Introduction
Heart failure is a clinical syndrome with
pathophysiological changes in heart caused by impaired ventricular
diastolic or systolic function due to damaged cardiac function or
abnormal internal structure of heart (1). Chronic heart failure (CHF), as one of
the leading causes of cardiovascular disease death, has a serious
impact on health and quality of life of patients (2). Studies have shown that myocardial
fibrosis (MF) is the most important pathological basis of CHF
(3). Other studies (4,5) show
that MF actually refers to a process in which a large amount of
collagen fibers in the heart matrix aggregate or collagen
compositions change.
Ivabradine hydrochloride (Iva), a highly specific If
channel drug with controling effect on sinus heart rate (6), also delay heart failure and improves
heart function (7). Due to its
unique dual action mechanism, Iva can control spontaneous diastolic
depolarization in sinoatrial node and regulate heart rate by
selectively and specifically inhibiting cardiac pacing If current,
providing a new therapeutic idea for heart failure (8). Trimetazidine, as a myocardial metabolic
drug, is also widely used in cardiovascular therapy due to its
anti-oxidation and anti-ischemia effects (9). It has been shown that trimetazidine has
inhibitory effects on the activities of myocardial fatty acids and
oxidation-related enzymes, which is conducive in increasing
myocardial productivity and thus improving ventricular function
(10). Although these two drugs are
currently used in CHF, studies on their effects on MF are still
rare. Therefore, CHF rat models were established and the effects of
Iva and trimetazidine on MF were observed in this study in order to
provide more theoretical data for the treatment of CHF.
Materials and methods
Animals and experimental
materials
A total of 50 Wistar male rats weighing 220.51±10.24
g were selected and purchased from Shanghai Slaccas Experimental
Animal Co., Ltd., with a production license of SCXK (Shanghai)
2012-0002, fed at a constant temperature of 22°C with normal
circadian rhythm and free diet. Iva was purchased from Servier
Laboratories. Trimetazidine was purchased from Beijing Wansheng
Pharmaceutical Co., Ltd. Sodium pentobarbital was purchased from
Hubei Hongyun Long Biological Technology Co. Ltd. Connective tissue
growth factor (CTGF) polyclonal antibody was purchased from
Invitrogen; Thermo Fisher Scientific, Inc. Superoxide dismutase
(SOD) kit was purchased from Dojindo Molecular Technologies, Inc.
Primary mouse anti-rat CTGF (cat. no. AMAB91366; dil, 1:1,000) and
β-actin monoclonal antibodies (cat. no. A1978; dil, 1:400),
secondary rabbit anti-mouse polyclonal antibody (cat. no.
SAB3701212; dil, 1:100) used in western blot analysis were
purchased from Sigma-Aldrich; Merck KGaA. BCA quantitative kit was
purchased from Beijing Solarbio Science & Technology Co., Ltd.
RT-qPCR kit and minScript reverse transcription kit were purchased
from Takara Biotechnology Co., Ltd. Biological signal recorder
system was purchased from Anhui Zhenghua Biological Instrument
Equipment Co., Ltd.
The study was approved by the Ethics Committee of
The Affiliated Hospital of Jining Medical University (Jining,
China).
Establishment and grouping of animal
models
Ten rats were randomly selected as sham operation
group, and the other rats were all used to construct CHF rat model
by constricting the abdominal aorta. The specific steps were: All
rats were fasted for more than 12 h before surgery, then 3%
pentobarbital sodium solution was prepared and injected
intraperitoneally (0.15 ml/100 g) to anesthetize the rats. After
anesthesia, a 2.5 cm long incision was made in the abdominal skin
along the anterior midline at the costal arch, then the left renal
artery was freed from the abdominal aorta at the upper part by
entering the abdominal cavity. Next, the abdominal aorta was placed
in parallel with needle 7 and ligated with thread 0, the needle was
taken out afterwards. Abdomen was closed layer by layer after
confirming that the blood flow of the abdominal aorta was
unobstructed. Rats in the sham operation group were only given
abdominal aorta separation, not ligated. All rats received 20,000
units of penicillin injections in abdominal cavity for 3
consecutive days after operation. After 4 weeks of continuous
feeding, 10 CHF rats were randomly selected for hemodynamic
detection. Left ventricular end-diastolic pressure (LVEDP) ≥15 mmHg
indicated the modeling was successful. Forty rats with successful
modeling were randomly divided into model, Iva, trimetazidine and
combined drug group (Iva + trimetazidine) with 10 rats each. Rats
in the sham operation and model group were given 10 mg/kg of normal
saline daily, rats in the Iva group were given 10 mg/kg of Iva
daily, rats in the trimetazidine group were given 10 mg/kg of
trimetazidine daily, and rats in the combined drug group were given
Iva (10 mg/kg) and trimetazidine (10 mg/kg) daily for 12 weeks of
continuous treatment. The changes of hemodynamic indexes, heart
rate, CTGF and SOD levels as well as transforming growth factor β1
(TGF-β1) and collagen I (COL-I) expression levels in myocardial
tissue in each group were detected.
Index detection method
Hemodynamic index detection
After the treatment, the right common carotid artery
was retrograde intubated to the left ventricle, and then the other
end was connected to the pressure transducer containing multimedia
biological signal recorder system in order to record maximum rising
and decreasing rate of the left ventricular end-diastolic pressure
(LVEDP) and the left ventricular pressure
(±dp/dtmax).
Detection of SOD and CTGF
The myocardial tissue was homogenized by a
high-speed homogenizer, then the expression of SOD was detected
using enzyme-linked immunosorbent assay (ELISA), and the specific
operation strictly followed the kit instructions. The content of
CTGF in the myocardial tissue was detected by western blot
analysis. The specific method was as follows: Total protein in the
myocardial tissue was extracted, and separated with 10% SDS-PAGE,
then transferred to PVDF membrane. The membrane was blocked with 5%
skimmed milk at room temperature for 1 h, then incubated overnight
at 4°C with primary mouse anti-rat CTGF (cat. no. AMAB91366; dil,
1:1,000) and β-actin monoclonal antibodies (cat. no. A1978; dil,
1:400) both from Sigma-Aldrich; Merck KGaA and incubated at 37°C
for 1 h with secondary rabbit anti-mouse polyclonal antibody (cat.
no. SAB3701212; dil, 1:100; Sigma-Aldrich; Merck KGaA). The protein
bands on the membrane were developed with DAB developer.
Detection of TGF-β1 mRNA and COL-I mRNA expression
by RT-qPCR. The myocardial tissue of rats was cut and then added
with TRIzol reagent to extract total RNAs. The purity and
concentration of RNAs were detected by an ultraviolet
spectrophotometer. According to the instructions of reverse
transcription kit, 1 µg of total RNA was reverse transcribed to
cDNA, with reaction parameters of 30°C for 10 min, 42°C for 30 min
and 95°C for 5 min. The transcribed cDNA was used for qPCR
amplification, and the system was as follows: 10 µl of Taqman PCR
Master Mix II, 0.25 µl of Takara Ex Taq, 0.5 µl of each upstream
and downstream primer, ddH2O complemented to 20 µl.
β-actin was used as an internal reference, and the primer sequences
are shown in Table I. qPCR reaction
conditions: Pre-denaturation at 94°C for 2 min, then 95°C for 30
sec and 60°C for 30 sec for 40 circles, then extension at 72°C for
1 min. Real-time quantitative PCR detection was carried out with
qPCR instrument, and the experiment was repeated 3 times. The
results were analyzed using the 2−ΔΔCq method (11).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Factor | Upstream primer | Downstream
primer |
|---|
| TGF-β1 |
5′-TGGACCGCAACAACGCAATCTA-3′ |
5′-CACCTCGACGTTTGGGACTGATC-3 |
| COL-I |
5′-ATGTCTGGTTTGGAGAGAGCA-3′ |
5′-GAGGAGCAGGGACTTCTTGAG-3′ |
| β-actin |
5′-GAGAGGGAAATCGTGCGTGAC-3′ |
5′-CATCTGCTGGAAGGTGGACA-3′ |
Statistical analysis
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was used to
analyze the experimental data. The measurement data were expressed
as mean ± standard deviation (SD). One-way analysis of variance
(ANOVA) was used to compare the differences among groups, and the
Dunnett's t-test was used for the following pairwise comparison.
The difference was statistically significant with P<0.05.
Results
Expression of hemodynamic indexes in
each group
Compared with the sham operation group, the LVEDP in
the model group increased significantly, while the
±dp/dtmax decreased significantly (P<0.05). Whereas,
compared with the model group, the LVEDP in the Iva, trimetazidine
and combined drug group decreased significantly, while the
±dp/dtmax increased significantly (P<0.05). The
changes in the combined drug group were the most notable
(P<0.05), and the indexes in the Iva and trimetazidine group had
no significant difference (P>0.05) (Table II).
 | Table II.Expression of hemodynamic indexes in
each group. |
Table II.
Expression of hemodynamic indexes in
each group.
| Index | Sham operation group
(n=10) | Model group
(n=10) | Iva group (n=10) | Trimetazidine group
(n=10) | Combined drug group
(n=10) | F value | P-value |
|---|
| LVEDP (mmHg) |
6.12±0.14a | 21.37±0.79 |
12.21±0.68a,b |
12.63±0.59a,b |
7.09±0.11a | 1252 | <0.001 |
| +dp/dtmax
(mmHg/sec) |
5210.71±119.75a | 4182.55±51.92 |
4713.91±111.25a,b |
4698.75±112.14a,b |
5122.76±118.34a | 149.7 | <0.001 |
| -dp/dtmax
(mmHg/sec) |
4351.83±129.54a | 2875.31±60.88 |
3451.26±113.79a,b |
3398.18±114.16a,b |
4268.81±125.33a | 317.0 | <0.001 |
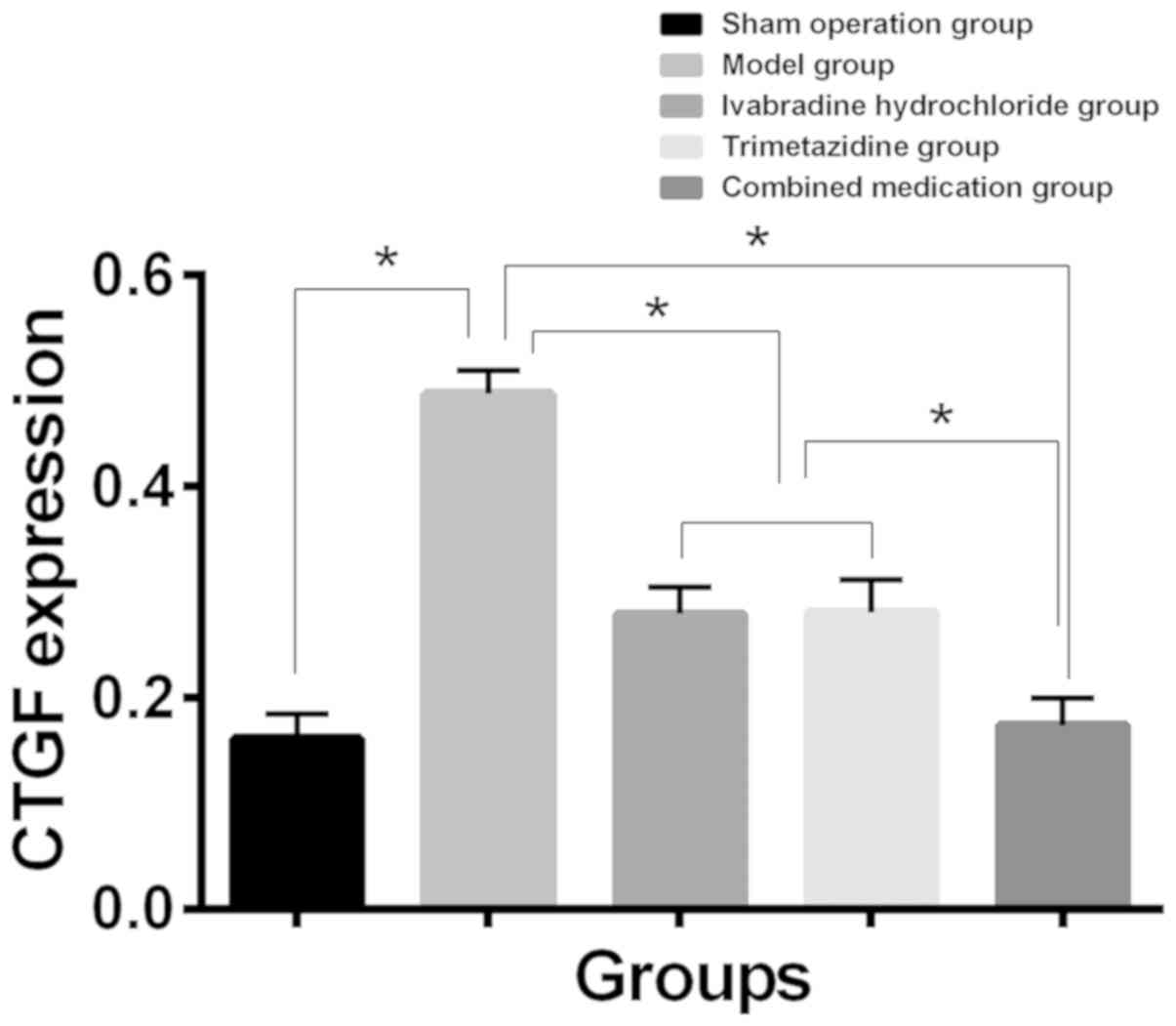
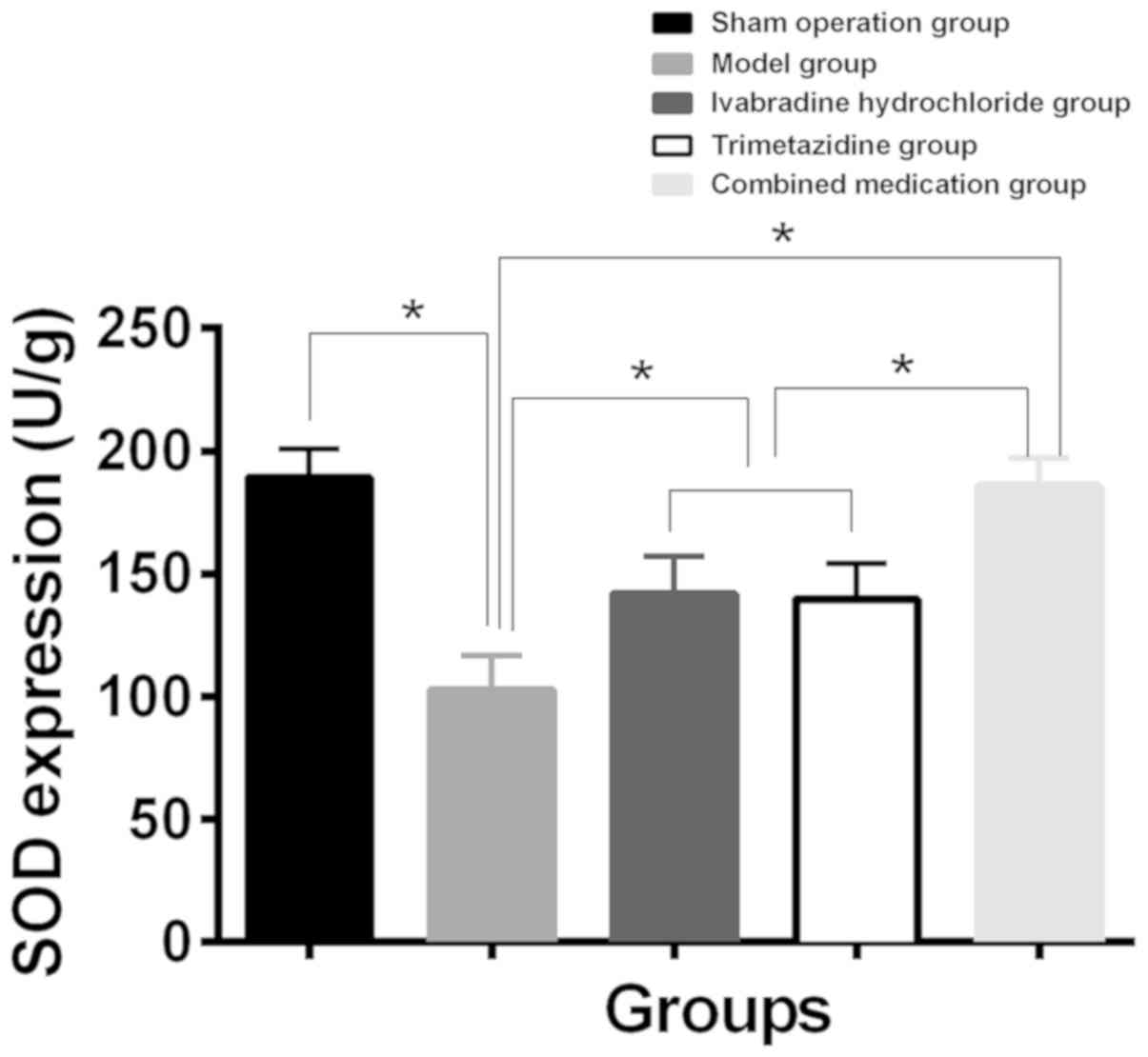
Expression of CTGF and SOD in
myocardial tissue of each group
Compared with the sham operation group, SOD content
decreased and CTGF expression increased in myocardial tissue of the
model group. While compared with the model group, SOD content
increased and CTGF expression level decreased in myocardial tissue
of the Iva, trimetazidine and combined drug group (P<0.01). The
changes in the combined drug group were the most notable
(P<0.05), and the expression levels of CTGF and SOD in the Iva
and trimetazidine group had no significant difference (P>0.05)
(Table III, Figs. 1 and 2).
 | Table III.Expression of CTGF and SOD in
myocardial tissue of each group. |
Table III.
Expression of CTGF and SOD in
myocardial tissue of each group.
| Index | Sham operation group
(n=10) | Model group
(n=10) | Iva group (n=10) | Trimetazidine group
(n=10) | Combined drug group
(n=10) | F value | P-value |
|---|
| CTGF |
0.162±0.023a | 0.488±0.022 |
0.276±0.025a,b |
0.281±0.031a,b |
0.174±0.026a | 261.0 | <0.001 |
| SOD (U/g) |
189.33±11.71a | 102.65±14.23 |
141.82±15.31a,b |
139.77±14.52a,b |
185.69±11.39a | 71.30 | <0.001 |
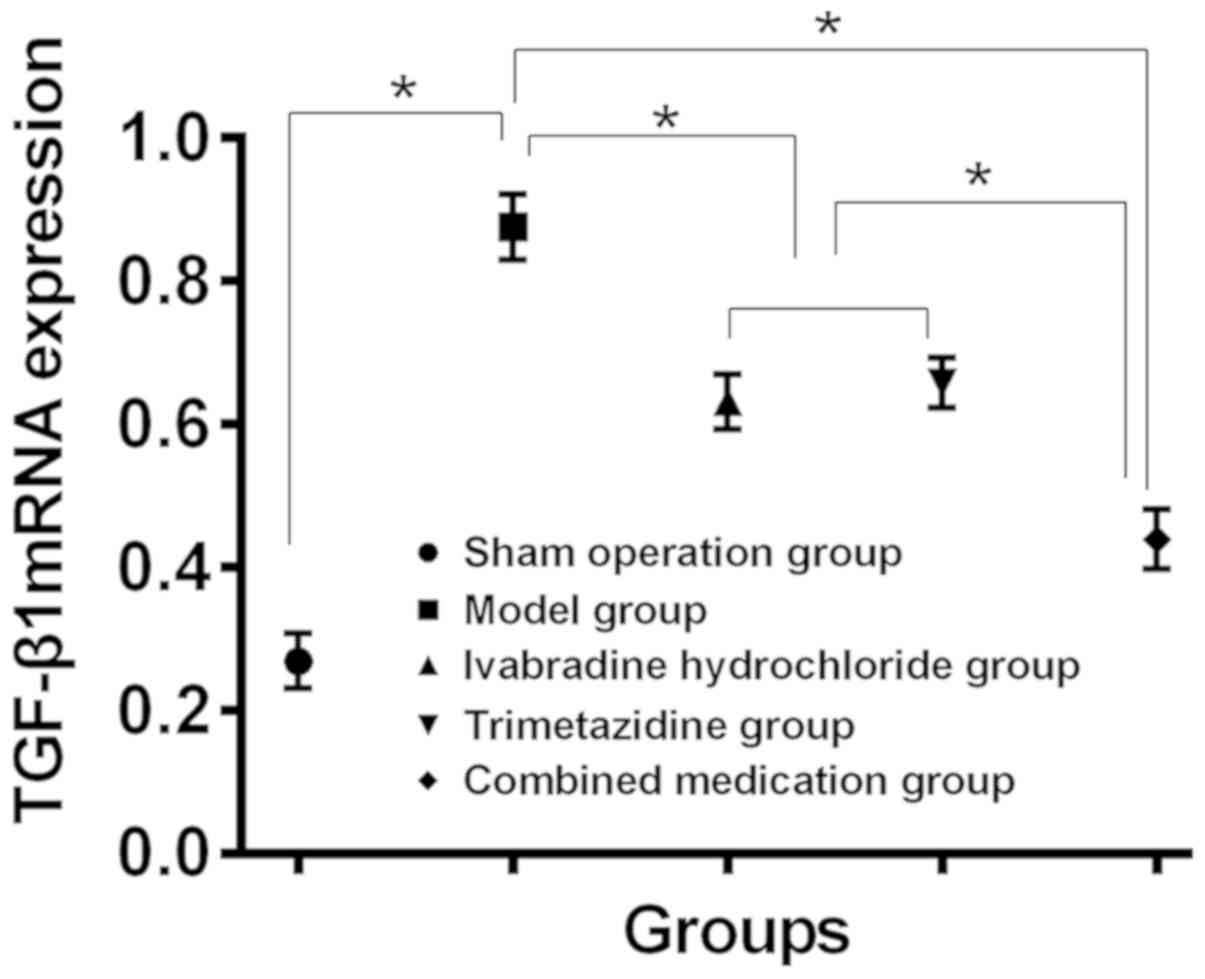
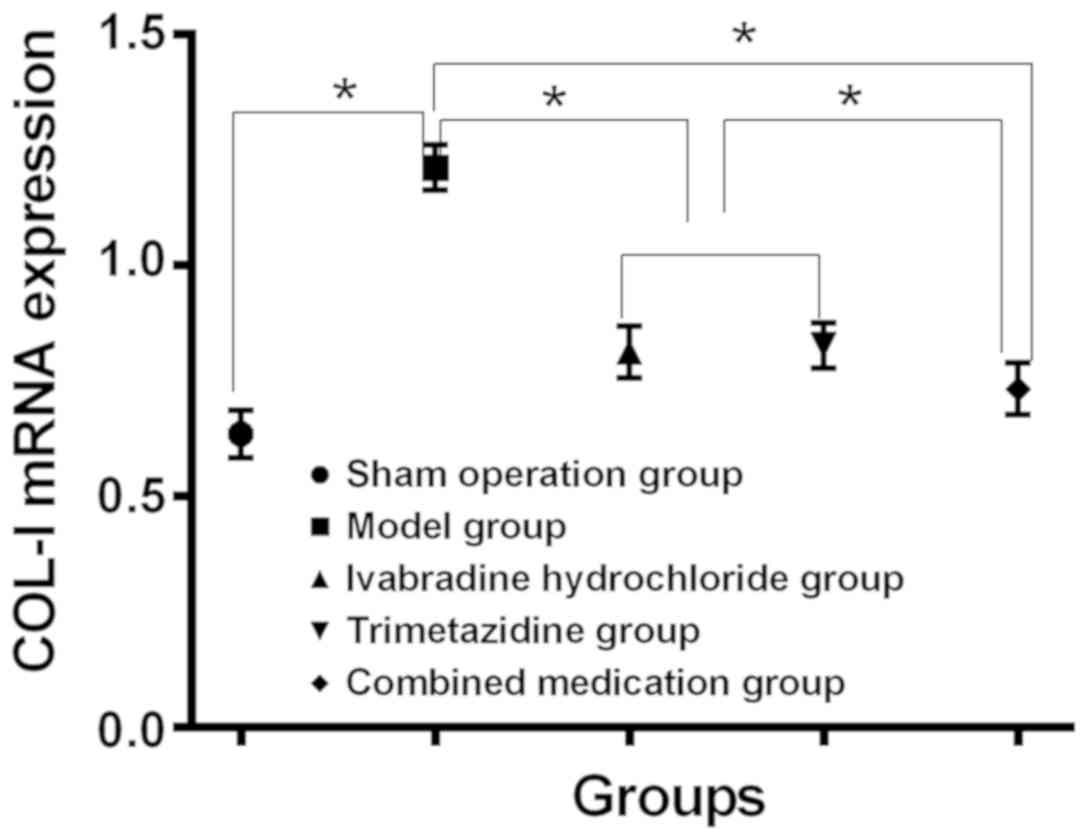
Expression levels of TGF-β1 and COL-I in myocardial
tissue of each group. Compared with the sham operation group, the
expression levels of TGF-β1 mRNA and COL-I mRNA in myocardial
tissue of model group increased, while compared with the model
group, the expression levels decreased in myocardial tissues of the
Iva, trimetazidine and combined drug group (P<0.05). Changes in
the combined drug group were the most notable (P<0.05), and the
expression of TGF-β1 mRNA and COL-I mRNA in the Iva and
trimetazidine group had no significant difference (P>0.05)
(Table IV, and Figs. 3 and 4).
 | Table IV.Expression of TGF-β1 mRNA and COL-I
mRNA in myocardial tissue of each group. |
Table IV.
Expression of TGF-β1 mRNA and COL-I
mRNA in myocardial tissue of each group.
| Index | Sham operation
group (n=10) | Model group
(n=10) | Iva group
(n=10) | Trimetazidine group
(n=10) | Combined drug group
(n=10) | F value | P-value |
|---|
| TGF-β1 mRNA |
0.369±0.038a | 0.875±0.046 |
0.631±0.039a,b |
0.657±0.035a,b |
0.439±0.042a | 328.4 | <0.001 |
| COL-I mRNA |
0.635±0.051a | 1.211±0.049 |
0.812±0.055a,b |
0.826±0.048a,b |
0.723±0.056a | 178.5 | <0.001 |
Discussion
The high incidence of hypertension, diabetes and
coronary heart diseases caused by accelerated aging of the
population and the improvement of living standards leads to a
rising number of heart failure patients (12). In recent years, diuretics, β receptor
blockers are mostly used in clinical treatment of heart failure but
achieving unsatisfactory efficacy and still high mortality rates
(13,14). A study pointed out that the decisive
mechanism of CHF was ventricular remodeling, of which MF is the
most significant (15). MF is
reported to be an important pathological basis of heart failure,
mainly referring to the remodeling of intermyocardial collagen
network (ICN) (16). In this study,
the CHF rat models were prepared by constricting the abdominal
aorta, and the effects of Iva and trimetazidine alone and in
combination on MF in CHF rats were explored.
LVEDP is often used to express cardiac volume load
clinically. Some studies show that its increase leads to increased
mitochondria and decreased adenosine triphosphate (ATP) in cardiac
myocytes, promotes the synthesis of collagen fibers and eventually
accelerates the process of MF in CHF rats (16,17). Our
experimental results showed that, compared with the sham operation
group, the LVEDP in the model group increased significantly while
the ±dp/dtmax decreased significantly (P<0.05), which
indicated that the model group rats developed MF. Compared with the
model group, the LVEDP in the Iva group, trimetazidine group and
combined drug group decreased significantly while the
±dp/dtmax increased significantly (P<0.05). Moreover,
the changes in the combined drug group were the most notable
(P<0.05). The results suggested that both Iva and trimetazidine
improved MF in rats, and the combined medication achieved a more
obvious improvement, which was suspected to be related to the
maximum rising and decreasing rate of left ventricular pressure in
rats. A previous study (18) also
reached the same conclusion as ours when discussing the effect of
trimetazidine on MF in CHF rats, but there is no relevant study to
explain it for the time being. SOD is an important antioxidant
enzyme and a major substance for eliminating free radicals in
vivo. CTGF is a factor that can induce fibroblast proliferation
with obvious mitogen and chemotaxis. Both SOD and CTGF are
important factors for evaluating MF (19,20). Our
results showed that, compared with the sham operation group, SOD
content decreased and CTGF expression increased in myocardial
tissue of the model group. Compared with the model group, SOD
content increased and CTGF expression level decreased in myocardial
tissue of the Iva group, trimetazidine group and combined drug
group (P<0.05), but the changes in the combined drug group were
the most notable (P<0.05). The results suggested that Iva and
trimetazidine affected MF process through CTGF and SOD in
myocardial tissue of CHF rats. A study (21) has shown that Iva could inhibit the
oxidative stress reaction of myocardial cells in CHF rats, and
another study (22) also showed that
trimetazidine, as a new anti-myocardial ischemia drug, also had the
function of oxygen free radical inhibition, oxidation resistance
and apoptosis, which partially explained the changes in SOD
expression in our conclusion. It was considered that (23) TGF-β1, a multifunctional regulatory
factor, could affect MF through Smads-dependent and
Smads-independent pathways. There is also a study (24) showing that excessive synthesis and
deposition of COL-I promoted MF in patients with hypertension and
heart failure. Therefore, the expression levels of TGF-β1 mRNA and
COL-I mRNA in myocardial tissues were also detected in this study.
The results showed that compared with the sham operation group, the
expression levels of both TGF-β1 mRNA and COL-I mRNA in myocardial
tissues of the model group were increased. Compared with the model
group, the expression levels decreased in myocardial tissues of the
Iva group, trimetazidine group and combined drug group (P<0.05),
and changes in the combined drug group were the most notable
(P<0.05). The results suggested that both Iva and trimetazidine
could inhibit the expression levels of TGF-β1 and COL-I, and the
inhibition effect was most pronounced when used in combination. The
above results all suggest that Iva and trimetazidine can
effectively inhibit MF in CHF rats, and the effect of combined
administration is greater than that of the single drug. However, at
present, there is no related research on the synergistic effect of
the two drugs through different mechanisms.
Collectively, Iva combined with trimetazidine can
inhibit MF in CHF rats by regulating LVEDP, SOD content and CTGF
expression in myocardial tissue, and by inhibiting TGF-β1w and
COL-I expression, and the inhibition effect is most pronounced when
used in combination. However, in our study, the specific mechanism
of Iva and trimetazidine in regulating MF and the possible toxic
and side effects of combined medication were not fully addressed,
thus further studies are anticipated.
Acknowledgements
Not applicable.
Funding
This study was supported by Technology boosting and
technology translation project of Jining City (No.
2017ZDGH031).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
DM wrote the manuscript. TX, GC and XW performed
qPCR and ELISA. ZL and XL were responsible for western blot
analysis. JL and NY contributed to analysis of observation indexes.
All the authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Jining Medical University (Jining,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Edelmann F, Knosalla C, Mörike K, Muth C,
Prien P and Störk S: Chronic heart failure. Dtsch Arztebl Int.
115:124–130. 2018.PubMed/NCBI
|
|
2
|
Füller M, von Bodman G, Kopf Dr, Brömsen
J, Sodian R and Block M: Chronic heart failure with reduced
ejection fraction: Standard treatment and new therapeutic options.
MMW Fortschr Med. 154:63–70. 2012.(In German).
|
|
3
|
Lena A, Coats AJS and Anker MS: Metabolic
disorders in heart failure and cancer. ESC Heart Fail. 5:1092–1098.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gyöngyösi M, Winkler J, Ramos I, Do QT,
Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, et al:
Myocardial fibrosis: Biomedical research from bench to bedside. Eur
J Heart Fail. 19:177–191. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curtis LH, Whellan DJ, Hammill BG,
Hernandez AF, Anstrom KJ, Shea AM and Schulman KA: Incidence and
prevalence of heart failure in elderly persons, 1994–2003. Arch
Intern Med. 168:418–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hohendanner F, Messroghli D, Bode D,
Blaschke F, Parwani A, Boldt LH and Heinzel FR: Atrial remodelling
in heart failure: Recent developments and relevance for heart
failure with preserved ejection fraction. ESC Heart Fail.
5:211–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards AM: ST2 and prognosis in chronic
heart failure. J Am Coll Cardiol. 72:2321–2323. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu DY, Huang DJ, Yuan ZY, Zhao RP, Yan XW
and Wang MH; Chinese Investigators of SHIFT Study, : Efficacy and
safety analysis of ivabradine hydrochloride treatment of Chinese
patients with chronic heart failure: Subgroup analysis of Chinese
patients in the SHIFT study. Zhonghua Xin Xue Guan Bing Za Zhi.
45:190–197. 2017.(In Chinese). PubMed/NCBI
|
|
9
|
Ke Y, Xu D, Li M, Wu Z and Huang Y:
Effects of bisoprolol in combination with trimetazidine on the
treatment of heart failure and concomitant chronic obstructive
pulmonary disease. Pak J Med Sci. 32:1208–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fragasso G, Palloshi A, Puccetti P,
Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O and
Margonato A: A randomized clinical trial of trimetazidine, a
partial free fatty acid oxidation inhibitor, in patients with heart
failure. J Am Coll Cardiol. 48:992–998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bernardi L, Spadacini G, Bellwon J, Hajric
R, Roskamm H and Frey AW: Effect of breathing rate on oxygen
saturation and exercise performance in chronic heart failure.
Lancet. 351:1308–1311. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suzuki T, Palus S and Springer J: Skeletal
muscle wasting in chronic heart failure. ESC Heart Fail.
5:1099–1107. 2018.PubMed/NCBI
|
|
14
|
Li CC, Chang SR and Shun SC: The self-care
coping process in patients with chronic heart failure: A
qualitative study. J Clin Nurs. 28:509–519. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
López B, Querejeta R, González A, Sánchez
E, Larman M and Díez J: Effects of loop diuretics on myocardial
fibrosis and collagen type I turnover in chronic heart failure. J
Am Coll Cardiol. 43:2028–2035. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang E-W, Jia X-S, Ruan C-W and Ge Z-R:
miR-487b mitigates chronic heart failure through inhibition of the
IL-33/ST2 signaling pathway. Oncotarget. 8:51688–51702.
2017.PubMed/NCBI
|
|
17
|
Boivin-Jahns V and Jahns R:
GPCR-autoantibodies in chronic heart failure. Front Biosci.
23:2065–2081. 2018. View
Article : Google Scholar
|
|
18
|
Brennan EJ: Chronic heart failure nursing:
Integrated multidisciplinary care. Br J Nurs. 27:681–688. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vergaro G and Iacoviello M: Is there a
‘renal paradox’ in chronic heart failure? Int J Cardiol.
267:139–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Canepa M, Ameri P, Lucci D, Nicolosi GL,
Marchioli R, Porcu M, Tognoni G, Franzosi MG, Latini R, Maseri A,
et al GISSI-HF Investigators, : Modes of death and prognostic
outliers in chronic heart failure. Am Heart J. 208:100–109. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Freitas P, Ferreira AM and Aguiar C:
Comparison of prognostic scores in chronic heart failure. JACC
Heart Fail. 6:887–888. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simonavičius J, Knackstedt C and
Brunner-La Rocca HP: Loop diuretics in chronic heart failure: How
to manage congestion? Heart Fail Rev. 24:17–30. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wolfson AM, Fong M, Grazette L, Rahman JE
and Shavelle DM: Chronic heart failure management and remote
haemodynamic monitoring. Heart. 104:1910–1919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fudim M, Ganesh A, Green C, Jones WS,
Blazing MA, DeVore AD, Felker GM, Kiefer TL, Kong DF, Boortz-Marx
RL, et al: Splanchnic nerve block for decompensated chronic heart
failure: splanchnic-HF. Eur Heart J. 39:4255–4256. 2018. View Article : Google Scholar : PubMed/NCBI
|