Introduction
Brain arteriovenous malformation (BAVM) refers to a
cluster of direct connections between arteries and draining veins
without an intervening capillary bed. AVMs have the following major
components: One or more feeding arteries, a nidus that forms the
site of the arteriovenous shunt and draining venous structures
(1,2). The arteries that supply blood to a BAVM
mainly arise from the internal carotid artery (ICA) and vertebral
basilar artery (VBA) systems (3,4).
However, when the BAVM is adjacent to cerebral
convexities or intracranial dural structures, the arteries that
supply the BAVM may also originate or even be wholly derived from
the arteries supplying the meninges. These latter structures are
called BAVM with transdural blood supply (TBS) (5,6). The TBS
of BAVM may be from the external carotid artery (ECA) or the
meningeal branches of the ICA and the VBA system (7).
To date, a relatively limited number of studies have
reported on BAVM with TBS; thus, the current knowledge is
insufficient. Furthermore, no comprehensive review has been
previously published. Hence, for the present article, a literature
search was performed using the PubMed database to review BAVM with
TBS.
Incidence
BAVM with TBS has long been in the spotlight of
research, while incidences of BAVM with TBS are significantly
different among published studies. For instance, according to the
study by Willinsky et al (8),
the incidence was 29%. Newton and Cronqvist (9) reported an incidence of 27%. However,
Miyachi et al (10) reported
an incidence of 65%.
Compared with that reported by Miyachi et al
(10), the studies by Willinsky
et al (8) and Newton and
Cronqvist (9) reported a lower
incidence of TBS, as they included whole-brain BAVM in their
studies. The study by Miyachi et al (10) mainly focused on superficial-area
BAVM, and as BAVM with TBS usually occurs in subpial areas and the
cortex, they estimated a relatively higher incidence of BAVM with
TBS than the other studies.
Among whole-brain studies of BAVM, the study by
Bervini et al (7) appears to
have assessed the incidence of TBS in the most suitable manner.
This study included 769 cases of BAVM; of these, 51 cases had a
TBS, resulting in an incidence of 6.6% (7). The incidence of TBS in other studies
was higher, perhaps due to confusion regarding the classification
of BAVM with TBS during the early years of research or differences
in angiographic techniques, as well as the location of the BAVMs
included (11).
Pathogenesis
The mechanisms by which TBS forms in a BAVM with TBS
are mainly divided into the congenital and acquired type.
Congenital factors
The congenital formation theory of TBS has
demonstrated that TBS developed upon generation of the BAVM,
according to which BAVM with TBS form during embryonic development
of the cerebral circulation (7).
Miyachi et al (10) indicated
that in early embryos, the epidural blood supply originates from
pial-dural anastomotic channels. Hence, during the embryonic
development of a BAVM, the BAVM may acquire a TBS.
Acquired factors
In recent years, TBS of BAVM has been more
frequently regarded as acquired (5,12–14).
This may occur in two situations: i) The formation of the BAVM is
congenital, and only the formation of TBS is acquired; ii) the
formation of the BAVM and the TBS is acquired (15). Acquired mechanisms mainly attribute
to sinus thrombosis and hypertension, including trauma, radiation,
infection, surgery and transarterial embolization (6,15–18).
When the above factors induce sinus thrombosis or thrombosis, the
endogenous dysplastic dural vessels in the sinus further develop
and directly induce an artery-to-sinus communication, thus forming
a BAVM with TBS (6,13,17,19–21).
In addition, the inflammation caused by the use of
glue or the Onyx Liquid Embolic System (Medtronic Plc) during
embolization may stimulate the formation of a BAVM in a superficial
area, resulting in the formation of a TBS (5). Subsequent to the establishment of BAVM,
high wall shear stress can cause angiogenesis and arteriogenesis to
recruit the TBS that directly supplies the BAVM (7).
Angioarchitecture
The characteristics of the angioarchitecture of BAVM
with TBS include its size, location, arterial blood supply artery
(including a TBS), nidus structure and drainage vein (22).
Location
The nidus of a BAVM with TBS is usually located in a
superficial area, including the subpial area or the cortex, but may
occasionally appear around the tentorium, skull base or posterior
fossa (22). Koo et al
(11) reported that BAVM with TBS
was most commonly observed in the temporal lobe (34.4%), followed
by the occipital lobe (28.1%), parietal lobe (18.8%) and frontal
lobe (12.5%). Soderman et al (6) also indicated that BAVM with TBS most
commonly occurs in the temporal lobe, followed by the parietal lobe
and frontal lobe. The reason why the temporal lobe is a relatively
common location may be due to this site being the major region
supplied by blood from the middle meningeal artery (MMA) and
occipital artery (6,11).
Feeding artery and draining vein
The major supplying arteries of a BAVM with TBS
include the non-meningeal branches of the ICA and VBA system; the
meningeal branches of the ECA, ICA and VBA systems may also be
involved in BAVM that acquire a TBS (23,24). In
rare cases, a BAVM will exclusively receive its blood from a TBS
(7,25). Of all types of TBS, those from the
MMA and occipital artery are the most common, accounting for 86% of
all types (23). Due to the presence
of a TBS for the BAVM, the venous drainage from the BAVM may be
similar to that of a dural arteriovenous fistula, which may have
cortical venous retrograde drainage, and the drainage veins of a
BAVM may be tortuous or variceal and exhibit aneurysmal dilation
(22,26).
Nidus
BAVMs are divided into large vs. small and compact
vs. diffuse types according to the size and compactness of the
nidus, respectively (27–29). In a BAVM with TBS, a larger nidus is
associated with a higher probability of a TBS. The reasons for this
association may be that larger BAVMs are more frequently close to
the dura mater and associated with a more complex source of
arterial blood supply (6,7,10).
For instance, Koo et al (11) indicated that the probability of a TBS
occurring in a BAVM with an AVM volume in the nidus of >10
cm3 was higher than that for a BAVM with a nidus of 4–10
cm3. In addition, it is more likely for a TBS to appear
in a diffuse-type BAVM (30).
Miyachi et al (10) and Koo
et al (11) reported that
diffuse-type BAVMs were associated with a significantly higher
incidence of TBS.
Clinical manifestations
The clinical manifestations of BAVM with TBS are
similar but not identical to those of BAVM without TBS. The
blood-supplying artery of a BAVM with TBS involves the dura; thus,
the clinical manifestations of the BAVM share the characteristics
of the dura (31). However, the
major clinical manifestations are associated with the size and
location of the BAVM.
General characteristics
BAVM with TBS is mostly observed in elderly
patients, and its incidence increases with age (6,7,10). Dahl et al (32) reported that the maximum age at onset
of BAVM with TBS is 50–60 years. Bervini et al (7) reported that the average age of patients
with BAVM with TBS was 43 years, which is obviously higher than
that of BAVM patients without TBS (with an average age of 37
years), perhaps due to a BAVM taking a certain time to acquire a
TBS.
Whether the incidence of BAVM with TBS is associated
with gender has not been clearly determined by previous studies. It
has been noted that BAVM with TBS occurring in the anterior cranial
fossa is more common in males than in females, an effect that may
be linked to factors including trauma (33). By contrast, BAVM with TBS in other
locations is more likely to occur in females (34).
Intracranial hemorrhage
The most important clinical manifestation of BAVM
with TBS is intracranial hemorrhage, which is life-threatening in
severe cases (35). For instance,
nine of the 30 cases of BAVM with TBS reported by Jin et al
(36) were caused by intracranial
hemorrhage (incidence, 30.0%). Intracranial hemorrhage may occur at
the BAVM nidus or from aneurysmal dilated drained veins, with the
latter being more common than the former, and is characterized by
intracranial hematoma and subarachnoid hemorrhage (37).
Subdural hematoma is associated with bleeding of
BAVM with TBS. As BAVM with TBS tend to be located at the surface
of the brain, ruptures in the small blood supply arteries between
the dura mater and cortex seep into the subdural region (38), with trauma being a likely cause. For
instance, Kominato et al (39) reported on a female patient who died
of acute subdural hematoma, and autopsy confirmed that the patient
had a BAVM with TBS in the falx cerebri.
Epilepsy
Epilepsy is the major non-hemorrhage symptom of BAVM
with TBS. Epilepsy occurs in affected patients due to the location
of the BAVM nidus usually involving the cerebral cortex. Thus, the
BAVM stimulates abnormal discharges in the pia mater and cortex,
thereby causing epilepsy (40).
Furthermore, vein pulsations, which are caused by vein hypertension
and are increased by vein resistance, may occur and increase the
incidence of epileptic seizures (41,42).
Other symptoms
Regarding other clinical symptoms, BAVM with TBS is
associated with chronic headache. For instance, Koo et al
(11) determined that chronic
headache occurred 3.7 times more frequently in patients with BAVM
with TBS than in those with BAVM without TBS, suggesting that TBS
itself is associated with chronic headache. Increased intracranial
pressure is another common clinical manifestation of BAVM with TBS.
The mechanism underlying this symptom may be an increase in
pressure and the flow of arterialized meningeal sinuses (43,44).
In addition, the hemodynamic factors associated with
BAVM with TBS may also cause corresponding symptoms. For instance,
the diversion of blood flow from the ophthalmic artery to the BAVM
may cause amaurosis fugax (37).
Other rare symptoms include cranial nerve palsy and trigeminal
neuralgia, and these symptoms are considered to be caused by
cranial nerve compression by enlarged and serpiginous draining
veins (45,46).
Imaging examinations
The clinical manifestations of BAVM with TBS are
nonspecific. Therefore, imaging, including digital subtraction
angiography (DSA), computed tomography (CT) angiography and
magnetic resonance angiography are the major methods used to
diagnose BAVM with TBS. Among these, DSA is the gold standard for
diagnosis (26). However, for BAVM
with TBS, selective angiographies, including selective ECA and ICA
angiogram, or superselective angiography, are important.
The characteristics of BAVM with TBS that may be
clearly observed on selective and superselective angiography
include the following: A nidus located close to the surface of the
brain, blood-supplying arteries originating from both epidural and
intracranial arteries, and venous drainage to the dural sinus
and/or leptomeningeal vein (24,47).
Retrograde leptomeningeal venous drainage is frequently tortuous,
variceal or frankly aneurysmal (5).
Treatment
The treatment plan for BAVM with TBS generally
includes surgical resection, endovascular treatment (EVT),
stereotactic radiosurgery and combined treatment (22,48–51). The
choice of treatment components is a multidisciplinary decision that
requires an evaluation of the anatomical location of the BAVM,
Spetzler-Martin classification and hemodynamics, and the decision
should be made by local experts and the patient (52).
Surgical resection
Complete surgical resection is the most effective
treatment for BAVM with TBS, but as it involves numerous and
complex arrangements of feeding arteries and drainage veins, the
operation is difficult to perform. TBS in particular increases the
risk of the operation (53).
Intra-operatively separating the dura mater from the BAVM surface
may tear the corresponding dural blood supply arteries, causing
catastrophic hemorrhage. To prevent this from occurring, it is
suggested that the dura and all of its blood-supplying arteries
connected to the BAVM should be resected (54).
In cases of BAVM with TBS caused by hemorrhaging of
the expanded drainage vein, simple surgical interruption of the
draining vein may be a successful treatment. However, to prevent
rebleeding, the focal point and nidus should also be amputated
(55,56).
Endovascular treatment
At present, EVT is one of the methods used for
treating BAVM with TBS. The advantage of EVT is its low procedural
invasiveness and preservation of brain functionality (52). Performing EVT in BAVM with TBS mainly
involves transarterial embolization, which is performed via either
a dural blood supply approach or a pial blood supply approach
(non-dural blood supply approach) (36).
Among these, the pial blood supply approach in EVT
may effectively embolize the BAVM and prevent rebleeding (57). However, the dural blood supply
approach is safer; furthermore, by reducing the blood flow of the
brain surface, certain clinical symptoms may be improved, including
epilepsy and headache from dural feeding pulsation, particularly in
unruptured BAVM with TBS (36).
During the dural blood supply approach, the use of a dual-lumen
balloon catheter is helpful to inject the liquid embolic material
into the BAVM to prevent reflux (58,59).
Combination of surgical and
endovascular approaches
During surgical resection of BAVM with TBS, the
corresponding dural blood-supplying arteries should be controlled
prior to performing the nidus resection to reduce the risk of
intra-operative bleeding (60,61).
Thus, in earlier studies, it was a feasible choice to perform ECA
ligation (53). The combination of
surgical and endovascular methods for intracranial neurovascular
diseases in a hybrid operating room is a novel and promising trend
(62,63), and in modern case series, numerous
TBS were selectively embolized as a surgical adjunct to make
surgical resection safer.
Stereotactic radiosurgery
Stereotactic radiosurgery is generally not the first
choice for BAVM with TBS and is frequently used as a complementary
treatment after surgical treatment and intravascular intervention.
The aim of stereotactic radiosurgery in those cases is to further
and more completely remove the nidus (64). However, a previous study described
the successful treatment of BAVM with TBS by stereotactic
radiosurgery alone (51). In 1993,
Chandler and Friedman (49) reported
on one case of BAVM with TBS located in the anterior cranial fossa
that was treated with 3,000 cGy to the 80% isodose line of an 18-mm
collimator, and angiography performed after 3 years indicated that
the BAVM had disappeared.
Prognosis
As BAVM with TBS is not frequently reported, the
prognosis of BAVM with TBS is uncertain. It is now agreed that, due
to BAVM with TBS involving a complex supply of arteries, the
therapeutic outcomes are generally poor. Shima et al
(56) reported that the surgical
cure rate for BAVM with TBS was low, while its morbidity and
mortality were high. If resection is not complete, the BAVM nidus
may expand or cause disastrous intracerebral hemorrhage (56).
Soderman et al (6) reported on EVT performed on 54 cases of
BAVM with TBS, 22% of whom required retreatment by EVT after 3
years of follow-up, indicating that the imaging-based cure rate was
not ideal. However, with the continuous enhancement of the
understanding BAVM with TBS, treatment outcomes are also gradually
improving (65).
Conclusions
BAVM with TBS are different from those observed in
common BAVM. The mechanisms by which TBS may form in a BAVM are
mainly divided into the congenital and acquired type, the acquired
factor may be popular (5,12–14).
BAVM with TBS is common in elderly patients. DSA is the gold
standard for diagnosing BAVM with TBS. Superselective angiography
is also important. Treatments for BAVM with TBS include surgical
resection, EVT, stereotactic radiosurgery and combined treatment.
Surgical resection is difficult to perform. EVT has become the
major method for treating BAVM with TBS due to its low procedural
invasiveness. Combination of surgical resection and EVT may be a
good option. In addition, stereotactic radiosurgery is frequently
used as a complementary treatment to surgical and endovascular
interventions. The prognosis of BAVM with TBS is not
optimistic.
Typical case of BAVM with TBS
The patient, a 65-year-old female, with no history
of hypertension or diabetes mellitus was admitted the First
Hospital of Jilin University (Changchun, China) in March 2018 due
to presentation of a sudden headache and vomiting for 1 h. After
its onset, the patient was mentally lucid and had flexible limbs.
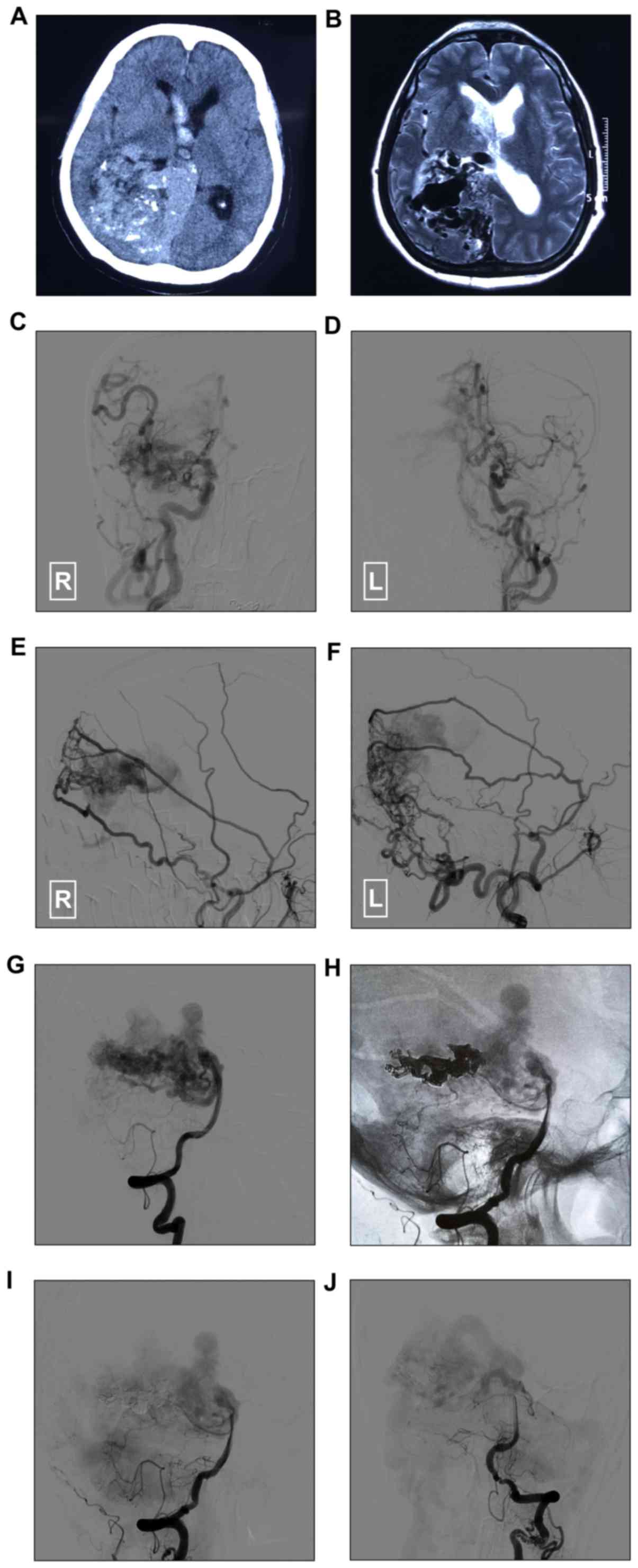
Head CT and magnetic resonance imaging revealed a BAVM near the
right temporal occipital lobe and corpus callosum, and right
ventricular hemorrhage (Fig. 1A-B).
DSA revealed a BAVM with TBS of the bilateral ECA and ICA systems
(Fig. 1C-G). To treat the BAVM near
the ventricle, Onyx embolization was performed via the posterior
cerebral artery, and targeted embolization was performed to treat
the BAVM near the hemorrhage point (Fig.
1H-J). The patient recovered well after surgery and exhibited
no new neurological deficits (Fig.
1).
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY and KX conceived and designed the study. TJ and
YG acquired the data. JP drafted the manuscript. All of the authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
This case study was approved by the Ethics Committee
of the First Hospital of Jilin University (Changchun, China). The
patient signed informed consent for use of clinical data and
images.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of data/information.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Josephson CB, Rosenow F and Al-Shahi
Salman R: Intracranial vascular malformations and epilepsy. Semin
Neurol. 35:223–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mouchtouris N, Jabbour PM, Starke RM,
Hasan DM, Zanaty M, Theofanis T, Ding D, Tjoumakaris SI, Dumont AS,
Ghobrial GM, et al: Biology of cerebral arteriovenous malformations
with a focus on inflammation. J Cereb Blood Flow Metab. 35:167–175.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanborn MR, Park MS, McDougall CG and
Albuquerque FC: Endovascular approaches to pial arteriovenous
malformations. Neurosurg Clin N Am. 25:529–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crowley RW, Ducruet AF, McDougall CG and
Albuquerque FC: Endovascular advances for brain arteriovenous
malformations. Neurosurgery. 1 (Suppl 74):S74–S82. 2014. View Article : Google Scholar
|
|
5
|
Heros RC: Editorial. Transdural arterial
recruitment to brain arteriovenous malformations. J Neurosurg.
127:47–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soderman M, Rodesch G and Lasjaunias P:
Transdural blood supply to cerebral arteriovenous malformations
adjacent to the dura mater. AJNR Am J Neuroradiol. 23:1295–1300.
2002.PubMed/NCBI
|
|
7
|
Bervini D, Morgan MK, Stoodley MA and
Heller GZ: Transdural arterial recruitment to brain arteriovenous
malformation: Clinical and management implications in a prospective
cohort series. J Neurosurg. 127:51–58. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Willinsky R, Lasjaunias P, Terbrugge K and
Pruvost P: Brain arteriovenous malformations: Analysis of the
angio-architecture in relationship to hemorrhage (based on 152
patients explored and/or treated at the hopital de Bicetre between
1981 and 1986). J Neuroradiol. 15:225–237. 1988.(In English,
French). PubMed/NCBI
|
|
9
|
Newton TH and Cronqvist S: Involvement of
dural arteries in intracranial arteriovenous malformations.
Radiology. 93:1071–1078. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyachi S, Negoro M, Handa T and Sugita K:
Contribution of meningeal arteries to cerebral arteriovenous
malformations. Neuroradiology. 35:205–209. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koo HW, Jo KI, Yeon JY, Kim KH, Jeon P,
Kim JS, Hong SC, Shin HJ and Lee JI: Clinical features of
superficially located brain arteriovenous malformations with
transdural arterial communication. Cerebrovasc Dis. 41:204–210.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaudhary MY, Sachdev VP, Cho SH, Weitzner
I Jr, Puljic S and Huang YP: Dural arteriovenous malformation of
the major venous sinuses: An acquired lesion. AJNR Am J
Neuroradiol. 3:13–19. 1982.PubMed/NCBI
|
|
13
|
Russell EJ and Berenstein A: Meningeal
collateralization to normal cerebral vessels associated with
intracerebral arteriovenous malformations: Functional angiographic
considerations. Radiology. 139:617–622. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nabors MW, Azzam CJ, Albanna FJ, Gulya AJ,
Davis DO and Kobrine AI: Delayed postoperative dural arteriovenous
malformations. Report of two cases. J Neurosurg. 66:768–772. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sturiale CL, Puca A, Sebastiani P, Gatto
I, Albanese A, Di Rocco C, Maira G and Pola R: Single nucleotide
polymorphisms associated with sporadic brain arteriovenous
malformations: Where do we stand? Brain. 136:665–681. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herman JM, Spetzler RF, Bederson JB,
Kurbat JM and Zabramski JM: Genesis of a dural arteriovenous
malformation in a rat model. J Neurosurg. 83:539–545. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Houser OW, Campbell JK, Campbell RJ and
Sundt TM Jr: Arteriovenous malformation affecting the transverse
dural venous sinus-an acquired lesion. Mayo Clin Proc. 54:651–661.
1979.PubMed/NCBI
|
|
18
|
Jeffree RL and Stoodley MA: Postnatal
development of arteriovenous malformations. Pediatr Neurosurg.
45:296–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JY, Kim OJ, Joo YJ and Joo JY: Dural
arteriovenous malformation occurring after craniotomy for pial
arteriovenous malformation. J Clin Neurosci. 10:134–136. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe A, Takahara Y, Ibuchi Y and
Mizukami K: Two cases of dural arteriovenous malformation occurring
after intracranial surgery. Neuroradiology. 26:375–380. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ugrinovski J, Vrcakovski M and Lozance K:
Dural arteriovenous malformation secondary to meningioma removal.
Br J Neurosurg. 3:603–607. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis AI, Rosenblatt SS and Tew JM Jr:
Surgical management of deep-seated dural arteriovenous
malformations. J Neurosurg. 87:198–206. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martins C, Yasuda A, Campero A, Ulm AJ,
Tanriover N and Rhoton A Jr: Microsurgical anatomy of the dural
arteries. Neurosurgery 56 (2 Suppl). S211–S251. 2005.
|
|
24
|
Davidson AS and Morgan MK: The embryologic
basis for the anatomy of the cerebral vasculature related to
arteriovenous malformations. J Clin Neurosci. 18:464–469. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosnik EJ, Hunt WE and Miller CA: Dural
arteriovenous malformations. J Neurosurg. 40:322–329. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Awad IA, Little JR, Akarawi WP and Ahl J:
Intracranial dural arteriovenous malformations: Factors
predisposing to an aggressive neurological course. J Neurosurg.
72:839–850. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Solomon RA and Connolly ES Jr:
Arteriovenous malformations of the brain. N Engl J Med.
376:1859–1866. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crimmins M, Gobin YP, Patsalides A and
Knopman J: Therapeutic management of cerebral arteriovenous
malformations: A review. Expert Rev Neurother. 15:1433–1444. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lawton MT, Rutledge WC, Kim H, Stapf C,
Whitehead KJ, Li DY, Krings T, terBrugge K, Kondziolka D, Morgan
MK, et al: Brain arteriovenous malformations. Nat Rev Dis Primers.
1:150082015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohata K, Takami T, El-Naggar A, Morino M,
Nishio A, Inoue Y and Hakuba A: Posterior approach for cervical
intramedullary arteriovenous malformation with diffuse-type nidus.
Report of three cases. J Neurosurg 91 (Soppl 1). S105–S111. 1999.
View Article : Google Scholar
|
|
31
|
Stein KP, Moenninghoff C, Kneist A,
Sandalcioglu IE, Forsting M and Sure U: Transdural blood supply in
cerebral arteriovenous malformations: A systematic evaluation of
angioarchitecture. AJNR Am J Neuroradiol. 39:2307–2312. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dahl RE and Kline DG: Intraparenchymal
arteriovenous malformations with predominant external carotid
artery contribution. J Neurosurg. 41:681–687. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reul J, Thron A, Laborde G and Bruckmann
H: Dural arteriovenous malformations at the base of the anterior
cranial fossa: Report of nine cases. Neuroradiology. 35:388–393.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lasjaunias P and Berenstein A: Surgical
neuroangiography. 2:Endovascular treatment of craniofacial lesions.
(Berlin). Springer-Verlag. 1987.273–3. View Article : Google Scholar
|
|
35
|
Farhat HI: Cerebral arteriovenous
malformations. Dis Mon. 57:625–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jin H, Qiu H, Chen C, Ge H, Li Y and He H:
Embolization of feeding arteries and symptom alleviation of mixed
dural-pial arteriovenous malformations. Chin Neurosurg J. 4:52018.
View Article : Google Scholar
|
|
37
|
Vinuela F, Fox AJ, Pelz DM and Drake CG:
Unusual clinical manifestations of dural arteriovenous
malformations. J Neurosurg. 64:554–558. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rengachary SS and Szymanski DC: Subdural
hematomas of arterial origin. Neurosurgery. 8:166–172. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kominato Y, Matsui K, Hata Y, Matsui K,
Kuwayama N, Ishizawa S and Takizawa H: Acute subdural hematoma due
to arteriovenous malformation primarily in dura mater: A case
report. Leg Med (Tokyo). 6:256–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kader A, Young WL, Pile-Spellman J, Mast
H, Sciacca RR, Mohr JP and Stein BM: The influence of hemodynamic
and anatomic factors on hemorrhage from cerebral arteriovenous
malformations. Neurosurgery. 34:801–807; discussion 807–808. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ding D, Starke RM, Quigg M, Yen CP,
Przybylowski CJ, Dodson BK and Sheehan JP: Cerebral arteriovenous
malformations and epilepsy, Part 1: Predictors of seizure
presentation. World Neurosurg. 84:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ding D, Quigg M, Starke RM, Yen CP,
Przybylowski CJ, Dodson BK and Sheehan JP: Cerebral arteriovenous
malformations and epilepsy, Part 2: Predictors of seizure outcomes
following radiosurgery. World Neurosurg. 84:653–662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lamas E, Lobato RD, Esperarza J and
Escudero L: Dural posterior fossa AVM producing raised sagittal
simus pressure. Case report. J Neurosurg. 46:804–810. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Obrador S, Soto M and Silvela J: Clinical
syndromes of arteriovenous malformations of the transverse-sigmoid
sinus. J Neurol Neurosurg Psychiatry. 38:436–451. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yamamoto M, Fukushima T, Sakamoto S,
Hashimoto T, Tomonaga M and Goto K: Isolated trochlear nerve palsy
caused by mixed dural-pial arteriovenous malformation of the
anterior cranial fossa: A case report. No Shinkei Geka. 21:177–181.
1993.(In Japanese). PubMed/NCBI
|
|
46
|
Ito M, Sonokawa T, Mishina H, Iizuka Y and
Sato K: Dural arteriovenous malformation manifesting as tic
douloureux. Surg Neurol. 45:370–375. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoshida S and Yamamoto T: Intracerebral
arteriovenous malformation supplied by ethmoidal arteries-case
report. Neurol Med Chir (Tokyo). 33:166–169. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lewis AI, Tomsick TA and Tew JM Jr:
Management of tentorial dural arteriovenous malformations:
Transarterial embolization combined with stereotactic radiation or
surgery. J Neurosurg. 81:851–859. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chandler HC Jr and Friedman WA: Successful
radiosurgical treatment of a dural arteriovenous malformation: Case
report. Neurosurgery. 33:139–141; discussion 141–132. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Halbach VV, Higashida RT, Hieshima GB,
Rosenblum M and Cahan L: Treatment of dural arteriovenous
malformations involving the superior sagittal sinus. AJNR Am J
Neuroradiol. 9:337–343. 1988.PubMed/NCBI
|
|
51
|
Hidaka H, Terashima H, Tsukamoto Y, Nakata
H and Matsuoka S: Radiotherapy of dural arteriovenous malformation
in the cavernous sinus. Radiat Med. 7:160–164. 1989.PubMed/NCBI
|
|
52
|
Flynn TH, McSweeney S, O'Connor G, Kaar G
and Ryder DQ: Dural AVM supplied by the ophthalmic artery. Br J
Neurosurg. 21:414–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Houkin K, Sato M, Echizenya K and Nakagawa
T: Mixed pial-dural arteriovenous malformation. Case report. No
Shinkei Geka. 12 (3 Suppl):S347–S352. 1984.(In Japanese).
|
|
54
|
Morgan MK, Davidson AS, Assaad NNA and
Stoodley MA: Critical review of brain AVM surgery, surgical results
and natural history in 2017. Acta Neurochir (Wien). 159:1457–1478.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gliemroth J, Nowak G and Arnold H: Dural
arteriovenous malformation in the anterior cranial fossa. Clin
Neurol Neurosurg. 101:37–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shima T, Yamane K, Nishida M, Hatayama T
and Yamanaka C: Successful surgical treatment of a large mixed
pial-dural arteriovenous malformation. J Clin Neurosci. 1 (Suppl
7):S30–S32. 2000.
|
|
57
|
Reul J: Neuro-interventional treatment of
cerebrovascular malformations. Aktuelle Radiol. 8:47–57. 1998.(In
German). PubMed/NCBI
|
|
58
|
Borota L, Mahmoud E, Nyberg C, Lewen A,
Enblad P and Ronne-Engstrom E: Dual lumen balloon catheter-An
effective substitute for two single lumen catheters in treatment of
vascular targets with challenging anatomy. J Clin Neurosci.
51:91–99. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Paramasivam S, Niimi Y, Fifi J and
Berenstein A: Onyx embolization using dual-lumen balloon catheter:
Initial experience and technical note. J Neuroradiol. 40:294–302.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Samson DS and Welch BG: Editorial:
Arteriovenous malformation and embolization. J Neurosurg.
118:967–968; discussion 968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zipfel GJ: Editorial: Arteriovenous
malformations and embolization. J Neurosurg. 122:1490–1491. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shi L, Li W, Xu K, Guo YB and Yu JL:
Current status of combined surgical and endovascular methods for
intracranial neurovascular diseases in a hybrid operating room. Int
J Clin Exp Med. 9:20741–20753. 2016.
|
|
63
|
Yu JL, Guo YB, Xu BF, Chen X and Xu K:
Onyx embolization and surgical removal as a treatment for
hemorrhagic AVM in a hybrid operating room. Int J Clin Exp Med.
9:22494–22501. 2016.
|
|
64
|
Ratliff J and Voorhies RM: Arteriovenous
fistula with associated aneurysms coexisting with dural
arteriovenous malformation of the anterior inferior falx. Case
report and review of the literature. J Neurosurg. 91:303–307. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Maki Y, Funaki T, Takahashi JC, Takagi Y,
Ishii A, Kikuchi T, Yoshida K, Makino Y and Miyamoto S: ‘True’
mixed pial-dural arteriovenous malformation: A case report. No
Shinkei Geka. 42:745–750. 2014.(In Japanese). PubMed/NCBI
|