Introduction
MicroRNAs (miRNAs) are a recently identified group
of small, single-stranded RNAs that do not encode proteins. The
roles of miRNAs in the immune system have been investigated
extensively. They are not only involved in innate immunity but also
regulate adaptive immunity (1). Our
previous study revealed that active peptides can perform biological
functions by targeting certain miRNAs (2,3).
Therefore, it has been suggested that inhibiting or restoring the
function of a miRNA can provide a therapeutic benefit in certain
diseases (4,5). However, this process requires an
efficient and nontoxic miRNA delivery system. It has been reported
that active peptides may also act as a delivery vector for miRNAs
(6). In summary, in addition to
targeting miRNAs, active peptides are also involved in the delivery
of miRNAs, which are promising functional molecules. miRNA
(miR)-155 was among the first miRNAs linked to immunity by virtue
of its potent expression upregulation in multiple immune cell
lineages, including T lymphocytes, B lymphocytes, macrophages and
dendritic cells (7). In addition to
the involvement of miR-155 in T lymphocyte activation, miR-155 can
also be involved in T cell development, differentiation and
responses (8,9). Studies have shown that, in a
miR-155-knockout mouse model, T cell-dependent antibody responses
and cytokine production are reduced (10). In addition to being involved in T
cell-related reactions, miR-155 is also involved in B
lymphocyte-related reactions. Studies have shown that the high
expression of the transcription factor PU.1 leads to B cell
dysfunction, including loss of the production of IgG1 antibodies,
TNF-α and lymphotoxin-α/β in miR-155-knockout animals (11). A high expression of miR-155 in T and
B lymphocytes can mediate specific immunity and regulate the
lymphocyte transcriptome and other biological functions, but can
also mediate natural killer (NK) cell-associated nonspecific
immunity (12). NK cells are a type
of innate immune lymphocyte with anti-infection and antitumor
effects (13). The molecular
mechanism underlying NK cell activation remains to be fully
elucidated. Following activation by cytokines, human and mouse NK
cells significantly upregulate the expression of miR-155 (14). The mechanism by which miR-155 is
regulated during NK cell activation is complex. miR-155 can set the
threshold of activation and the degree of activation in mature NK
cells, and it can be used as a dynamic regulator to regulate the
activation of NK cells (14).
A wide variety of immunologically relevant target
genes of miR-155 have been reported, and among these genes,
suppressor of cytokine signaling 1 (SOCS1) can be targeted directly
by miR-155 (15). SOCS is a type of
inducible intracellular protein that serves important roles in
immune and nonimmune functions. The SOCS family is now known to
have eight member proteins, and SOCS1 and SOCS3 synergistically
regulate the differentiation and response of T cells in the immune
system (16). Whole lymphocyte
transcriptome analysis has revealed that miRNAs serve a key role in
lymphocyte subsets, including B lymphocytes and CD8+ and
CD4+ T cells [including T helper (Th)1, Th2, Th17 cells
and regulatory T (Treg) cells] (17). Th17 cells can secrete IL-17A to
promote inflammation, and Treg cells can secrete IL-10 and TGF-β1
to inhibit inflammation. Studies have shown that miR-155-knockout
Treg cells cause increased expression of SOCS1. Researchers have
speculated that miR-155 can promote the differentiation of
Treg/Th17 cells by directly inhibiting SOCS1 and enhance the
function of Th17 cells, which may be involved in the regulation of
inflammatory diseases, at least partially through the regulation of
SOCS1 (18,19). The present study aimed to verify this
hypothesis.
Bioactive peptides are a group of the most popular
polypeptide proteins in current food research. They are peptide
compounds that are beneficial to the life activities of living
organisms or have physiological functions (20). These peptides are composed of several
or tens of amino acids and are arranged in different ways. Their
molecular weights are <6,000 kDa, and these polypeptides have
several biological functions, including hormonal effects, immune
regulation, and antithrombotic, antihypertensive,
cholesterol-lowering, antibacterial, antiviral and anticancer
effects (21). Studies have
confirmed that bioactive peptides are absorbed and utilized more
readily than free amino acids (22).
Modern biometabolism studies have found that the proteins ingested
by humans are digested by various enzymes in the digestive tract;
these digested proteins are not absorbed in the form of amino acids
as previously suggested, but are absorbed more directly in the form
of small peptides (composed of 2–15 amino acid residues), and
dipeptides (composed of two amino acid residues) and tripeptides
(composed of three amino acid residues) are absorbed more quickly
than amino acids of the same composition (23). Bioactive peptides from animal and
plant species are widely distributed. The active peptides used in
the present study were derived from the liver of healthy goats. The
peptides were prepared by Professor Su's research group at Inner
Mongolia Medical University (Hohhot, China) using a bioengineering
method. The extraction method has been established successfully,
and the quality is controllable (24). Our previous studies have shown that
these peptides can inhibit cancer cell growth, enhance cancer cell
sensitivity to chemotherapeutic drugs and reduce toxic side effects
on normal cells (2,3).
The present study aimed to evaluate the effects of
bioactive hepatic peptide (BHP) on the immune function of healthy
mice and immunosuppressed mice and examine whether changes in the
expression of SOCS1/miR-155 mediate the effects of BHP on mouse
immune function. The results may provide a theoretical basis for
the development of new health products.
Materials and methods
Preparation of BHP
Under relatively sterile conditions, the liver of
two Boer goats (2-year-old females; Mengyang Animal Husbandry Co.,
Ltd.) was obtained and rapidly pulverized. The Boer goats were
housed at 22–24°C, with humidity at 45–50%, good ventilation, and a
natural light/dark cycle. The goats were given sufficient water
four times a day and were fed four times a day (8:30 am, 12:30 am,
4:30 pm and 8:30 pm) with crude feed 1–1.25 kg/goat per day and
fine feed 0.25 kg/goat. The mixture was homogenized with
physiological saline at a ratio of 0.5–2 g/4 ml, frozen and thawed
under low temperature conditions (−80°C) three times, filtered,
centrifuged (14,000 × g, 4°C, 20 min), ultrafiltered and
sterilized. The final filtrate was a pale yellow liquid, which was
referred to as BHP solution. According to SDS-PAGE analysis, the
molecular weight of the BHP was ~8,000 kDa (25). In addition, the concentration of the
BHP solution was 42 mg/ml. A BCA kit (Bradford) was used to
determine the concentration of BHP at 450 nm on an enzyme label
instrument.
Body weight and organ index
determinations
A total of 40 male and 40 female Kunming mice
(purchased from Beijing Weitong Lihua) with body weights of 18–22 g
(6–8 weeks old) were housed for 5 days. The housing conditions were
as follows: Indoor temperature, 18–22°C; relative humidity, 50–70%;
12/12 h light/dark cycles between 8:00 am-8:00 pm; free access to
drinking water and food; and then randomly divided into eight
groups of 10 mice. All animals were treated according to the
protocol approved by the Institutional Animal Care and Use
Committee (IACUC) of Inner Mongolia Medical University. The groups
included the BHP (provided by the Clinical Research Center of the
Affiliated Hospital of Inner Mongolia Medical University) high-dose
group (100 mg/kg, HBHP), BHP mid-dose group (50 mg/kg, MBHP), BHP
low-dose group (25 mg/kg, LBHP), cyclophosphamide (Cy)-treated
immunosuppressed group (40 mg/kg Cy, Jiangsu Hengrui Pharmaceutical
Co., Ltd.), Cy high-dose peptide group (40 mg/kg Cy, 100 mg/kg BHP;
Cy + HBHP), Cy mid-dose peptide group (40 mg/kg Cy; 50 mg/kg BHP;
Cy + MBHP), Cy low-dose peptide group (40 mg/kg Cy; 25 mg/kg BHP;
Cy + LBHP), and a control group (intraperitoneal injection of the
same volume of saline daily; Con). The mice in the immunosuppressed
Cy combined with peptide groups were intraperitoneally injected
with Cy three times (every other day) in advance. On day 4, the
remaining mice were administered with the appropriate dose of BHP
twice daily, and the mice in the Cy groups were injected
intraperitoneally every other day. During the administration
period, the body weights were measured and recorded every 3 days at
a fixed time point (9:00 a.m.). All mice were harvested 4 weeks
later; the mice were sacrificed by cervical dislocation. The spleen
and thymus gland were weighed under aseptic conditions, and the
correlation indices were calculated according to following
formulas: Spleen weight index=spleen weight/body weight ×100; and
thymus index=thymus weight/body weight ×100.
Determination of lymphocyte
percentages
Blood was collected from the posterior ocular vein
following anesthetizing the mice with ether, and the mice were then
sacrificed with carbon dioxide (CO2) followed by
cervical dislocation. Flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA) was used to determine the total number of
lymphocytes and the percentages of NK cells, and Th and killer or
cytotoxic T cell (Tc) lymphocytes. BD Multitest CD3/CD8/CD45/CD4
reagent and BD Multitest CD3/CD16+CD56/CD45/CD19 reagent were used
for the lymphocyte percentage determinations. Various
fluorescein-labeled monoclonal antibodies were added to the whole
blood and combined with the corresponding antigens on the
leukocytes. The blood was then subjected to hemolysis with 1X BD
FACS Lysing Solution (cat. no. 349202; BD Biosciences) for 10 min
at room temperature, fixation with 0.1% glutaraldehyde for 15 min
at room temperature and washing with PBS, and was then analyzed
with a flow cytometer. The percentages of the lymphocyte subsets
were obtained. The fluorescein labeling and lymphocyte surface
antigen distribution are shown in Table
I. According to the differences in the CD molecule expression
on lymphocyte membranes, flow cytometry can distinguish lymphocytes
and their various subpopulations, and the percentages of lymphocyte
subsets can be calculated with computer software. All procedures
were performed according to the reagent instructions (BD
Biosciences).
 | Table I.Determination of lymphocyte
percentage. |
Table I.
Determination of lymphocyte
percentage.
| Kit | Contents | Lymphocyte
subset | CD molecules |
|---|
| BD Multitest
CD3/CD8/CD45/CD4 reagent | FITC-labeled
CD3, | Lymphocyte |
CD45+ |
|
| PE-labeled
CD8, | T helper cell | CD3+,
CD4+, CD45+ |
|
| PerCP-labeled
CD45, | Cytotoxic T
cell | CD3+,
CD8+, CD45+ |
|
| APC-labeled
CD4 |
|
|
| BD Multitest
CD3/CD16+CD56/CD45/CD19 reagent | FITC-labeled
CD3, | B lymphocyte | CD3−,
CD19+, CD45+ |
|
| PE-labeled
CD16, | Natural killer
cell | CD3-,
CD56+, CD45+ |
|
| PE-labeled
CD56, |
|
|
|
| PerCP-labeled
CD45, |
|
|
|
| APC-labeled
CD19 |
|
|
Determination of the phagocytic
index
Three SPF chickens [8-week-old males reared at 27°C,
60% humidity and a 9/15 h light/dark cycle (light, 8:00 am-3:00
pm)] were fed six times a day with cold and white boiled water were
purchased from the China Experimental Animal Information Network.
All procedures were approved by the IACUC of Inner Mongolia Medical
University. A blood collection needle (Beijing Haide Venture
Biotechnology Co., Ltd.) and EDTA-K2 anticoagulation blood
collection tube (Guangzhou Bangbiao Medical Instrument Co., Ltd.)
were used for chicken wing vein blood collection. Blood was
collected (1 ml) and added to 2 ml sterile saline followed by
centrifugation at 1,760 × g for 10 min at room temperature. The
supernatant was discarded, and the blood cells were suspended in
physiological saline, following which the precipitate was
centrifuged in the same manner as above. The red blood cells were
washed three times, and the 0.05% chicken red cell suspension was
prepared using sterile physiological saline. At 12:00 p.m. on day
27, the mice were injected intraperitoneally with 1 ml of
preprepared 0.5% starch solution, and fasted with water overnight.
At 6:00 a.m. on day 28, each mouse was injected with 0.05% chicken
red blood cells prepared in advance, and the abdomen of the mice
was then gently massaged to disperse the chicken red blood cells.
The mice were anesthetized at 12:00 p.m. on day 28 and sacrificed
with CO2 asphyxiation, followed by cervical dislocation.
The abdominal cavity of the mice was washed with 1 ml of
physiological saline, and ~0.5 ml of peritoneal lavage fluid was
sampled and stained 1 h at 37°C with Wright's staining reagent
(Beijing Solarbio Science & Technology Co., Ltd., CAS:
68988-92-1, reagent no. G1040). Wright's staining is commonly used
for staining blood (mainly white blood cells and red blood cells)
and bone marrow smears during biochemical research (26–28). The
number of phagocytic cells engulfing the chicken erythrocytes and
the number of chicken erythrocytes phagocytosed by 100 phagocytic
cells were counted under an oil-immersion objective (Olympus
Corporation, Tokyo, Japan) and calculated according to following
formulas: Percentage of phagocytosis (%)=number of macrophages that
engulfed chicken red blood cells/100 phagocytes ×100; and
phagocytosis index=number of chicken erythrocytes engulfed/100
phagocytes ×100.
Determination of lymphocyte
proliferation
A spleen cell suspension was prepared and counted
under a microscope (Olympus Corporation), and the cell density was
adjusted to 2×105/ml for use. A total of 100 µl of
spleen cell suspension was added to each well of a ٩٦-well
flat-bottomed culture plate. The final concentration of
concanavalin A (ConA; Nanjing Jinyibai Biotechnology Co., Ltd.) was
7.5 µg/µl, and 100 µl of RPMI 1640 medium (Gibco, Thermo Fisher
Scientific, Inc.) was added to the control wells. Three repetitions
were performed in parallel for the experimental and control wells.
The culture plate was placed in a 37°C, 5% CO2 incubator
and incubated for 66 h. The state of the cells was observed once a
day. The plate was removed and 20 µl of CCK-8 (Dojindo Institute of
Japan) was added to each well and incubated for a further 2.5 h.
Following incubation, the absorbance (A) was measured with a
microplate reader (Molecular Devices, LLC) at a wavelength of 450
nm. The splenic lymphocyte proliferation rate and stimulation index
were calculated according to the following formulas: Proliferation
rate=(AConA-ARPMI1640)/ARPMI1640;
and stimulus index=AConA/ARPMI1640.
H&E staining
Excised specimens were fixed with 4%
paraformaldehyde (Linyi URB Chemical Co., Ltd.), dehydrated and
embedded in paraffin. H&E staining was performed on 4-µm-thick
paraffin sections containing a representative, well-preserved
splenic tissue sample. The sections were examined under a light
microscope at ×100 and ×400 magnification. The slides of all
treatment groups were examined and images were captured.
Determination of the expression of
miR-155 in the mouse spleen
RNA extraction was performed using TRIzol reagent
(CWBio Beijing Kangwei Century Biotechnology Co., Ltd.). The
reverse transcription primers (Sangon Biotech Co., Ltd., Shanghai,
China) were as follows: miR-155,
5′-GCACTTCAGTGTCGTGGTCAGTGACGGCAATTTGAAGTGCACCCCTAT-3′ and U6,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The RT reaction mixture was included
in the PrimeScript 1st Strand cDNA Synthesis kit (Thermo Fisher
Scientific, Inc.). All procedures followed the manufacturer's
instructions. Samples were incubated at 25°C for 5 min, 42°C for 60
min, 70°C for ٥ min and then maintained at 4°C. When the instrument
was at 4°C, the cDNA was removed and stored in a −86°C freezer.
Real-time fluorescence quantitative polymerase chain reaction
(qPCR) analysis was used to determine the level of miR-155. The
RT-qPCR protocol was as previously described by Ji et al
(29). The sequences of the primers
used (Sangon Biotech Co., Ltd., Shanghai, China) were as follows:
miR-155 forward: 5′-CAGACGACCATCAGTTAATGCTAATTGTGAT-3′, miR-155
reverse: 5′-CGTCGTCACTGACCACGACACTG-3′, U6 forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ and U6 reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′. Applied Biosystems™ SYBR™
Green mix (Thermo Fisher Scientific, Inc.) was used, and all
procedures were performed following the manufacturer's
instructions. PCR was performed with the ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.) The
quantification of PCR results was performed using the
2−ΔΔCq method, as described by Ji et al (29).
Immunohistochemical detection of the
expression of SOCS1 in the mouse spleen
The excised specimens were fixed with 4%
paraformaldehyde (Linyi URB Chemical Co., Ltd.) at room temperature
for a week, dehydrated (70, 80, 90 and 100% alcohol for 5 min per
gradient) and embedded in paraffin. A standard immunohistochemical
staining procedure was performed using 4-µm-thick paraffin sections
containing a representative, well-preserved splenic tissue sample,
as recommended by the primary antibody supplier. Tissues were
rehydrated in decreasing concentrations of graded ethanol and
double distilled water. Endogenous peroxidase/phosphatase activity
was blocked with 3% hydrogen peroxide for 10 min at room
temperature. Non-specific expression was blocked with ready-to-use
normal goat serum (cat. no. AR0009; Boster Biotechnology Co., Ltd.)
at room temperature for 10 min. SOCS1 antigens were retrieved by
heating at high pressure in a pressure cooker with 1 mmol/l EDTA
(pH 8.0) for 7 min at a temperature of 98°C at 90 kPa. Sections
were incubated with anti-SOCS1 primary antibodies (cat. no.
PA5-27239; dilution of 1:500; Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C for 2 h, rinsed three times (3 min/time) with PBS,
and incubated with horseradish peroxidase-conjugated goat
anti-rabbit immunoglobulin G secondary antibodies (cat. no. G21234;
dilution of 1:1,000; Invitrogen; Thermo Fisher Scientific, Inc.)
for 10 min at room temperature. The sections were washed with PBS
three times (3 min/time), then incubated with 3,3′-diaminobenzidine
for 5 min at room temperature. Tissues were counterstained with
hematoxylin for 5 min at room temperature, and dehydrated in
increasing concentrations of graded ethanol and xylene. Staining
intensity for SOCS1 expression was assessed under an optical
microscope. The method of scoring immunohistochemical staining was
as described by Zhang et al (30); the cell staining density and the
staining intensity under an optical microscope at a magnification
of ×100 were used to establish the semi-quantitative scoring method
for the immunohistochemical results. The positive cell density
scoring method was as follows: 0, no positive cells; 1, 1–10%
positive cells; 2, 10–50% positive cells; 3, 50–80% positive cells;
and 4, 80–100% positive cells. The color intensity of the positive
cells was scored as follows: 0, negative; 1, light color (light
brownish yellow); 2, moderate coloring (brown); and 3, strong
staining (dark brownish yellow). The product of the color density
and the intensity was used as the final score result.
Determination of the levels of
cytokines IL-17A, IL-10 and TGF-β1
The splenic tissue was stored in a −86°C freezer.
The levels of IL-10, IL-17A and TGF-β1 in the splenic tissues were
determined using commercial enzyme-linked immunosorbent assay
(ELISA) kits (Wuhan Xinqidi Biological Technology Co., Ltd.)
according to the manufacturer's protocols. All samples were tested
in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS
version 23.0 (IBM, Corp.). Each experiment was performed at least
in triplicate. One-way analysis of variance was used. Significant
differences among the treatment means were determined at a 5%
confidence level using Duncan's Multiple Range test.
Results
Successful establishment of an
immunosuppressed mouse model
Compared with the mice in the Con group, there were
no significant differences in body weight among the groups
(Fig. 1A). However, an upward trend
was observed when comparing the Cy group with groups treated with
Cy and increasing doses of BHP. In addition, the spleen and thymus
indices of the Cy group were significantly decreased (Fig. 1B and C). The percentage of total
lymphocytes, Tc cell percentage, Th cell percentage and
phagocytosis index of mice in the Cy group were significantly
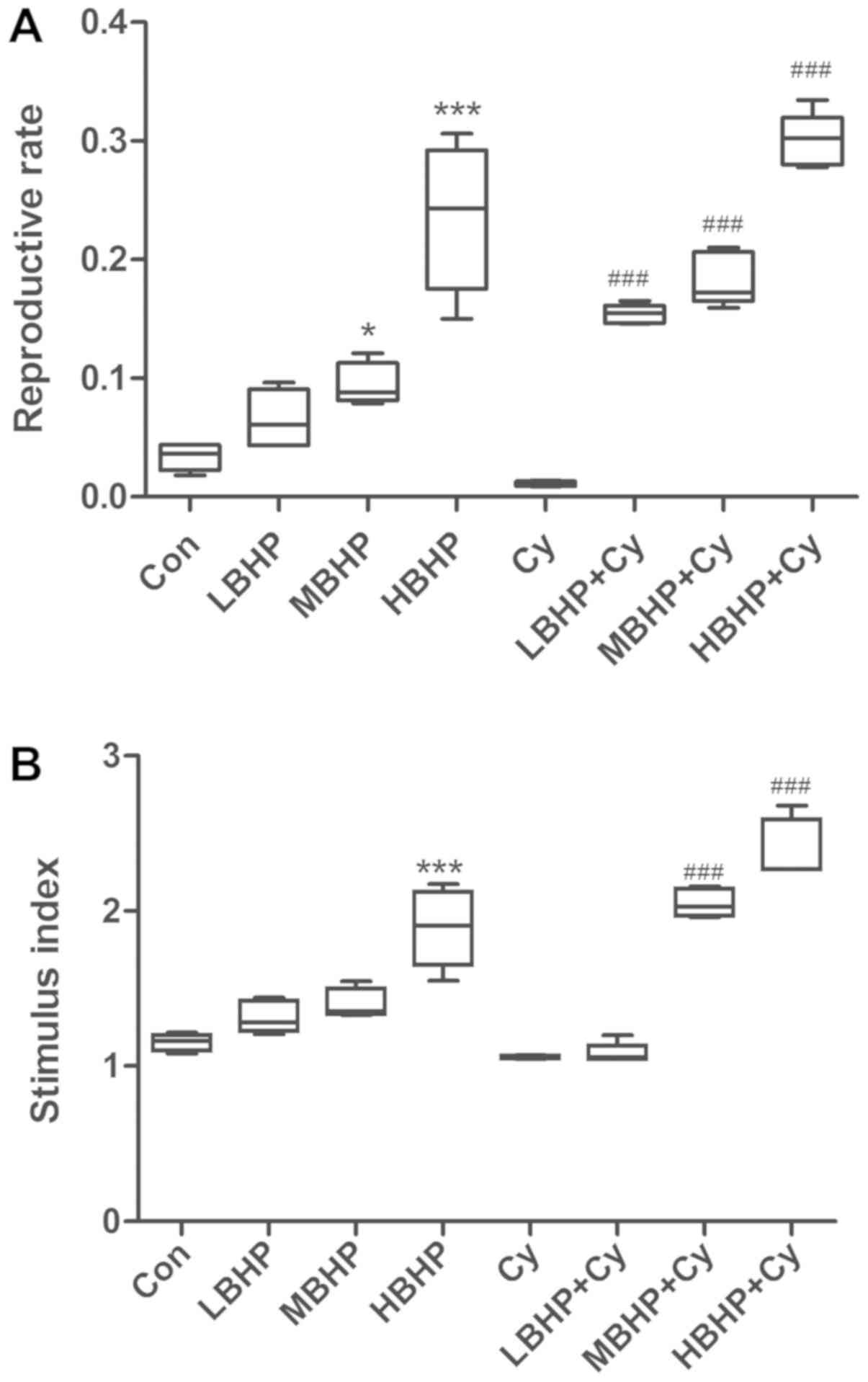
decreased compared with those of mice in the Con group (Fig. 2A-D). The level of phagocytosis by
peritoneal macrophages in mice of the Cy group was also
significantly lower than that of mice in the Con group (Fig. 3A-C). Although there was no
significant difference in the proliferation of splenic lymphocytes
from the mice in vitro, there was a downward trend in the Cy
group (Fig. 4A and B). Therefore,
the immunosuppressed mouse model was considered to be successfully
established. The H&E results showed that the splenic tissue
samples from the Cy group, the LBHP + Cy group, the MBHP + Cy group
and the HBHP + Cy group all exhibited abnormalities to varying
degrees (Fig. 5). Lymphocytes were
sparse, and the splenic structure was ambiguous and irregularly
arranged. The number of lymphocytes was decreased, red veins were
engorged with small veins, the splenic sinus was marginally
expanded, and lymphoid follicles and necrotic cells were visible in
the Cy groups compared with the Con group. The Cy group exhibited
the most marked changes, which indicated that Cy had a certain
damaging effect on the mouse spleen. In summary, establishment of
the immunosuppressed mouse model was successful.
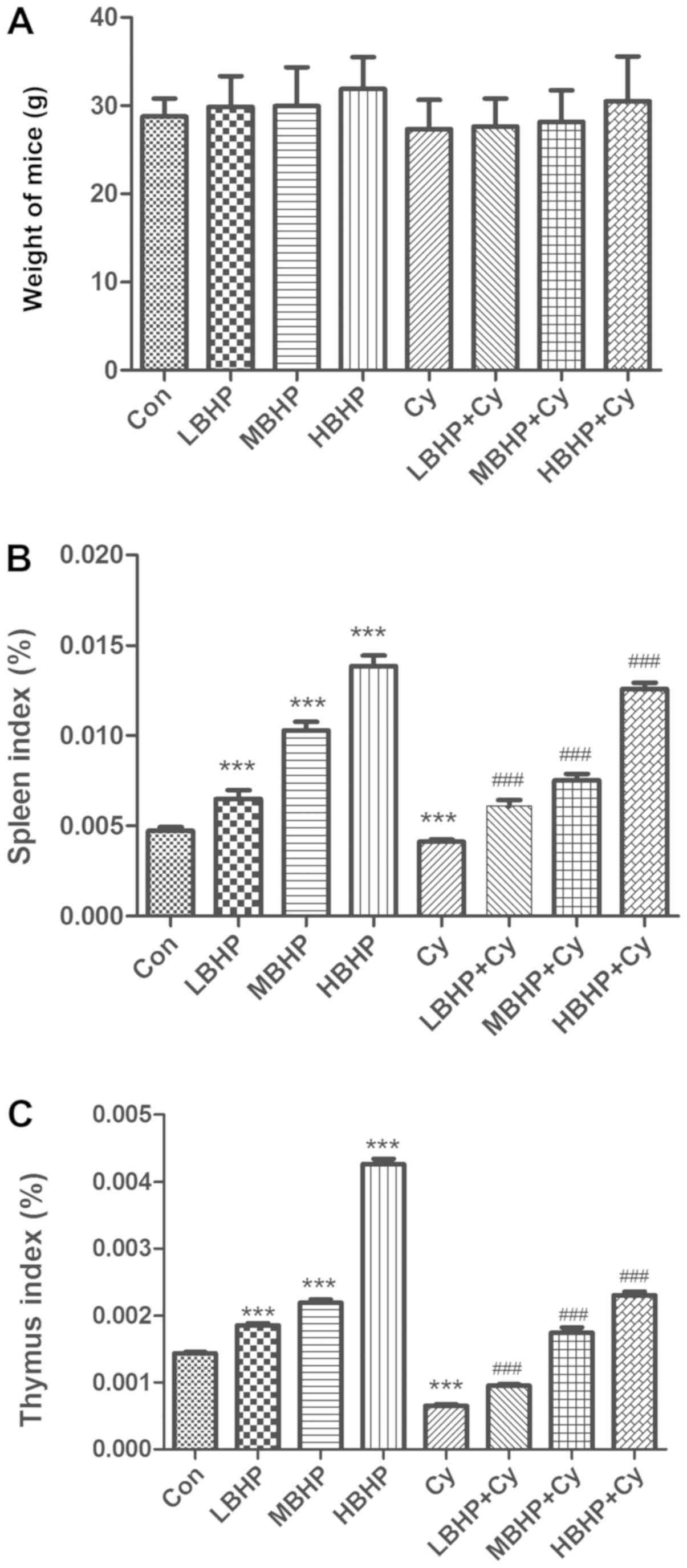 | Figure 1.Weight, spleen index and thymus index
of normal untreated mice and normal and immunosuppressed mice
treated with BHP. (A) Weights of mice. There was an increased trend
in the BHP group, although no significant differences between
groups were observed. (B) Spleen index of the mice. Significant
differences were observed when comparing the Cy, LBHP, MBHP or HBHP
group with the Con group. Significant increases were observed in
the LBHP + Cy, MBHP + Cy and HBHP + Cy groups compared with the Cy
group. (C) Thymus index of the mice. Significant differences were
observed when comparing the Cy, LBHP, MBHP or HBHP group with the
Con group. Significant differences were observed when comparing the
LBHP + Cy, MBHP + Cy or HBHP + Cy group with the Cy group.
***P<0.0001 vs. Con group; ###P<0.0001 vs. Cy group. All data
were from two independent experiments, each of which was repeated
in parallel twice. BHP, bioactive hepatic peptide; LBHP, low-dose
BHP; MBHP, mid-dose BHP; HBHP, high-dose BHP; Cy, cyclophosphamide;
Con, control. |
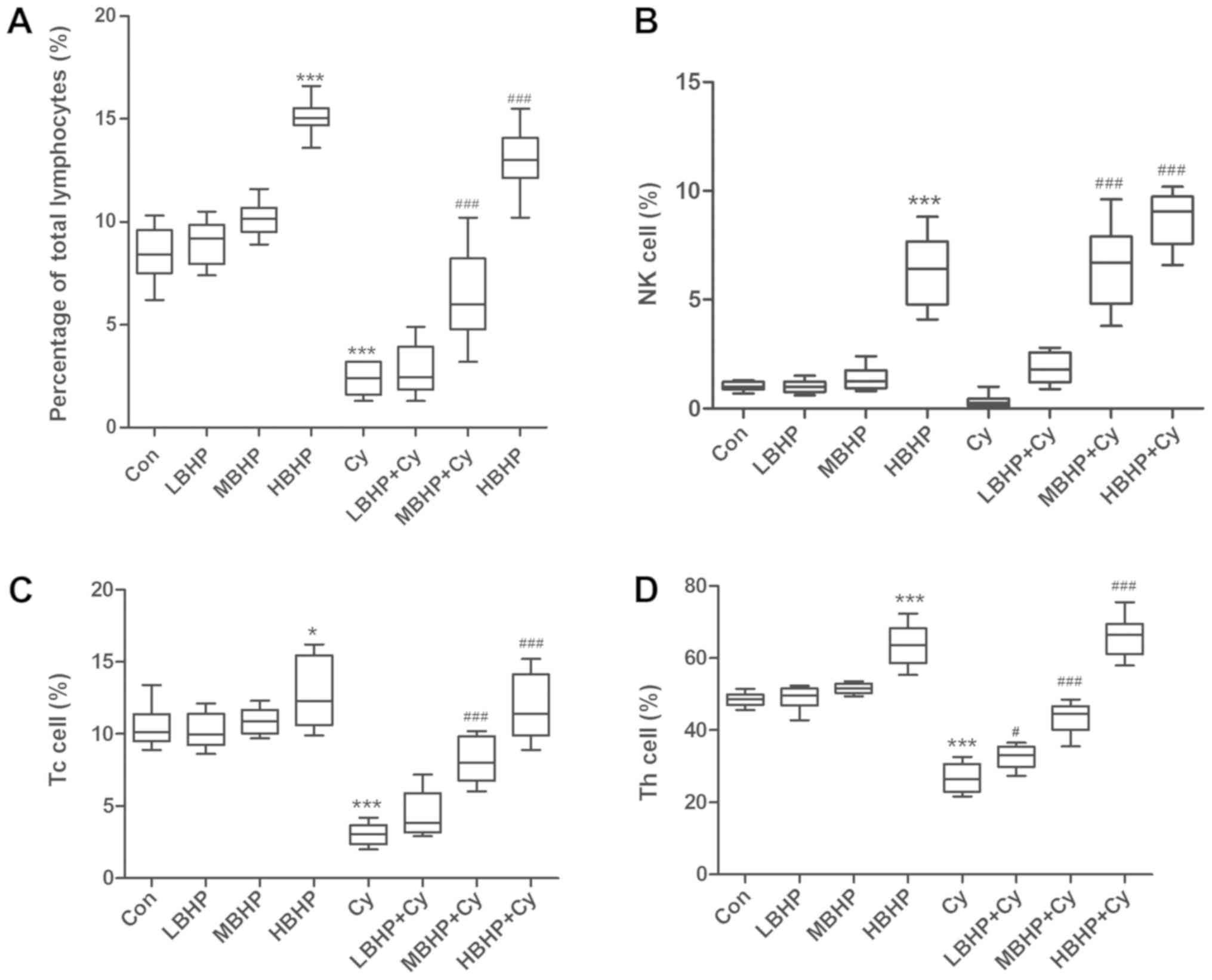 | Figure 2.Percentages of lymphocytes in normal
untreated mice and normal and immunosuppressed mice treated with
BHP. (A) Percentages of total lymphocytes were increased in the
HBHP group and significantly decreased in the Cy group compared
with that in the Con group, and were increased significantly in the
MBHP + Cy and HBHP + Cy groups compared with that in the Cy group.
(B) Percentages of NK cells. NK cell percentage was increased
significantly in the HBHP group compared with that in the Con
group. NK cell percentages were increased significantly in the MBHP
+ Cy and HBHP + Cy groups compared with that in the Cy group. (C)
Percentages of Tc cells. Tc cell percentage was increased in the
HBHP group and significantly decreased in the Cy group compared
with that in the Con group. Percentages of Tc cells were increased
significantly in the MBHP + Cy and HBHP + Cy groups compared with
that in the Cy group. (D) Percentage of Th cells was increased in
the HBHP group and significantly decreased in the Cy group compared
with that in the Con group. Percentages of Th cells were increased
significantly in the LBHP + Cy, MBHP + Cy and HBHP + Cy groups
compared with that in the Cy group. *P<0.01 vs. Con group;
***P<0.0001 vs. Con group; #P<0.01 vs. Cy group;
###P<0.0001 vs. Cy group. All data were from two independent
experiments, each of which was repeated in parallel twice. BHP,
bioactive hepatic peptide; LBHP, low-dose BHP; MBHP, mid-dose BHP;
HBHP, high-dose BHP; Cy, cyclophosphamide; Con, control; NK,
natural killer; Tc, cytotoxic T cell; Th, T helper cell. |
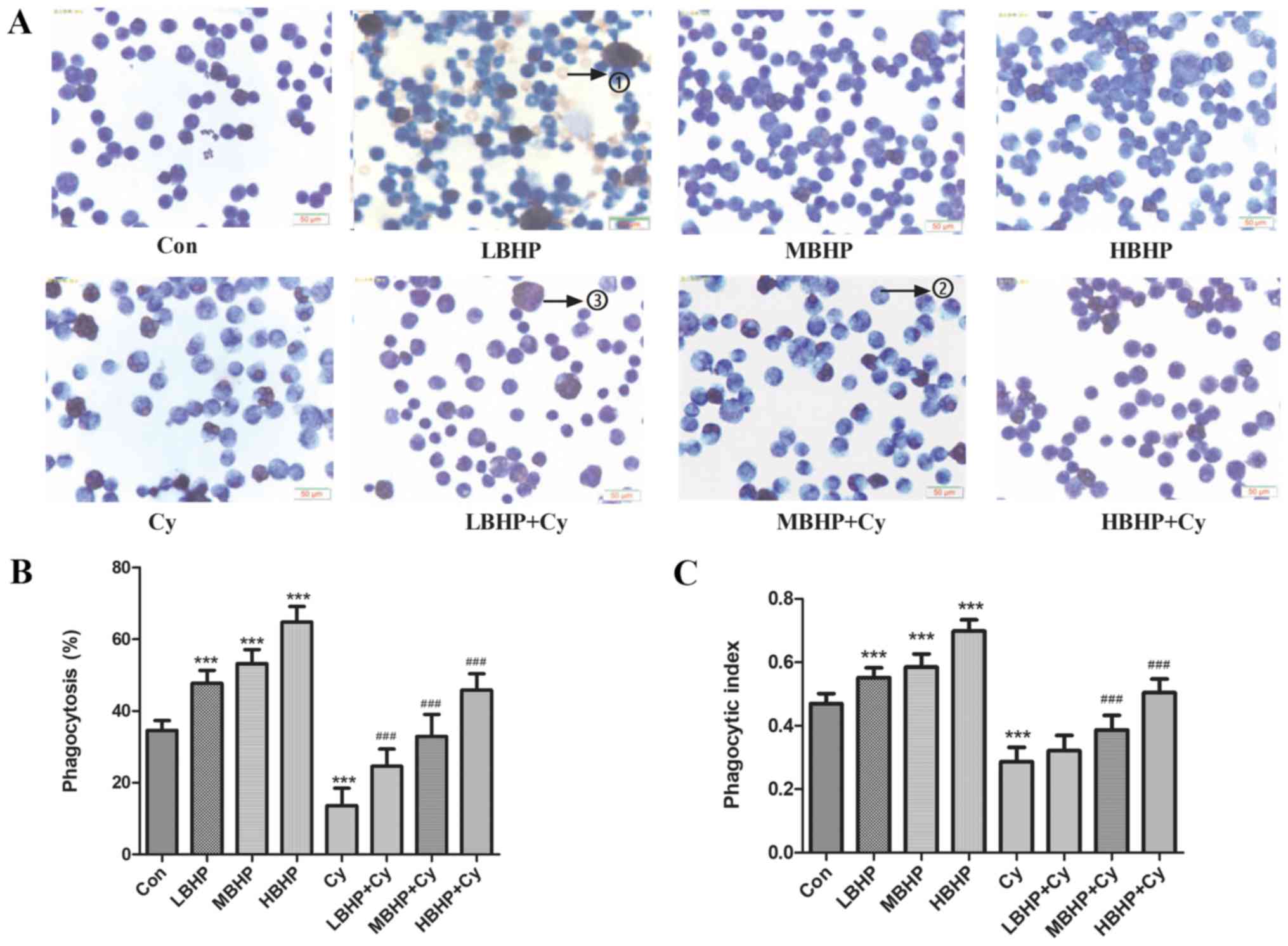 | Figure 3.Macrophage phagocytosis in normal
untreated mice and normal and immunosuppressed mice treated with
BHP. (A) Wright's staining results (×400 magnification with a light
microscope). Due to the pre-injection of starch, as a
non-self-contained substance, such stimulation can produce more
macrophages. Numerous macrophages, macrophages that have engulfed
chicken red blood cells, and a small number of unphagocytosed
chicken red blood cells were observed under oil microscope. The
numbers on the images represent the following: 1) unphagocytosed
chicken red blood cells; 2) macrophages that have not engulfed
chicken red blood cells, and 3) macrophages that had engulfed
chicken red blood cells. (B) Percentage of phagocytosis.
Phagocytosis was increased significantly in the LBHP, MBHP and HBHP
groups and decreased significantly in the Cy group compared with
that in the Con group. Phagocytosis was increased significantly in
the LBHP + Cy, MBHP + Cy and HBHP + Cy groups compared with that in
the Cy group. (C) Phagocytic index. The index was increased
significantly in the LBHP, MBHP and HBHP groups and decreased
significantly in the Cy group compared with that in the Con group.
The index was increased significantly in the MBHP + Cy and HBHP +
Cy groups compared with that in the Cy group. ***P<0.0001 vs.
Con group; ###P<0.0001 vs. Cy group. All data were from two
independent experiments, each of which was repeated in parallel
twice. BHP, bioactive hepatic peptide; LBHP, low-dose BHP; MBHP,
mid-dose BHP; HBHP, high-dose BHP; Cy, cyclophosphamide. |
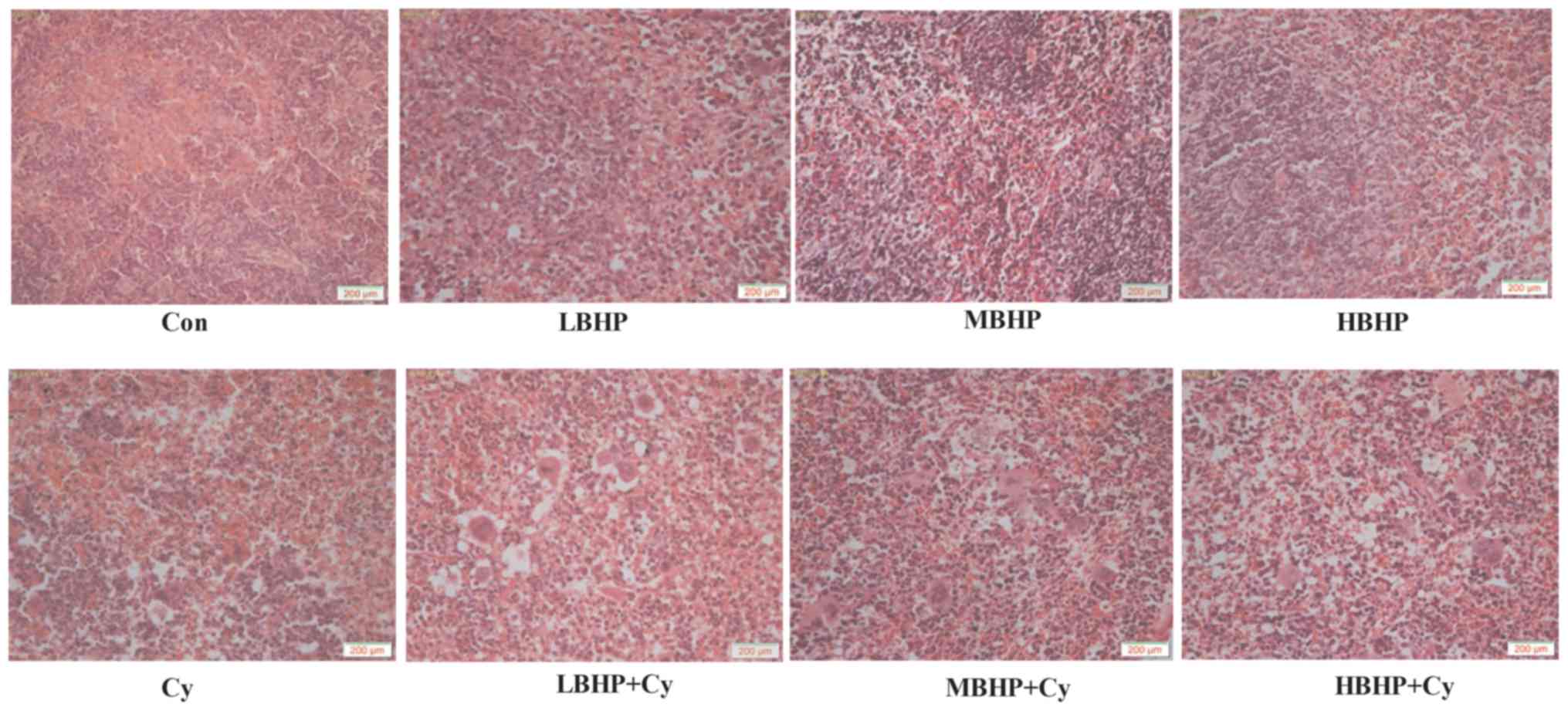 | Figure 5.Hematoxylin-eosin staining of the
mouse spleen. Images were captured at ×100 magnification with a
light microscope. The spleen of the mice in the Cy group, LBHP + Cy
group, MBHP + Cy group and HBHP + Cy group all exhibited
abnormalities to varying degrees compared with that in the Con
group. The lymphocyte arrangement was sparse, and the structure was
ambiguous and irregularly arranged. The number of lymphocytes was
decreased, red veins were engorged with small veins, the splenic
sinus was marginally expanded, and lymphoid follicles and necrotic
cells were visible. The Cy group exhibited the most marked changes.
No substantial changes were found among the LBHP group, MBHP group
and HBHP group. The LBHP + Cy group, MBHP + Cy group and HBHP + Cy
group had relatively neatly arranged lymphocytes and relatively
clear cell structure. All data were from two independent
experiments, each of which was repeated in parallel twice. BHP,
bioactive hepatic peptide; LBHP, low-dose BHP; MBHP, mid-dose BHP;
HBHP, high-dose BHP; Cy, cyclophosphamide. |
BHP improves immunity in normal
mice
Compared with those of mice in the Con group, the
body weights of mice in the LBHP, MBHP and HBHP groups were
increased in a dose-dependent manner, although there were no
significant differences. In addition, the spleen and thymus indices
of mice in the LBHP, MBHP and HBHP groups were significantly
increased (Fig. 1B and C). The
percentage of total lymphocytes, NK cell percentage, Tc cell
percentage and Th cell percentage in the HBHP group were
significantly increased compared with those in the Con group
(Fig. 2A-D). These percentages
increased in a dose-dependent manner; however, there were no
significant differences between the LBHP group and the MBHP group
(Fig. 2A-D). Furthermore, the
phagocytosis percentage and phagocytic index of macrophages from
the mice were significantly increased in the LBHP, MBHP and HBHP
groups (Fig. 3A-C). The
proliferation rate and proliferation index of the splenocytes from
the mice in the MBHP and HBHP groups were significantly increased
in vitro (Fig. 4A and B). The
H&E staining result shows no obvious changes in morphology
among the LBHP, MBHP and HBHP groups (Fig. 5). This indicates that HBP can improve
immunity in normal mice and does not cause damage to the spleen
morphology.
BHP improves immunity in
immunosuppressed mice
Compared with Cy mice, the body weights of mice in
the LBHP + Cy group, MBHP + Cy group and HBHP + Cy group increased
in a dose-dependent manner, although differences were not
significant (Fig. 1A). However, the
spleen and thymus indices of mice in the LBHP + Cy group, MBHP + Cy
group and HBHP + Cy group were significantly increased (Fig. 1B and C). The percentage of total
lymphocytes, NK cell percentage, Tc cell percentage and Th cell
percentage in the MBHP + Cy group and HBHP + Cy group were
significantly increased compared with those in the Cy group
(Fig. 2A-D). In addition, the
percentage of macrophage phagocytosis and phagocytic index of
macrophages were increased significantly in mice in the MBHP + Cy
group and HBHP + Cy group, and the former was also increased
significantly in the LBHP + Cy group (Fig. 3A-C). The proliferation rate and
proliferation index of splenic cells from mice in MBHP + Cy group
and HBHP + Cy group were increased significantly in vitro
(Fig. 4A and B). The H&E
staining result showed relatively neatly arranged lymphocytes and
relatively intact cell structure in the LBHP + Cy, MBHP + Cy and
HBHP + Cy groups compared with the Cy group, but no pathological
inflammatory lesions were found, which indicated that BHP can
effectively protect against the splenic tissue damage caused by
immunosuppressive agents (Fig.
5).
miR-155/SOCS1 mediates the regulation
of immune function in mice induced by BHP
The relative expression of miR-155 was upregulated
in the LBHP, MBHP and HBHP groups but decreased in the Cy group
compared with that in the Con group (Fig. 6). In addition, the expression of
miR-155 was increased in the MBHP + Cy group and the HBHP + Cy
group compared with that in the Cy group to a certain degree. The
expression of SOCS1 was downregulated in the groups of mice treated
with LBHP, MBHP or HBHP but increased in the Cy group compared with
that in the Con group (Fig. 7). In
addition, the expression of SOCS1 was decreased in the LBHP + Cy,
MBHP + Cy and HBHP + Cy groups compared with that in the Cy group.
Therefore, the expression of miR-155/SOCS1 may mediate the
regulation of immune function in mice induced by BHP.
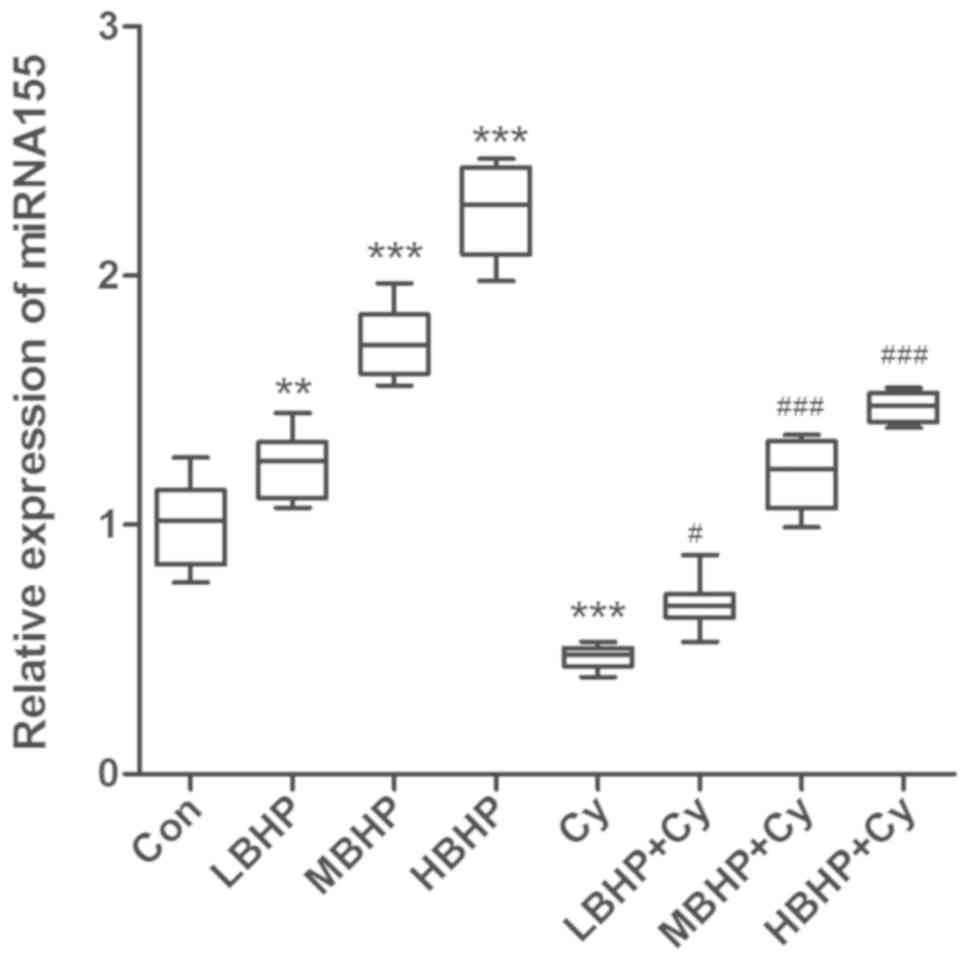 | Figure 6.Relative expression of miR-155 in the
spleen of untreated normal mice and normal and immunosuppressed
mice treated with BHP. Expression was increased significantly in
the LBHP, MBHP and HBHP groups and decreased significantly in the
Cy group compared with that in the Con group. Expression was
increased significantly in the LBHP + Cy, MBHP + Cy and HBHP + Cy
groups compared with that in the Cy group. **P<0.001 vs. Con
group, ***P<0.0001 vs. Con group; #P<0.01 vs. Cy group,
###P<0.0001 vs. Cy group. All data were from two independent
experiments, each of which was repeated in parallel twice. BHP,
bioactive hepatic peptide; LBHP, low-dose BHP; MBHP, mid-dose BHP;
HBHP, high-dose BHP; Cy, cyclophosphamide; miR/miRNA, microRNA. |
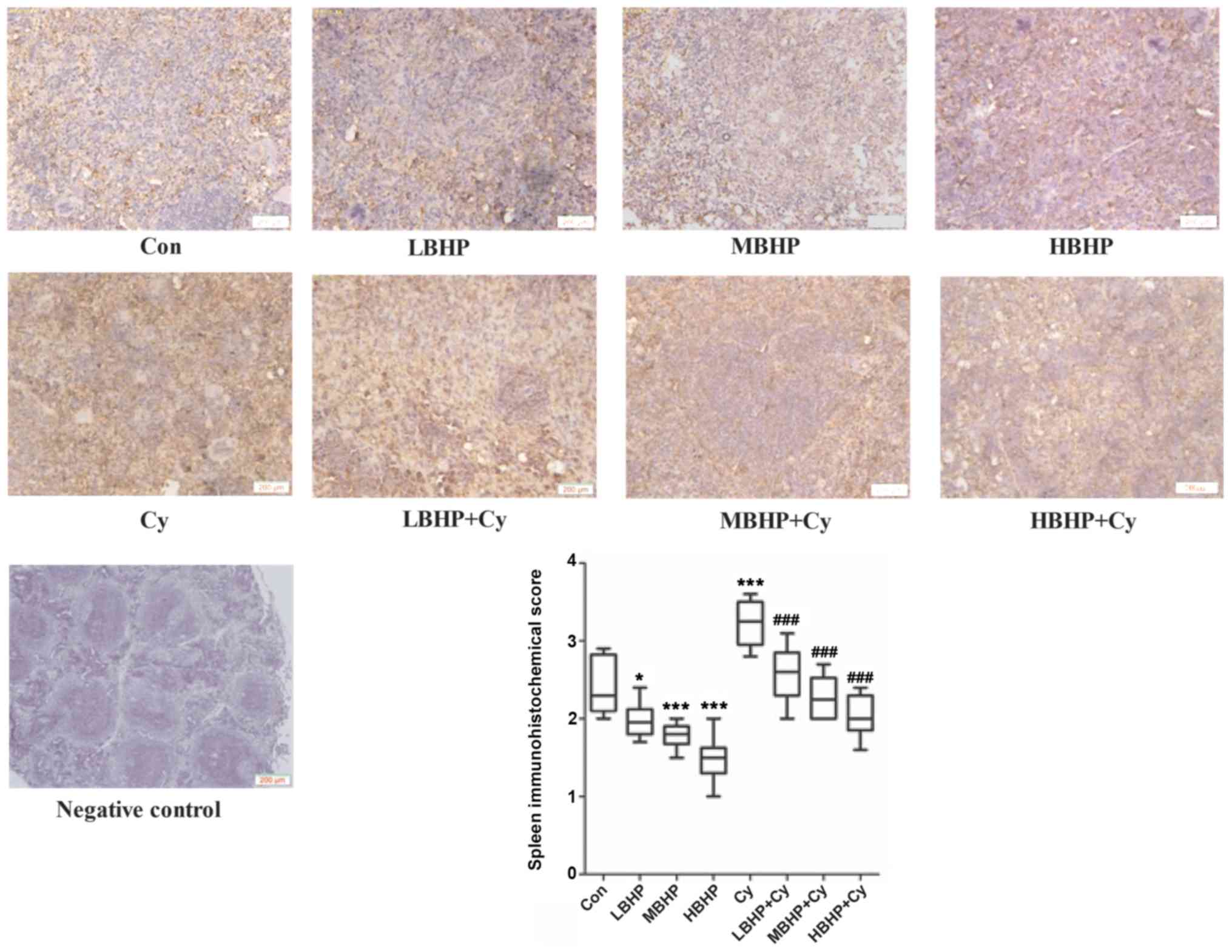 | Figure 7.Expression of SOCS1 in the spleen of
untreated normal mice and normal and immunosuppressed mice treated
with BHP. Expression was decreased significantly in the LBHP, MBHP
and HBHP groups, and increased significantly in the Cy group
compared with that in the Con group. Expression was decreased
significantly in the LBHP + Cy, MBHP + Cy and HBHP + Cy groups
compared with that in the Cy group. *P<0.01 vs. Con group;
***P<0.0001 vs. Con group, ###P<0.0001 vs. Cy group. All data
were from two independent experiments, each of which was repeated
in parallel twice. BHP, bioactive hepatic peptide; LBHP, low-dose
BHP; MBHP, mid-dose BHP; HBHP, high-dose BHP; Cy, cyclophosphamide;
SOCS1, suppressor of cytokine signaling 1. |
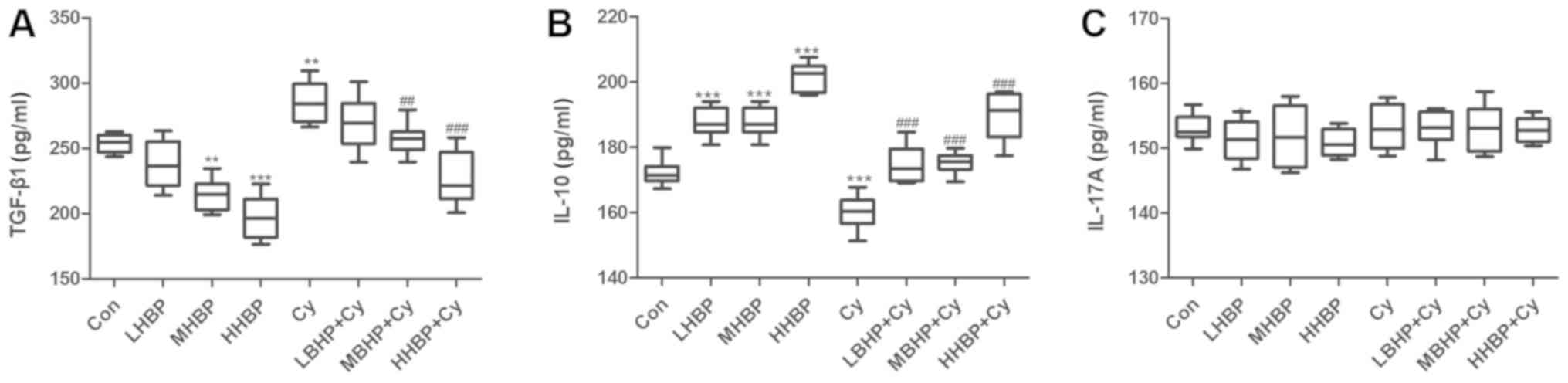
TGF-β1 and IL-10, but not IL-17A, are
involved in the regulation of immune function in mice induced by
BHP
Following treatment with BHP, the secretion of
TGF-β1 in the spleen of the normal mice and immunosuppressed mice
was significantly decreased (Fig.
8A), and the level of IL-10 secretion was significantly
increased (Fig. 8B). No significant
difference in the level of IL-17A was observed among the groups
(Fig. 8C).
Discussion
The immune system, which is mainly composed of
immune organs, immune cells and immunologically active substances,
is important in immune surveillance, defense and regulation. The
spleen is the largest immune organ and the thymus is the main
immune organ. Therefore, the spleen index and thymus index are
generally used to measure immune function (31). According to the results of the
present study, the spleen index and thymus index of the mice
treated with BHP were significantly higher than those of the mice
in the normal control group (P<0.01), and there was a
correlation between the intensity of the spleen index and the dose
of BHP.
T lymphocytes and B lymphocytes are derived from
pluripotent stem cells in the bone marrow. The lymphoid stem cells
that differentiate from pluripotent stem cells can migrate in the
blood, move to the thymus gland and mature within the thymus;
therefore, these cells are termed thymus-dependent lymphocytes or T
lymphocytes. Bone marrow cells that mature in the bone marrow are
known as bone marrow-dependent lymphocytes, which are B lymphocytes
(32). T and B lymphocytes
stimulated by an antigen or mitogen in vitro can be
converted into larger, metabolically competent, mitotic
lymphoblasts (33). ConA can
stimulate the conversion and proliferation of T lymphocytes. The
degree of T cell transformation and proliferation can reflect the
cellular immune function of the body. A previous study also showed
that lipopolysaccharides can stimulate the transformation of B
lymphocytes, reflecting humoral immunity in the body (34). The spleen is the largest peripheral
lymphoid organ in the body and is the site where T and B
lymphocytes reside. T lymphocytes account for ~35% of the
splenocyte population, and B lymphocytes account for ~55%.
Therefore, the level of lymphocyte activity in the spleen
represents its immune function ability (35). In the present study, ConA was used as
a stimulus for the transformation and proliferation of splenic T
lymphocytes in each experimental mouse. The stimulation index and
proliferation rate of the lymphocytes in the splenic tissues of
each experimental group were measured using a microplate reader.
The results showed that the in vitro proliferation rate and
proliferation index of the splenocytes from mice in the HBHP group
were increased significantly compared with those from mice in the
Con group (P<0.0001), and similar results in the
immunosuppressed BHP treatment group indicated that BHP effectively
promoted the transformation ability of splenic lymphocytes. These
results indicate that BHP can enhance splenic lymphocyte
proliferation in normal mice and immunosuppressed mice and are
consistent with the findings of Yu et al (36). T cells are the main lymphocytes and
have a variety of biological functions. T cells can be divided into
three subgroups, adjuvant or induced T cells (Th), Tc and
suppressor T cells (37). Th cells
are CD4+ T lymphocytes, which express CD4 on the cell
surface. These cells are activated by recognizing polypeptide
antigens presented by major histocompatibility complex (MHC)II.
MHCII is expressed on the surface of antigen-presenting cells
(APCs). Cytokines can be secreted by APCs and are secreted by T
cells following activation. Lymphokines promote the differentiation
of B cells into effector B cells, thereby modulating or assisting
the immune response (38). Tc cells
are CD8+ T lymphocytes that express CD8 on the cell
surface; CD8 can bind directly to an antigen through MHCI, leading
to the release of perforin. The killing of target cells by factors
including perforin and granzymes, and the initiation of target cell
apoptosis also explain why these cells are referred to as cytotoxic
cells (39). In the present study,
the percentage of Th lymphocytes and the percentage of Tc
lymphocytes were determined. The results showed that the percentage
of Th lymphocytes in the HBHP group was significantly increased
compared with that in the Con group (P<0.0001). The percentages
of Tc lymphocytes in the LBHP group and the MBHP group were
increased compared with that in the control group, although the
difference was not significant. The percentage of Tc lymphocytes in
the HBHP group was increased significantly (P<0.01). The
percentages of Th lymphocytes in the LBHP + Cy group (P<0.01),
the MBHP + Cy group and the HBHP + Cy group (P<0.0001) were
significantly increased compared with that in the Cy group, and
these increases were correlated with the BHP dose. These findings
indicate that BHP can increase the numbers of Th lymphocytes and Tc
cells in normal mice and immunosuppressed mice to a certain extent.
These findings are similar to the results of previous studies
(40–42).
NK cells are known as natural killer cells due to
their killing activity, which is not MHC restricted and is
independent of antibodies. Although it is generally considered that
NK cells are derived from the bone marrow, there is currently no
definite evidence to explain their source. NK cells are mainly
distributed in the peripheral blood, therefore, venous blood from
the mouse eye was used in the present study. NK cells can
nonspecifically recognize target cells and exert their killing
effect through perforin, NK cytotoxic factor and TNF (43). When NK cells are activated, they can
secrete various cytokines, thereby contributing to physiological
functions, including antiviral infection responses, immune
surveillance and the killing of mutant tumor cells (44). NK cells are granular lymphocytes and
important immune cells. Therefore, the number of NK cells is an
important indicator of immune function. In the present study, mouse
immune cells were evaluated by measuring the percentage of mouse NK
cells. The results showed that, compared with that in the Con
group, the percentage of NK cells in the HBHP group was
significantly increased (P<0.0001) and the percentages in the
mice treated with MBHP + Cy or HBHP + Cy were significantly
increased (P<0.0001) compared with that in mice in the
immunosuppressed model group. The increase was correlated with the
BHP dose. These results show that BHP can increase the number of NK
cells in normal and immunosuppressed mice.
The types of immunity comprise mainly nonspecific
immunity and specific immunity, and the latter is divided into
cellular immunity and humoral immunity. Nonspecific immunity, also
known as innate immunity, refers to the natural ability of the body
to establish protection and includes the barrier function of the
mucosal system and the phagocytosis of foreign bodies by phagocytic
cells. Innate immunity is the basis of adaptive immunity, and
immune substances produced by adaptive immune system components can
enhance the role of the innate immune system (45). Macrophages, which are nonspecific
immune cells derived from the proliferation and differentiation of
leukocytes, are the most important phagocytes in the immune system.
They are responsible for phagocytosing, processing and treating
antigenic substances, transmitting antigens to lymphocytes and
stimulating lymphocytes to produce antibodies or lymphokines to
enhance the ability to kill target cells. In turn, the production
of antibodies and lymphokines also enhances macrophage chemotaxis,
activation and phagocytosis (46).
Therefore, the phagocytic ability of macrophages is directly
related to the strength of immune function. In the present study,
macrophage phagocytosis was evaluated by measuring the phagocytic
index and the percentage of phagocytosis in each experimental group
of mice to evaluate changes in immune function. The results showed
that the percentage of phagocytosing macrophages and the phagocytic
index in the LBHP, MBHP and HBHP groups were significantly
increased compared with those in the control group (P<0.0001).
Additionally, the percentage of phagocytosing macrophages and the
phagocytic index of mice in the LBHP + Cy, MBHP + Cy and HBHP + Cy
groups were significantly increased compared with those of the mice
in the immunosuppressed model group, and the intensity of these
effects was correlated with the BHP dose (P<0.0001), indicating
that BHP can increase the phagocytic ability of macrophages in
normal mice and immunosuppressed mice. These findings are similar
to those of Yu et al (36).
Additionally, Cy is a member of the class of
alkylating agents widely used for the treatment of cancer. It
mainly exerts its killing mechanism by interfering with DNA and RNA
synthesis in cells. As with most chemotherapeutics, it kills tumor
cells and a certain proportion of normal cells. This killing
effect, particularly in the thymus, spleen and other immune organs,
is potent and can reduce lymphocyte antibody production, resulting
in low immune function (47). To
assess this feature in the present study, mice were treated with an
intraperitoneal injection of 40 mg/kg Cy every other day, which led
to varying degrees of weight loss. The immune organ indices also
decreased to a certain extent, and the percentage of lymphocytes
was relatively low in the Cy group compared with that in the normal
control group. These results are consistent with those in the
literature, indicating the modeling was successful (48).
The results of the present study also showed that
the expression of miR-155 was significantly upregulated in the MBHP
and HBHP groups compared with that in the Con group (P<0.0001).
The expression of miR-155 was also significantly higher in the LBHP
group (P<0.01), LBHP + Cy group (P<0.001), MBHP + Cy group
and HBHP + Cy group (P<0.0001) compared with that in the Cy
group (P<0.0001). The expression of SOCS1 was significantly
downregulated in the LBHP group, MBHP group and HBHP group compared
with that in the Con group. Compared with the Cy group,
significantly downregulated expression of SOCS1 was observed in the
MBHP and HBHP + Cy groups (P<0.0001). These results indicate
that the expression of miR-155/SOCS1 may mediate the regulation of
immune function in mice induced by BHP. These results are
consistent with the results of other studies on miR-155/SOCS1
(49–52).
Finally, TGF-β1 is mainly secreted by activated T
and B lymphocytes and macrophages, and is mainly produced by
lymphocytes. TGF-β1 is involved not only in the development of the
thymus but also in the maturation of T cells (53). TGF-β1 is a multifunctional cytokine
that affects a variety of biological processes, including
inflammation, cell differentiation and immune responses (54). IL-10 is primarily derived from
monocytes, macrophages and Th cells. In addition, dendritic cells
(DCs), B cells, mast cells, Tc cells, NK cells, eosinophils and
neutrophils secrete IL-10 (55).
IL-10 has a dual role in regulating the immune system, as it can
promote B cell proliferation, differentiation and antibody
production but can also inhibit inflammation (56,57).
Activated Th17 cells can secrete IL-17A, which is an inflammatory
factor that promotes inflammatory responses. In the present study,
the secretion of IL-10 in the mouse spleen was significantly
increased following BHP treatment in the normal mice and the
immunosuppressed mice, and this finding indicates that IL-10
mediates the BHP-induced regulation of immune function in mice.
IL-10 has a positive role in regulating immune function in mice.
However, the majority of studies have focused on the negative
regulatory role of IL-10, and even consider it and TGF-β1 to be
immunosuppressive factors secreted by Treg cells (58). The results of the present study
showed that the levels of TGF-β1 in the spleen of normal and
immunosuppressed mice were significantly decreased following BHP
treatment, indicating that TGF-β1 mediates the BHP-induced
regulation of immune function in mice. In addition, TGF-β1 has a
negative regulatory role in the immune function of mice. The
results of the present study are similar to those reported
previously (59). IL-17A is a
recognized inflammatory factor, and there was no significance
difference in IL-17A levels among the groups. Therefore, it was
possible to conclude that the establishment of the immunosuppressed
model with Cy did not cause splenic inflammation in mice, and this
finding is consistent with the H&E staining results. In
addition, if BHP mediates the maintenance of the Treg/Th17 balance,
IL-10 and TGF-β1 would act as immunosuppressive factors
simultaneously, however, the results of the present study overturn
this conjecture. In addition, others have shown that miR-155 can
downregulate the level of TGF-β1 (60). The results of the present study
appear to show similar trends, although further experiments are
required to verify these findings. Therefore, BHP regulates the
immune function in mice but not by improving inflammation. BHP has
a regulatory role in the immune system of mice by upregulating the
expression of immune-stimulating factors (including IL-10) or
downregulating the expression of certain immunosuppressive factors
(including TGF-β1) and ultimately achieves the effect of improving
immune function.
However, there are certain limitations to the
present study, including insufficient fresh blood samples for
Treg/Th17 cell ratio determination and insufficient tissue
specimens for further experiments, including western blot analysis.
In addition, the BHP used in the present study was only compared
with the traditional immunosuppressive drug (Cy), whereas the
classic immune protection drug Transfer Factor Oral Solution
(Jinhua, Xi'an, China) was used in our subsequent experiments
(unpublished data). The present study was performed in normal mice
and immunosuppressive model mice; further experiments are required
on the immunosuppressive mouse model based on the tumor mouse
model, and related tumor cell lines require verification. The
regulatory mechanism of BHP on immune function also requires
further investigation.
In conclusion, data from in vivo and in
vitro experiments in the present study show that BHP can
significantly improve immune function in healthy and
immunosuppressed mice, suggesting that the development of BHP into
a healthcare product suitable for healthy individuals or
chemotherapy-treated cancer patients has an optimistic future.
However, further large-sample basic research and preclinical
validation studies are required. In addition, the relative
expression of SOCS1/miR-155 and the secreted levels of TGF-β1 and
IL-10 are regulated by BHP, which means that BHP has the potential
to improve immunity as a health supplement. A limitation of this
study is that it discusses BHP preliminarily at the molecular
level. The next step is to investigate whether the intestinal flora
affects the BHP-mediated regulation of immune function.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81450047 and
81660468) and Inner Mongolia Autonomous Region Higher Education
Research Project of China (grant no. NJZY19106).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CC performed the experiments and wrote the
manuscript. XS designed the study, and drew and edited the figure.
ZH analyzed the experimental data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animals were treated according to the protocol
approved by the Institutional Animal Care and Use Committee of
Inner Mongolia Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
McKenzie CG, Guo L, Freedman J and Semple
JW: Cellular immune disfunction inimmune thrombocytopenia (ITP). Br
J Haematol. 163:10–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xing Z, Yu L, Li X and Su X: Anticancer
bioactive peptide-3 inhibits human gastric cancer growth by
targeting miR-338-5p. Cell Biosci. 6:532016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su X, Dong C, Zhang J, Su L, Wang X, Cui H
and Chen Z: Combination therapy of anti-cancer bioactive peptide
with Cisplatin decreases chemotherapy dosing and toxicity to
improve the quality of life in xenograft nude mice bears human
gastric cancer. Cell Biosci. 4:7–19. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Słotwiński R, Lech G and Słotwińska SM:
Therapeutic microRNAs in human cancer. Cent Eur J Immunol.
43:314–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prabahar A and Natarajan J: ImmunemiR-A
database of prioritized immune miRNA disease associations and its
interactome. Microrna. 6:71–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Köllmer M, Buhrman JS, Tang MY
and Gemeinhart RA: Arginine-rich, cell penetrating
peptide-anti-microRNA complexes decrease glioblastoma migration
potential. Peptides. 58:83–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao C and Rajewsky K: MicroRNA control in
the immune system: Basic principles. Cell. 136:26–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Escobar T, Yu CR, Muljo SA and Egwuagu CE:
STAT3 activates miR-155 in Th17 cells and acts in concert to
promote experimental autoimmune uveitis. Invest Ophthalmol Vis Sci.
54:4017–4025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Preston GC, Sinclair LV, Kaskar A,
Hukelmann JL, Navarro MN, Ferrero I, MacDonald HR, Cowling VH and
Cantrell DA: Single cell tuning of Myc expression by antigen
receptor signal strength and interleukin-2 in T lymphocytes. EMBO
J. 34:2008–2024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobhkhez M, Joensen LL, Tollersrud LG,
Strandskog G, Thim HL and Jørgensen JB: A conserved inhibitory role
of suppressor of cytokine signaling 1 (SOCS1) in salmon antiviral
immunity. Dev Comp Immunol. 67:66–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ma C, Wang Y, Shen A and Cai W:
Resveratrol upregulates SOCS1 production by
lipopolysaccharide-stimulated RAW264.7 macrophages by inhibiting
miR-155. Int J Mol Med. 39:231–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yokoyama WM, Kim S and French AR: The
dynamic life of natural killer cells. Annu Rev Immunol. 22:405–429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sullivan RP, Fogel LA, Leong JW, Schneider
SE, Wong R, Romee R, Thai TH, Sexl V, Matkovich SJ, Dorn GW II, et
al: miR-155 tunes both the threshold and extent of NK cell
activation via targeting of multiple signaling pathways. J Immunol.
191:5904–5913. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu LF, Thai TH, Calado DP, Chaudhry A,
Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K and
Rudensky AY: Foxp3-dependent microRNA155 confers competitive
fitness to regulatory T cells by targeting SOCS1 protein. Immunity.
30:80–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banerjee A, Schambach F, DeJong CS,
Hammond SM and Reiner SL: Micro-RNA-155 inhibits IFN-gamma
signaling in CD4+ T cells. Eur J Immunol. 40:225–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okoye IS, Czieso S, Ktistaki E, Roderick
K, Coomes SM, Pelly VS, Kannan Y, Perez-Lloret J, Zhao JL,
Baltimore D, et al: Transcriptomics identifieda critical role for
Th2 cell-intrinsic miR-155 in mediating allergy andantihelminth
immunity. Proc Natl Acad Sci USA. 111:E3081–E3090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X
and Liao YH: MicroRNA-155 modulates treg and Th17 cells
differentiation and Th17 cell function by targeting SOCS1. PLoS
One. 7:e460822012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y,
Lu S and Wang Z: Inhibition of microRNA-155 ameliorates
experimental autoimmune myocarditis by modulating Th17/Treg immune
response. J Mol Med (Berl). 94:1063–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Babicz-Zielinska E and Jezewska-Zychowicz
M: Conceptual model of consumer's willingness to eat functional
foods. Rocz Panstw Zakl Hig. 68:33–41. 2017.PubMed/NCBI
|
|
21
|
Bose U, Hodson MP, Shaw PN, Fuerst JA and
Hewavitharana AK: Two peptide, cycloaspeptide A and nazumamide A
from a sponge associated marine actinobacterium Salinispora sp. Nat
Prod Commun. 9:545–546. 2014.PubMed/NCBI
|
|
22
|
Pessione E and Cirrincione S: Bioactive
molecules released in food by lactic acid bacteria: Encrypted
peptides and biogenic amines. Front Microbiol. 7:8762016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silk DB, Grimble GK and Rees RG: Protein
digestion and amino acid and peptide absorption. Proc Nutr Soc.
44:63–72. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Su X, Chen C, Liu Q, She Y, Yan M and Hou
J: Preparation method of anti-gastric cancer bioactive peptide.
China Patent ZL96122236.0. Filed November 4, 1996; issued September
4, 2000.
|
|
25
|
Li L, Yu L, Hu J, et al: Establishment of
separation and identification methods for bioactive peptide protein
components. Sheng Wu Ji Shu Tong Xun. 25:682–686. 2014.
|
|
26
|
Crago SS, Prince SJ, Pretlow TG, McGhee JR
and Mestecky J: Human colostral cells. I. Separation and
characterization. Am J Clin Exp Immunol. 38:585–597. 1979.
|
|
27
|
Shen QC, Lin Y, Wang SY, et al:
Identification and culture of Buffalo peripheral blood mononuclear
macrophage. China Animal Husbandry & Veterinary Medicine.
38:31–35. 2011.
|
|
28
|
Stevenson HC, Katz P, Wright DG, Contreras
TJ, Jemionek JF, Hartwig VM, Flor WJ and Fauci AS: Human blood
monocytes: Characterization of negatively selected human monocytes
and their suspension cell culture derivatives. Scand J Immunol.
14:243–256. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji H, Tian D, Zhang B, Zhang Y, Yan D and
Wu S: Overexpression of miR-155 in clear-cell renal cell carcinoma
and its oncogenic effect through targeting FOXO3a. Exp Ther Med.
13:2286–2292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang SP, Wang W, Gu Y, Jin L and Zhou F:
Study on the correlation between ultrasonic imaging features in
breast cancer and the expression of ER, PR, HER-2 and nm23.
Biomedical Res. 28:5925–5929. 2017.
|
|
31
|
Ke M, Wang H, Zhou Y, Li J, Liu Y, Zhang
M, Dou J, Xi T, Shen B and Zhou C: SEP enhanced the antitumor
activity of 5-fluorouracil by up-regulating NKG2D/MICA and
reversedimmune suppressionvia inhibiting ROS and caspase-3 in mice.
Oncotarget. 7:49509–49526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou G, Wang D, Liu D, Qi D and Liu Z:
Expression of B and T lymphocyte attenuator in patients with severe
community-acquired pneumonia and the effect of steroid therapy in a
mouse model. Clin Lab. 62:2367–2377. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pavić I, Katalinić-Janković V,
Čepin-Bogović J, Rešić A and Dodig S: Discordance between
tuberculin skin test and interferon-γ release assay in children
younger than 5 years who have been vaccinated with bacillus
calmette-guérin. Lab Medicine. 46:200–206. 2015. View Article : Google Scholar
|
|
34
|
Janossy G, Snajdr J and Simakellis M:
Patterns of B-lymphocyte gene expression elicited by
lipopolysaccharide mitogen. Immunology. 30:799–810. 1976.PubMed/NCBI
|
|
35
|
Smialek M, Tykalowski B, Dziewulska D,
Stenzel T and Koncicki A: Immunological aspects of the efficiency
of protectotype vaccination strategy against chicken infectious
bronchitis. BMC Vet Res. 13:1–44. 2017.PubMed/NCBI
|
|
36
|
Yu Y, Wang H, Meng X, Hao L, Fu Y, Fang L,
Shen D, Yu X and Li J: Immunomodulatory effects of cinobufagin on
murine lymphocytes and macrophages. Evid Based Complement Alternat
Med. 2015:8352632015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bernard M, Furlong SJ, Power Coombs MR and
Hoskin DW: Differential inhibition of T lymphocyte proliferation
and cytokine synthesis by [6]-Gingerol, [8]-Gingerol, and
[10]-Gingerol. Phytother Res. 29:1707–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nicholson LB: The immune system. Essays
Biochem. 60:275–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsai CY, Allie SR, Zhang W and Usherwood
EJ: MicroRNA miR-155 affects antiviral effector and effector
memoryCD8 T cell differentiation. J Virol. 87:2348–2351. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shi J, Bo S and Miao M: Effect of
Astragalus polysaccharide on immunological function of
immunosuppressive mice induced by cyclophosphamide. Zhong Yi Xue
Bao. 31:243–246. 2016.
|
|
41
|
Cai K, Wang X, Zhang B, et al: Effects of
Curculigo chinensis polysaccharides on immune function in
immunosuppressed mice induced by cyclophosphamide. Zhong Hua Zhong
Yi Yao Za Zhi. 148–152. 2016.
|
|
42
|
Zhu X, Xu D, Chen X, et al:
Immunomodulatory effects of black chicken peptide on
cyclophosphamide-induced immunocompromised mice. Shi Pin Yu Fa Jiao
Gong Ye. 42:44–49. 2016.
|
|
43
|
Zak DE, Tam VC and Aderem A: Systems-level
analysis of inna-teImmunity. Annu Rev Immunol. 32:547–577. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Leong JW, Sullivan RP and Fehniger TA:
microRNA managem-of NK-cell developmental and functional programs.
Eur J Immunol. 44:2862–2868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Seregin SS and Amalfitano A: Improving
adenovirus based gene transfer: Strategies to accomplish immune
evasion. Viruses. 2:2013–2036. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gemperle C, Schmid M, Herova M, Marti-Jaun
J, Wuest SJ, Loretz C and Hersberger M: Regulation of the formyl
peptide receptor 1 (FPR1) gene in primary human macrophages. PLoS
One. 7:1–6. 2012. View Article : Google Scholar
|
|
47
|
Galon J, Costes A, Sanchez-Cabo F,
Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M,
Berger A, Wind P, et al: Type, density, and location of immune
cells within human colorectal tumors predict clinical outcome.
Science. 313:1960–1964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cortelazzo S, Tarella C, Gianni AM,
Ladetto M, Barbui AM, Rossi A, Gritti G, Corradini P, Di Nicola M,
Patti C, et al: Randomized trial comparing R-CHOP versus high-dose
sequential chemotherapy in high-risk patients with diffuse large
B-cell lymphomas. J Clin Oncol. 34:4015–4022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang S, Zheng Q, Tian F, et al: Changes of
microRNA expression profiles during NKT cell development. Zhong Guo
Mian Yi Xue Za Zhi. 33:979–984. 2017.
|
|
50
|
Shaohua S: Research progress in the
regulation of SOCS1 on immune response in the body. Chin J Med
Officer. 35:1035–1037. 2010.
|
|
51
|
Liu Y: Mechanism of MiR-155 regulating T
cell activation. PhD dissertation, Di Er Jun Yi Da Xue. CNKI;
Beijing: 2011
|
|
52
|
Liu Y and Cheng X: Research Progress of
MicroRNA on host immunity regulation. Xian Dai Mian Yi Xue.
432–435. 2011.
|
|
53
|
Zhang Y, Alexander PB and Wang XF: TGF-β
family signaling in the control of cell proliferation and survival.
Cold Spring Harb Perspect Biol. 9(pii): a0221452017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Komai T, Okamura T, Yamamoto K and Fujio
K: The effects of TGF-βs on immune responses. Nihon Rinsho Meneki
Gakkai Kaishi. 39:51–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Saraiva M and Garra A: The regulation of
IL-10 production by immune Cells. Nat Rev Immunol. 10:170–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
MacNeil IA, Suda T, Moore KW, Mosmann TR
and Zlotnik A: IL-10, a novel growth cofactor for mature and
immature T cells. J Immunol. 145:4167–4173. 1990.PubMed/NCBI
|
|
57
|
Rutz S and Ouyang W: Regulation of
interleukin-10 expression. Adv Exp Med Biol. 941:89–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cao W, Chen W, Liang X, Zhou J, Wei C, Cui
S and Liu J: All-trans-retinoic acid ameliorates the inflammation
by inducing transforming growth factor beta 1 and interleukin 10 in
mouse epididymitis. Am J Reprod Immunol. 71:312–321. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang X, Jiang Z, Gu Y, Liu Y, Cao X and
Han Y: Inflammation-induced CD69+Kupffer cell feedback inhibits T
cell proliferation via membrane-bound TGF-β1. Sci China Life Sci.
59:1259–1269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta⁄SMAD
signal by MiR-155 is involved in arsenic trioxideinduced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1549.
2014. View Article : Google Scholar : PubMed/NCBI
|