Introduction
Mycoplasma pneumonia is an atypical bacterial
pneumonia that can damage human organs and systems through local
respiratory tract infections (1).
Mycoplasma pneumonia is one of the most common community-acquired
pneumonia (CAP) among children and adolescents (2), accounting for 40% of CAP in children
and 18% of hospitalized patients. It has a high infectious rate.
Once the patients are infected, the condition becomes more serious
and the course of disease also becomes longer. If not treated in
time, it may lead to a series of complications and seriously affect
the physical and mental health of the children (3). At present, the treatment of this
disease is mainly drug therapy (4).
Azithromycin is a macrolide antibiotic with good tolerance and long
half-life, which can shorten the course of treatment (5). Pidotimod is a new peptide
immunomodulator, which, not only promotes the non-specific immune
response, but also promotes the specific immune response, acting on
the different stages of the immune response (6). It can also activate the immune system
to eliminate pathogenic microorganisms (7). At present, there is little research on
the combination of the two drugs on the treatment of mycoplasma
pneumonia. Although it is now widely used, the mechanism of its
action is still unclear.
Interleukin-10 (IL-10) is an important factor
involved in airway inflammation in asthma (8). IL-10 is an anti-inflammatory medium
that reduces host response through T cells, resists excessive
inflammatory responses caused by pro-inflammatory factors, and
reduces body damage (9,10). In recent years, it has been shown
that IL-10 may play an important role in the occurrence and
development of Mycoplasma pneumoniae asthmatic attack
(11). Granulocyte
colony-stimulating factor (G-CSF) is produced by macrophage, T
cell, epithelium and fibroblast activated by IL-1, IL-6 and TNF-α.
It is a glycoprotein that plays an important role in anti-infective
non-immune cellular immune response and can enhance granulocyte
maturation and regulate the differentiation and proliferation of
medulla progenitor cells (12). The
production of G-CSF is mainly stimulated and regulated by bacteria
and endotoxin. When the body is infected, the level of G-CSF is
significantly upregulated (13). At
present, the effect of pidotimod combined with azithromycin on the
expression levels of IL-10 and G-CSF in serum of children with
mycoplasma pneumonia needs to be further studied.
In this study, the efficacy of pidotimod combined
with azithromycin in the treatment of mycoplasma pneumonia in
children and the expression levels of IL-10 and G-CSF in serum
before and after treatment were detected in order to provide a more
sufficient theoretical basis for the treatment of mycoplasma
pneumonia in children.
Patients and methods
General materials
The clinical data of 149 children with mycoplasma
pneumonia admitted to Zhangqiu District Maternal and Child Health
Care Hospital (Jinan, China) from May 2014 to May 2018 were
collected. Among them, 70 children were treated with azithromycin
sequential therapy as control group (38 males and 32 females; mean
age: 5.86±1.34 years; course of disease: 8.19±1.82 days), and 79
children with pidotimod combined with azithromycin as observation
group (44 males and 35 females; mean age: 5.98±1.27 years; course
of disease: 8.23±1.79 days).
Inclusion criteria: Patients with complete clinical
data and good compliance; patients finally diagnosed by biochemical
and imaging examinations; none of the patients had been treated
with hormone or immunomodulators.
Exclusion criteria: Patients allergic to drugs used
in this study; patients with other respiratory diseases; patients
with family history of mental illness; patients with liver disease
or abnormal liver functions, who had used other antibiotics in the
course of treatment; patients with cardiovascular disease and viral
pneumonia, congenital malformations, bacterial pneumonia and
tuberculosis.
This study was approved by the Ethics Committee of
Zhangqiu District Maternal and Child Health Care Hospital and
subjects' experimental contents were described in detail. Patients
who participated in this research had complete clinical data. The
signed informed consents were obtained from the patients or the
guardians.
Treatment method
Patients in both groups were given routine treatment
on the basis of medication. Control group: azithromycin sequential
therapy was used; intravenous drip of 10
mg·kg−1·day−1 of azithromycin (Jiangsu
Jichuan Pharmaceutical Co., Ltd., SFDA approval no. H20030782) for
3–5 days; changed to 10 mg·kg−1·day−1 of oral
azithromycin tablet (Zhejiang Asia-Pacific Pharmaceutical Co.,
Ltd., SFDA approval no. H10980289) after patient's condition was
stable. The patients were treated for 3 days and stopped for 4
days, which was a course. The patients were administered the course
again for 3 consecutive days, then stopped for four days for the
next course of treatment. The patients needed to take 2 to 4
consecutive courses of treatment. Corticosteroids were given to
patients with pulmonary dilatation and pulmonary interstitial
fibrosis.
Observation group: Pidotimod was given orally on the
basis of the control group (Jiangsu Wu Chinese Medicine Group Co.,
Ltd. Suzhou Pharmaceutical Factory, SFDA approval no. H20030463),
0.4 g/time, twice a day for 2 weeks.
During the course of treatment, the clinical
efficacy and adverse reactions of the two groups were closely
observed.
Index detection
Fasting venous blood (2 ml) was collected 24 h
before treatment and 2 weeks after treatment, and was centrifuged
for 10 min with 2,300 × g at 4°C. The supernatant was then
collected and placed at −80°C for testing, avoiding repeated
freezing and thawing. The expression levels of IL-10 and G-CSF in
serum were measured by double antibody sandwich enzyme-linked
immunosorbent assay (ELISA). BS-1101 enzyme label analyzer,
purchased from Beijing Linmao Technology Co., Ltd., was used. IL-10
and G-CSF ELISA kits were purchased from Shanghai Yuanmu
Biotechnology Co., Ltd. (art. no. YM-S0066, YM-S0040). Standard
sample (50 µl) was added to the reaction well coated with enzyme.
Sample diluent (40 µl) was added to the sample well and then 10 µl
of sample to be tested was added (sample dilution multiple ×5).
During the procedure, touching the well wall was avoided, followed
by gentle agitation. The reaction well was sealed with the sealing
plate membrane, and then incubated in a water bath or thermostat at
37°C for 30 min. Then the sealing membrane was carefully opened,
the liquid was discarded, drying with water-absorbing paper, and
each reaction well was filled with washing fluid, after which the
membrane was left to stand for 30 sec. This step was repeated five
times and then the membrane was patted dry. Enzyme reagent (50 µl)
was added to each well, except the blank well (for the blank
control well the same steps as above were carried out, but without
enzyme reagent and sample), and incubated at 37°C. After 30 min,
the membrane was washed. Substrate A and B (50 µl) was added to
each well and the mixture was kept in the dark for 15 min at 37°C.
Termination fluid (50 µl) was added to each well and a blank well
was used as zero. The optical density value (OD value) of each well
was detected at the wavelength of 450 nm in 25 min. The expression
levels of IL-10 and G-CSF in serum were then calculated.
Observation indices
Chest X-ray inflammatory absorption time, fever
clearance time, disappearance time of cough and pulmonary rales
were recorded in the two groups. Therapeutic efficacy and adverse
reactions between the two groups were compared. The changes of
IL-10 and G-CSF levels in serum before and after treatment in the
two groups were observed. The correlation of IL-10 and G-CSF in
serum was analyzed.
Therapeutic evaluation criteria (14) were classified as ineffective,
markedly effective and cure. Ineffective: after 2 weeks of
treatment, chest X-ray was aggravated or did not improve, and the
symptoms and signs of the children were aggravated or did not
improve. Markedly effective: Within 2 weeks of treatment, chest
X-ray showed that most of the absorbed shadows were significantly
reduced, and the symptoms and signs of the children were improved.
Cure: within 2 weeks, Chest X-ray examination and blood routine
examination showed normal, the symptoms and signs of the children
returned to normal. Total effective rate = cure rate + markedly
effective rate.
Statistical analysis
SPSS20.0 (IBM Corp.) was used for statistical
analysis. Enumeration data were expressed as [n (%)]. Chi-square
test was used for inter-group comparison. Measurement data were
expressed as mean ± SD. Independent sample t-test was used for
comparison between groups. Paired t-test was used for intra-group
comparison. Pearson's correlation coefficient was used for
bivariate normal distribution data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of clinical data between
two groups
The general clinical data of the two groups of
children were collected (Table I).
There was no significant difference in sex, age, course of disease,
average weight, anorexia, severity of illness, leukocytes,
platelets, fibrinogen and glutamic-oxaloacetic transaminase between
observation and control group (P>0.05).
 | Table I.Comparison of clinical data between
two groups (mean ± SD)/[n (%)]. |
Table I.
Comparison of clinical data between
two groups (mean ± SD)/[n (%)].
| Factors | Observation group
(n=79) | Control group
(n=70) | χ2/t
value | P-value |
|---|
| Sex |
|
| 0.030 | 0.863 |
| Male | 44 (55.70) | 38 (54.29) |
|
|
|
Female | 35 (44.30) | 32 (45.71) |
|
|
| Average age
(years) | 5.98±1.27 | 5.86±1.34 | 0.561 | 0.576 |
| Age (years) |
|
| 0.019 | 0.891 |
|
<5 | 42 (53.16) | 38 (54.29) |
|
|
| ≥5 | 37 (46.84) | 32 (45.71) |
|
|
| Course of disease
(days) | 8.23±1.79 | 8.19±1.82 | 0.135 | 0.893 |
| Average weight
(kg) | 18.27±0.65 | 18.19±0.72 | 0.713 | 0.477 |
| Anorexia |
|
| 0.013 | 0.911 |
|
Yes | 66 (83.54) | 58 (82.86) |
|
|
| No | 13 (16.46) | 12 (17.14) |
|
|
| Severity of
illness |
|
| 0.014 | 0.993 |
|
Mild | 23 (29.11) | 21 (30.00) |
|
|
|
Moderate | 48 (60.76) | 42 (60.00) |
|
|
|
Severe | 8
(10.13) | 7
(10.00) |
|
|
| Leukocytes
(109/l) | 10.79±7.68 | 10.83±7.92 | 0.031 | 0.975 |
| Platelets
(109/l) | 292.19±110.28 | 290.18±109.82 | 0.111 | 0.912 |
| Fibrinogen
(g/l) | 3.39±0.96 | 3.42±0.89 | 0.197 | 0.844 |
|
Glutamic-oxaloacetic transaminase
(U/l) | 40.38±27.29 | 42.28±28.53 | 0.415 | 0.679 |
Comparison of time for improvement of
symptoms between two groups
The time for improvement of symptoms of the two
groups were collected, as shown in Table II. Chest X-ray inflammatory
absorption time, fever clearance time, disappearance time of cough
and pulmonary rales in observation group were significantly shorter
than those in control group (P<0.05).
 | Table II.Comparison of time for improvement of
symptoms of the two groups (mean ± SD)/(days). |
Table II.
Comparison of time for improvement of
symptoms of the two groups (mean ± SD)/(days).
| Groups | Chest X-ray
inflammatory absorption time | Fever clearance
time | Disappearance time
of cough | Disappearance time
of pulmonary rales |
|---|
| Observation
(n=79) |
8.29±2.02 | 3.17±0.76 |
7.24±1.68 |
9.89±1.97 |
| Control (n=70) | 10.12±2.98 | 5.01±0.67 | 10.06±1.77 | 11.03±2.07 |
| χ2
value | 4.430 | 15.590 | 9.972 | 3.442 |
| P-value | <0.001 | <0.001 | <0.001 | <0.001 |
Comparison of therapeutic effects
between two groups
The therapeutic effects of the two groups were
collected. After treatment, in the observation group, 65 cases were
cured, 10 cases were markedly effective, 4 cases were ineffective,
and the total effective rate was 94.94%. In the control group, 40
cases were cured, 17 cases were markedly effective and 13 cases
were ineffective, and the total effective rate was 81.43%. The
total effective rate after treatment in the observation group was
significantly higher than that in the control group
(χ2=9.280, P<0.05) (Table III).
 | Table III.Comparison of therapeutic effects
between two groups [n (%)]. |
Table III.
Comparison of therapeutic effects
between two groups [n (%)].
| Groups | Cure | Markedly
effective | Ineffective | Total effective
rate |
|---|
| Observation
(n=79) | 65 (82.28) | 10 (12.66) | 4 (5.06) | 94.94% |
| Control (n=70) | 40 (57.14) | 17 (24.29) | 13 (18.57) | 81.43% |
| χ2
value |
|
|
| 9.280 |
| P-value |
|
|
| 0.002 |
Expression levels of IL-10 and G-CSF
in serum
The expression levels of IL-10 and G-CSF in serum
before and after treatment in the two groups were detected. There
was no significant difference in expression levels of IL-10 and
G-CSF in serum between the two groups before treatment (P>0.05).
After treatment, the expression levels of IL-10 and G-CSF in the
two groups were significantly lower than those before treatment,
and the difference was statistically significant (P<0.05). After
treatment, the levels of IL-10 and G-CSF in serum of the
observation group were significantly lower than those of the
control group (Table IV).
 | Table IV.Expression levels of IL-10 and G-CSF
in serum (mean ± SD). |
Table IV.
Expression levels of IL-10 and G-CSF
in serum (mean ± SD).
| Groups | IL-10 (pg/ml) | G-CSF (pg/ml) |
|---|
| Observation
(n=79) |
| Before
treatment | 21.78±2.09 | 158.98±17.78 |
| After
treatment |
6.21±0.87 | 46.32±7.27 |
| t
value | 58.910 | 53.420 |
|
P-value | <0.001 | <0.001 |
| Control (n=70) |
| Before
treatment | 21.97±2.17 | 160.98±16.87 |
| After
treatment |
13.56±1.12a |
79.43±6.85a |
| t
value | 29.950 | 36.400 |
|
P-value | <0.001 | <0.001 |
Comparison of adverse reactions
between the two groups
The adverse reactions of the two groups were
collected, as shown in Table V. The
incidence of adverse reactions in observation group (7.60%) was
significantly lower than that in control group (20.00%) (t=5.980,
P<0.05).
 | Table V.Comparison of adverse reactions
between the two groups [n (%)]. |
Table V.
Comparison of adverse reactions
between the two groups [n (%)].
| Groups | Nausea and
vomiting | Erythra | Diarrhoea | Abdominal Pain | Gastrointestinal
reaction | Incidence |
|---|
| Observation
(n=79) | 2 (2.53) | 0 (0.00) | 2 (2.53) | 1 (1.27) | 1 (1.27) |
7.60% |
| Control (n=70) | 5 (7.14) | 1 (1.43) | 3 (4.29) | 2 (2.86) | 3 (4.29) | 20.00% |
| χ2
value |
|
|
|
|
| 5.980 |
| P-value |
|
|
|
|
| 0.015 |
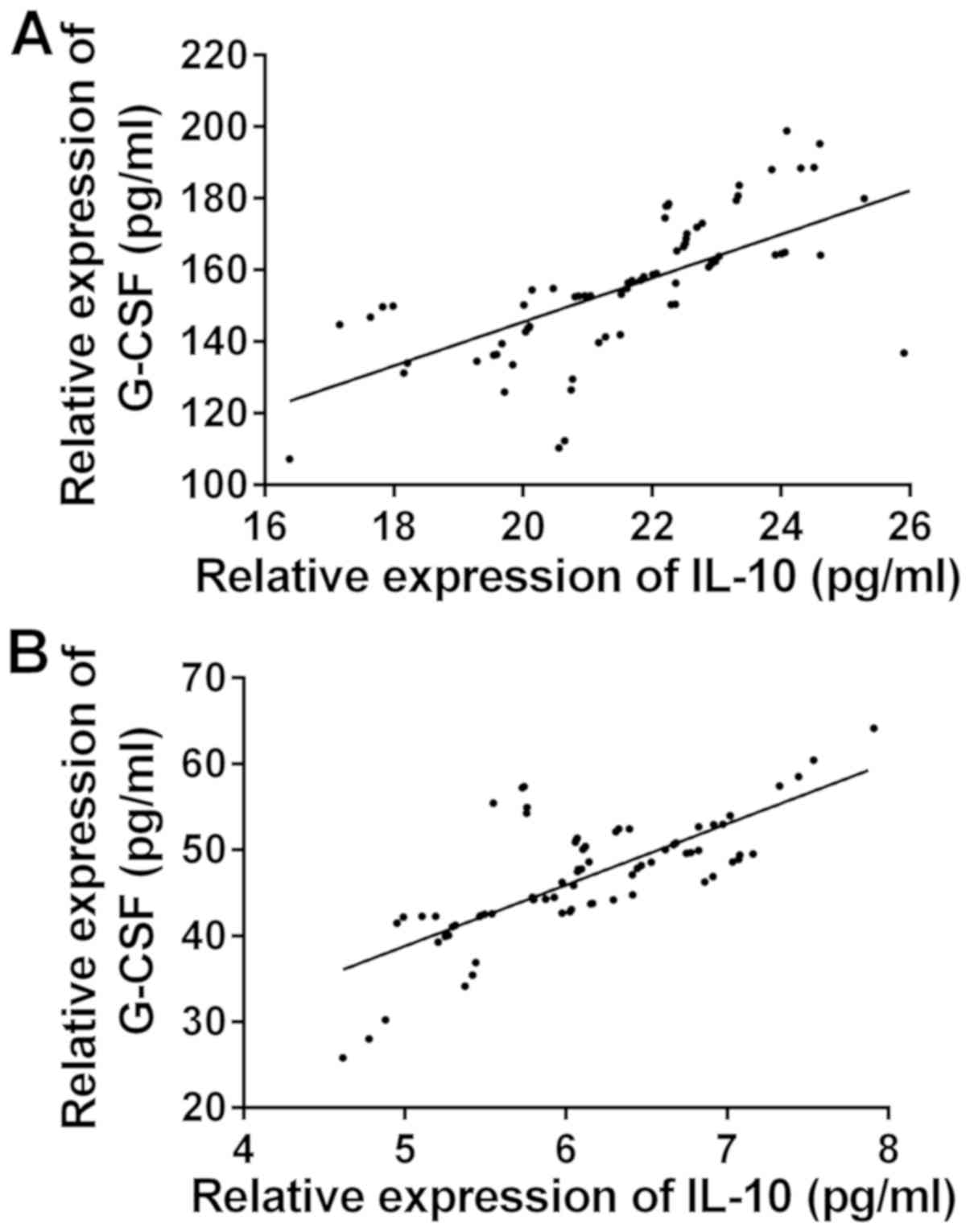
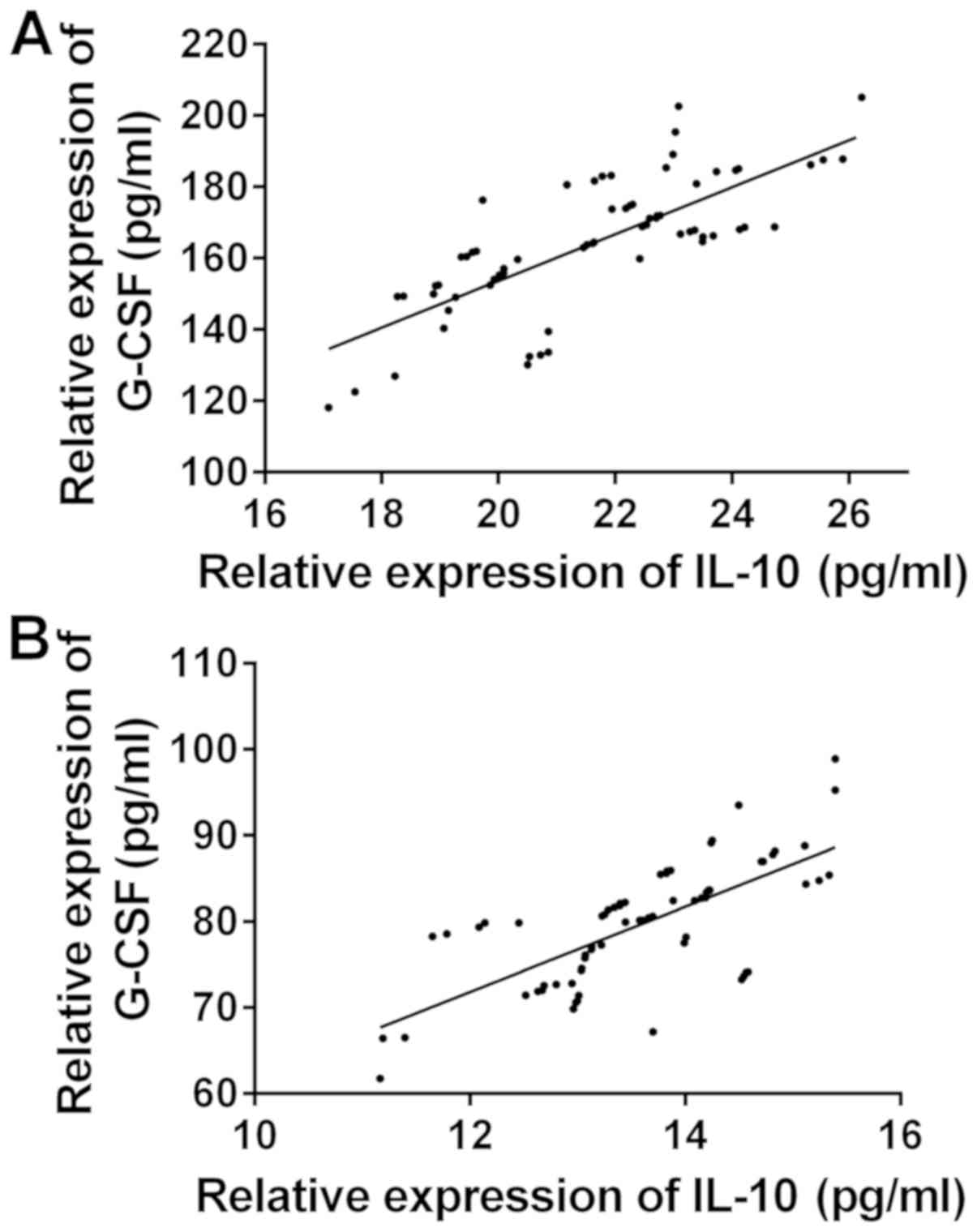
Correlation of IL-10 and G-CSF
expression levels in serum
The correlation of the expression levels of IL-10
and G-CSF between the two groups before and after treatment was
observed (Fig. 1). There was a
significant positive correlation between the expression levels of
IL-10 and G-CSF before and after treatment in the observation group
(r=0.6713, 0.7387, P<0.05) (Fig.
2). There was a significant positive correlation between the
expression levels of IL-10 and G-CSF before and after treatment in
the control group (r=0.7547, 0.7034, P<0.05).
Discussion
Mycoplasmal pneumonia is very common in clinical
pediatrics, it can occur in all seasons, and is mostly subacute
onset, mainly showing cough, pharynx pain, headache and other
clinical symptoms (15). The results
of epidemiological studies show that the incidence of the disease
has been increasing year by year (16). Clinical treatment of Mycoplasma
pneumoniae is mainly based on oxygen inhalation, asthma relief,
cough relief, atomization inhalation, and combined with antibiotics
(17). Azithromycin is the first
choice for the treatment of Mycoplasma pneumoniae pneumonia
in children and can effectively achieve the role of bacteriostatic
and bacteriological cleaning (18,19).
Children with mycoplasma pneumonia not only have mycoplasma
infection, but also have obvious airway hyperreactivity and
inflammatory reaction. Simple anti-infective therapy can control
the condition of children to a certain extent, but its therapeutic
effect is limited, children are prone to recurrence after
treatment, affecting their prognosis (20). Pidotimod is an immunologically active
synthetic dipeptide molecule, which can activate the proliferation
and expression of a variety of lymphocytes and regulate Th17/CD4
CD25 Treg balance in children with mycoplasma pneumonia. Pidotimod
enhances specific immune function by stimulating T helper cells,
promoting the production of IFN-γ and IL-2. Non-specific immune
function can be enhanced by the activation of phagocytes and
natural killer cells (21,22).
IL-10 is a class of Th2 type anti-inflammatory
cytokine produced by innate immune cells and acquired immune cells,
playing an important role in the immune regulation of the body, and
its main immunomodulatory effect is achieved by inhibiting
inflammatory reaction (23,24). It can inhibit the antigen
presentation function of macrophages, the response of Th1 cells,
and the immune response of the body by down-regulating the
costimulatory signal of CD28 (25).
Some studies have shown that IL-10 is related to the severity and
treatment outcome of Mycoplasma pneumoniae pneumonia in
children, and can be used in the diagnosis of this disease
(26). The biological function of
G-CSF is to regulate the proliferation, differentiation and mature
release of neutral granulocyte progenitor cells, inhibit cell
apoptosis and enhance its function by binding to effector
surface-specific receptowr (G-CSFR), thus to aggravate inflammatory
injury (27). In vitro
studies have shown that G-CSF promotes the proliferation and
migration of tumor cells by binding to G-CSFR and activating
multiple cellular signal transduction pathways such as JAK2/STAT3
and ERK1/2 (28–30). Some findings have shown that the
level of G-CSF in the diagnosis of pneumonia in children has a
certain clinical value (31).
The time for improvement of symptoms of the two
groups were recorded in this study. The results showed that chest
X-ray inflammatory absorption time, fever clearance time,
disappearance time of cough and pulmonary rales in observation
group were significantly shorter than those in control group. Zhao
et al showed that the clinical symptoms, the duration of
signs and the length of hospitalization in patients treated with
pidotimod granules combined with azithromycin sequential therapy
were significantly lower than those treated with Azithromycin
sequential therapy alone (32).
Results of that study were similar to those obtained in this study.
All the results showed that pidotimod combined with azithromycin
could significantly improve the clinical symptoms of children with
mycoplasma pneumonia. The total effective rate of children in the
observation group (94.94%) was significantly higher than that in
the control group (81.43%), indicating that pidotimod combined with
azithromycin had a good curative effect in the treatment of
mycoplasma pneumonia in children. The results are similar to those
of Waites et al and they suggest that the treatment of
mycoplasma pneumonia with pidotimod can significantly increase the
expression of Foxp3 gene and the proportion of Treg cells in
children with mycoplasma pneumonia, and enhance the immunity
(33). Studies have shown that
(34) G-CSF levels are low in normal
healthy people, and when bacterial infections occur, G-CSF levels
rise rapidly, and with the control of infection conditions, the
level of G-CSF in vivo also decreased to normal.
It is reported that IL-10 is highly expressed in
mycoplasma pneumonia and may be involved in the pathogenesis of
mycoplasma pneumonia (35). The
expression levels of IL-10 and G-CSF in serum before and after
treatment in the two groups were detected. There was no significant
difference in expression levels of IL-10 and G-CSF in serum between
the two groups before treatment. After treatment, the expression
levels of IL-10 and G-CSF in the two groups were significantly
lower than those before treatment, the levels of IL-10 and G-CSF in
serum of the observation group were significantly lower than those
of the control group (P<0.05). The results showed that pidotimod
combined with azithromycin could significantly decrease the
expression levels of IL-10 and G-CSF in serum, effectively kill
pathogenic microorganisms and inhibit or resist the growth of
pathogenic bacteria; it can also inhibit the release of
inflammatory transmitters to reduce the expression of inflammatory
factors. The study of Lei et al on the mechanism of
sequential treatment of azithromycin combined with Tanreqing on
mycoplasma pneumonia in children found that the treatment could
significantly downregulate the levels of serum sTREM-1, CK, G-CSF
and IL-10, so as to achieve therapeutic effect (36). In the present study, pidotimod also
played this role, and the effect of combined drugs was better. Our
results showed that the incidence of adverse reactions in the
observation group was significantly lower than that in the control
group. The results are similar to those of Hakansson et al,
suggesting that pidotimod combined with azithromycin is safe in the
treatment of mycoplasma pneumonia in children (37). At present, there are few studies on
the correlation between IL-10 and G-CSF. The present study showed
that there was a significant positive correlation between the
expression levels of IL-10 and G-CSF in serum before and after
treatment in both groups, suggesting that G-CSF may have the
function of promoting inflammation.
The effect of pidotimod combined with azithromycin
on the expression levels of IL-10 and G-CSF in children with
mycoplasma pneumonia was comprehensively described in this study.
However, due to the bias of data collection, there are some
limitations. Whether IL-10 and G-CSF can be used to evaluate the
efficacy and prognosis of drugs needs to be further studied.
In conclusion, compared with sequential treatment
with azithromycin alone, pidotimod combined with azithromycin in
the treatment of mycoplasma pneumonia in children can significantly
reduce the levels of IL-10 and G-CSF, improve the efficacy, reduce
the occurrence of adverse reactions, and has high application value
in clinic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HS and LL were responsible for ELISA. XL and LS
contributed to the analysis of observation indexes. HS wrote the
manuscript. The final version was read and adopted by all the
authors. All the authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Zhangqiu District Maternal and Child Health Care Hospital (Jinan,
China) and subjects' experimental contents were described in
detail. Patients who participated in this research had complete
clinical data. The signed informed consents were obtained from the
patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gong L, Zhang C-L and Zhen Q: Analysis of
clinical value of CT in the diagnosis of pediatric pneumonia and
mycoplasma pneumonia. Exp Ther Med. 11:1271–1274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waites KB and Talkington DF: Mycoplasma
pneumoniae and its role as a human pathogen. Clin Microbiol
Rev. 17:697–728. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L: Comparison of the effect of
erythromycin combined with azithromycin sequential therapy and
azithromycin in treatment of mycoplasma pneumonia in children.
China Med Her. 13:173–176. 2016.(In Chinese).
|
|
4
|
Bajantri B, Toolsie O, Venkatram S and
Diaz-Fuentes G: Mycoplasma pneumoniae pneumonia: Walking
pneumonia can cripple the susceptible. J Clin Med Res. 10:891–897.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bajantri B, Venkatram S and Diaz-Fuentes
G: Mycoplasma pneumoniae: A potentially severe infection. J
Clin Med Res. 10:535–544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JH, Kwon JH, Lee JY, Lee JS, Ryu JM,
Kim SH, Lim KS and Kim WY: Clinical features of Mycoplasma
pneumoniae coinfection and need for its testing in influenza
pneumonia patients. J Thorac Dis. 10:6118–6127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin X, Zhu Y, Zhang Y, Chen J, Rong L and
Zhao X: Assessment of levels of D-dimer and interferon-γ in
pediatric patients with Mycoplasma pneumoniae pneumonia and
its clinical implication. Exp Ther Med. 16:5025–5030.
2018.PubMed/NCBI
|
|
8
|
Mathur S, Fuchs A, Bielicki J, Van Den
Anker J and Sharland M: Antibiotic use for community-acquired
pneumonia in neonates and children: WHO evidence review. Paediatr
Int Child Health. 38 (Suppl 1):S66–S75. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medjo B, Atanaskovic-Markovic M, Nikolic
D, Radic S, Lazarevic I, Cirkovic I and Djukic S: Increased serum
interleukin-10 but not interleukin-4 level in children with
Mycoplasma pneumoniae pneumonia. J Trop Pediatr. 63:294–300.
2017.PubMed/NCBI
|
|
11
|
Akashi Y, Hayashi D, Suzuki H, Shiigai M,
Kanemoto K, Notake S, Ishiodori T, Ishikawa H and Imai H: Clinical
features and seasonal variations in the prevalence of
macrolide-resistant Mycoplasma pneumoniae. J Gen Fam Med.
19:191–197. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mehta HM, Malandra M and Corey SJ: G-CSF
and GM-CSF in neutropenia. J Immunol. 195:1341–1349. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liongue C and Ward AC: Granulocyte
colony-stimulating factor receptor mutations in myeloid malignancy.
Front Oncol. 4:932014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dutow P, Lingner S, Laudeley R, Glage S,
Hoymann HG, Dittrich AM, Fehlhaber B, Müller M, Braun A and Klos A:
Severity of allergic airway disease due to house dust mite allergen
is not increased after clinical recovery of lung infection with
Chlamydia pneumoniae in mice. Infect Immun. 81:3366–3374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hall MW, Geyer SM, Guo CY,
Panoskaltsis-Mortari A, Jouvet P, Ferdinands J, Shay DK, Nateri J,
Greathouse K, Sullivan R, et al Pediatric Acute Lung Injury, Sepsis
Investigators (PALISI) Network PICFlu Study Investigators, : Innate
immune function and mortality in critically ill children with
influenza: A multicenter study. Crit Care Med. 41:224–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trikha P and Carson WE III: Signaling
pathways involved in MDSC regulation. Biochim Biophys Acta.
1846:55–65. 2014.PubMed/NCBI
|
|
17
|
Liang H, Liu Y and Mo L: Clinical study of
using additives qianjin weijing decoction in treating children with
phlegm and blood stasis mutual junction type mycoplasma pneumonia.
J Sichuan Trad Med. 7:81–83. 2017.
|
|
18
|
Fleming-Dutra KE, Demirjian A, Bartoces M,
Roberts RM, Taylor TH Jr and Hicks LA: Variations in antibiotic and
azithromycin prescribing for children by geography and
specialty-United States, 2013. Pediatr Infect Dis J. 37:52–58.
2018.PubMed/NCBI
|
|
19
|
He HX and Zhang XQ: Clinical observation
on self-made yiqi xiaozhi decoction and azithromycin in the
sequential treatment of mycoplasma pneumonia in children. J Sichuan
Trad Med. 7:43–46. 2018.
|
|
20
|
Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng
Q, Wang Y, Yuan W and Ma J: G-CSF is a key modulator of MDSC and
could be a potential therapeutic target in colitis-associated
colorectal cancers. Protein Cell. 7:130–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Salvatore CM, Techasaensiri C, Tagliabue
C, Katz K, Leos N, Gomez AM, McCracken GH and Hardy RD: Tigecycline
therapy significantly reduces the concentrations of inflammatory
pulmonary cytokines and chemokines in a murine model of
Mycoplasma pneumoniae pneumonia. Antimicrob Agents
Chemother. 53:1546–1551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Taher TE, Bystrom J, Ong VH, Isenberg DA,
Renaudineau Y, Abraham DJ and Mageed RA: Intracellular B lymphocyte
signalling and the regulation of humoral immunity and autoimmunity.
Clin Rev Allergy Immunol. 53:237–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mavropoulos A, Varna A, Zafiriou E,
Liaskos C, Alexiou I, Roussaki-Schulze A, Vlychou M, Katsiari C,
Bogdanos DP and Sakkas LI: IL-10 producing Bregs are impaired in
psoriatic arthritis and psoriasis and inversely correlate with
IL-17- and IFNγ-producing T cells. Clin Immunol. 184:33–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morris KT, Castillo EF, Ray AL, Weston LL,
Nofchissey RA, Hanson JA, Samedi VG, Pinchuk IV, Hudson LG and
Beswick EJ: Anti-G-CSF treatment induces protective tumor immunity
in mouse colon cancer by promoting protective NK cell, macrophage
and T cell responses. Oncotarget. 6:22338–22347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, You X, Peng Z, Zhang H, Gao S, Zeng
Y, Yu M and Zhu C: Mycoplasma pneumoniae capsular
polysaccharides bind to DC-SIGN and promote the secretion of IL-10.
Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 29:10–13. 2013.(In Chinese).
PubMed/NCBI
|
|
26
|
Zhang X, Ma X, An H, Xu C, Cao W, Yuan W
and Ma J: Upregulation of microRNA-125b by G-CSF promotes
metastasis in colorectal cancer. Oncotarget. 8:50642–50654.
2017.PubMed/NCBI
|
|
27
|
Guilbert TW and Denlinger LC: Role of
infection in the development and exacerbation of asthma. Expert Rev
Respir Med. 4:71–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morris KT, Khan H, Ahmad A, Weston LL,
Nofchissey RA, Pinchuk IV and Beswick EJ: G-CSF and G-CSFR are
highly expressed in human gastric and colon cancers and promote
carcinoma cell proliferation and migration. Br J Cancer.
110:1211–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takahashi H, Kimura T, Yuki N and Yoshioka
A: An adult case of recurrent Guillain-Barré syndrome with
anti-galactocerebroside antibodies. Intern Med. 57:409–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chakraborty A, White SM and Guha S:
Granulocyte colony-stimulating receptor promotes
beta1-integrin-mediated adhesion and invasion of bladder cancer
cells. Urology. 68:208–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He J, Liu M, Ye Z, Tan T, Liu X, You X,
Zeng Y and Wu Y: Insights into the pathogenesis of Mycoplasma
pneumoniae (Review). Mol Med Rep. 14:4030–4036. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao JL, Wang X and Wang YS: Relationships
between Th1/Th2 cytokine profiles and chest radiographic
manifestations in childhood Mycoplasma pneumoniae pneumonia.
Ther Clin Risk Manag. 12:1683–1692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Waites KB, Xiao L, Liu Y, Balish MF and
Atkinson TP: Mycoplasma pneumoniae from the respiratory
tract and beyond. Clin Microbiol Rev. 30:747–809. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeong YC, Yeo MS, Kim JH, Lee HB and Oh
JW: Mycoplasma pneumoniae infection affects the serum levels
of vascular endothelial growth factor and interleukin-5 in atopic
children. Allergy Asthma Immunol Res. 4:92–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun J, Xiao Y, Zhang M, Ao T, Lang S and
Wang J: Serum inflammatory markers in patients with adenovirus
respiratory infection. Med Sci Monit. 24:3848–3855. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lei WT, Lin HH, Tsai MC, Hung HH, Cheng
YJ, Liu SJ, Lin CY and Yeh TL: The effects of macrolides in
children with reactive airway disease: A systematic review and
meta-analysis of randomized controlled trials. Drug Des Devel Ther.
12:3825–3845. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hakansson AP, Orihuela CJ and Bogaert D:
Bacterial-host interactions: Physiology and pathophysiology of
respiratory infection. Physiol Rev. 98:781–811. 2018. View Article : Google Scholar : PubMed/NCBI
|