|
1
|
Callen DJ, Shroff MM, Branson HM, Lotze T,
Li DK, Stephens D and Banwell BL: MRI in the diagnosis of pediatric
multiple sclerosis. Neurology. 72:961–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moore MM, Gustas CN, Choudhary AK,
Methratta ST, Hulse MA, Geeting G, Eggli KD and Boal DK: MRI for
clinically suspected pediatric appendicitis: An implemented
program. Pediatr Radiol. 42:1056–1063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hartman JH, Bena J, McIntyre S and Albert
NM: Does a photo diarydecrease stress and anxiety in children
undergoing magnetic resonance imaging? A randomized, controlled
study. J Radiol Nurs. 28:122–128. 2009. View Article : Google Scholar
|
|
4
|
McJury M and Shellock FG: Auditory Noise
associated with MR procedures: A review. J Magn Reson Imaging.
12:37–45. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li BL, Ni J, Huang JX, Zhang N, Song XR
and Yuen VM: Intranasal dexmedetomidine for sedation in children
undergoing transthoracic echocardiography study-a prospective
observational study. Paediatr Anaesth. 25:891–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuen VM, Li BL, Cheuk DK, Leung MKM, Hui
TWC, Wong IC, Lam WW, Choi SW and Irwin MG: A randomised controlled
trial of oral chloral hydrate vs. intranasal dexmedetomidine before
computerised tomography in children. Anaesthesia. 72:1191–1195.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Millar K, Bowman AW, Burns D, McLaughlin
P, Moores T, Morton NS, Musiello T, Wallace E, Wray A and Welbury
RR: Children's cognitive recovery after day-case general
anesthesia: A randomized trial of propofol or isoflurane for dental
procedures. Paediatr Anaesth. 24:201–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiaretti A, Benini F, Pierri F, Vecchiato
K, Ronfani L, Agosto C, Ventura A, Genovese O and Barbi E: Safety
and efficacy of propofol administered by paediatricians during
procedural sedation in children. Acta Paediatr. 103:182–187. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
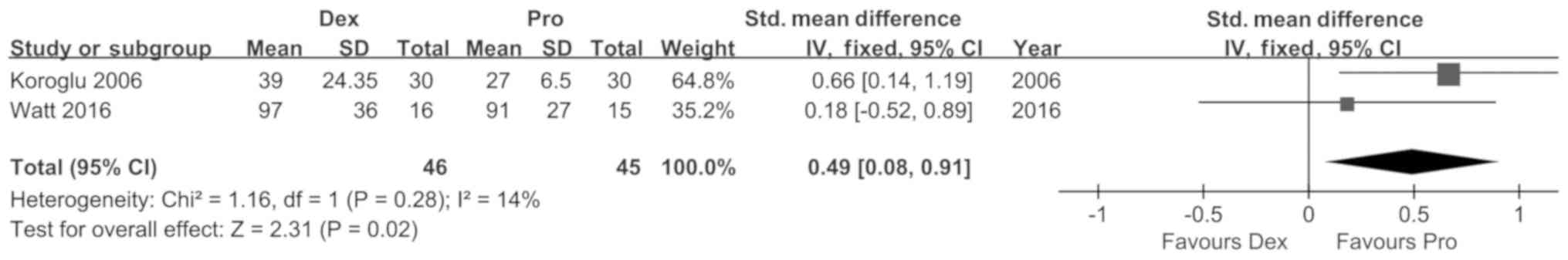
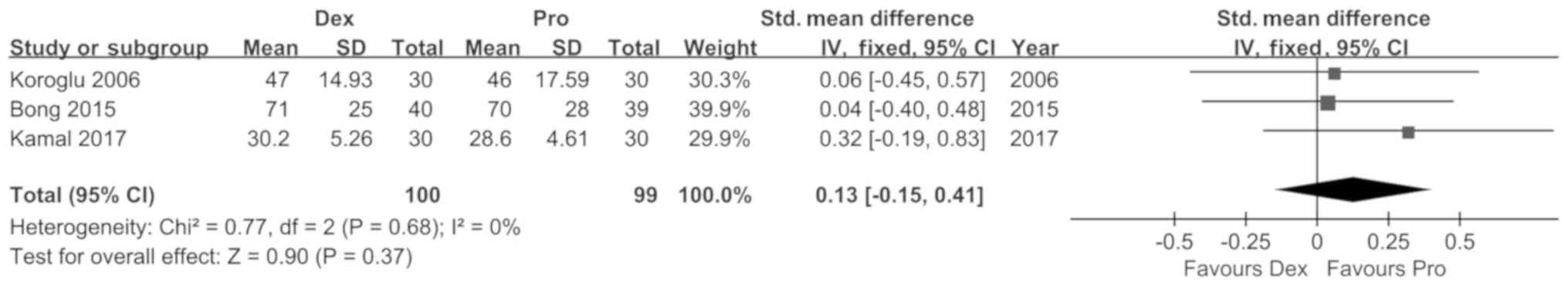
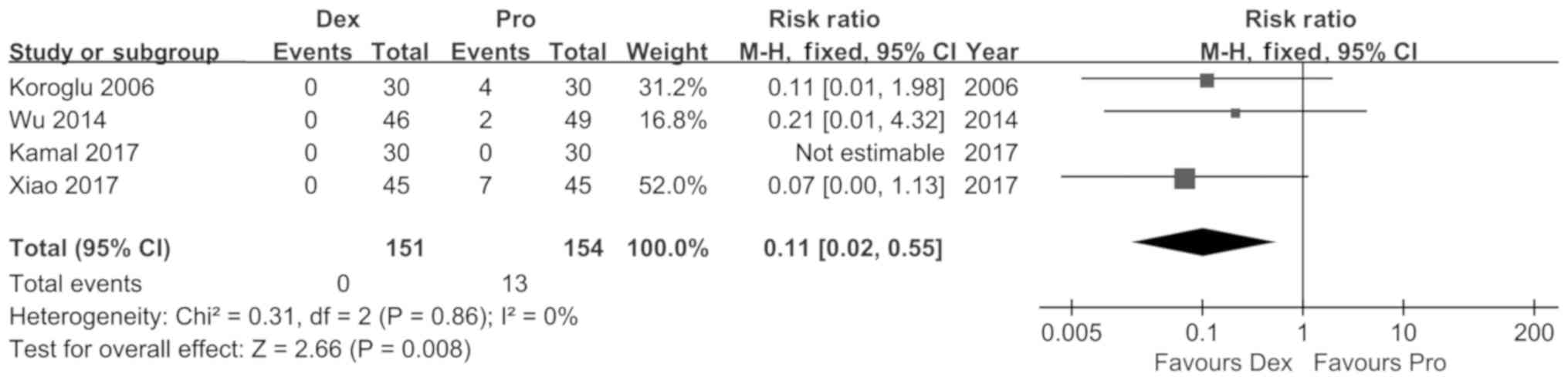
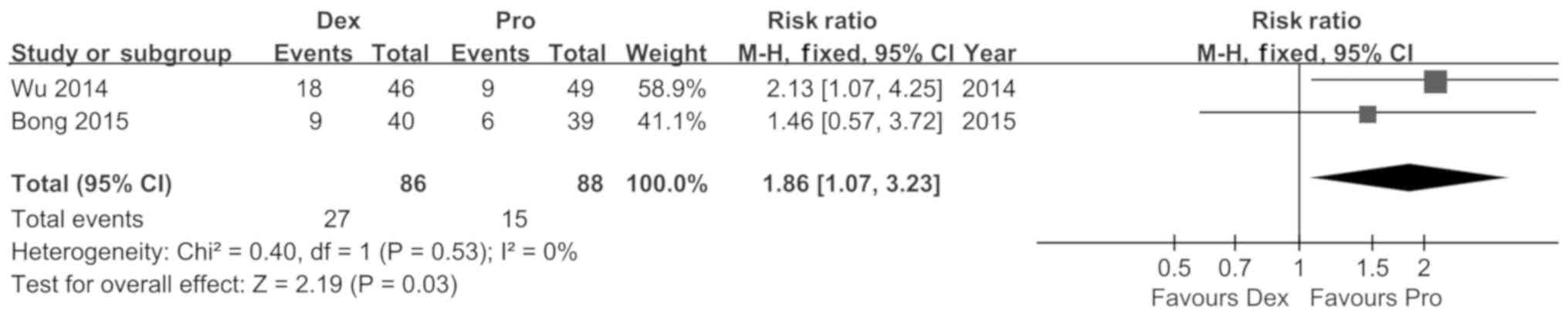
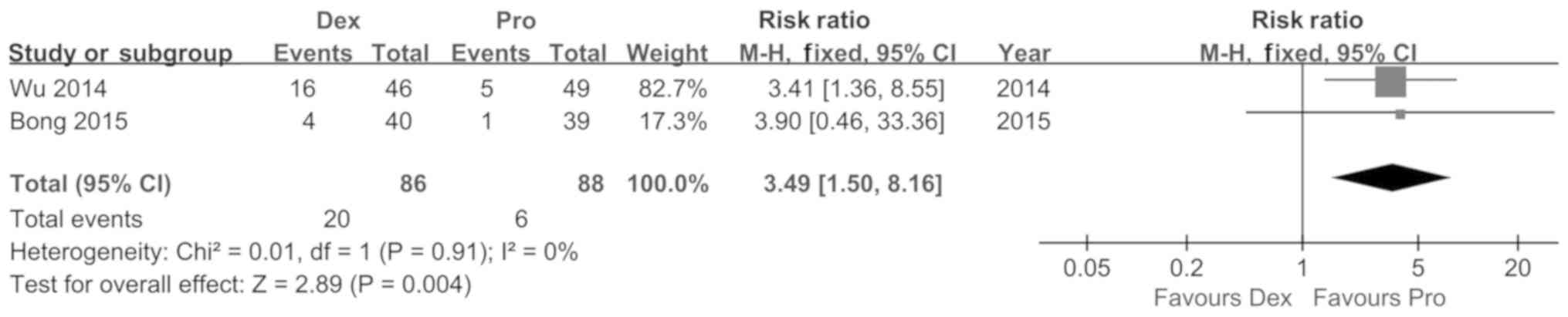
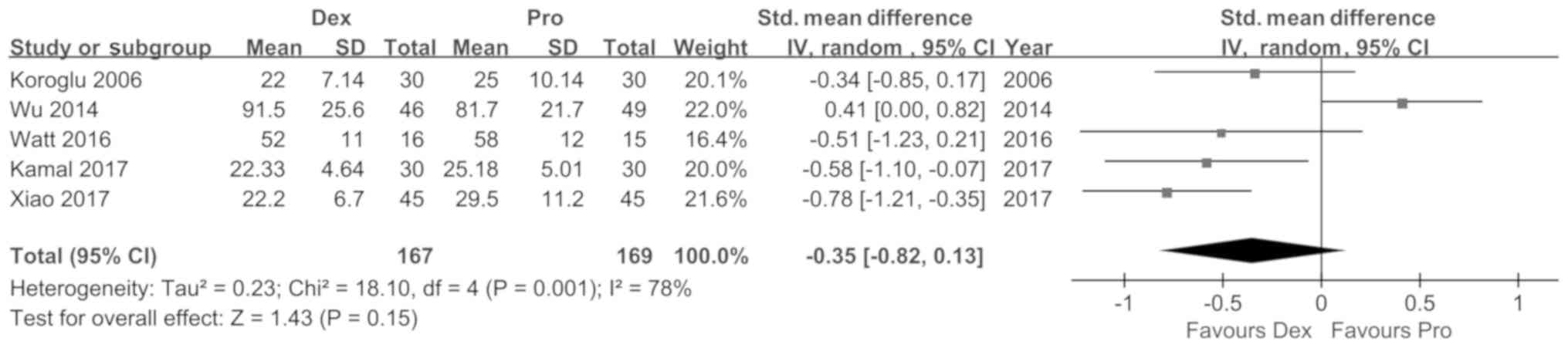
Koroglu A, Demirbilek S, Teksan H, Sagir
O, But AK and Ersoy MO: Sedative, haemodynamic and respiratory
effects of dexmedetomidine in children undergoing magnetic
resonance imaging examination: Preliminary results. Br J Anaesth.
94:821–824. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
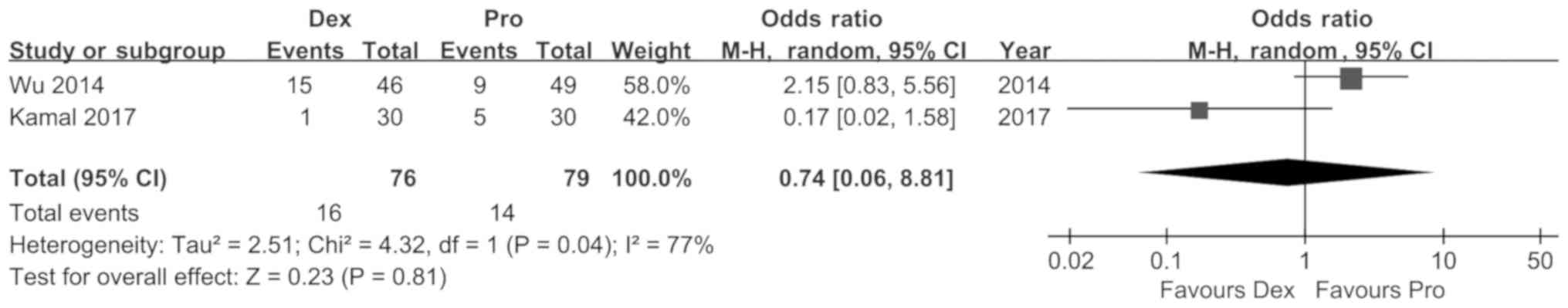
Wu J, Mahmoud M, Schmitt M, Hossain M and
Kurth D: Comparison of propofol and dexmedetomedine techniques in
children undergoing magnetic resonance imaging. Paediatr Anaesth.
24:813–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Q, Shen L, Zhang X, Li J and Tang Y:
Dexmedetomidine versus propofol on the sedation of pediatric
patients during magnetic resonance imaging (MRI) scanning: A
meta-analysis of current studies. Oncotarget. 8:102468–102473.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watt S, Sabouri S, Hegazy R, Gupta P and
Heard C: Does dexmedetomidine cause less airway collapse than
propofol when used for deep sedation? J Clin Anesth. 35:259–267.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiao Y, He P, Jing G, Wang Q and Wen J:
Comparison of sedative effect of dexmedetomide injection and
propofol injection in pediatric patients undergoing magnetic
resonance imaging. Zhongguo Lin Chuang Yao Li Xue Za Zhi.
33:1764–1767. 2017.(In Chinese).
|
|
14
|
Knobloch K, Yoon U and Vogt PM: Preferred
reporting items for systematic reviews and meta-analyses (PRISMA)
statement and publication bias. J Craniomaxillofac Surg. 39:91–92.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ringblom J, Wahlin I and Proczkowska M: A
psychometric evaluation of the Pediatric Anesthesia Emergence
Delirium scale. Paediatr Anaesth. 28:332–337. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bong CL, Lim E, Allen JC, Choo WL, Siow
YN, Teo PB and Tan JS: A comparison of single-dose dexmedetomidine
or propofol on the incidence of emergence delirium in children
undergoing general anaesthesia for magnetic resonance imaging.
Anaesthesia. 70:393–399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamal K, Asthana U, Bansal T, Dureja J,
Ahlawat G and Kapoor S: Evaluation of efficacy of dexmedetomidine
versus propofol for sedation in children undergoing magnetic
resonance imaging. Saudi J Anaesth. 11:163–168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang H, Yang L, Wang X and Zhu H: Clinical
efficacy of dexmedetomidine versus propofol in children undergoing
magnetic resonance imaging: A meta-analysis. Int J Clin Exp Med.
8:11881–11889. 2015.PubMed/NCBI
|
|
20
|
Chandler JR, Myers D, Mehta D, Whyte E,
Groberman MK, Montgomery CJ and Ansermino JM: Emergence delirium in
children: A randomized trial to compare total intravenous
anesthesia with propofol and remifentanil to inhalational
sevoflurane anesthesia. Paediatr Anaesth. 23:309–315. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Isik B, Arslan M, Tunga AD and Kurtipek O:
Dexmedetomidine decreases emergence agitation in pediatric patients
after sevoflurane anesthesia without surgery. Paediatr Anaesth.
16:748–753. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
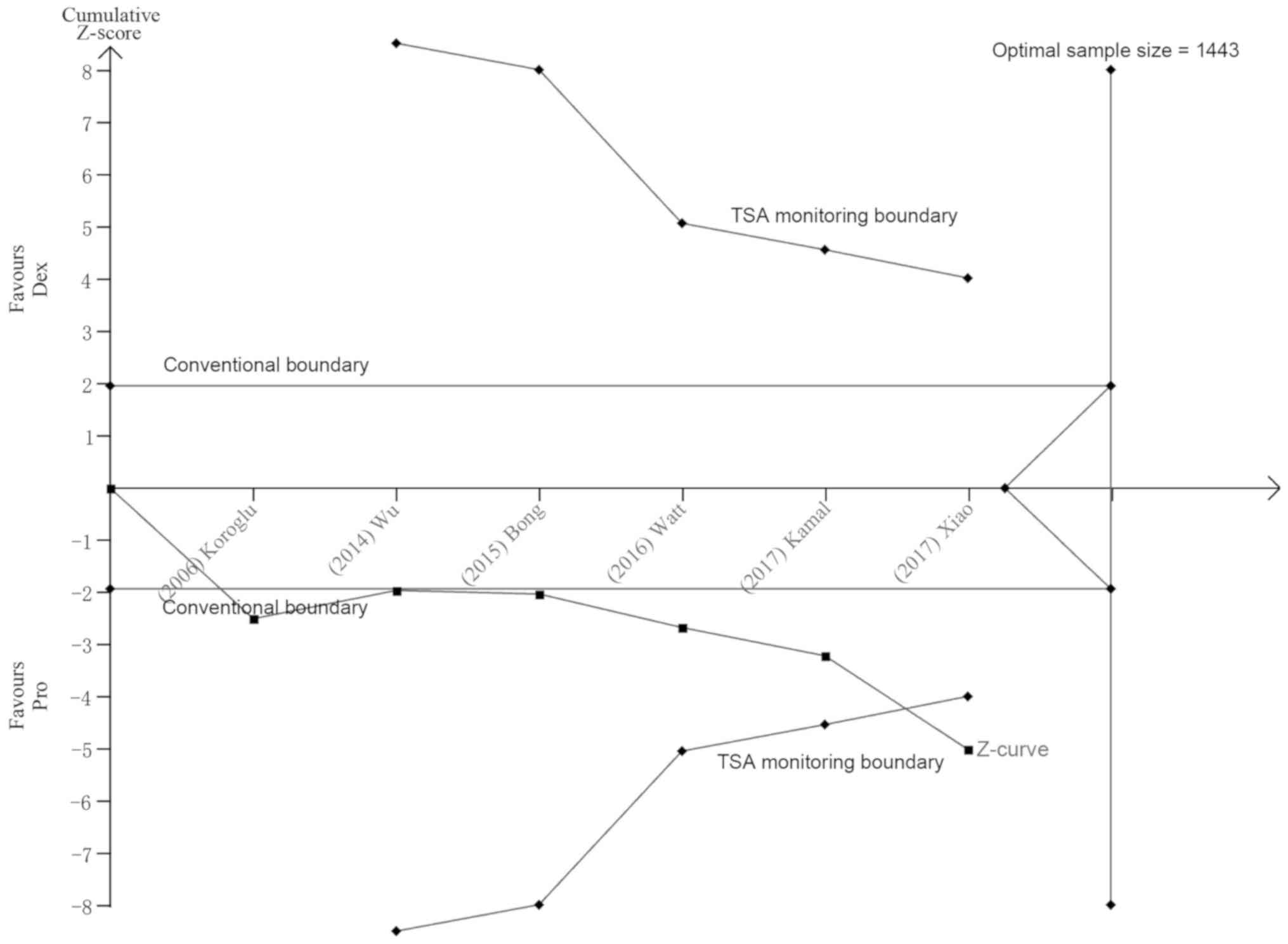
Wetterslev J, Jakobsen JC and Gluud C:
Trial sequential analysis in systematic reviews with meta-analysis.
BMC Med Res Methodol. 17:392017. View Article : Google Scholar : PubMed/NCBI
|