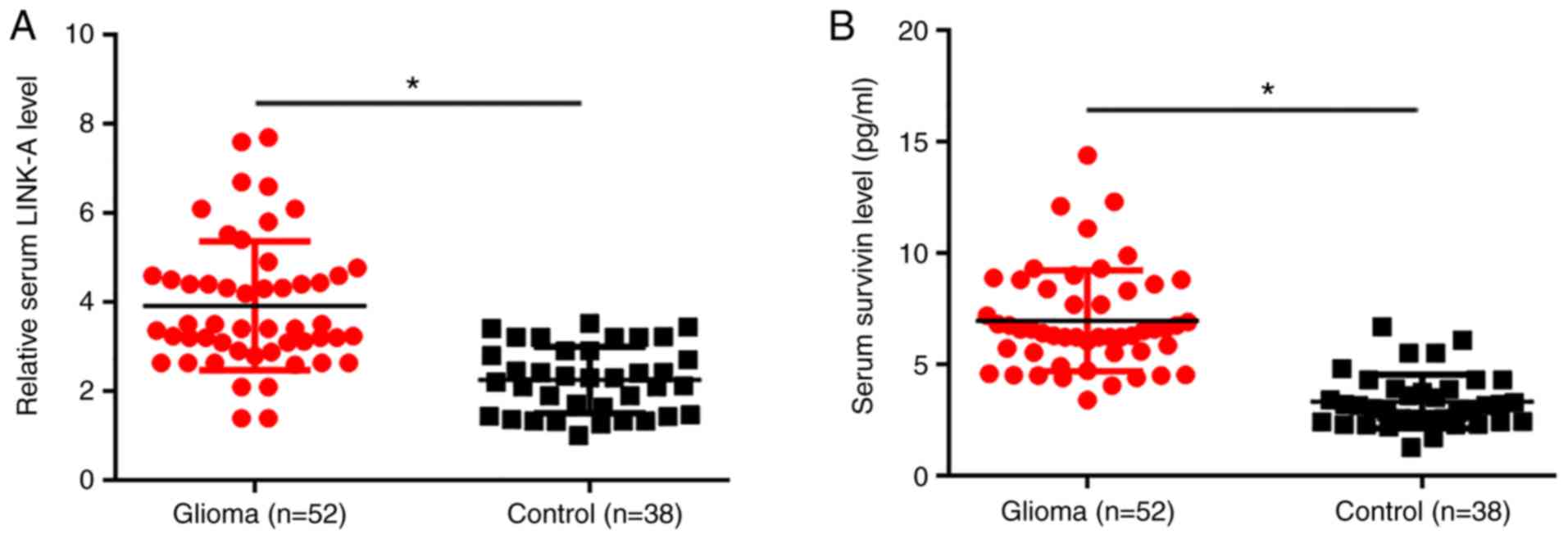
RT-qPCR results showed that, compared with healthy
controls, serum levels of LINK-A lncRNA were significantly
increased in patients with glioma (P<0.05; Fig. 1A). ELISA results showed that the
serum level of survivin was also significantly higher in patients
with glioma compared with healthy controls (P<0.05; Fig. 1B).
Serum levels of LINK-A lncRNA and
survivin are positively correlated in patients with glioma
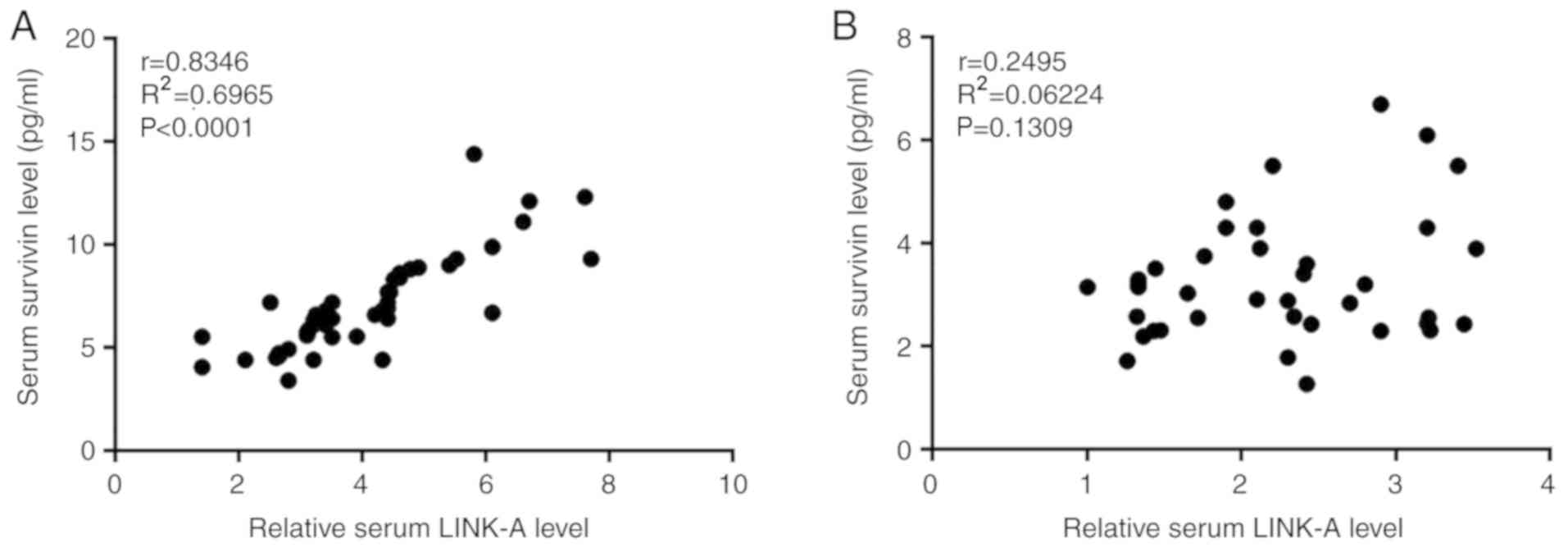
Serum levels of LINK-A lncRNA and survivin were
analyzed using Pearson's correlation coefficient. As presented in
Fig. 2A, a significant positive
correlation between serum levels of LINK-A lncRNA and survivin was
observed in patients with glioma. By contrast, no correlation was
observed in healthy controls (Fig.
2B).
Increased serum levels of LINK-A
lncRNA distinguish patients with glioma from healthy controls
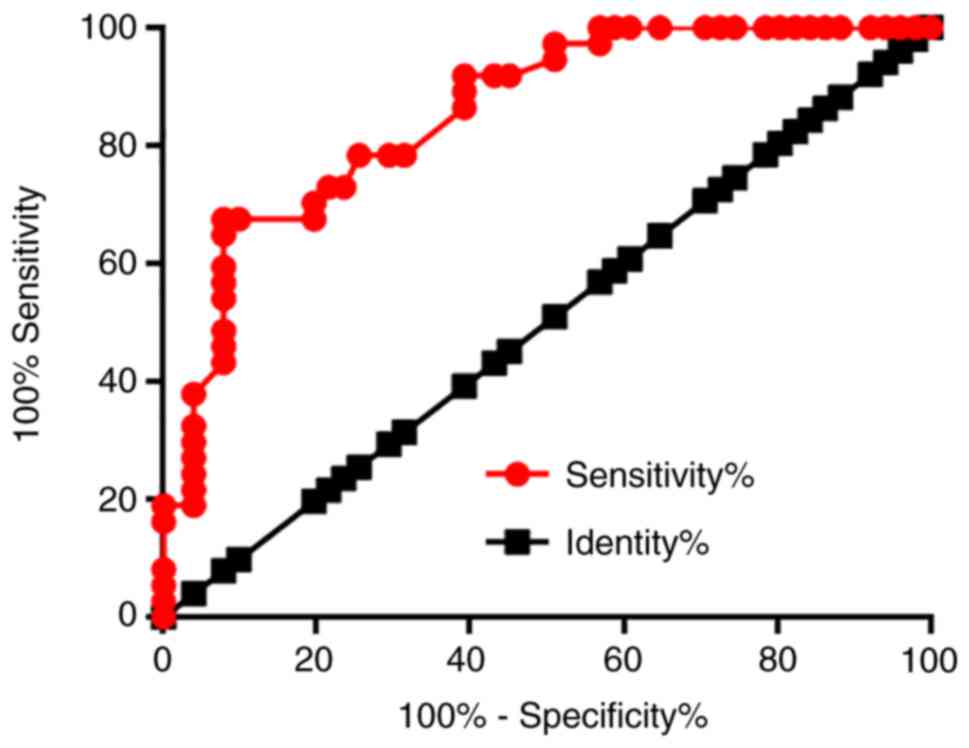
The diagnostic value of serum LINK-1 lncRNA for
glioma was evaluated by ROC curve analysis using glioma patients as
true positive cases and healthy controls as true negative cases. As
presented in Fig. 3, the area under
the curve was 0.8543 (standard error, 0.0394; 95% confidence
interval, 0.7770–0.9315; P<0.001).
LINK-A lncRNA is an upstream activator
of survivin in glioma cells
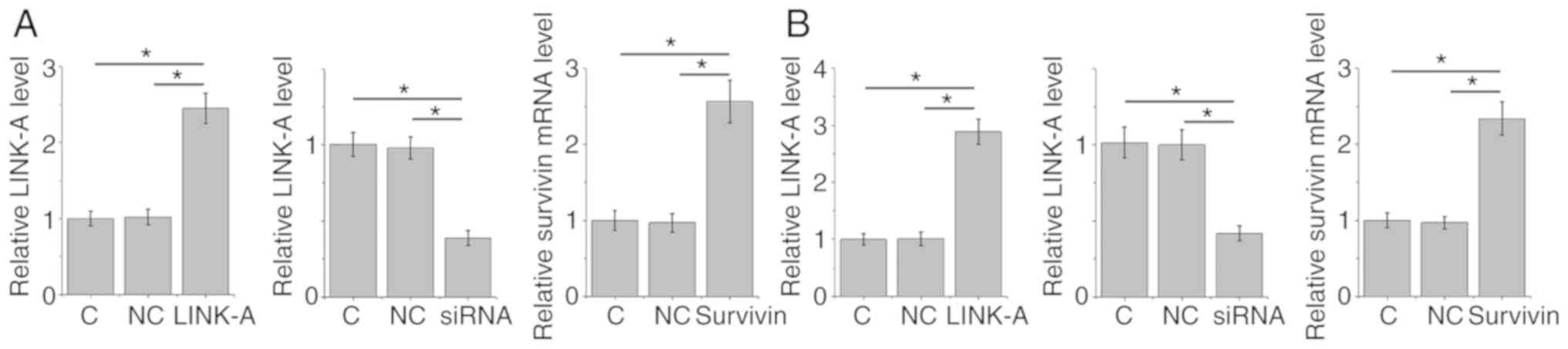
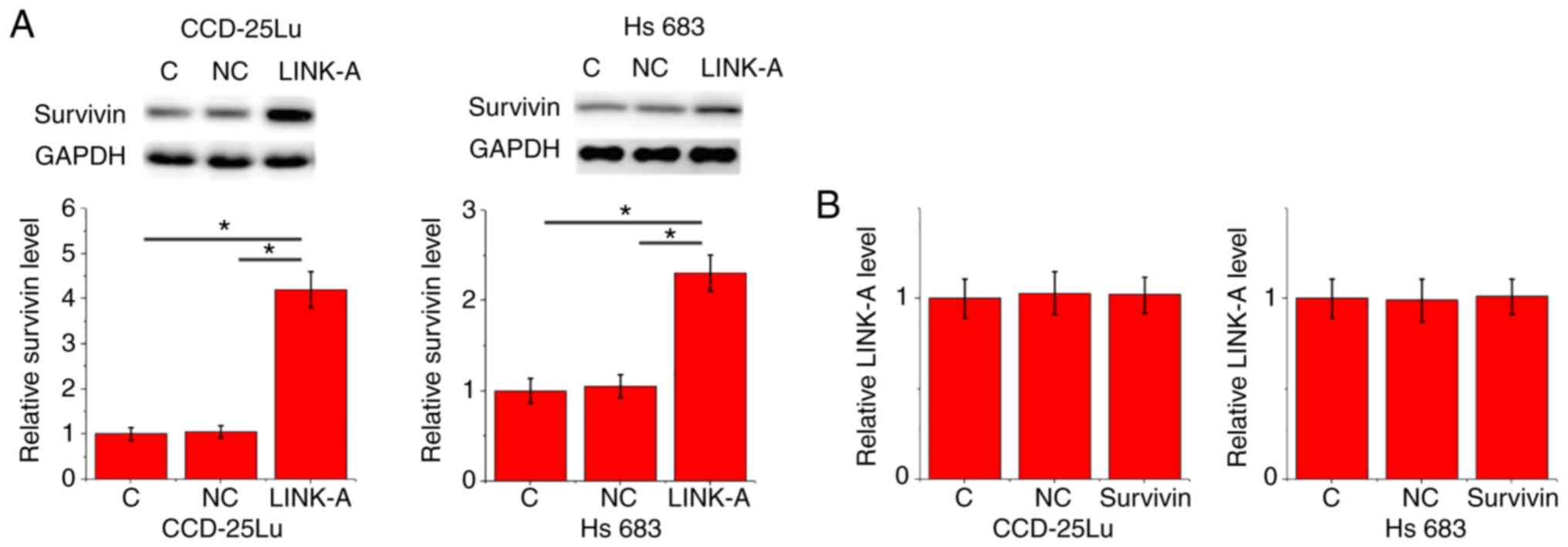
Confirmation of LINK-A overexpression and knockdown
and survivin overexpression in CCD-25Lu and Hs 683 human glioma
cell lines following transfection is presented in Fig. 4 (P<0.05). After transfection, the
expression levels of LINK-A lncRNA and survivin were detected by
RT-qPCR and western blotting, respectively. Compared with the
control and negative control groups, LINK-A lncRNA overexpression
resulted in significantly upregulated expression of survivin in
CCD-25Lu and Hs 683 human glioma cell lines (P<0.05; Fig. 5A). Compared with the control and
negative control groups, survivin overexpression did not
significantly affect LINK-A expression (P<0.05; Fig. 5B).
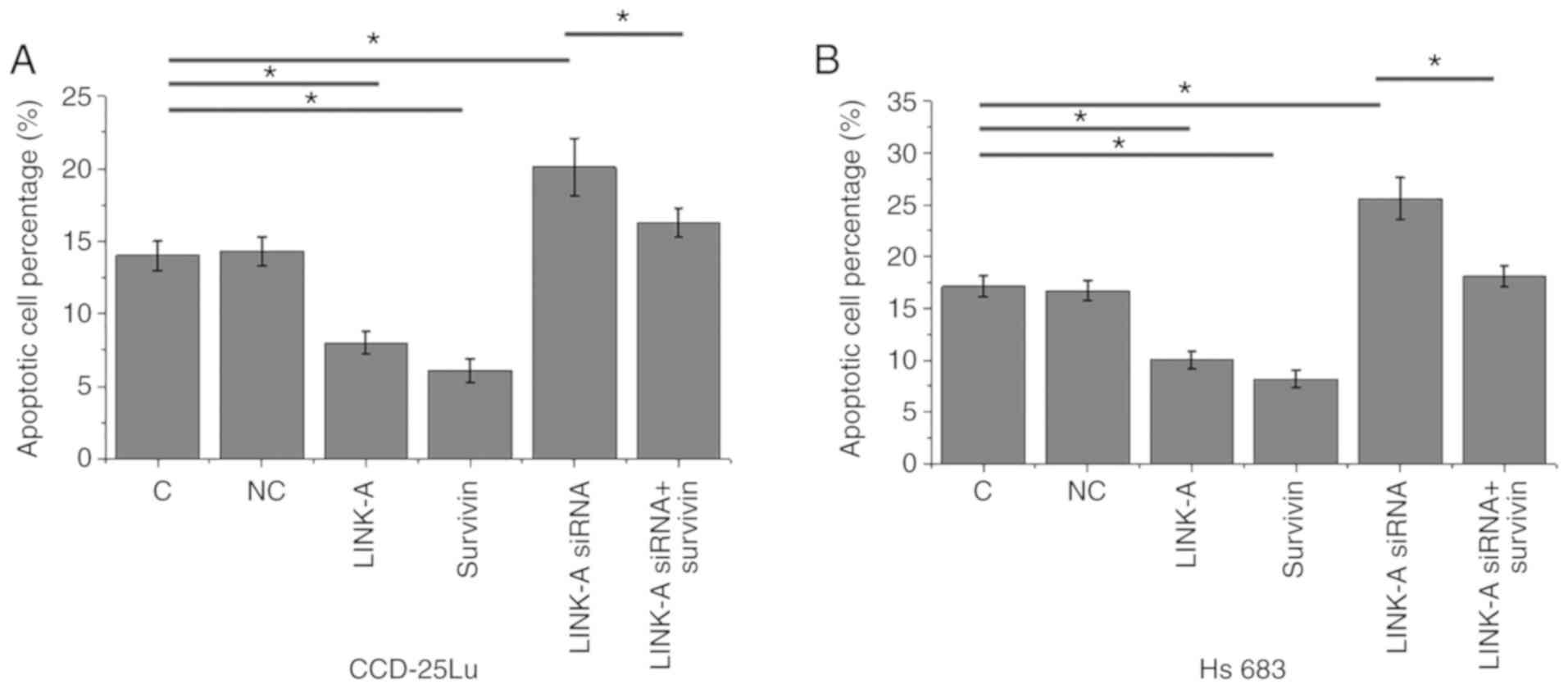
LINK-A lncRNA overexpression inhibits
apoptosis glioma cell through survivin
Cell apoptosis was detected following transfection.
Compared with the control and negative control groups, LINK-A
lncRNA and survivin overexpression significantly inhibited
apoptosis in CCD-25Lu and Hs 683 human glioma cell lines
(P<0.05; Fig. 6). The
siRNA-mediated knockdown of LINK-A lncRNA resulted in significantly
increased apoptosis of CCD-25Lu and Hs 683 glioma cell lines
(P<0.05). In addition, survivin overexpression attenuated the
inducing effect of LINK-A knockdown on glioma cell apoptosis
(P<0.05).
Discussion
A recent study reported that LINK-A lncRNA serves a
role in triple negative breast cancer as an oncogene by regulating
energy metabolism (13). However,
the involvement of LINK-A in other human diseases is unknown. In
the present study, the role of LINK-A lncRNA in glioma as an
oncogene was investigated. The effects of LINK-A lncRNA in glioma
cells may be mediated through the upregulation of survivin, which
is a key player in cancer development and progression (7–10).
Survivin has been found to be overexpressed during
the development of different types of human cancers, suggesting it
may serve a regulatory role in cancer cell apoptosis (7). In glioma, the overexpression of
survivin has been associated with accelerated cancer cell
proliferation and inhibited cancer cell apoptosis in glioma
(15). Detection of survivin
expression in tumor tissue is informative in estimating the
prognosis of glioma patients (16).
Survivin inhibitors are considered as potential therapeutic drugs
for the treatment of human cancer, including glioma (8,9,17). Consistent with previous studies
(8,9,17), the
present study showed that the serum level of survivin was
significantly higher in patients with glioma compared with healthy
controls. In addition, the overexpression of survivin significantly
inhibited the apoptosis of glioma cells in vitro. These data
further confirm the oncogenic role of survivin in glioma.
LncRNAs serve important roles in glioma (18–21).
Differentially expressed lncRNAs in glioma promote or inhibit
cancer development (18,19). In triple negative breast cancer,
LINK-A lncRNA was found to be overexpressed, and potentially acts
as an oncogene (13). In the present
study, LINK-A lncRNA expression was observed to be significantly
upregulated in patients with glioma compared with healthy controls.
Additionally, upregulation of LINK-A in serum distinguished
patients with glioma from healthy controls, indicating that it may
have potential as a biomarker in the diagnosis of glioma.
Additionally, LINK-A lncRNA overexpression inhibited, while
siRNA-mediated knockdown of LINK-A promoted the apoptosis in
cultured glioma cells in vitro. Therefore, inhibition of
LINK-A lncRNA may serve as a potential therapeutic target for
glioma.
A significant correlation was observed between the
expression levels of LINK-A lncRNA and survivin in serum of
patients with glioma indicating that there may be a potential
interaction between them. In vitro experiments demonstrated
that LINK-A lncRNA may be an upstream activator of survivin in the
regulation of apoptosis in glioma cells. However, the mechanism of
the role of LINK-A lncRNA in the regulation of survivin expression
is unknown. Disease-related mediators may exist, as no correlation
was observed between LINK-A lncRNA and survivin in the serum of
healthy controls in the present study. It is known that LINK-A
lncRNA promotes cancer development by activating normoxic HIF1α
signaling in triple negative breast cancer (13). However, LINK-A lncRNA overexpression
failed to significantly affect HIF1α in glioma cells (data not
shown). Since a previous study found that LINK-A lncRNA can
interact with phosphatidylinositol-3,4,5-trisphosphate, which may
also participate in cancer biology (22), future studies will focus on the
interactions between LINK-A lncRNA and
phosphatidylinositol-3,4,5-trisphosphate in glioma. A limitation of
the present study is that it did not measure LINK-A lncRNA
expression in tumor tissues; however, circulating RNA levels are
able to reflect gene expression in tumors (23).
In conclusion, LINK-A lncRNA and survivin were
upregulated in serum from patients with glioma. In addition, LINK-A
lncRNA may inhibit apoptosis in glioma cells by upregulating
survivin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XH designed experiments. XH and GL performed
experiments. ZL and ZN collected and analyzed data. XH drafted this
manuscript. All authors read and approved this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the General Hospital of Ningxia Medical University.
All patients and healthy volunteers provided written informed
consent prior to their inclusion in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goodenberger ML and Jenkins RB: Genetics
of adult glioma. Cancer Genet. 205:613–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mamelak AN and Jacoby DB: Targeted
delivery of antitumoral therapy to glioma and other malignancies
with synthetic chlorotoxin (TM-601). Expert Opin Drug Deliv.
4:175–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macartney G, Harrison MB, VanDenKerkhof E,
Stacey D and McCarthy P: Quality of life and symptoms in pediatric
brain tumor survivors: A systematic review. J Pediatr Oncol Nurs.
31:65–77. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rice T, Lachance DH, Molinaro AM,
Eckel-Passow JE, Walsh KM, Barnholtz-Sloan J, Ostrom QT, Francis
SS, Wiemels J, Jenkins RB, et al: Understanding inherited genetic
risk of adult glioma-a review. Neurooncol Pract. 3:10–16.
2016.PubMed/NCBI
|
|
5
|
Radner H, el-Shabrawi Y, Eibl RH, Brüstle
O, Kenner L, Kleihues P and Wiestler OD: Tumor induction by ras and
myc oncogenes in fetal and neonatal brain: Modulating effects of
developmental stage and retroviral dose. Acta Neuropathol.
86:456–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jaiswal PK, Goel A and Mittal RD:
Survivin: A molecular biomarker in cancer. Indian J Med Res.
141:389–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Duan N, Zhang C and Zhang W:
Survivin and tumorigenesis: Molecular mechanisms and therapeutic
strategies. J Cancer. 7:314–323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Yang F, Li X, Gong ZJ and Wang LW:
Long noncoding RNA LNC473 inhibits the ubiquitination of survivin
via association with USP9X and enhances cell proliferation and
invasion in hepatocellular carcinoma cells. Biochem Biophys Res
Commun. 499:702–710. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang R, Qu S, Liang H, Chen X, Zhang C and
Guo H: Long noncoding RNA H19 regulates survivin expression in
bladder cancer as sponge of miR-138-5p. Eur Urol Suppl.
16:e1464–e1465. 2017. View Article : Google Scholar
|
|
13
|
Lin A, Li C, Xing Z, Hu Q, Liang K, Han L,
Wang C, Hawke DH, Wang S, Zhang Y, et al: The LINK-A lncRNA
activates normoxic HIF1α signalling in triple-negative breast
cancer. Nat Cell Biol. 18:213–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang F, Chu J and Wang F: Expression and
clinical significance of cyclooxygenase 2 and survivin in human
gliomas. Oncol Lett. 14:1303–1308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lv S, Dai C, Liu Y, Shi R, Tang Z, Han M,
Bian R, Sun B and Wang R: Retraction note to: The impact of
survivin on prognosis and clinicopathology of glioma patients: A
systematic meta-analysis. Mol Neurobiol. 54:23762017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jane EP, Premkumar DR, Sutera PA, Cavaleri
JM and Pollack IF: Survivin inhibitor YM155 induces mitochondrial
dysfunction, autophagy, DNA damage and apoptosis in Bcl-xL silenced
glioma cell lines. Mol Carcinog. 56:1251–1265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Sun S, Pu JK, Tsang AC, Lee D,
Man VO, Lui WM, Wong ST and Leung GK: Long non-coding RNA
expression profiles predict clinical phenotypes in glioma.
Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang P, Ren Z and Sun P: Overexpression of
the long non-coding RNA MEG3 impairs in vitro glioma cell
proliferation. J Cell Biochem. 113:1868–1874. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Wei DL, Wan L, Yan SF and Sun YH:
Highly expressed lncRNA CCND2-AS1 promotes glioma cell
proliferation through Wnt/β-catenin signaling. Biochem Biophys Res
Commun. 482:1219–1225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: LncRNA CASC2 interacts with miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin A, Hu Q, Li C, Xing Z, Ma G, Wang C,
Li J, Ye Y, Yao J, Liang K, et al: The LINK-A lncRNA interacts with
PtdIns(3,4,5)P3 to hyperactivate AKT and confer
resistance to AKT inhibitors. Nat Cell Biol. 19:238–251. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kopreski MS, Benko FA, Kwak LW and Gocke
CD: Detection of tumor messenger RNA in the serum of patients with
malignant melanoma. Clin Cancer Res. 5:1961–1965. 1999.PubMed/NCBI
|