Introduction
Pruritus (itch) is the hallmark of atopic dermatitis
(AD) that can severely impair patient quality of life (1). Therefore, the ultimate aim of AD
management is to successfully treat itch. However, treatment is
difficult due to a lack of information and an insufficient
understanding of itch etiology within AD (2). A number of mediators have been
implicated in the pathogenesis of AD itch, most notably including
histamine, which has been extensively studied (3). Histamine is released from mast cells
when tissues are inflamed or stimulated by allergens and functions
to induce itch by triggering the excitation of a subset of
unmyelinated C-fibers (4). Previous
studies have identified four subtypes of histamine receptor coupled
to guanine nucleotide-binding proteins, which include histamine
receptor subtype I (H1R), histamine receptor subtype II, histamine
receptor subtype III and histamine receptor subtype IV (H4R). H1R
and H4R have been extensively studied and are considered to be the
primary receptors that mediate AD itch, leading to the extensive
use of their antagonists when managing and alleviating itch
symptoms (5,6). Histamine has been previously
demonstrated to stimulate the release of various cytokines,
including interleukin (IL)-1α and IL-6, which have also been
indicated to serve a role in itch (7,8). The
signaling pathway of histamine production involves phospholipase C
(PLC), phosphatidylinositol 3-kinase and protein kinase C (PKC)
(8). PLCγ catalyzes
phosphatidylinositol 4,5-bisphosphate hydrolysis, yielding
diacylglycerol and inositol trisphosphate, which respectively
results in PKC activation and the liberation of intracellular
calcium (8). These signals
subsequently lead to mast cell degranulation (8). Cytokines have also been demonstrated to
induce itch and activate neuropeptide release from sensory nerves
located in the skin of patients with AD (9,10).
Previous studies have revealed a cytokine (IL-31) of the
glycoprotein130/IL-6 family, which directly serves a role in AD
development in mice and humans (11,12). The
results of the aforementioned studies indicate that transgenic mice
that overexpress IL-31 or wild-type mice administered with
recombinant IL-31 protein, develop characteristic skin phenotypes
which mimic that of mice with AD. Itch in AD has also been
associated with IL-31 expression in mice. In a previous study,
IL-31 mRNA was expressed in NC/Nga AD model mice, who experienced
itch (13). These results indicate
the importance of IL-31 in the pathogenesis of AD and particularly
in itch. Until recently, various treatments have been used to
relieve itch in patients with AD (14). The use of immunomodulators and
topical applications of corticosteroids result in the cessation of
itch (14). However, long-term
applications may result in serious and imminent side effects such
as stretch marks, small red/purple spots, small and dilated blood
vessels on the surface of the skin and skin thinning, atrophy
(14). Chronic itch is difficult to
treat as current therapeutic options are frequently ineffective,
which emphasizes the requirement for more effective therapeutic
approaches (15). Furthermore,
difficulties are experienced due to the failure of most
antihistamines used in AD treatment, as histamine is not the sole
mediator of itch (16). The
requirement for new therapies for itch treatment are therefore
essential.
Recently, natural plant extracts and phytochemicals
have been reported to potentially prevent and treat several
diseases, including AD. Commiphora myrrha (Myrrh), a member
of the Burseraceae plant family, is an indigenous tree native to
Somalia, Ethiopia and northern Kenya (17). Myrrh has been traditionally used in
perfumes, balms for mummification, skin disease treatments and for
healing wounds (18). Myrrh is also
used as an anti-inflammatory and antimicrobial agent for the
treatment of oral ulcers, gingivitis, sinusitis,
glomerulonephritis, brucellosis and parasitic infections (19). In Germany, a herbal preparation
containing myrrh, coffee and chamomile flower extracts with a
well-known safety profile and has been used for >50 years in
treatments for gastrointestinal disorders (20). A randomized clinical trial involving
the same herbal preparation demonstrated that it was well tolerated
in patients and demonstrated a good safety profile, while
demonstrating potential efficacy for the treatment of ulcerative
colitis (21). Previous studies have
reported that myrrh contains biologically active metabolites,
including volatile oils (eugenol, cuminic aldehyde, metacresol,
pinene, diterpenes, limonene and sesquiterpenes), steroids,
flavonoids, terpenoids, tannins, saponins and carbohydrates
(22,23). Despite the anti-inflammatory
properties of myrrh extracts, their effect on itch-associated IL-31
cytokine and histamine secretion has not yet, to the best of our
knowledge, been investigated. The current study hypothesized that
myrrh may inhibit IL-31 and histamine secretion in
phorbol-12-myristate 13-acetate (PMA) and calcium ionophore
(A23187) stimulated human mast cells (HMC-1).
Materials and methods
Chemicals
Antibodies against IL-31 (cat. no. sc-515415),
ERK1/2 (cat. no. sc-135900), phospho-ERK1/2 (cat. no. sc-81492),
p38 (cat. no. sc-81621), phospho-p38 (cat. no. sc-166182), JNK
(cat. no. sc-7345), phospho-JNK (cat. no. sc-293136), NF-κB/p65
(cat. no. sc-8008) and phospho-NF-κB/p65 (cat. no. sc-136548) were
purchased from Santa Cruz Biotechnology, Inc. Antibodies against
β-actin (cat. no. 612657) were purchased from Bioscience. PMA and
A23187 were purchased from Sigma-Aldrich (Merck KGaA). Western
blotting reagent and western blotting buffer were purchased from
Bio-Rad Laboratories, Inc. and Thermo Fisher Scientific, Inc.,
respectively. ELISA kits for IL-31 (cat. no. 445704) and histamine
(cat. no. ENZKIT140-0001) were purchased from Biolegend, Inc. and
Enzo Life Sciences, Inc., respectively. All subsequent chemicals
used in the current study were reagent grade and have been listed
within the text.
Plant extraction
Commiphora myrrha (Myrrh) was purchased from
Omniherb (cat. no. MYR-1601). The plant was authenticated by
Professor Kim Hong-Jun of the College of Oriental Medicine, Woosuk
University (Jeollabuk-do, Republic of Korea) and a reference sample
was stored in the laboratory. Following the method of a previous
study (24), myrrh was extracted in
80% (V/V) ethanol in 2,000 ml water for 72 h at room temperature on
a shaker. The extracted sample was passed through filter paper with
a pore size of 0.45 µm (Advantec Co., Ltd.), prior to being
concentrated under reduced pressure in a vacuum (<1 bar). The
concentrated sample was then lyophilized to obtain a powder, which
was stored at −20°C for subsequent use. The percentage yield of
myrrh extract was 20% (w/w).
Cell culture and treatment with
myrrh
HMC-1 cells were cultured in Iscove's modified
Dulbecco's medium (Gibco; Thermo Fisher Scientific) at 37°C with 5%
CO2 in a humidified incubator. Culture media was
supplemented with 10% heat-inactivated FBS (Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin antibiotics
(Sigma-Aldrich; Merck KGaA). Cells (5×105 cells/ml) were
seeded into sterile six or 24-well plates for 16 h at 37°C and
treated with or without myrrh. This was performed 1 h prior to cell
stimulation with 50 nM PMA and 1 µM A23187 for the indicated time
periods. The control group was neither treated with myrrh extract
nor stimulated with PMA+A23187. For treatment, myrrh extracts were
dissolved in dimethyl sulfoxide (DMSO). The final concentration of
DMSO in cells undergoing treatment was below the <0.01%
non-toxicity level.
Cell viability assay
A Water-soluble Tetrazolium salts (WST) assay was
used to determine cell viability. Cells were pre-treated with
various concentrations of myrrh (0, 3.125, 6.25, 12.5, 25, 50 and
100 µg/ml) for 24 h at 37°C. A total of 0.01 ml EZ-Cytox reagent
(DoGenBio) was added and cells were subsequently incubated at 37°C
for 4 h. Absorbance was measured at 540 nm using a microplate
reader (Tecan Group, Ltd.).
Reverse transcription-quantitative
(RT-q)-PCR analysis
HMC-1 cells (5×105 cells/ml) were
cultured in sterile six-well dishes, pre-treated with or without
myrrh at 25 and 50 µ/ml for 1 h and then stimulated with 50 nM PMA
and 1 µM A23189 for 3 h at 37°C. Total RNA was isolated and
purified using an RNeasy Mini extraction kit (Qiagen GmbH),
according to the manufacturer's protocol, and stored at −20°C. A
total of 1 µg RNA from each sample was reverse transcribed to cDNA
using a PrimeScript™ RT Master Mix (Takara Biotechnology Co., Ltd.)
with a T100TM Thermal Cycler according to the manufacturer's
protocol (Bio-Rad Laboratories, Inc.). Using specific primers for
IL-31 (forward, 5′-TGTGCCAACAGACACCCATG-3′ and reverse,
3′-TGTTGGGCTCCAGAGGTCAA-5′) and GADPH (forward,
5′-CACTCCTCCACCTTTGACGC-3′ and reverse, 3′-TCCACCACCCTGTTGCTGTA-5′)
as a loading control, qPCR was performed using SYBR Premix Ex Taq™
(Takara Bio Inc.). The thermocycling conditions for RT-qPCR were as
follows: 95°C for 3 min followed by 45 cycles of 95°C for 30 sec,
60°C for 30 sec and 72°C for 3 sec. The final extension performed
at 72°C for 5 min. Fluorescence data was analyzed using the
2−ΔΔCq method for relative quantification with GAPDH as
a control (25).
Western blot analysis
HMC-1 cells (5×105 cells/ml) were
cultured in sterile six-well dishes, pre-treated with or without
myrrh at 25 and 50 µg/ml for 1 h and stimulated with 50 nM PMA with
the addition of 1 µM A23189 for 6 h. Whole cell proteins were
extracted using radioimmunoprecipitation assay lysis buffer
purchased from Thermo Fisher Scientific, Inc. The proteins were
quantified using Quick Start™ Bradford 1× dye reagent
(Bio-Rad Laboratories, Inc.). Whole cell protein lysate (20 µg) was
separated via electrophoresis on 12% Tris-glycine gels and
transferred to polyvinylidene difluoride membranes (Immobilon; EMD
Millipore). Membranes were blocked with 5% non-fat dry milk in TBST
(0.05% Tween-20 in TBS; pH 7.4) for 1 h at room temperature and
incubated with IL-31 (1:100), ERK1/2 (1:100), phospho-ERK1/2
(1:100), p38 (1:100), phospho-p38 (1:100), JNK (1:100), phospho-JNK
(1:100), NF-κB/p65 (1:100), or phospho-NF-κB/p65 (1:100) overnight
at 4°C. Blots were washed with TBST and incubated with mouse IgGκ
light chain binding protein (m-IgGκ BP) conjugated to horseradish
peroxidase secondary antibodies (1:10,000; cat. no. sc-516102;
Santa Cruz Biotechnology, Inc.) for 2 h at room temperature.
Antibody-specific proteins were then visualized using an enhanced
chemiluminescence detection kit (GE Healthcare). To ensure equal
protein loading, the membranes were stripped and reprobed with
anti-β-actin antibodies (1:5,000). The density of each immunoblot
band was analyzed using ImageJ (v64-bit Java 1.8.0_112) gel
analysis software (National Institutes of Health).
Measurement of cytokine and histamine
production
HMC-1 cells (5×105 cells) were cultured
in sterile 24-well plates and pre-treated with or without at 25 and
50 µg/ml myrrh for 1 h at 37°C. After stimulating cells with
PMA+A23187 for 12 h, the cells were centrifuged at 142 × g for 2
min at 4°C to obtain the supernatants. The concentration of IL-31
and histamine were subsequently determined using ELISA kits. For
the standard curve, recombinant IL-31 and histamine standards were
run alongside the samples to calculate the concentration of IL-31
and histamine. All steps were performed at room temperature and all
standards and samples were assayed in triplicate.
Statistical analysis
All data from the current study was analyzed using
one-way analysis of variacnce with a Duncan's multiple range test,
which were performed using SPSS version 20.0 statistical software
(IBM Corp). P<0.05 was considered to indicate a statistically
significant result.
Results
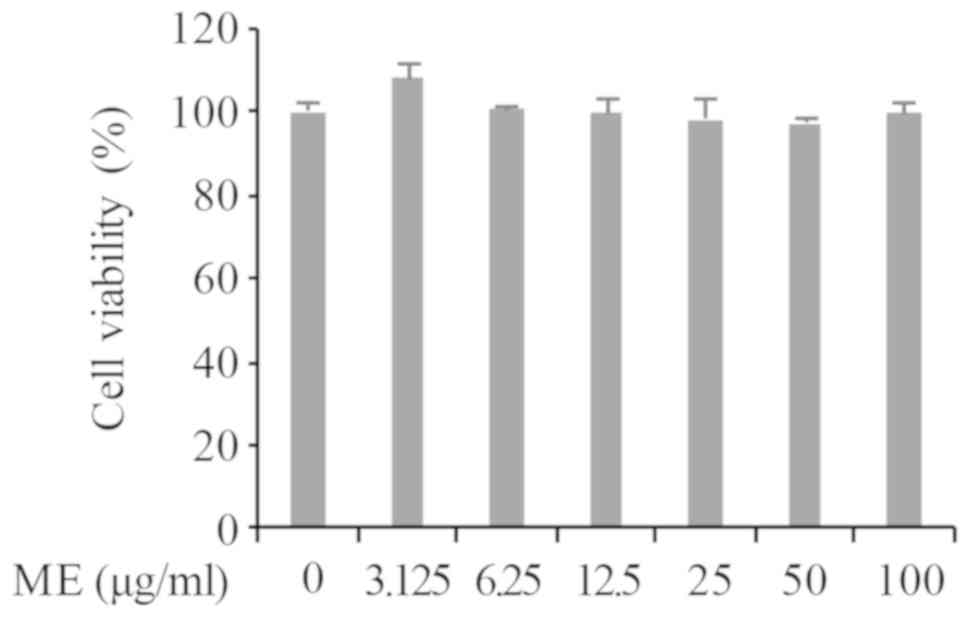
Myrrh extract exhibits no cytotoxicity
to pre-treated HMC-1
A WST cell viability assay was performed to evaluate
the cytotoxic effects of myrrh extract in HMC-1 cells. As presented
in Fig. 1, the viability of cells
were assessed 4 h after stimulation with the WST cytotoxic reagent
in the absence or presence of myrrh (0, 3.125, 6.25, 12.5, 25, 50
and 100 µg/ml). The results demonstrated that HMC-1 cell
pre-treatment with myrrh extract did not significantly affect cell
viability. Based on preliminary RT-qPCR results for IL-31 mRNA
expression the concentrations of 25 and 50 µg/ml were chosen for
subsequent experimentation.
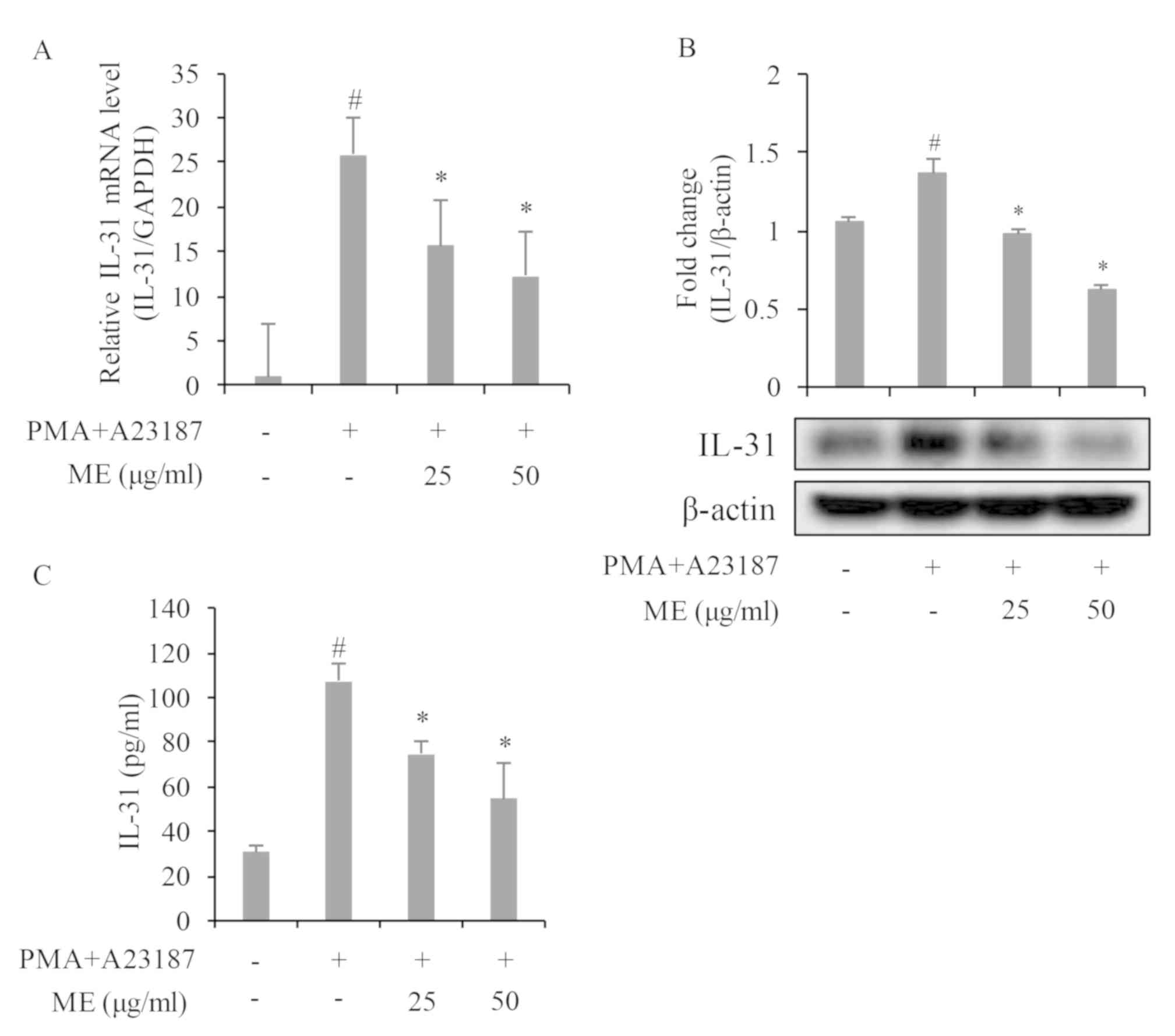
Myrrh extract suppresses IL-31 mRNA
expression
To investigate if Myrrh extract regulates IL-31 gene
expression in PMA+A23187-stimulated HMC-1, cells were pre-treated
with myrrh extract at 25 and 50 µg/ml for 1 h and subsequently
stimulated with 50 nM PMA and 1 µM A23187 for 3 h. As presented in
Fig. 2A, PMA+A23187 significantly
stimulated IL-31 mRNA expression in HMC-1 cells, while
pre-treatment with myrrh extract dose-dependently and significantly
inhibited PMA+A23187-induced IL-31 gene expression.
Myrrh extract suppresses IL-31 protein
expression
IL-31 protein expression was investigated following
treatment of PMA+A23187-stimulated HMC-1 cell samples with myrrh
extract at 25 and 50 µg/ml. Protein expression was detected 6 h
after stimulation. As presented in Fig.
2B, PMA+A23187 significantly stimulated IL-31 protein
expression in HMC-1 cells while pre-treatment with myrrh extract
significantly and dose-dependently inhibited IL-31 protein
expression.
Myrrh extract suppresses the
production of IL-31 cytokine
The release of IL-31 in cell culture media was
investigated to confirm the effect of myrrh extract on IL-31 gene
and protein expression. As presented in Fig. 2C, treatment with PMA+A23187
significantly (at 12 h) stimulated IL-31 release from HMC-1 cells
into the cell culture media. However, pre-treatment with myrrh at
25 and 50 µg/ml inhibited PMA+A23187-induced IL-31 release.
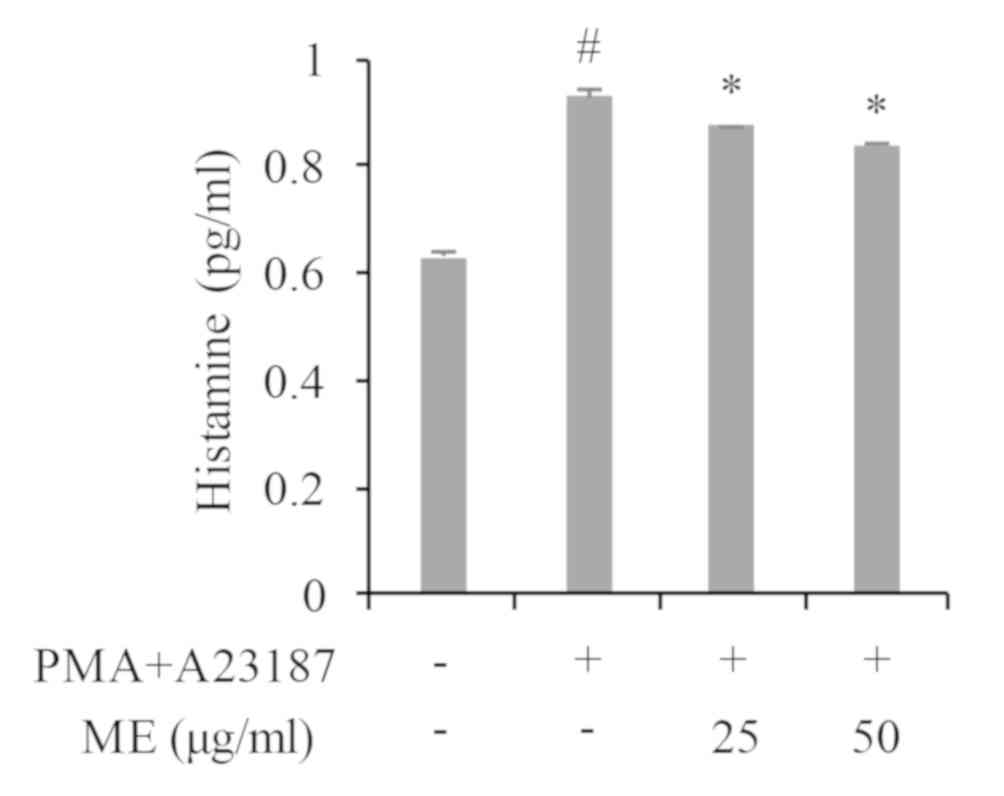
Myrrh extract inhibits histamine
production
As histamine has been widely implicated in AD itch,
the effect of myrrh extract on histamine release was also
investigated in the current study. As presented in Fig. 3, treatment with PMA+A23187
significantly stimulated histamine release in HMC-1 cells. However,
cells treated with myrrh extract (25 and 50 µg/ml) exhibited a
dose-dependent and significant decrease in histamine release.
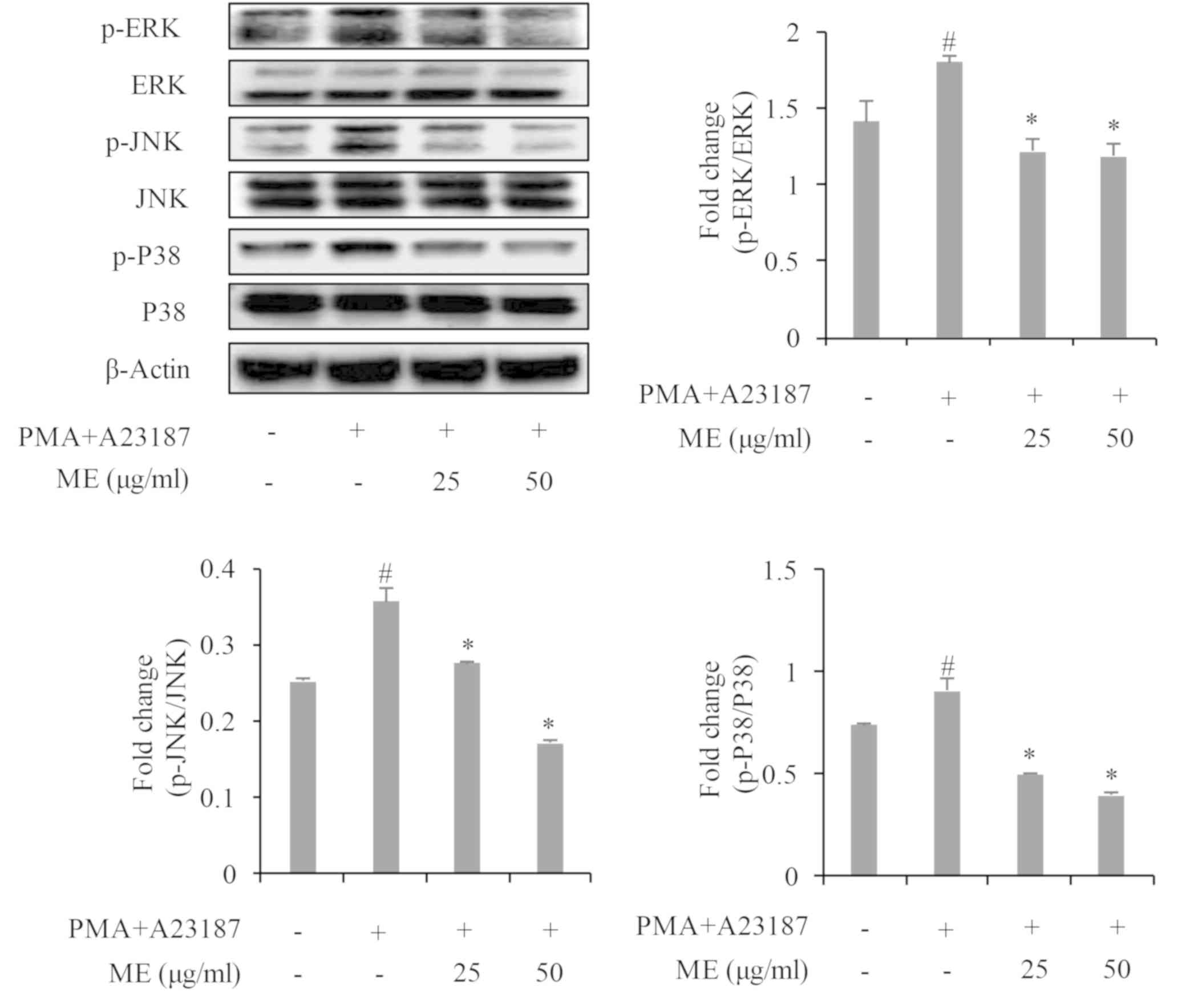
Myrrh extract suppresses
mitogen-activated protein kinases (MAPK) and NF-κB activation
To assess the mechanism of action of myrrh extract
in the suppression of IL-31 gene expression and cytokine release,
the activation of MAP kinases (p38, ERK and JNK) and NF-κB/p65 was
determined. The results revealed that PMA+A23187 significantly
stimulated the phosphorylation of p38, ERK and JNK in HMC-1 cells
within 30 min of stimulation (Fig.
4). However, pre-treatment with myrrh extract significantly and
dose-dependently suppressed the phosphorylation of p38, ERK, JNK
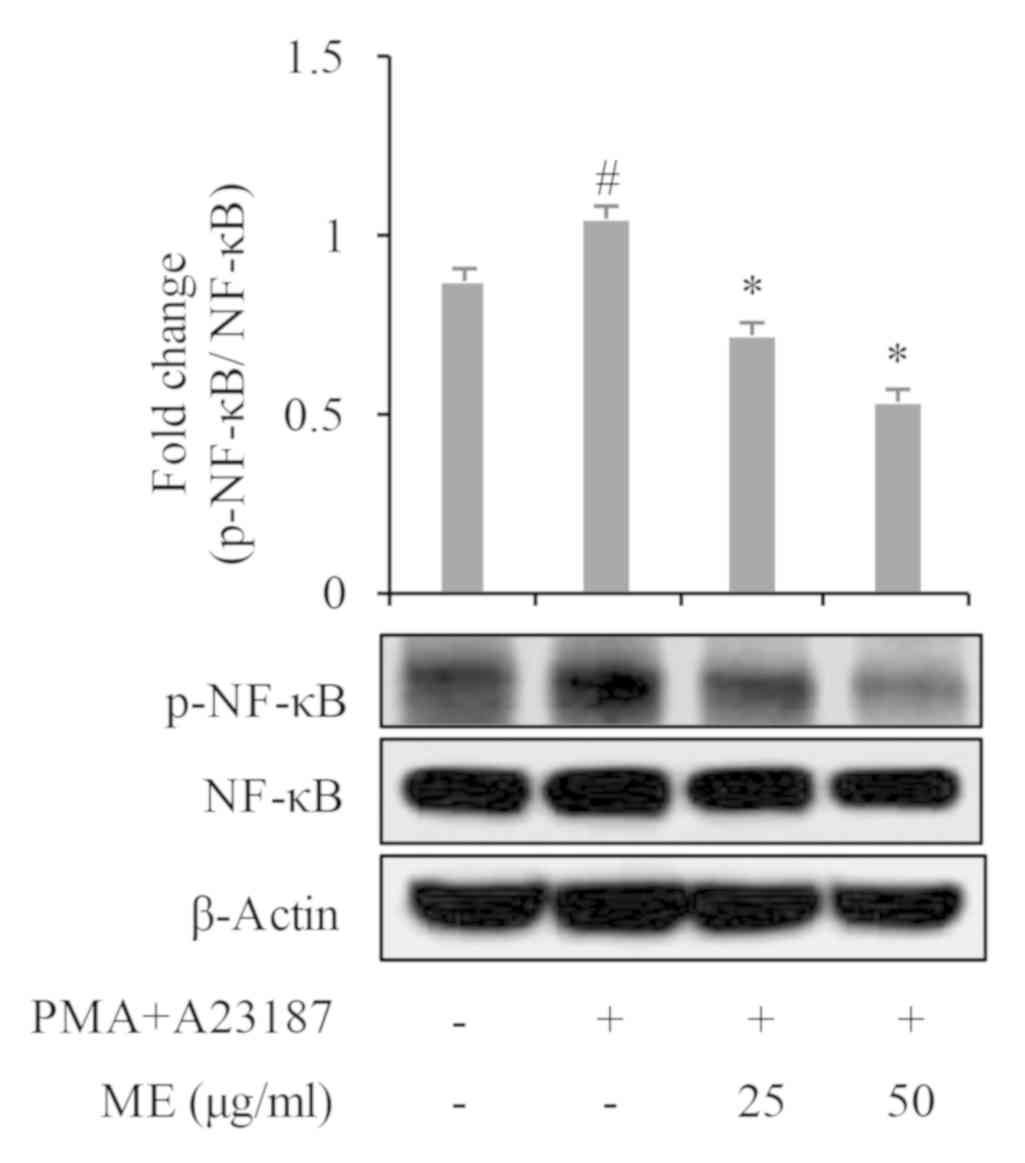
and NF-κB (Figs. 4 and 5).
Discussion
The majority of drugs used for the treatment of
itch, particularly in AD, have been demonstrated to be ineffective.
This is due to the sole targeting of the histamine pathways for
which histamine is not the only mediator of itch (15). As such, there is an urgent
requirement to develop drugs that will target histamine-dependent
and histamine-independent itch in AD. Drugs that are used to treat
histamine-dependent itch also are typically associated with
long-term side effects such as stretch marks, dilated blood vessels
on the surface of the skin, skin thinning and atrophy (14). Natural extracts and compounds are
known for their significant contribution to the treatment and
prevention of certain diseases (26,27). The
current study investigated if myrrh, known for its
anti-inflammatory and antibacterial activity, can inhibit
itch-associated IL-31 expression and histamine release in human
mast cell lines stimulated with PMA+A23187.
The results of the current study demonstrated that
myrrh extract exhibited no evidence of cytotoxicity to HMC-1 in
treatments up to 100 µg/ml. As a result, myrrh extract <100
µg/ml (25 and 50 µg/ml) were used to demonstrate the effects on
stimulated-HMC-1 cells. The results of the present study indicated
that myrrh extract significantly inhibited the expression of IL-31
mRNA in comparison with the control group. The effect of myrrh on
IL-31 protein expression and release in HMC-1 cells was also
investigated. Myrrh was demonstrated to inhibit the expression and
release of IL-31 in HMC-1 cells. The results also demonstrated that
the inhibitory effects of myrrh in IL-31 production start at the
level of gene expression. Myrrh and myrrh oils have been previously
demonstrated to exhibit anti-inflammatory effects by inhibiting the
production of prostaglandin E2, nitrous oxide and proinflammatory
cytokines including tumor necrosis factor (TNF)-α, IL-6 and IL-8,
in other cell lines including peripheral macrophages, human
gingival fibroblasts and epithelial cells within in vivo
studies (28–30). To understand the mechanisms of action
used by myrrh extract in inhibiting itch-associated IL-31 further,
the effects of myrrh extract on NF-κB and MAPK activation was
assessed. The MAPK cascade is a signaling pathway of the immune
response and serves an essential role in the intracellular signal
network, while also regulating cytokine expression (31). NF-κB serves a role in the regulation
of cell survival genes and the coordination of proinflammatory
cytokine expression including in TNF-α and IL-6 (32). MAPK has also been implicated in NF-κB
activation (33). It has been
previously demonstrated that MAPK and NF-κB/p65 activation drive
IL-31 production and secretion, as potent inhibitors of MAPK and
NF-κB block IL-31 release in HMC-1 cells (34). A novel NF-κB-binding element within
the IL-31 promoter has also been revealed to mediate IL-31
expression in human T helper 2 cells (35). Major compounds found in myrrh, which
include flavonoids, sesquiterpene, terpenoids and eugenol, have
been revealed to inhibit the activation of the MAPK and NF-κB
pathways (36–39). In the current study, the results
indicated that myrrh extract inhibited the PMA+A23187-induced
phosphorylation of JNK, ERK and p38. Myrrh extract also inhibited
the phosphorylation of NF-κB, indicating that the action of myrrh
extract in the inhibition of PMA+A23187-induced production of IL-31
is mediated by its inhibition of the MAPK and NF-κB signaling
pathways.
Since histamine is released from mast cells when
tissues are inflamed or stimulated by AD allergens, serving to
mediate the histamine-induced itch response, the current study also
assessed the effect of myrrh extract on histamine release from mast
cells stimulated with PMA+A23187. PMA+A23187 has been previously
demonstrated to stimulate the release of histamine in HMC-1 cells
(40). In the present study, the
PMA+A23187 stimulation of mast cell histamine release was revealed
and myrrh extract was demonstrated to inhibit this release. The
results of the current study may raise the awareness of myrrh and
the ability of its active compounds to target histamine-dependent
and histamine-independent itch, particularly in cases where
histamine receptor blockers alone are ineffective in alleviating
itch.
In the present study, it was demonstrated that an
anti-inflammatory effect of myrrh is the regulation of the
itch-associated IL-31 cytokine release. It was also demonstrated
that myrrh may be associated with the reduction of intracellular
MAPK and NF-κB/p65 activation in PMA+A23187-activated HMC-1 cells.
The results also revealed that myrrh inhibits histamine release in
activated HMC-1 cells. The current study provides new evidence as
to the anti-itch mechanism of myrrh, which has been previously used
as a treatment for other skin infections, inflammatory conditions,
periodontal, diarrhea and allergic diseases (41). Conclusively, it can be expected that
myrrh and its active compounds may be considered a viable candidate
in AD itch treatment due to its action on mast cells. However, the
current study was performed on a single cell line (HMC-1). Further
studies involving other associated cell lines, including primary
mast cells and in vivo experiments mimicking symptoms like
itch in AD, are therefore required to ascertain these claims.
Acknowledgements
Not applicable.
Funding
The present study was supported by project for
Cooperative R&D between Industry, Academy and Research
Institute funded Korea Ministry of SMEs and Startups in 2017 (grant
no. C0506751).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BC and SJ designed the current study and analyzed
the data. JS, DC, HK and JK performed the experiments. JS, DC and
BC wrote the manuscript. SJ managed the research project.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yosipovitch G and Papoiu AD: What causes
itch in atopic dermatitis? Curr Allergy Asthma Rep. 8:306–311.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanders KM, Nattkemper LA and Yosipovitch
G: Advances in understanding itching and scratching: A new era of
targeted treatments. F1000Res. 5:F1000 Faculty Rev-2042. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mollanazar NK, Smith PK and Yosipovitch G:
Mediators of chronic pruritus in atopic dermatitis: Getting the
itch out? Clin Rev Allergy Immunol. 51:263–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tani E, Shiosaka S, Sato M, Ishikawa T and
Tohyama M: Histamine acts directly on calcitonin gene-related
peptide- and substance P-containing trigeminal ganglion neurons as
assessed by calcium influx and immunocytochemistry. Neurosci Lett.
115:171–176. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dunford PJ, Williams KN, Desai PJ,
Karlsson L, McQueen D and Thurmond RL: Histamine H4 receptor
antagonists are superior to traditional antihistamines in the
attenuation of experimental pruritus. J Allergy Clin Immunol.
119:176–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simons FE, Simons KJ, Becker AB and Haydey
RP: Pharmacokinetics and antipruritic effects of hydroxyzine in
children with atopic dermatitis. J Pediatr. 104:123–127. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stempelj M and Ferjan I: Signaling pathway
in nerve growth factor induced histamine release from rat mast
cells. Inflamm Res. 54:344–349. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Metcalfe DD, Peavy RD and Gilfillan AM:
Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin
Immunol. 124:639–646; quiz 647–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nemeth K, Wilson T, Rada B, Parmelee A,
Mayer B, Buzas E, Falus A, Key S, Masszi T, Karpati S and Mezey E:
Characterization and function of histamine receptors in human bone
marrow stromal cells. Stem Cells. 30:222–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nordlind K, Chin LB, Ahmed AA, Brakenhoff
J, Theodorsson E and Liden S: Immunohistochemical localization of
interleukin-6-like immunoreactivity to peripheral nerve-like
structures in normal and inflamed human skin. Arch Dermatol Res.
288:431–435. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stevens SR, Hanifin JM, Hamilton T, Tofte
SJ and Cooper KD: Long-term effectiveness and safety of recombinant
human interferon gamma therapy for atopic dermatitis despite
unchanged serum IgE levels. Arch Dermatol. 134:799–804. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Putheti P, Zhou Q, Liu Q and Gao
W: Structures and biological functions of IL-31 and IL-31
receptors. Cytokine Growth Factor Rev. 19:347–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takaoka A, Arai I, Sugimoto M, Yamaguchi
A, Tanaka M and Nakaike S: Expression of IL-31 gene transcripts in
NC/Nga mice with atopic dermatitis. Eur J Pharmacol. 516:180–181.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weidinger S, Beck LA, Bieber T, Kabashima
K and Irvine AD: Atopic dermatitis. Nat Rev Dis Primers. 4:12018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CH: Progress of pruritus research in
atopic dermatitis. Biomol Ther. 18:246–256. 2010. View Article : Google Scholar
|
|
16
|
Rukwied R, Lischetzki G, McGlone F, Heyer
G and Schmelz M: Mast cell mediators other than histamine induce
pruritus in atopic dermatitis patients: A dermal microdialysis
study. Br J Dermatol. 142:1114–1120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Başer KHC, Demirci B, Dekebo A and Dagne
E: Essential oils of some Boswellia spp., Myrrh and Opopanax.
Flavour Fragr J. 18:153–156. 2003. View
Article : Google Scholar
|
|
18
|
Ljaljević Grbić M, Unković N, Dimkić I,
Janaćković P, Gavrilović M, Stanojević O, Stupar M, Vujisić L,
Jelikić A, Stanković S and Vukojević J: Frankincense and myrrh
essential oils and burn incense fume against micro-inhabitants of
sacral ambients. Wisdom of the ancients? J Ethnopharmacol.
219:1–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mohamed AA, Ali SI, El-Baz FK, Hegazy AK
and Kord MA: Chemical composition of essential oil and in vitro
antioxidant and antimicrobial activities of crude extracts of
Commiphora myrrha resin. Industr Crops Prod. 57:10–16. 2014.
View Article : Google Scholar
|
|
20
|
Albrecht U, Muller V, Schneider B and
Stange R: Efficacy and safety of a herbal medicinal product
containing myrrh, chamomile and coffee charcoal for the treatment
of gastrointestinal disorders: A non-interventional study. BMJ Open
Gastroenterol. 1:e0000152015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Langhorst J, Varnhagen I, Schneider SB,
Albrecht U, Rueffer A, Stange R, Michalsen A and Dobos GJ:
Randomised clinical trial: A herbal preparation of myrrh, chamomile
and coffee charcoal compared with mesalazine in maintaining
remission in ulcerative colitis-a double-blind, double-dummy study.
Aliment Pharmacol Ther. 38:490–500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shameem I: Phytochemical & therapeutic
potentials of Murr Makki (Commiphora myrrha): A review. Indian J
Appl Res. 8:102–104. 2018.
|
|
23
|
Hanus LO, Rezanka T, Dembitsky VM and
Moussaieff A: Myrrh-Commiphora chemistry. Biomed Papers Med Faculty
Univ Palacky, Olomouc, Czechoslovakia. 149:3–27. 2005. View Article : Google Scholar
|
|
24
|
Baek SJ and Kim DH: The Study on
anti-obesity of Myrrh ethanol extract. Korea J Herbol. 31:11–18.
2016. View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jamshidi-Kia F, Lorigooini Z and
Amini-Khoei H: Medicinal plants: Past history and future
perspective. J Herbmed Pharmacol. 7:1–7. 2018. View Article : Google Scholar
|
|
27
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim MS, Bae GS, Park KC, Koo BS, Kim BJ,
Lee HJ, Seo SW, Shin YK, Jung WS, Cho JH, et al: Myrrh inhibits
LPS-induced inflammatory response and protects from cecal ligation
and puncture-induced sepsis. Evid Based Complement Alternat Med.
2012:2787182012.PubMed/NCBI
|
|
29
|
Tipton DA, Lyle B, Babich H and Dabbous
MKh: In vitro cytotoxic and anti-inflammatory effects of myrrh oil
on human gingival fibroblasts and epithelial cells. Toxicol In
Vitro. 17:301–310. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fatani AJ, Alrojayee FS, Parmar MY,
Abuohashish HM, Ahmed MM and Al-Rejaie SS: Myrrh attenuates
oxidative and inflammatory processes in acetic acid-induced
ulcerative colitis. Exp Ther Med. 12:730–738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawakami Y, Hartman SE, Holland PM, Cooper
JA and Kawakami T: Multiple signaling pathways for the activation
of JNK in mast cells: Involvement of Bruton's tyrosine kinase,
protein kinase C and JNK kinases, SEK1 and MKK7. J Immunol.
161:1795–1802. 1998.PubMed/NCBI
|
|
32
|
Park HJ, Lee HJ, Choi MS, Son DJ, Song HS,
Song MJ, Lee JM, Han SB, Kim Y and Hong JT: JNK pathway is involved
in the inhibition of inflammatory target gene expression and
NF-kappaB activation by melittin. J Inflamm (Lond). 5:72008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Craig R, Larkin A, Mingo AM, Thuerauf DJ,
Andrews C, McDonough PM and Glembotski CC: p38 MAPK and NF-kappa B
collaborate to induce interleukin-6 gene expression and release.
Evidence for a cytoprotective autocrine signaling pathway in a
cardiac myocyte model system. J Biol Chem. 275:23814–23824. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Che DN, Cho BO, Shin JY, Kang HJ, Kim YS
and Jang SI: Fisetin inhibits IL-31 production in stimulated human
mast cells: Possibilities of fisetin being exploited to treat
histamine-independent pruritus. Life Sci. 201:121–129. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Maier E, Werner D, Duschl A, Bohle B and
Horejs-Hoeck J: Human Th2 but not Th9 cells release IL-31 in a
STAT6/NF-κB-dependent way. J Immunol. 193:645–654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Zhou B, Lu J, Chen Q, Ti H, Huang
W, Li J, Yang Z, Jiang Z and Wang X: Inhibition of influenza virus
via a sesquiterpene fraction isolated from Laggera pterodonta by
targeting the NF-κB and p38 pathways. BMC Complement Altern Med.
17:252017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salminen A, Lehtonen M, Suuronen T,
Kaarniranta K and Huuskonen J: Terpenoids: Natural inhibitors of
NF-kappaB signaling with anti-inflammatory and anticancer
potential. Cell Mol Life Sci. 65:2979–2999. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deepak V, Kasonga A, Kruger MC and Coetzee
M: Inhibitory effects of eugenol on RANKL-induced osteoclast
formation via attenuation of NF-κB and MAPK pathways. Connect
Tissue Res. 56:195–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karunaweera N, Raju R, Gyengesi E and
Münch G: Plant polyphenols as inhibitors of NF-κB induced cytokine
production-a potential anti-inflammatory treatment for Alzheimer's
disease? Front Mol Neurosci. 8:242015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Je IG, Kim HH, Park PH, Kwon TK, Seo SY,
Shin TY and Kim SH: SG-HQ2 inhibits mast cell-mediated allergic
inflammation through suppression of histamine release and
pro-inflammatory cytokines. Exp Biol Med (Maywood). 240:631–638.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nomicos EY: Myrrh: Medical marvel or myth
of the Magi? Holist Nurs Pract. 21:308–323. 2007. View Article : Google Scholar : PubMed/NCBI
|