Introduction
In an aging European population, osteoporosis has
become a major public health problem. It increases morbidity and
mortality (1–3) and it leads to high direct and indirect
medical costs (4,5) for society due to its hallmark clinical
manifestation: Fragility fractures, particularly vertebral and hip
fractures. The population at risk includes post-menopausal females,
the elderly, patients with long-term glucocorticoid treatment and
patients with chronic inflammatory diseases, e.g. rheumatoid
arthritis (RA). Patients with this auto-immune inflammatory disease
constitute a special subpopulation of patients prone to
osteoporosis, since they possess multiple risk factors for systemic
bone loss: Chronic inflammation of uncontrolled disease activity
(6), higher prevalence of early
menopause (7), increased smoking and
alcohol consumption due to RA-associated stress and depression
(8–10), physical disability and sedentary
behavior caused by RA radiographic progression (11,12) and
treatment with glucocorticoids (13), which are independently involved in
lowering bone mineral density (BMD). Complex management principles
of RA should include strategies to reduce these risk factors and
should promote osteoporosis screening among RA patients.
Unfortunately, a large fraction of patients is still being
diagnosed with osteoporosis at the late stage, after a fragility
fracture. Thus, early detection of osteoporosis and prevention of
its complications are important and require a reliable and
reproducible method, accessible to rheumatologists in all clinical
settings. Dual-energy X-ray absorptiometry (DXA) is the current
validated ‘gold standard’ for the diagnosis of osteoporosis,
fracture risk estimation and follow-up of anti-osteoporotic
treatment (14–16), since it is non-invasive, simple,
precise, fast, less expensive than other imaging techniques,
including computed tomography and magnetic resonance imaging, and
more sensitive than quantitative ultrasound (QUS) (17). DXA and QUS offer the advantage of
body composition estimation. However, QUS may have particular
advantages over DXA, which make it suitable for a potentially wider
use in the primary screening or pre-screening of osteoporosis and
the identification of cases requiring DXA scanning (18,19): It
is less expensive, does not use ionising radiation, uses smaller
hardware and is portable. A recently introduced QUS method that
integrates ultrasound imaging and radiofrequency signals is being
used for the diagnosis of osteoporosis and it produced results
comparable to those obtained with the DXA method (20,21). To
the best of our knowledge, this technique has never been used in a
controlled study environment to assess BMD in Romanian RA patients
and healthy controls. Therefore, the objective of the present study
was to observe whether this innovative QUS technique is reliable
compared to DXA in a typical sample of Romanian patients. In the
first stage of the present study, Romanian RA patients and healthy
controls were pre-screened for osteoporosis using a novel QUS
machine, in order to compare the two groups in terms of bone
parameters and to observe the influence of body composition on BMD
and osteoporosis outcomes.
Patients and methods
Patients and variables
The present study prospectively included
post-menopausal females diagnosed with RA, who also fulfilled the
latest classification criteria (22). Age-matched controls were recruited
from healthy post-menopausal females presenting at the rheumatology
department in the same time period due to osteoarthritis or
occupational musculoskeletal complaints. All of the subjects were
examined between January and June 2018 in a random order of arrival
at two rheumatology clinics of university hospitals with national
addressability (Rheumatology Department of Sfânta Maria Clinical
Hospital and ‘Dr I. Cantacuzino’ Clinical Hospital). Neither the RA
patients nor the controls had been subjected to a DXA scan or had a
known diagnosis of osteoporosis prior to inclusion in the study;
therefore, none of them were receiving any active pharmacologic
treatment (bisphosphonates, hormone replacement therapy,
teriparatide, denosumab, strontium ranelate or vitamin D
supplements). Prior to any study procedure, each patient provided
written informed consent and the study was approved by the Ethics
Committee of Sfanta Maria Clinical Hospital (no. 21440;
22.12.2017). The patients were evaluated for the following
exclusion criteria that were applied: Age <18 years; overlap
syndromes of RA with other chronic auto-immune inflammatory
rheumatologic disorders or their presence in the control group;
causes of secondary osteoporosis, either in the patient's history
or diagnosed during the study by each attending physician (cancer,
celiac disease, chronic liver disease, chronic pancreatitis,
chronic renal disease, chronic obstructive pulmonary disease,
Cushing's disease, cystic fibrosis, diabetes mellitus,
hemoglobinopathies, heparin treatment, hypogonadism,
hyperthyroidism, inflammatory bowel disease, immobility,
malabsorption). Body height and weight were measured in an upright
anatomical position, with light clothing and no shoes, using a
mechanical scale (0.1 kg maximal error) and a stadiometer (0.3 cm
maximal error). The body mass index (BMI) was calculated by
dividing the body weight by the square of the height and the
following weight categories were defined (23): Underweight (BMI<18.5
kg/m2), normal weight (18.5≤BMI<25 kg/m2),
overweight (25≤BMI<30 kg/m2) and obese (BMI≥30
kg/m2).
BMD measurement
All of the measurements were performed by a single
trained technologist using an EchoS machine (Echolight®;
Echolight SRL), which uses quantitative ultrasonometry to estimate
bone density and structure. The system is equipped with a convex
3.5 MHz transducer, which simultaneously detects B-mode ultrasound
signals and unprocessed radiofrequency signals. Those which are
analyzed by the machine's statistical algorithm
(EchoStudio®; Echolight SRL) for each region of interest
(ROI), taking into account the patient's ethnicity, age, sex and
BMI and comparing them to a database of ~10,000 subjects (18,20,24–26). The
ROI for each patient included the usual sites for the diagnosis of
osteoporosis: The lumbar spine (L1-L4 vertebrae approached from the
abdomen) and the femoral necks. The machine's software outputs the
following measurements: 10-year risk of major osteoporotic and hip
fractures (Fracture Risk Assessment Tool; FRAX®),
calculated according to the established algorithm (27), BMD (g/cm2) with T and Z
scores [standard deviations (SD)], body fat percentage and basal
metabolic rate [BMR (kcal/day)]. The producer reported the
following quality and precision (15,28)
parameters for the machine: Minimal detectable change of 0.013
g/cm2 for the spine and 0.008 g/cm2 for the
hip; intra-operator reproducibility of 0.4% for the spine and 0.3%
for the hip; inter-operator reproducibility of 0.54% for the spine
and 0.41% for the hip; diagnostic correlation with DXA of 93.1% for
the spine and 94.2% for the hip. In the present study, osteoporosis
was defined by a T score for the spine or either hip of ≤-2.5
SD.
Statistics
Normality of distribution of data was assessed using
descriptive statistics, normality plots and Lillefors corrected
Kolmogorov-Smirnov tests. Normally distributed continuous variables
are expressed as the mean ± standard deviation, while non-normally
distributed variables are expressed as the median
(minimum-maximum). Qualitative variables are expressed as absolute
frequency (fraction of subgroup). The correlations of normally
distributed continuous variables were assessed using 2-tailed
partial correlations controlling for recorded confounders. The
difference of continuous variables between subgroups (RA and
controls or RA patients with or without osteoporosis) was assessed
by independent-samples t-tests or Mann-Whitney U-tests, depending
on their normality of distribution, while differences of nominal
variables were assessed with χ2 tests. The distribution
of BMD measurements among RA patients according to weight category
was assessed using independent-samples Kruskal-Wallis tests.
P<0.05 was considered to indicate statistical significance.
Statistical analysis was performed using SPSS v.20 for Windows (IBM
Corp.).
Results
General characteristics
The study included 225 subjects: 106 RA patients
(47.1%), with an average age of 65±8 years and 119 controls (52.9%)
with an average age of 64±13 years (Table I). The median disease duration of RA
was 3.2 (1.5–42.0) years and all RA patients were receiving at
least one conventional synthetic disease-modifying anti-rheumatic
drug: Methotrexate (77.4%), leflunomide (18.9%), sulfasalazine
(12.3%) and/or hydroxychloroquine (8.5%). All of the RA patients
had received glucocorticoids for different periods of time during
the disease course, but 14 patients (13.2%) were still taking
glucocorticoids at the time of study inclusion. The mean dose of
glucocorticoids in the 3 months prior to study inclusion was 8.9
(4.0–15.0) mg of prednisone/day or equivalent.
 | Table I.Comparison of clinicopathological
characteristics between subjects with RA and controls. |
Table I.
Comparison of clinicopathological
characteristics between subjects with RA and controls.
| Parameter | Controls
(n=119) | RA (n=106) | P-value |
|---|
| Age (years) | 64±13 | 65±8 | 0.448a |
| Menopause
(years) | 10 (1–42) | 17 (1–45) | 0.223b |
| Height (m) | 1.6±0.1 | 1.6±0.1 | 0.166a |
| Weight (kg) | 68
(42–109) | 65 (45–95) | 0.011b |
| BMI
(kg/m2) | 27
(18–41) | 26 (18–38) | 0.039b |
| Obesity (%) | 34
(28.6%) | 25
(23.6%) | 0.396c |
| Body fat (%) | 35.4±5.5 | 35.5±6.6 | 0.257a |
| BMR (1,000
kcal/day) | 1.3
(0.9–2.8) | 1.2
(0.8–2.6) | 0.038b |
| Spine T-score
(SD) | –1.8
(−3.2–1.5) | –1.9
(−4.2–1.2) | 0.110b |
| Spine BMD
(g/cm2) | 0.89±0.13 | 0.87±0.09 | 0.035a |
| Spine FRAX-mof
(%) | 4.1
(1.2–6.2) | 6.3
(1.9–15.2) |
<0.001b |
| Spine FRAX-hf
(%) | 0.8 (0–8.2) | 2.1
(0.8–12.5) |
<0.001b |
| Left femoral neck
T-score (SD) | –1.7
(−2.8–1.2) | –2.0
(−3.9–0.8) | 0.039b |
| Left femoral neck
BMD (g/cm2) | 0.71±0.13 | 0.67±0.12 | 0.021a |
| Left hip FRAX-mof
(%) | 4.9 (0–8.1) | 6.6 (1–13.2) |
<0.001b |
| Left hip FRAX-hf
(%) | 1.2 (0–5.2) | 1.8
(0.9–11.6) |
<0.001b |
| Right femoral neck
T-score (SD) | –1.7
(−2.7–1.5) | –2.0
(−3.7–1.2) | 0.030b |
| Right femoral neck
BMD (g/cm2) | 0.71±0.12 | 0.67±0.12 | 0.041a |
| Right hip FRAX-mof
(%) | 4.6 (0–5.1) | 6.4 (1–12.8) |
<0.001b |
| Right hip FRAX-hf
(%) | 1.0 (0–4.8) | 1.7
(1.1–10.2) |
<0.001b |
| Osteoporosis
(%) | 16
(13.4%) | 30
(28.3%) | 0.006c |
Inter-group comparison
Comparing the two groups, significant differences
became apparent: The RA patients had a significantly lower body
weight and BMI and BMR than the controls, although the prevalence
of obesity and body fat percentage differed insignificantly
(Table I). Regarding bone
measurements, RA patients had a significantly lower spine and hip
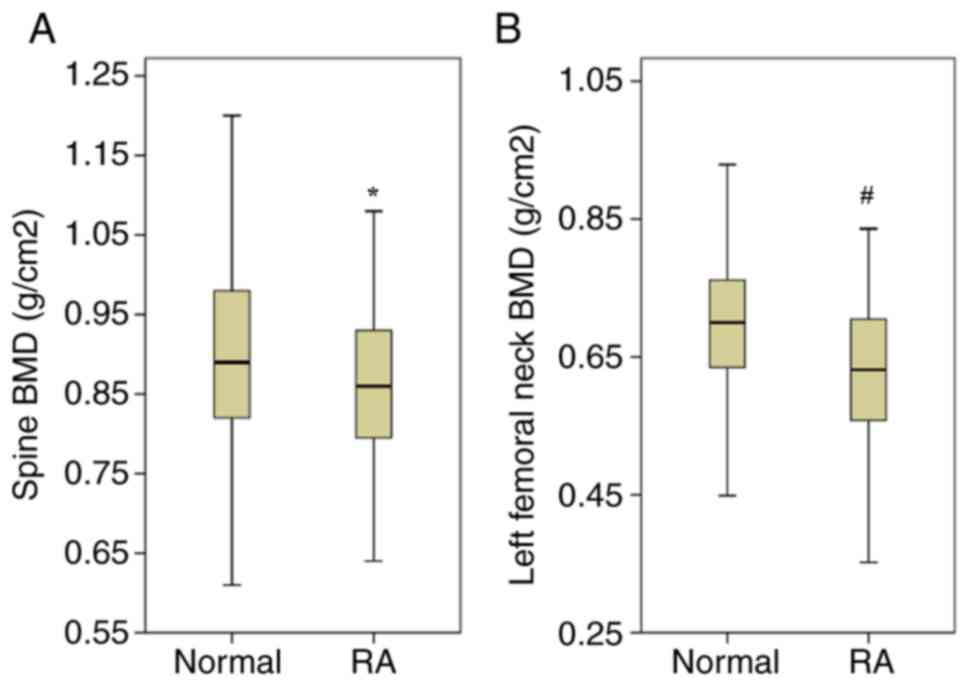
BMD (Fig. 1), higher fracture risks
according to FRAX scores and higher prevalence of T score-defined
osteoporosis (Table I).
RA subgroup analysis
RA patients were also studied independently
regarding their osteoporosis status: Patients with or without
osteoporosis were compared (Table
II). Compared to non-osteoporotic RA patients, RA patients with
osteoporosis were significantly older and had a longer menopause
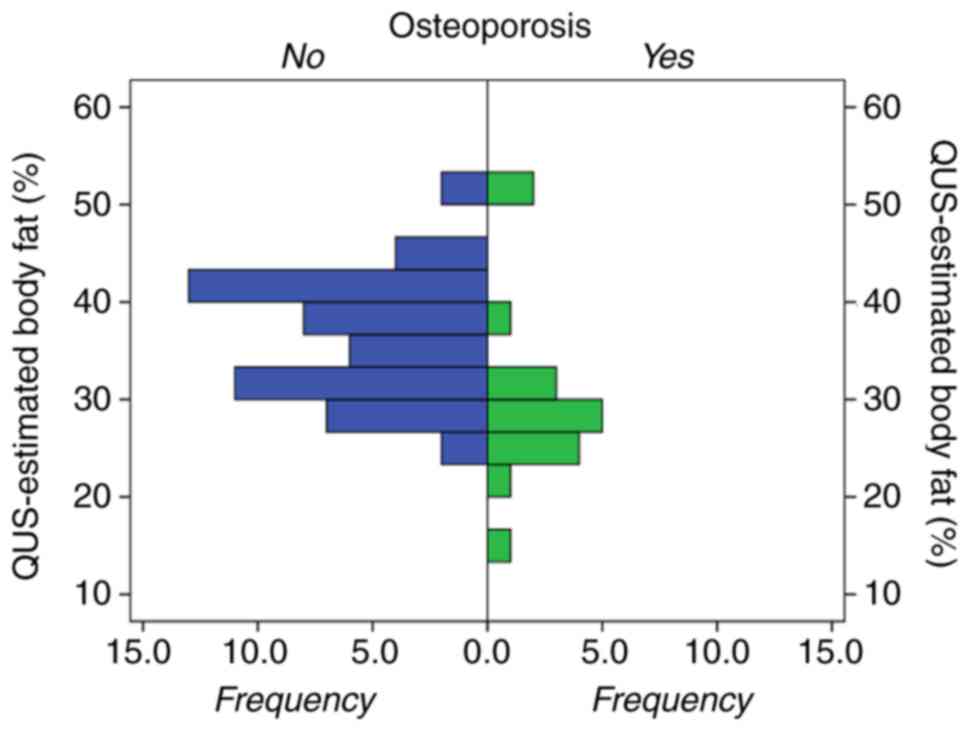
duration, but they had a significantly lower percentage of body fat
(28% compared to 37% respectively; P<0.001; Fig. 2), BMI, prevalence of obesity and BMR
(Table II). Median BMD values
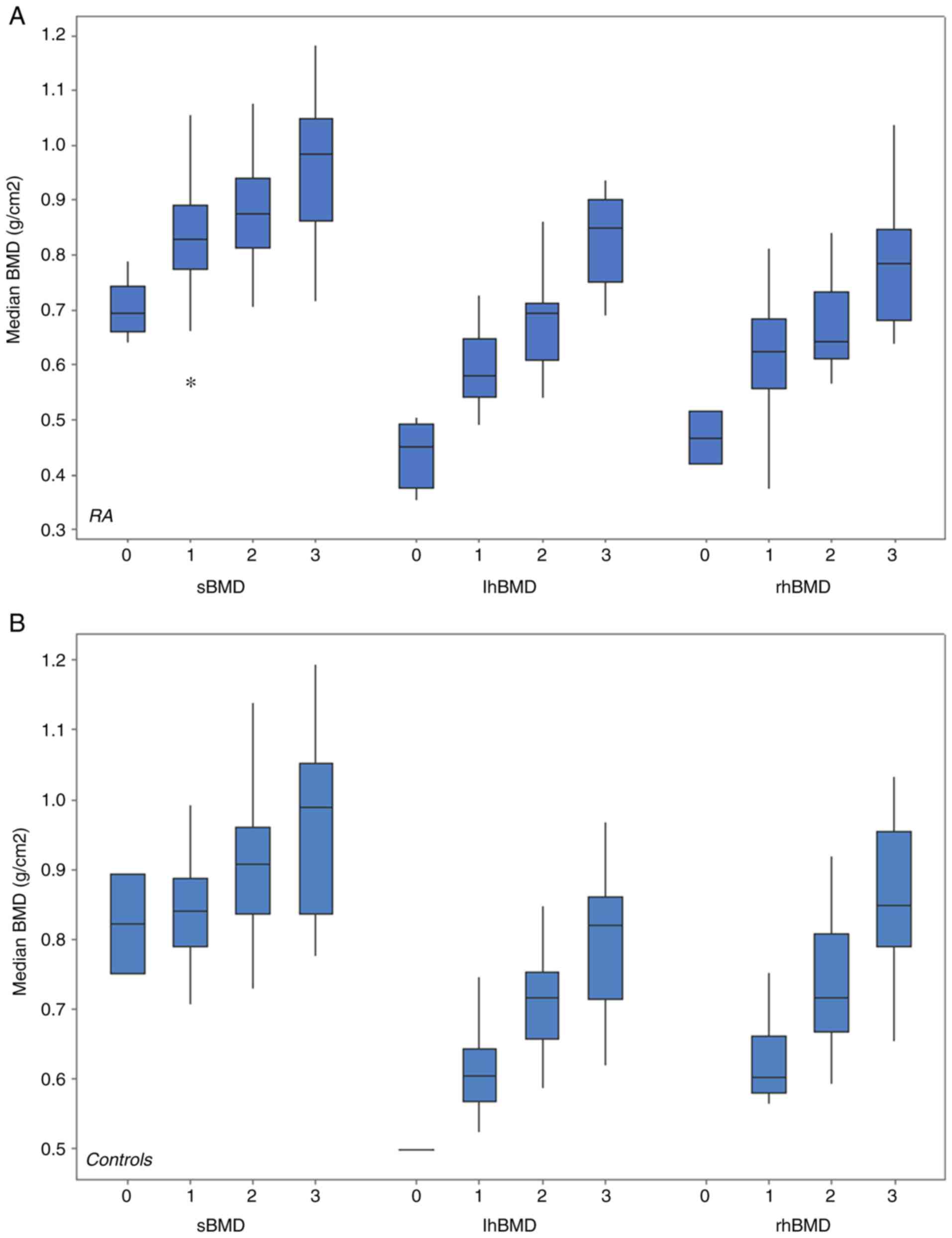
increased proportionally and significantly with weight category
(Fig. 3). Body fat and BMD at all
three scanning sites were significantly and positively correlated
when controlling for age, menopause duration and BMI (Table III).
 | Table II.Comparison of clinicopathological
parameters between RA patients with and without osteoporosis. |
Table II.
Comparison of clinicopathological
parameters between RA patients with and without osteoporosis.
|
| Osteoporosis |
|
|---|
|
|
|
|
|---|
| Parameter | No (n=76) | Yes (n=30) | P-value |
|---|
| Age (years) | 60 (42–82) | 69 (36–84) |
<0.001a |
| Menopause
(years) | 13 (1–38) | 26 (1–42) |
<0.001a |
| Height (cm) | 161 (151–185) | 160 (152–182) |
<0.001a |
| Weight (kg) | 70 (46–95) | 55 (42–82) |
<0.001a |
| BMI
(kg/m2) | 27 (18–36) | 22 (18–31) |
<0.001a |
| Obesity (%) | 23 (21.7%) | 2 (1.9%) | 0.010b |
| Body fat (%) | 37 (30–61) | 28 (28–52) | 0.001a |
| BMR (1,000
kcal/day) | 1.3 (0.8–2.8) | 1.1 (0.8–2.5) |
<0.001a |
 | Table III.Correlation of body fat with BMD
controlling for age, menopause duration and body mass index. |
Table III.
Correlation of body fat with BMD
controlling for age, menopause duration and body mass index.
|
| All (n=225) | RA group
(n=106) | Control group
(n=119) |
|---|
|
|
|
|
|
|---|
|
| r | P-value | r | P-value | r | P-value |
|---|
| Spine BMD | 0.663 | <0.001 | 0.645 | <0.001 | 0.674 | <0.001 |
| Left hip BMD | 0.622 | <0.001 | 0.691 | <0.001 | 0.572 | <0.001 |
| Right hip BMD | 0.697 | <0.001 | 0.514 |
0.003 | 0.777 | <0.001 |
Discussion
Regarding the aims of the present study,
implementation of the novel QUS technique revealed a higher
prevalence of osteoporosis among RA patients compared to controls,
which in turn caused higher fragility fracture risks according to
FRAX, which is well documented in the literature in studies using
DXA (29–31). The fraction of RA patients with
osteoporosis was previously reported to be up to 50% (30), but the 28.3% prevalence of
osteoporosis among the RA patients in the present study is similar
to that determined by other European DXA-defined studies on the
prevalence of osteoporosis: For instance, Hauser et al
(29) reported that 29.9% of their
RA patients had osteoporosis, while Mobini et al (31) reported a slightly higher prevalence
of 32.3%. RA is highly associated with osteoporosis: The rate of
BMD decline in females with RA is significantly higher than that of
controls in all age groups (29) and
10% of females with RA with a normal initial BMD develop
osteoporosis within a decade (32).
Positive rheumatoid factor and anti-citrullinated protein
antibodies (31), RA disease
duration (30), quality of life
(30) and glucocorticoids (33) are specific risk factors for
osteoporosis in RA, proving that the association of the two
diseases is causal. This assumption is further documented by
genetic studies, which revealed >30 genetic factors of the
RA-osteoporosis association (34),
including the 14-3-3ε protein (35)
and polymorphisms of vitamin D receptors (36,37) and
of receptor activator of NF-κB (38). This strong link is the fundamental
reason for the requirement of intensive and extensive osteoporosis
screening and treatment of RA patients, a goal which may be
achieved by exploiting the physical advantages of QUS methods.
In terms of body composition, an insignificant
difference in body fat was observed among RA patients and controls,
as estimated by the novel QUS method, a result which replicates a
similar previous observation of our group using DXA (39). Considering the fact that the RA
patients of the present study had a significantly lower median body
weight and BMD in the selected ROI compared with those of the
controls, it may be hypothesized that the RA patients had a lower
muscle mass than the controls, which is in accordance with
literature reports of higher prevalence of cachexia and sarcopenia
among RA patients (40–42), and/or that there was a selection bias
of controls (the fact that these subjects solicited medical
attention may translate to a higher morbidity toll than the
remainder of the healthy Romanian population, including obese
individuals). Of note, in the present study, the RA patients with
osteoporosis had a significantly lower median BMI and QUS-estimated
body fat than the RA patients without osteoporosis. If this lower
BMI is explained by a lower body fat mass, this observation is
explainable by the trend reported in the general population of a
low BMI being associated with an increased risk of osteoporosis
(43,44), which translates into the lack of the
protective effect of adiposity against systemic bone loss. If the
observed lower BMI of RA patients with osteoporosis was not due to
their lower body fat mass, it must be assumed that these patients
had a loss of therapeutic control of their disease activity which
is expected to decrease BMI through cytokine production. In the
association of BMI and BMD, the significant inflexion point of
increasing BMD appears to be between BMI-defined overweight and
obese RA patients and between BMI-defined normal-weight and
overweight controls, which graphically demonstrates that overweight
RA patients experience the same BMD effect as normal-weight
controls, reaffirming the requirement to lower BMI cutoffs in RA
patients, since there is DXA evidence that the traditional 30
kg/m2 cutoff for obesity is too high for RA patients
(45). It may also suggest that
there is a critical adipose tissue mass [or its biochemical
products, including estrogens, androgens and leptin (46)], which is required counteract systemic
bone loss. Clinicians should be aware of body composition trends in
their RA patients, as evidence suggests that patients with early RA
tend to lose their lean body mass status and gain truncal fat
distribution (47), and that these
modifications in body composition may be significantly predicted by
RA-specific variables, including disease duration, disease activity
scores and radiographic progression (48). Complex management of RA (control of
disease activity and cardiovascular risk factors) should aim to
prevent or reverse these changes in body composition, with
additional long-term effects or lowering of mortality by decreasing
cardiovascular and fracture complications.
There are several limitations of the present study,
which may influence the relevance of the results, including its
cross-sectional design (which, at this time, did not allow for
follow-up QUS scans in order to assess the evolution in time and
the effect of therapeutic intervention), hospital-recruited study
samples (which may have been biased regarding the presence of
comorbidities) and the lack of data regarding disease activity
(including composite disease activity scores, which would have
allowed for evaluation of the association of QUS measurements and
the inflammation burden). Future research efforts will aim to bring
improvements to the design of the present preliminary study on the
novel QUS technique in detecting osteoporosis in post-menopausal
females with or without RA, including direct comparison with DXA
results, inclusion of more study groups with other rheumatic
diseases and, in a longer term, to evaluate the cost-effectiveness
of this portable QUS method in a wider screening program.
This innovative, non-ionizing technique
Radiofrequency Echographic Multi Spectrometry (REMS) used for
diagnosing osteoporosis was recently evaluated through a
multicenter study that involved 1914 women and the results obtained
with this method were significantly in agreement with those
obtained using the gold standard lumbar spine and femoral neck DXA
evaluation. Regarding its diagnostic capability, REMS evaluation
had a sensitivity and specificity of >90% for the two anatomic
sites that were measured (48). It
should be mentioned that on 21 September 2018, at the meeting of
the International Scientific Advisory Board of Echolight in
Florence, Prof. Dr. Jean-Yves Reginster, president of the European
Society for Clinical and Economic Aspects of Osteoporosis,
Osteoarthritis and Musculoskeletal Diseases (ESCEO), signed a
letter in the name of the organization, recommending the inclusion
of REMS technology into clinical practice guidelines for the
diagnosis and treatment of osteoporosis. The letter mentioned the
fact that ESCEO is considering to publish a position paper
discussing the potentialities of REMS technology for the early
diagnosis of osteoporosis and for monitoring treatment effects.
This method will be considered at the next revision of the ESCEO
clinical practice guidelines.
In conclusion, osteoporosis is prevalent among RA
patients, increasing their fragility fracture risk, and is part of
a more complex pathological process of alteration of body mass
composition, involving BMI and fat mass. The novel scanning
technique, which combines B-mode ultrasound and radiofrequency
signals, is able to replicate the results of the established DXA
measurements of BMD and is potentially suitable for the screening
of wide populations for osteoporosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
VCB was responsible for the study design,
ultrasonographic densitometric determinations and writing of the
manuscript. CCP was responsible for the statistical analysis and
writing of the manuscript. RDD and AD were responsible for the
recruitment of patients and performed the ultrasonographic
densitometric determinations. SMB and ARB made substantial
contributions to the conception or design of the work. MB was
responsible for the recruitment of patients, provided scientific
advice and critically reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Each patient provided written informed consent and
the study was approved by the Ethics Committee of Sfanta Maria
Clinical Hospital (no. 21440; 22.12.2017).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bliuc D, Alarkawi D, Nguyen TV, Eisman JA
and Center JR: Risk of subsequent fractures and mortality in
elderly women and men with fragility fractures with and without
osteoporotic bone density: The Dubbo Osteoporosis Epidemiology
Study. J Bone Miner Res. 30:637–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diamantopoulos AP, Hoff M, Skoie IM,
Hochberg M and Haugeberg G: Short- and long-term mortality in males
and females with fragility hip fracture in Norway. A
population-based study. Clin Interv Aging. 8:817–823. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gutzwiller JP, Richterich JP, Stanga Z,
Nydegger UE, Risch L and Risch M: Osteoporosis, diabetes, and
hypertension are major risk factors for mortality in older adults:
An intermediate report on a prospective survey of 1467
community-dwelling elderly healthy pensioners in Switzerland. BMC
Geriatr. 18:1152018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujiwara S, Zhao X, Teoh C, Jaffe DH and
Taguchi Y: Disease burden of fractures among patients with
osteoporosis in Japan: Health-related quality of life, work
productivity and activity impairment, healthcare resource
utilization, and economic costs. J Bone Miner Metab. 37:307–318.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weycker D, Li X, Barron R, Bornheimer R
and Chandler D: Hospitalizations for osteoporosis-related
fractures: Economic costs and clinical outcomes. Bone Rep.
5:186–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roux C: Osteoporosis in inflammatory joint
diseases. Osteoporos Int. 22:421–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banas T, Hajdyla-Banas I, Pitynski K,
Nieweglowska D, Juszczyk G, Ludwin A, Knafel A and Ludwin I: Age at
natural menopause in women on long-term methotrexate therapy for
rheumatoid arthritis. Menopause. 23:1130–1138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang K, Yang SM, Kim SH, Han KH, Park SJ
and Shin JI: Smoking and rheumatoid arthritis. Int J Mol Sci.
15:22279–22295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Matcham F, Rayner L, Steer S and Hotopf M:
The prevalence of depression in rheumatoid arthritis: A systematic
review and meta-analysis. Rheumatology (Oxford). 52:2136–2148.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matcham F, Rayner L, Steer S and Hotopf M:
The prevalence of depression in rheumatoid arthritis: A systematic
review and meta-analysis: Reply. Rheumatology (Oxford). 53:578–579.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bombardier C, Barbieri M, Parthan A, Zack
DJ, Walker V, Macarios D and Smolen JS: The relationship between
joint damage and functional disability in rheumatoid arthritis: A
systematic review. Ann Rheum Dis. 71:836–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Legge A, Blanchard C and Hanly JG:
Physical activity and sedentary behavior in patients with systemic
lupus erythematosus and rheumatoid arthritis. Open Access
Rheumatol. 9:191–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kan SL, Yuan ZF, Li Y, Ai J, Xu H, Sun JC
and Feng SQ: Alendronate prevents glucocorticoid-induced
osteoporosis in patients with rheumatic diseases: A meta-analysis.
Medicine (Baltimore). 95:e39902016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
DeVita MV and Stall SH: Dual-energy X-ray
absorptiometry: A review. J Ren Nutr. 9:178–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Maghraoui A and Roux C: DXA scanning in
clinical practice. QJM. 101:605–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanner SB and Moore CF Jr: A review of the
use of dual-energy X-ray absorptiometry (DXA) in rheumatology. Open
Access Rheumatol. 4:99–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Maghraoui A, Morjane F, Mounach A,
Ghazi M, Nouijai A, Achemlal L, Bezza A and Ghozlani I: Performance
of calcaneus quantitative ultrasound and dual-energy X-ray
absorptiometry in the discrimination of prevalent asymptomatic
osteoporotic fractures in postmenopausal women. Rheumatol Int.
29:551–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iida T, Chikamura C, Aoi S, Ikeda H,
Matsuda Y, Oguri Y, Ono Y, Katada K and Ishizaki F: A study on the
validity of quantitative ultrasonic measurement used the bone
mineral density values on dual-energy X-ray absorptiometry in young
and in middle-aged or older women. Radiol Phys Technol. 3:113–119.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pisani P, Renna MD, Conversano F, Casciaro
E, Muratore M, Quarta E, Paola MD and Casciaro S: Screening and
early diagnosis of osteoporosis through X-ray and ultrasound based
techniques. World J Radiol. 5:398–410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aventaggiato M, Conversano F, Pisani P,
Casciaro E, Franchini R, Lay-Ekuakille A, Muratore M and Casciaro
S: Validation of an automatic segmentation method to detect
vertebral interfaces in ultrasound images. IET Sci Measurement
Technol. 10:18–27. 2016. View Article : Google Scholar
|
|
21
|
Casciaro S, Peccarisi M, Pisani P,
Franchini R, Greco A, De Marco T, Grimaldi A, Quarta L, Quarta E,
Muratore M and Conversano F: An advanced quantitative echosound
methodology for femoral neck densitometry. Ultrasound Med Biol.
42:1337–1356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aletaha D, Neogi T, Silman AJ, Funovits J,
Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP,
Cohen MD, et al: 2010 rheumatoid arthritis classification criteria:
An American College of Rheumatology/European League Against
Rheumatism collaborative initiative. Arthritis Rheum. 62:2569–2581.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
World Health Organization (WHO), .
Physical status: The use and interpretation of anthropometry.
Report of a WHO Expert Committee. World Health Organ Tech Rep Ser.
854:1–452. 1995.PubMed/NCBI
|
|
24
|
Casciaro S, Conversano F, Pisani P and
Muratore M: New perspectives in echographic diagnosis of
osteoporosis on hip and spine. Clin Cases Miner Bone Metab.
12:142–150. 2015.PubMed/NCBI
|
|
25
|
Conversano F, Franchini R, Greco A,
Soloperto G, Chiriacó F, Casciaro E, Aventaggiato M, Renna MD,
Pisani P, Di Paola M, et al: A novel ultrasound methodology for
estimating spine mineral density. Ultrasound Med Biol. 41:281–300.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olszynski WP, Adachi JD, Hanley DA,
Davison KS and Brown JP: Comparison of speed of sound measures
assessed by multisite quantitative ultrasound to bone mineral
density measures assessed by Dual-energy X-Ray absorptiometry in a
large canadian cohort: The canadian multicentre osteoporosis study
(CaMos). J Clin Densitom. 19:234–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanis JA, McCloskey EV, Johansson H, Oden
A, Ström O and Borgström F: Development and use of FRAX in
osteoporosis. Osteoporos Int. 21 (Suppl 2):S407–S413. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Glüer CC, Lu Y and Engelke K: Quality and
performance measures in bone densitometry. Part 2: Fracture risk.
Osteoporos Int. 17:1449–1458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hauser B, Riches PL, Wilson JF, Horne AE
and Ralston SH: Prevalence and clinical prediction of osteoporosis
in a contemporary cohort of patients with rheumatoid arthritis.
Rheumatology (Oxford). 53:1759–1766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JH, Sung YK, Choi CB, Cho SK, Bang SY,
Choe JY, Hong SJ, Jun JB, Kim TH, Lee J, et al: The frequency of
and risk factors for osteoporosis in Korean patients with
rheumatoid arthritis. BMC Musculoskelet Disord. 17:982016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mobini M, Kashi Z and Ghobadifar A:
Prevalence and associated factors of osteoporosis in female
patients with rheumatoid arthritis. Caspian J Intern Med.
3:447–450. 2012.PubMed/NCBI
|
|
32
|
Hwang J, Lee EK, Ahn JK, Cha HS, Koh EM
and Lee J: Bone-density testing interval and transition to
osteoporosis in patients with rheumatoid arthritis. Osteoporos Int.
28:231–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coulson KA, Reed G, Gilliam BE, Kremer JM
and Pepmueller PH: Factors influencing fracture risk, T score, and
management of osteoporosis in patients with rheumatoid arthritis in
the Consortium of Rheumatology Researchers of North America
(CORRONA) registry. J Clin Rheumatol. 15:155–160. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou R, Lin X, Li DY, Wang XF, Greenbaum
J, Chen YC, Zeng CP, Lu JM, Ao ZX, Peng LP, et al: Identification
of novel genetic loci for osteoporosis and/or rheumatoid arthritis
using cFDR approach. PLoS One. 12:e01838422017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong X, Xu SQ, Wu Y, Ma CC, Qi S, Liu W
and Xu JH: Elevated serum 14-3-3η protein may be helpful for
diagnosis of early rheumatoid arthritis associated with secondary
osteoporosis in Chinese population. Clin Rheumatol. 36:2581–2587.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hussien YM, Shehata A, Karam RA, Alzahrani
SS, Magdy H and El-Shafey AM: Polymorphism in vitamin D receptor
and osteoprotegerin genes in Egyptian rheumatoid arthritis patients
with and without osteoporosis. Mol Biol Rep. 40:3675–3680. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mosaad YM, Hammad EM, Fawzy Z, Abdal Aal
IA, Youssef HM, ElSaid TO, Monir R and El-Deek BS: Vitamin D
receptor gene polymorphism as possible risk factor in rheumatoid
arthritis and rheumatoid related osteoporosis. Hum Immunol.
75:452–461. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mohamed RH, Mohamed RH and El-Shahawy EE:
Relationship Between RANK and RANKL gene polymorphisms with
osteoporosis in rheumatoid arthritis patients. Genet Test Mol
Biomarkers. 20:249–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Popescu C, Bojincă V, Opriş D and Ionescui
R: Dual X-ray absorptiometry whole body composition of adipose
tissue in rheumatoid arthritis. Rom J Intern Med. 53:237–247.
2015.PubMed/NCBI
|
|
40
|
Challal S, Minichiello E, Boissier MC and
Semerano L: Cachexia and adiposity in rheumatoid arthritis.
Relevance for disease management and clinical outcomes. Joint Bone
Spine. 83:127–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Doğan SC, Hizmetli S, Hayta E, Kaptanoğlu
E, Erselcan T and Güler E: Sarcopenia in women with rheumatoid
arthritis. Eur J Rheumatol. 2:57–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ngeuleu A, Allali F, Medrare L, Madhi A,
Rkain H and Hajjaj-Hassouni N: Sarcopenia in rheumatoid arthritis:
Prevalence, influence of disease activity and associated factors.
Rheumatol Int. 37:1015–1020. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mazocco L and Chagas P: Association
between body mass index and osteoporosis in women from northwestern
Rio Grande do Sul. Rev Bras Reumatol Engl Ed. 57:299–305. 2017.(In
English, Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cui R, Zhou L, Li Z, Li Q, Qi Z and Zhang
J: Assessment risk of osteoporosis in Chinese people: Relationship
among body mass index, serum lipid profiles, blood glucose, and
bone mineral density. Clin Interv Aging. 11:887–895. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guimarães M, da Costa Pinto MR, Raid R,
Andrade MV and Kakehasi AM: Which is the best cutoff of body mass
index to identify obesity in female patients with rheumatoid
arthritis? A study using dual energy X-ray absorptiometry body
composition. Rev Bras Reumatol. Feb 11–2016.(Epub ahead of
print).
|
|
46
|
Crepaldi G, Romanato G, Tonin P and Maggi
S: Osteoporosis and body composition. J Endocrinol Invest 30 (6
Suppl). S42–S47. 2007.
|
|
47
|
Book C, Karlsson MK, Nilsson JA, Akesson K
and Jacobsson LT: Changes in body composition after 2 years with
rheumatoid arthritis. Scand J Rheumatol. 40:95–100. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Popescu C, Bojinca V, Opris D and Ionescu
R: Disease activity predicts whole body and regional lean tissue in
rheumatoid arthritis-a cross-sectional study. Romanian J Rheumatol.
23:74–83. 2015.
|