Introduction
In arteriosclerosis obliterans (ASO), lipids are
persistently deposited in the intima of arteries to form
atheromatous plaques (1). The intima
and middle layers of the arteries are deteriorated and then
proliferate, leading to thickening, stiffness and distortion of
arterial walls (1,2). The gradual loss of elasticity,
enlargement of atherosclerotic plaque and secondary thrombosis
result in narrowing or even obstruction of the arterial lumen,
leading to corresponding ischemic symptoms at the distal end of
arteries (1,2).
There are numerous hypotheses regarding the etiology
of ASO, including lipid infiltration, thrombosis and inflammatory
injury response (3–5). Although these hypotheses do not
comprehensively explain all the pathological phenomena of ASO, they
do demonstrate that atherosclerosis (AS) is initiated by form
damaging stimuli, such as dysregulation of lipid metabolism,
hemodynamic damage, heredity, infection, and physical or chemical
stimuli (6,7). Multiple inflammatory factors and
associated cytokine networks co-operatively act on the vascular
wall, leading to a persistent state of vascular dysfunction
(8). The gradual formation and
development of AS plaques in blood vessels, accompanied by the
rupture of unstable plaques and thrombosis ultimately result in
different degrees of stenosis or occlusion of the arteries, leading
to clinical events of acute and chronic limb ischemia (8).
Recent studies have suggested that toll-like
receptor 4 (TLR4) and its associated signal transduction pathways
are critical for formation of AS (9,10). TLR4
expression is high in the atherosclerotic plaque, resulting in the
synthesis and release of various cytokines or chemokines associated
with AS (11,12). TLR4 activates nuclear translocation
of NF-κB by mediating the myeloid differentiation primary response
protein MyD88 (Myd88)-dependent early response pathway, thus
initiating a series of inflammatory responses by producing
pro-inflammatory factors and monocyte chemoattractants (12). Transforming growth factor-β (TGF-β)
is inhibited by the p38 mitogen activated protein kinase (MAPK)
pathway (13–15). Increased expression of monocyte
chemoattractant protein-1 (MCP-1) accelerates the progression of
AS, and its absence can slow the progression of AS (16). TGF-β downregulated the levels of
cytokines during the atherosclerotic inflammatory response,
including tumor-necrosis factor-α (TNF-α), interferon (IFN)γ and
interleukin (IL)-1 (17). IL-1β and
TNF-α are proinflammatory cytokines implicated in the pathogenesis
of autoimmune diseases such as rheumatoid arthritis, whereas TGF-β
is an anti-inflammatory cytokine, which has been reported to serve
an anti-inflammatory role in autoimmune diseases such as multiple
sclerosis and mediate the beneficial effect of IFNβ in multiple
sclerosis (18–20).
At present, the primary treatment options used for
treating ASO are drug therapy and surgical treatment for inhibiting
arterial intimal hyperplasia (1).
However, surgical treatment has certain risks such as angina
pectoris, myocardial infarction and pulmonary infection, is
expensive, and the middle-aged and elderly patients may refuse
surgery (21). Therefore patients
with ASO are frequently treated with drug therapy including
antiplatelet drugs, vasodilators and drugs that promote collateral
circulation. Rivaroxaban is a novel anticoagulant with the
advantages of easy absorption, a quick onset of effect and fewer
and less egregious side effects (22). Rivaroxaban is used at present to
prevent the formation of venous thrombosis and pulmonary embolism
after hip or knee joint replacement and may also be used to prevent
the recurrence of coronary artery syndrome (22). Rivaroxaban may reduce NF-κB activity
through the Myd88-dependent pathway of TLR4, thereby preventing AS
by reducing the expressions and release of downstream
pro-inflammatory cytokines (23).
The present study developed a rat model of ASO and the
pharmacological role of rivaroxaban was determined when used to
treat ASO.
Materials and methods
In vivo ASO model
A total of 60 adult male Sprague Dawley rats (age,
6–8 weeks; weight, 210–250 g), were obtained from Charles River
Laboratories. The rats were housed in a temperature-controlled room
(21±2°C) with 40–70% relative humidity and a 12-h light/dark cycle.
All rats had free access to water and food. The rats were randomly
assigned into three groups, a sham group, model group and the Riv
group, with 20 rats in each group. Rats in the sham group were fed
with a normal diet, whereas those in the model group and Riv group
were fed a high-fat diet for 8 weeks. After establishment of the
ASO model by surgery, rats in the Riv group were intragastrically
administered with 10 mg/kg rivaroxaban as described previously
(24), whereas those in sham group
and model group were administrated with the same volume of 0.9%
saline for 4 weeks. The present study was approved by The Animal
Ethics Committee of Zhejiang Chinese Medical University Animal
Center (Hangzhou, China).
Construction of ASO model in rats
After 8 weeks of high fat diet and prior to rav
administration, rats were anesthetized with intraperitoneal
injection of 10% chloral hydrate (300 mg/kg). After routine
disinfection of the bilateral inguinal region and the skin on the
inner side of the hind limbs, a longitudinal incision of ~2 cm in
length was made from the bilateral groin to the knee joint. The
surrounding tissue of the vascular nerve sheath was isolated, and
the femoral artery and its branches were ligated for 30 sec. Rats
in the sham group were only cut open but the ligation was not
performed. After the incision was closed, 2 ml saline was
subcutaneously injected for fluid infusion. Rats were returned to
the cage until their vital signs were stable.
Sample collection
After a total of 4 weeks of Rav/saline treatment,
the rats received anesthesia with 10% chloral hydrate (0.4 g/kg)
and were sacrificed by cervical dislocation prior to blood
collection. A 2.5-ml blood sample was harvested from the tail vein
and centrifuged at 1,000 × g for 15 min at 4°C The supernatant was
collected and preserved at −80°C. A part of rat femoral artery was
resected and washed with PBS. Half of the femoral artery was
preserved at −80°C, and the other half was fixed in 4%
paraformaldehyde or 2.5% glutaraldehyde at room temperature for 24
h.
Hematoxylin and eosin (H&E)
staining
The rat femoral artery fixed with 4%
paraformaldehyde was embedded in paraffin and sectioned into slices
(4-µm-thick). Artery slices were unfolded in warm water at room
temperature for 60 min and transferred on to a slide. After
staining with hematoxylin for 5 min at room temperature and eosin
for 3 min at room temperature, the femoral artery was observed
using a light microscope (magnification, ×400).
Transmission electron microscopy
Rat femoral arteries fixed with 2.5% glutaraldehyde
were washed with PBS and re-fixed with 1% osmic acid at room
temperature for 2 h. After washing with PBS and different
concentrations of ethanol (30–100%), the femoral artery was
dehydrated using 100% acetone and embedded in Epon 812 at 45°C for
12 h. The artery was sectioned in to 70-nm thick slices. Sections
were stained with lead citrate for 10 min and uranium acetate for
30 min at room temperature, and finally imaged using transmission
electron microscope (magnification, ×25,000).
Determination of the serum lipid
levels
Serum levels of total cholesterol (TC), triglyceride
(TG), low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) in rats were measured using an
automatic serum biochemical analyzer (BK-200; BIOBASE).
ELISA
Serum samples were warmed in room temperature. Serum
levels of IL-1 (cat. no. SRLB00), TNF-α (cat. no. SRTA00), MCP-1
(cat. no. DY3144-05) and TGF-β (cat. no. SMB100B) in rats were
determined using commercial ELISA kits (R&D Systems, Inc.).
Western blotting
The femoral artery tissues were homogenized after
the addition of RIPA lysis buffer (Beyotime Institute of
Biotechnology). Then the supernatant was centrifuged at 5,000 × g
for 10 min at 4°C. BCA assay kit (cat. no. p0011; Beyotime
Institute of Biotechnology) was used to determine total protein
content. A total of 10 µg protein was loaded per lane and separated
by SDS-PAGE (12% gel). After being separated, the proteins were
transferred to a PVDF membrane (Roche Diagnostics), which was
blocked with 5% skim milk for 1 h at room temperature. The specific
primary antibodies including TLR4 (1:500; cat. no. ab217274;
Abcam), NF-κB (1:500; cat. no. ab32360; Abcam), MCP-1 (1:500; cat.
no. ab25124; Abcam), TGF-β (1:500; cat. no. ab92486; Abcam) and
GAPDH (1:500; cat. no. ab181602; Abcam), were used to incubate with
the membrane overnight at 4°C. The membrane was washed with TBS
with Tween-20 (TBST) five times, and the goat anti-rabbit IgG
H&L secondary antibody (1:1,000; cat. no. ab150077; Abcam) was
used to incubate the membrane for 2 h at room temperature. After
washing with TBST for 1 min, SuperSignal West Pico PLUS
Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.) was
used to visualize the signals. Quantity One software (version 4.0,
Bio-Rad Laboratories, Inc.) was used for quantification.
Statistical analyses
All statistical analyses were performed in SPSS
version 17.0 (SPSS Inc.). Data are presented as the mean ± standard
deviation. Differences among multiple groups were analyzed by
one-way ANOVA, followed by a least significant difference post-hoc
test. Each experiment was repeated three times. P<0.05 was
considered to indicate a statistically significant difference.
Results
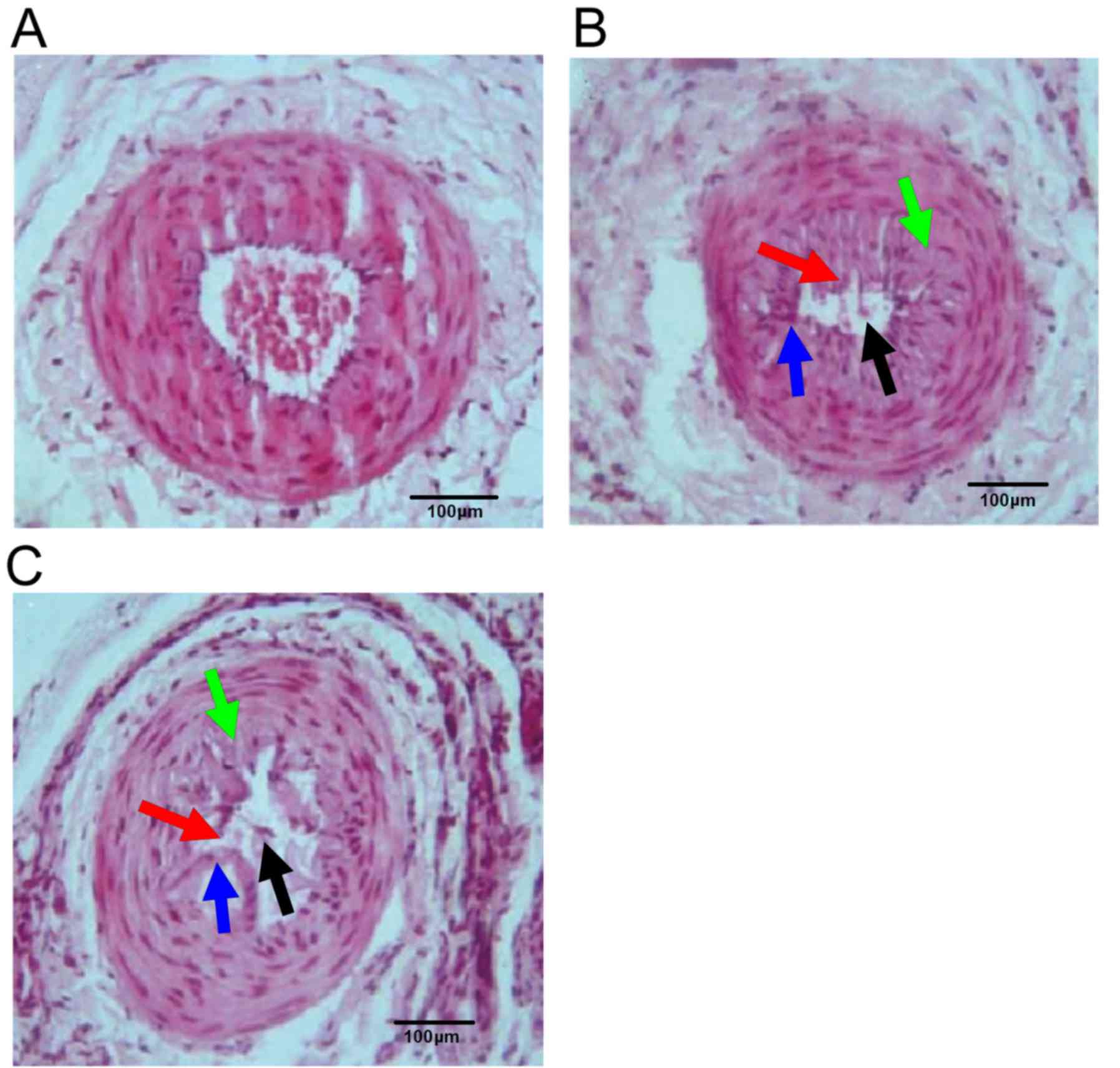
Pathological changes of rat femoral
artery
Femoral artery endothelial cells, inner elastic
plate and smooth muscle cells of rats in the sham group were
regularly arranged, and the vascular lumen was not narrowed
(Fig. 1A). Rats in model group
presented irregularly narrowed femoral artery lumen (black arrow),
disordered endothelial cells (red arrow), defective internal
elastic lamina (blue arrow) and proliferative smooth muscle cells
(green arrow) (Fig. 1B). These
characteristics were present in the Riv group, but to a lesser
extent compared with the model group indicating that the arterial
wall structure and stenosis of the femoral artery of rats in Riv
group were partially recovered (Fig.
1C).
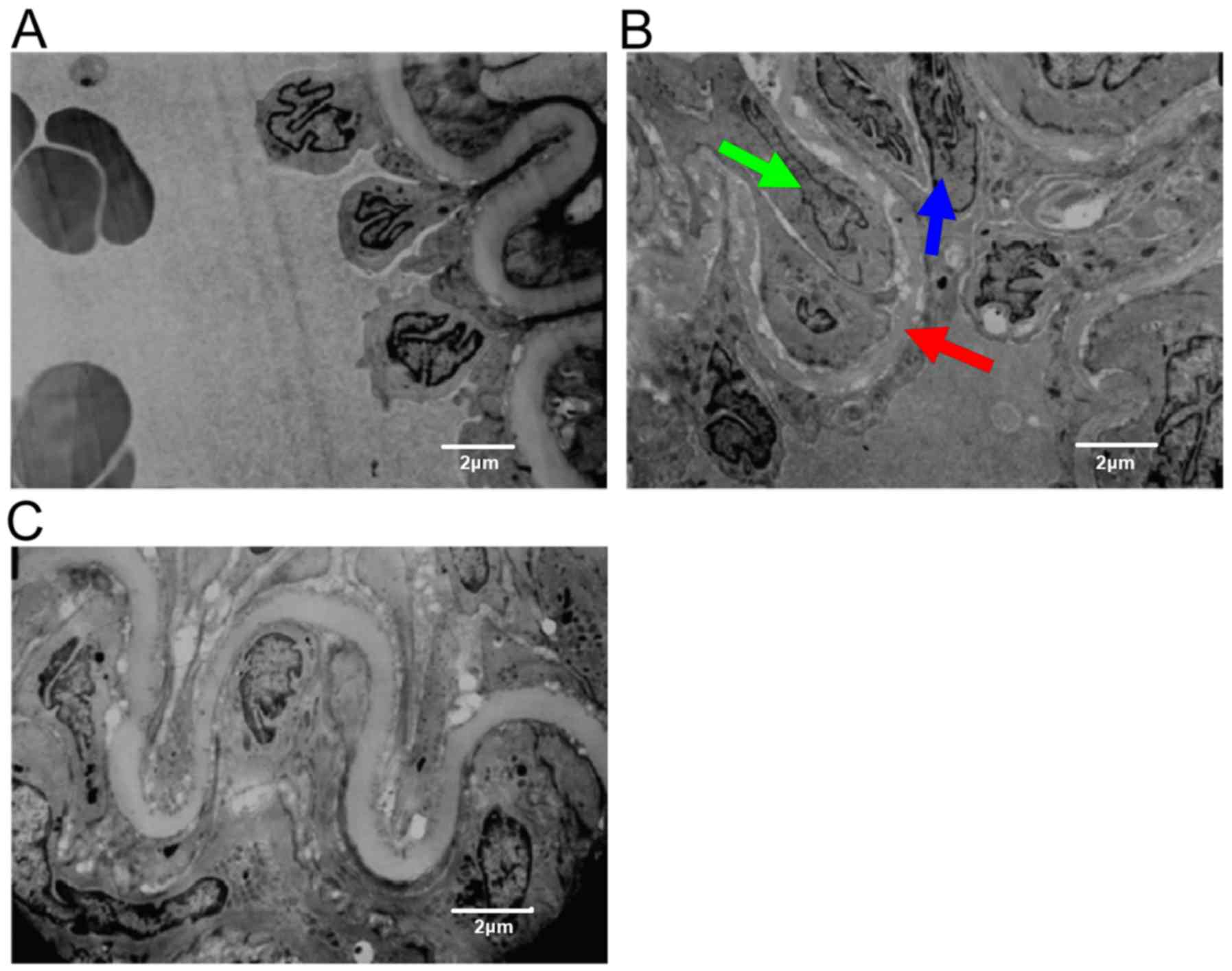
Transmission electron microscopy of
the rat femoral artery
Endothelial cells of rats in the sham group were
intact and covered the vascular surface. The internal elastic
lamina was clear and complete (Fig.
2A). In the model group, the endothelial cells were irregular
and the incomplete endothelium was exposed to the luminal surface
(blue arrow). The inner elastic plate was partially deformed (red
arrow). Smooth muscle cells, which had migrated to the vascular
intima, showed deformation and nuclear condensation (green arrow)
(Fig. 2B). Rats in Riv group
presented regular arterial endothelial cells and smooth muscle
cells, as well as a continuous elastic plate (Fig. 2C).
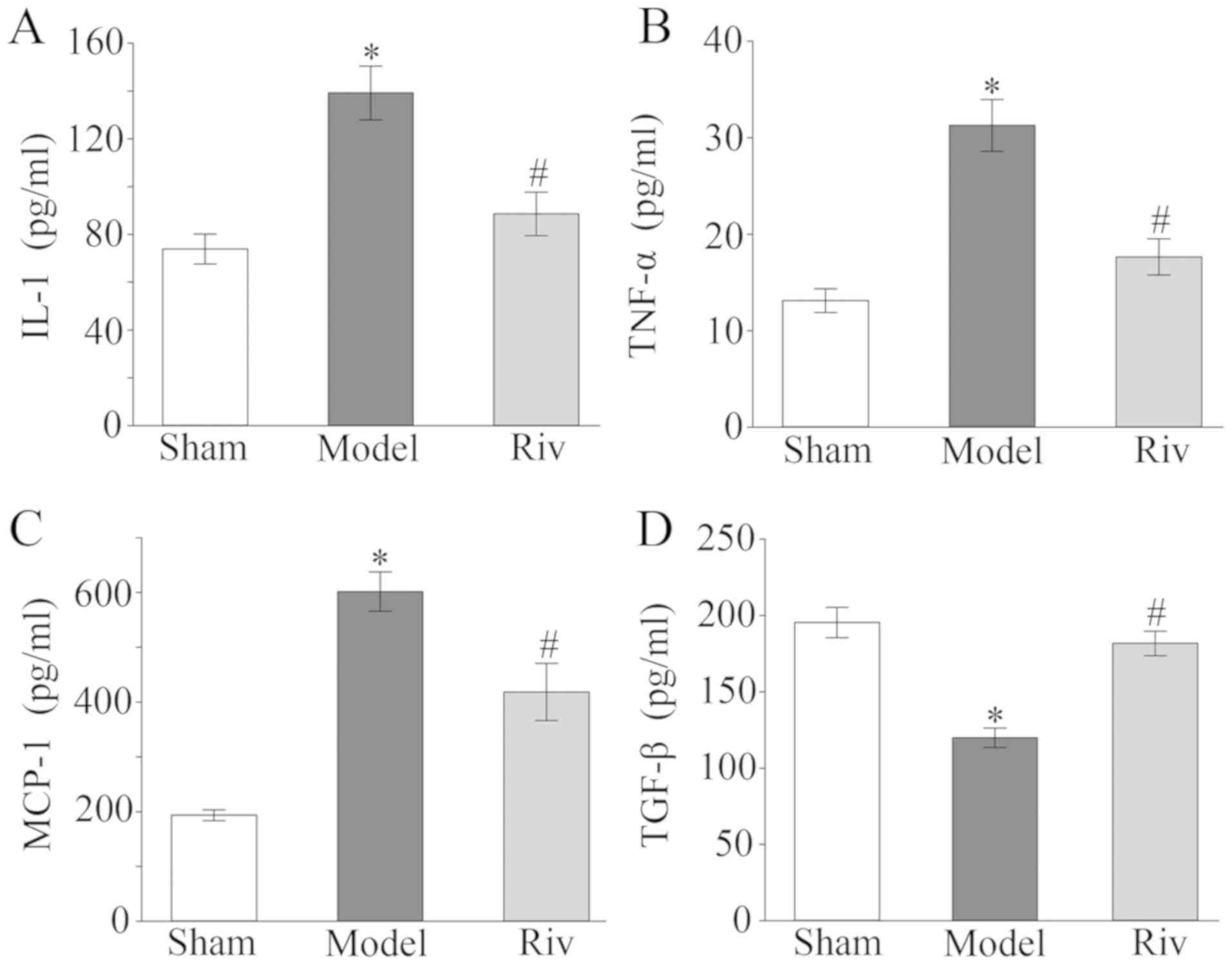
Serum lipid levels
Compared with the sham group, rats in the model
group exhibited significantly higher levels of TC, TG and LDL-C, as
well as significantly lower levels of HDL-C (all P<0.05;
Fig. 3). The levels of TC, TG and
LDL-C were significantly decreased, and the levels of HDL-C were
significantly increased in the Riv group compared with the model
group (all P<0.05; Fig. 3).
 | Figure 3.Serum levels of lipids. Serum levels
of (A) TC, (B) TG, (C) LDL-C, (D) HDL-C in rats of the different
groups *P<0.05, compared with sham group; #P<0.05,
compared with model group. TC, total cholesterol; TG, triglyceride;
LDL-C, low density lipoproteins; HDL-C, high density lipoproteins;
Riv, rivaroxaban. |
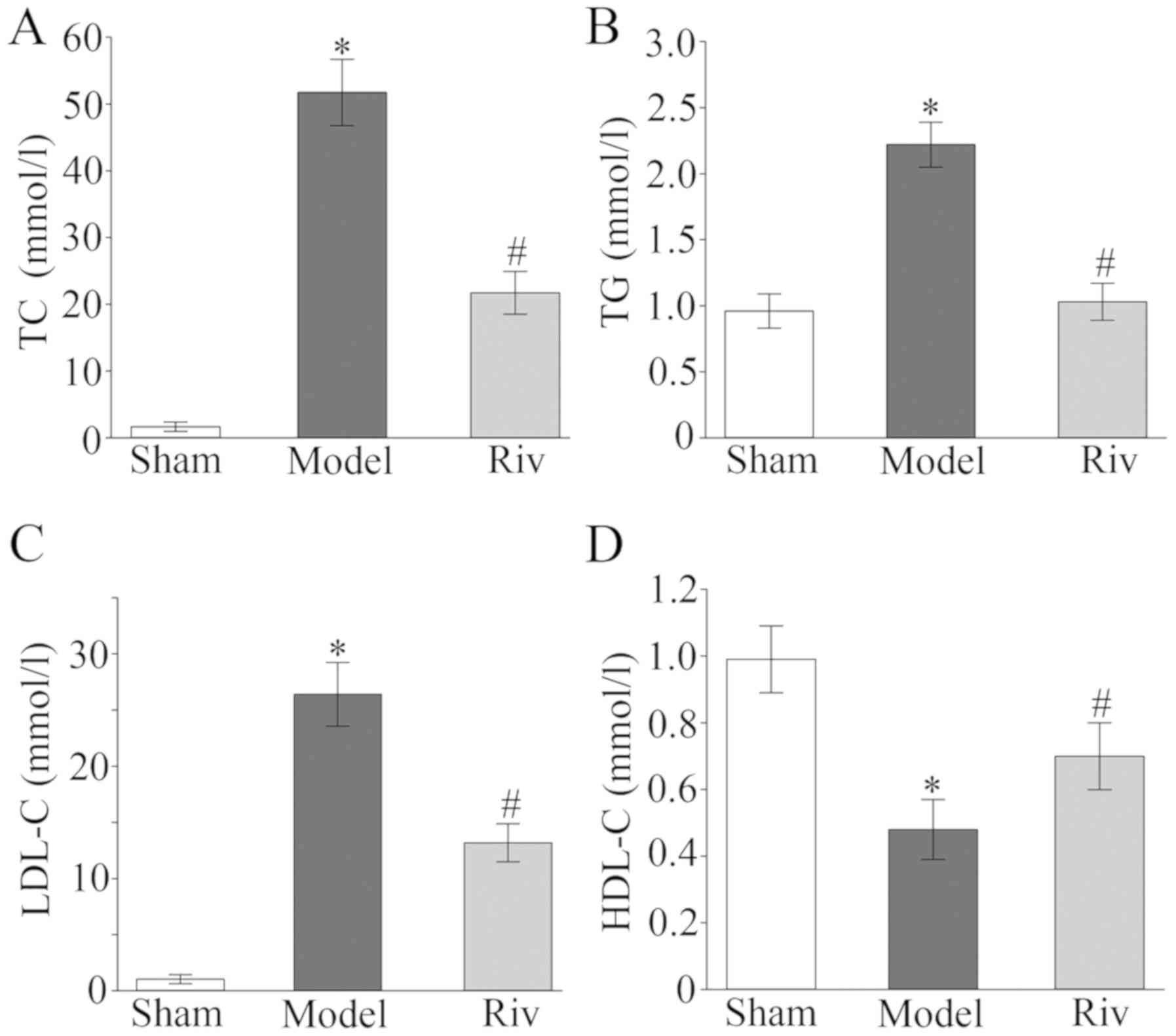
Serum levels of inflammatory factors
in rats of different groups
ELISA data demonstrated that rats in the model group
exhibited significantly higher serum levels of IL-1, TNF-α and
MCP-1 compared with the sham group, whereas the TGF-β level was
significantly lower (all P<0.05; Fig.
4). Rivaroxaban treatment significantly decreased the serum
levels of IL-1, TNF-α and MCP-1, and increased the serum levels of
TGF-β compared with the model group (all P<0.05; Fig. 4).
Protein expression of TLR4, NF-κB,
MCP-1 and TGF-β in rat femoral artery
Upregulated protein expression levels of TLR4, NF-κB
and MCP-1 were detected by western blot analysis the in femoral
artery tissues of rats in the model group compared with those in
sham group (all P<0.05; Fig. 5).
However, rats in the Riv group exhibited decreased expression of
TLR4, NF-κB and MCP-1 in the femoral artery tissues compared with
the model group (all P<0.05; Fig.
5). Protein expression levels of TGF-β were lower in the
femoral artery tissues of rats in the model group compared with the
sham group, and were increased in the Riv group compared to the
model group (all P<0.05, Fig.
5).
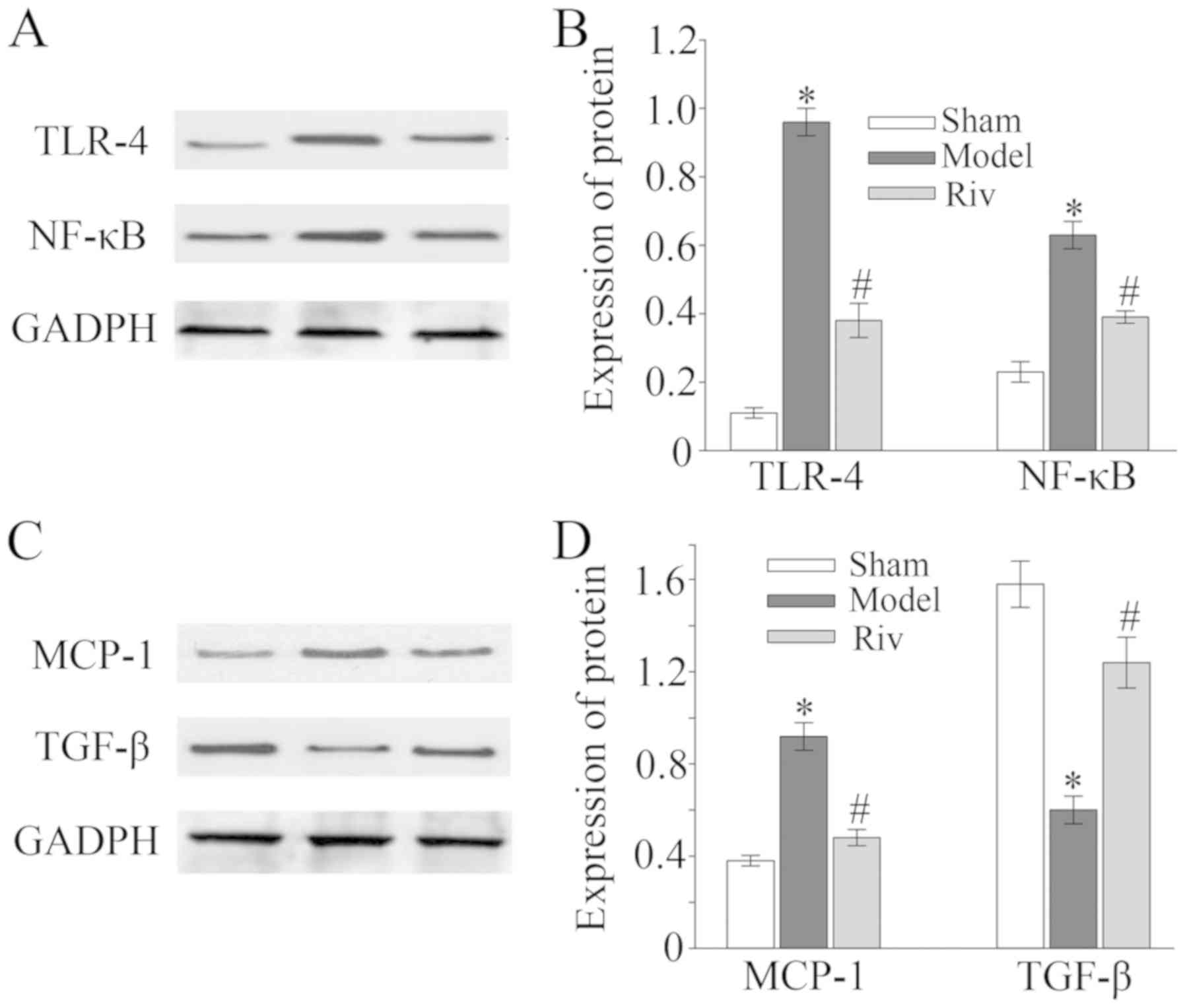 | Figure 5.Protein expression of TLR4, NF-κB,
MCP-1 and TGF-β in the rat femoral artery. Protein expression of
(A) TLR4, (B) NF-κB, (C) MCP-1 and (D) TGF-β in the rat femoral
artery *P<0.05, compared with sham group; #P<0.05,
compared with model group. TLR-4, toll-like receptor-4; NF-κB,
nuclear factor-κB; MCP-1, monocyte chemoattractant protein-1;
TGF-β, transforming growth factor-β. |
Discussion
The occurrence of ASO is a complex process, and its
cause has not been fully elucidated. Genetics, sex, age, abnormal
lipid metabolism, obesity, smoking, mechanical damage of blood
vessel walls and imbalance of trace elements are recognized as
factors affecting the occurrence of ASO (25). Additionally, long-term mental
stimulation and emotional stress result in contraction of the
arteries (26). Increased blood
pressure results in dystrophy of the blood vessel wall and
deposition of certain substances in the blood vessels, eventually
leading to the occurrence of ASO (25–27).
The inflammatory response is an important factor
affecting the occurrence of ASO. TLRs are not only key molecules of
the inflammatory process, but are also the initial link between the
recognition of exogenous antigens and initiation of the
inflammatory response (28). TLRs
are a family of receptors expressed on the cell membrane. As
transmembrane signal transduction receptors, TLRs link both innate
and specific immunity, when molecular components of certain
microorganisms are recognized (29).
In vitro studies have shown that TLR4 expression is lower in
human vascular endothelial cells under physiological conditions
(14,15). However, stimulation of inflammatory
factors markedly upregulates TLR-4 expression in tunica media
vascular smooth muscle cells, exerting a significant role in
vascular reconstruction (30). TLR4
is expression is increased in human atherosclerotic plaques, and is
involved in the proliferative regulation of smooth muscle cells
(28). Plaques subsequently migrate
to the tunica intima under stimulation of cytokines, which is the
primary step in the formation of an atherosclerotic plaque
(31,32). NF-κB is an essential multi-channel
nuclear transcription factor involved in the inflammatory process,
cell proliferation and differentiation (33,34).
TLR4 not only activates NF-κB, but also stimulates macrophage
aggregation and inflammatory response by upregulating MCP-1 through
the Myd88-dependent signaling pathway (16,35).
MCP-1 is involved in the formation and transformation of
macrophages and may promote the formation of atherosclerotic
plaques by regulating inflammatory factors (35).
Rivaroxaban is a highly selective oral drug that
directly inhibits factor Xa (FXa), which has an antithrombotic
effect in an in vivo arteriovenous thrombosis model.
Rivaroxaban not only inhibits free FXa, but also inhibits the
activity of FXa in the prothrombin complex (36). In the coagulation cascade, FXa is
involved in regulating the conversion of prothrombin to thrombin on
the surface of vascular cells (36).
A previous study demonstrated that FXa activates the acute
inflammatory response (37). In
endothelial cells, FXa can activate NF-κB, resulting in the release
of inflammatory factors such as IL-6 and MCP-1 (38). Activation of inflammatory pathways is
closely associated with the coagulation reaction (37,38).
Previous studies have found that anticoagulant therapy efficiently
inhibits coagulation activation and the inflammatory response,
suggesting that anticoagulant therapy may be applied in treating
ASO (39).
In the present study, the levels of IL-1, TNF-α,
MCP-1, TLR-4 and NF-κB in the rats of the model group were
significantly increased compared with those in the sham group,
whereas TGF-β levels decreased. TGF-β expression may be inhibited
by Myd88-dependent TLR4/NF-κB signal transduction by activating the
p38MAPK pathway, thus attenuating the anti-inflammatory effect of
TGF-β (9). Levels of IL-1, TNF-α,
MCP-1, TLR-4 and NF-κB in the Riv group were lower compared with
those in the model group, while the TGF-β level increased.
Therefore, rivaroxaban may suppress transcriptional activity of
NF-κB and synthesis of MCP-1 by inhibiting TLR4 expression. TGF-β
expression was increased in the Riv group, which in turn negatively
regulated Myd88-dependent TLR4/NF-κB signal transduction decreasing
the inflammatory response. Specific TLR4 inhibitors, such as
VGX-1027 and eritoranor, were used to treat a number of
inflammatory diseases, with positive results (40–42). The
results of the present study highlight the possibility of using
specific TLR4 inhibitors to treat ASO.
In conclusion, an ASO model in rats was developed by
crush injury of the femoral artery and feeding the rats with a
high-fat diet. The TLR4/NF-κB pathway and its downstream
inflammatory factors were inhibited following rivaroxaban
treatment. Therefore, rivaroxaban may prevent ASO through
inhibiting inflammatory response.
The TLR-4/NF-κB signaling pathway is an important
signal transduction mechanism and may be a key regulatory pathway
in AOS. Rivaroxaban may significantly inhibit inflammation and
serve an anti-atherosclerotic role by inhibiting the TLR-4/NF-κB
signaling pathway and downstream inflammatory factors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL and XY designed the study, performed the
experiments, analyzed the data, and prepared the manuscript. XL, JC
and ZY established the animal models and collected the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Ethics
Committee of Zhejiang Chinese Medical University Animal Center
(Hangzhou, China; approval no. 20180322).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akagi D, Hoshina K, Akai A and Yamamoto K:
Outcomes in patients with critical limb ischemia due to
arteriosclerosis obliterans who did not undergo arterial
reconstruction. Int Heart J. 59:1041–1046. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nau JY: Arteriosclerosis obliterans of
lower limbs: Early diagnosis before symptoms. Rev Med Suisse.
11:1458–1459. 2015.(In French). PubMed/NCBI
|
|
3
|
Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han
X, Tang D and Chen R: Research progress on the relationship between
atherosclerosis and inflammation. Biomolecules. 8(pii): E802018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Psychogios K, Stathopoulos P, Takis K,
Vemmou A, Manios E, Spegos K and Vemmos K: The pathophysiological
mechanism is an independent predictor of Long-Term outcome in
stroke patients with large vessel atherosclerosis. J Stroke
Cerebrovasc Dis. 24:2580–2587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyahara T and Shigematsu K: Epidemiology,
etiology, pathology, and pathophysiology of arteriosclerosis
obliterans. Nihon Rinsho. 74 (Suppl 2):S324–S327. 2016.(In
Japanese).
|
|
6
|
He XM, Zheng YQ, Liu SZ, Liu Y, He YZ and
Zhou XY: Altered Plasma MicroRNAs as novel biomarkers for
arteriosclerosis obliterans. J Atheroscler Thromb. 23:196–206.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Steffens S and Pacher P: The activated
endocannabinoid system in atherosclerosis: Driving force or
protective mechanism? Curr Drug Targets. 16:334–341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu XH, Zheng XL and Tang CK: Nuclear
Factor-kappaB activation as a pathological mechanism of lipid
metabolism and atherosclerosis. Adv Clin Chem. 70:1–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Chu Y, Wang L, Wang Y, Zhao X, He
W, Zhang P, Yang X, Liu X, Tian L, et al: Overexpression of CRY1
protects against the development of atherosclerosis via the
TLR/NF-kappaB pathway. Int Immunopharmacol. 28:525–530. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo H, Wang J, Qiao C, Ma N, Liu D and
Zhang W: Pycnogenol attenuates atherosclerosis by regulating lipid
metabolism through the TLR4-NF-kappaB pathway. Exp Mol Med.
47:e1912015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu R, Fan B, Cong H, Ikuyama S, Guan H
and Gu J: Pycnogenol Reduces Toll-Like receptor 4 signaling
pathway-mediated atherosclerosis formation in Apolipoprotein
E-Deficient mice. J Cardiovasc Pharmacol. 68:292–303. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kutikhin AG, Ponasenko AV, Khutornaya MV,
Yuzhalin AE, Zhidkova II, Salakhov RR, Golovkin AS, Barbarash OL
and Barbarash LS: Association of TLR and TREM-1 gene polymorphisms
with atherosclerosis severity in a Russian population. Meta Gene.
9:76–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pan S, Lei L, Chen S, Li H and Yan F:
Rosiglitazone impedes Porphyromonas gingivalis-accelerated
atherosclerosis by downregulating the TLR/NF-kappaB signaling
pathway in atherosclerotic mice. Int Immunopharmacol. 23:701–708.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhaskar S, Sudhakaran PR and Helen A:
Quercetin attenuates atherosclerotic inflammation and adhesion
molecule expression by modulating TLR-NF-kB signaling pathway. Cell
Immunol. 310:131–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schnittker D, Kwofie K, Ashkar A, Trigatti
B and Richards CD: Oncostatin M and TLR-4 ligand synergize to
induce MCP-1, IL-6, and VEGF in human aortic adventitial
fibroblasts and smooth muscle cells. Mediators Inflamm.
2013:3175032013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin J, Kakkar V and Lu X: Impact of MCP-1
in atherosclerosis. Curr Pharm Des. 20:4580–4588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen PY, Qin L, Li G, Tellides G and
Simons M: Smooth muscle FGF/TGFβ cross talk regulates
atherosclerosis progression. EMBO Mol Med. 8:712–728. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nikfar S, Saiyarsarai P, Tigabu BM and
Abdollahi M: Efficacy and safety of interleukin-1 antagonists in
rheumatoid arthritis: A systematic review and Meta-analysis.
Rheumatol Int. 38:1363–1383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mitoma H, Horiuchi T, Tsukamoto H and Ueda
N: Molecular mechanisms of action of anti-TNF-α agents-Comparison
among therapeutic TNF-α antagonists. Cytokine. 101:56–63. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicoletti F, Di Marco R, Patti F, Reggio
E, Nicoletti A, Zaccone P, Stivala F, Meroni PL and Reggio A: Blood
levels of transforming growth factor-beta 1 (TGF-beta1) are
elevated in both relapsing remitting and chronic progressive
multiple sclerosis (MS) patients and are further augmented by
treatment with interferon-beta 1b (IFN-beta1b). Clin Exp Immunol.
113:96–99. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kasashima S, Kawashima A, Endo M,
Matsumoto Y, Kasashima F, Zen Y and Nakanuma Y: A clinicopathologic
study of immunoglobulin G4-related disease of the femoral and
popliteal arteries in the spectrum of immunoglobulin G4-related
periarteritis. J Vasc Surg. 57:816–822. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Antoniou S: Rivaroxaban for the treatment
and prevention of thromboembolic disease. J Pharm Pharmacol.
67:1119–1132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hashikata T, Yamaoka-Tojo M, Namba S,
Kitasato L, Kameda R, Murakami M, Niwano H, Shimohama T, Tojo T and
Ako J: Rivaroxaban inhibits Angiotensin II-induced activation in
cultured mouse cardiac fibroblasts through the modulation of NF-kB
pathway. Int Heart J. 56:544–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flierl U, Fraccarollo D, Micka J,
Bauersachs J and Schafer A: The direct factor Xa inhibitor
Rivaroxaban reduces platelet activation in congestive heart
failure. Pharmacol Res. 74:49–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y, Nie H, Ren H, Li F, Tian C, Li H
and Zheng Y: Change of Serum Angiopoietin-like Protein 2 and its
significance in patients with Arteriosclerotic occlusion. Zhongguo
Yi Xue Ke Xue Yuan Xue Bao. 39:188–195. 2017.PubMed/NCBI
|
|
26
|
Wang SM and Yao C: Standardize the
endovascular treatment for arteriosclerosis obliterans. Zhonghua
Wai Ke Za Zhi. 54:564–567. 2016.(In Chinese). PubMed/NCBI
|
|
27
|
Hartman J and Frishman WH: Inflammation
and atherosclerosis: A review of the role of interleukin-6 in the
development of atherosclerosis and the potential for targeted drug
therapy. Cardiol Rev. 22:147–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Cui R, Li R, Lin H, Huang Z and
Lin L: Development of pristane induced mice model for lupus with
atherosclerosis and analysis of TLR expression. Clin Exp Rheumatol.
34:600–608. 2016.PubMed/NCBI
|
|
29
|
Zhong K: Curcumin Mediates a protective
effect Via TLR-4/NF-kB signaling pathway in rat model of severe
acute pancreatitis. Cell Biochem Biophys. 73:175–180. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roshan MH, Tambo A and Pace NP: The role
of TLR2, TLR4, and TLR9 in the pathogenesis of atherosclerosis. Int
J Inflam. 2016:15328322016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang YL, Jiang JH, Wang S, Liu Z, Tang XQ,
Peng J, Yang YZ and Gu HF: TLR4/NF-kB signaling contributes to
chronic unpredictable mild stress-induced atherosclerosis in
ApoE-/-mice. PLoS One. 10:e1236852015.
|
|
32
|
Xie X, Shi X and Liu M: The Roles of TLR
gene polymorphisms in atherosclerosis: A systematic review and
Meta-Analysis of 35,317 subjects. Scand J Immunol. 86:50–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu ZR, Li JY, Dong XW, Tan ZJ, Wu WZ, Xie
QM and Yang YM: Apple polyphenols decrease atherosclerosis and
hepatic steatosis in ApoE-/- Mice through the ROS/MAPK/NF-kB
pathway. Nutrients. 7:7085–7105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Chen Q, Pu H, Wei Q, Duan M, Zhang
C, Jiang T, Shou X, Zhang J and Yang Y: Adiponectin improves
NF-kappaB-mediated inflammation and abates atherosclerosis
progression in apolipoprotein E-deficient mice. Lipids Health Dis.
15:332016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei M, Li Z, Xiao L and Yang Z: Effects of
ROS-relative NF-kB signaling on high glucose-induced TLR4 and MCP-1
expression in podocyte injury. Mol Immunol. 68:261–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Imano H, Kato R, Tanikawa S, Yoshimura F,
Nomura A, Ijiri Y, Yamaguchi T, Izumi Y, Yoshiyama M and Hayashi T:
Factor Xa inhibition by rivaroxaban attenuates cardiac remodeling
due to intermittent hypoxia. J Pharmacol Sci. 137:274–282. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma J, Li X, Wang Y, Yang Z and Luo J:
Rivaroxaban attenuates thrombosis by targeting the NF-kB signaling
pathway in a rat model of deep venous thrombus. Int J Mol Med.
40:1869–1880. 2017.PubMed/NCBI
|
|
38
|
Zuo P, Zuo Z, Wang X, Chen L, Zheng Y, Ma
G and Zhou Q: Factor Xa induces pro-inflammatory cytokine
expression in RAW 264.7 macrophages via protease-activated
receptor-2 activation. Am J Transl Res. 7:2326–2334.
2015.PubMed/NCBI
|
|
39
|
Han Y, Gao C, Qin B, Xu H, Song X, Li B,
Peng B, Fan T and Cheng Z: The effect of anticoagulant therapy on
coagulation and inflammation markers in sepsis patients and its
significance. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 27:102–105.
2015.(In Chinese). PubMed/NCBI
|
|
40
|
Lee JC, Menacherry S, Diehl MC, Giffear
MD, White CJ, Juba R, Bagarazzi ML, Muthumani K, Boyer J, Agarwal
V, et al: Safety, bioavailability, and pharmacokinetics of
VGX-1027-A novel oral anti-inflammatory drug in healthy human
subjects. Clin Pharmacol Drug Dev. 5:91–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fagone P, Muthumani K, Mangano K, Magro G,
Meroni PL, Kim JJ, Sardesai NY, Weiner DB and Nicoletti F: VGX-1027
modulates genes involved in lipopolysaccharide-induced Toll-like
receptor 4 activation and in a murine model of systemic lupus
erythematosus. Immunology. 142:594–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stojanovic I, Cuzzocrea S, Mangano K,
Mazzon E, Miljkovic D. Wang M, Donia M, Al Abed Y, Kim J, Nicoletti
F, et al: In vitro, ex vivo and in vivo immunopharmacological
activities of the isoxazoline compound VGX-1027: Modulation of
cytokine synthesis and prevention of both organ-specific and
systemic autoimmune diseases in murine models. Clin Immunol.
123:311–323. 2007. View Article : Google Scholar : PubMed/NCBI
|