Introduction
Tumor growth serves pivotal roles in cancer
development and progression. Chemical drugs that inhibit tumor cell
growth are used in the treatment of cancer (1). Laryngeal squamous cell carcinoma (LSCC)
is a rare malignancy, accounting for ~2% of all cancer cases
(2). However, LSCC is considered to
be a major cause of cancer-associated mortality due to its
aggressive nature and rapid development (3). In spite of the efforts made to prevent
and treat LSCC, the survival rate of patients with this disease
remains poor (4). Therefore, novel
therapeutic approaches with higher efficacies are required to
improve treatment outcomes in patients with LSCC. It has been
reported that tumor size is associated with the prognosis of
patients with LSCC (5). Therefore,
the identification of factors that can regulate tumor growth in
patients with LSCC may provide guidance for the treatment of
LSCC.
Hexokinase 2 (HK-2) is an enzyme in the human body
that can phosphorylate glucose to produce glucose-6-phosphate,
which is typically the initial step in glucose metabolism (6). The overexpression of HK-2 is frequently
observed in the progression of different types of cancer, and the
upregulated expression of HK-2 in certain types of cancer
accelerates cell growth and promotes cancer progression (7–9). Thus,
the inhibition of HK-2 expression is considered to be a promising
therapeutic target for cancer treatment (10). HK-2 achieves its biological roles
through its interactions with different cellular factors, including
long (>200 nucleotides) non-coding RNAs (lncRNAs), which are a
heterogeneous group of RNA transcripts with pivotal roles in human
diseases due to their regulatory roles in gene expression (11). LncRNA loc285194 has been reported to
have tumor suppressive functions in certain types of malignancies
(12), while its involvement in LSCC
remains unclear. Our preliminary data of microarray revealed an
association between HK-2 and loc285194 (data not shown). The
present study aimed to assess the involvement of lncRNA loc285194
in the pathogenesis of LSCC via its interaction with HK-2.
Materials and methods
Subjects and specimens
The current study included 66 patients with LSCC,
who were diagnosed at the Affiliated Second Hospital Xinjiang
Medical University between January 2015 and March 2017. The
inclusion criteria were as follows: i) Patients diagnosed through
laryngeal biopsies; ii) patients received treatment for the first
time; iii) patients willing to join the study. The exclusion
criteria were as follows: i) Patients complicated with other
malignancies (102 patients were excluded); ii) patients who were
treated prior to admission (12 cases were excluded); iii) patients
with chronic diseases (45 patients were excluded); iv) blood
relatives (2 patients were excluded). Among those patients, 10
cases were in American Joint Committee on Cancer stage II, 12 in
stage III, 9 in stage IVA, 12 in stage IVB and 23 in stage IVC. The
patients included 36 males and 30 females with ages ranging between
24 and 66 years, and a mean age of 44.5±6.6 years. During the same
time period, 92 people received laryngeal biopsies to detect
suspected laryngeal lesions and laryngeal lesions were excluded in
39 cases. Among these 39 cases, laryngeal biopsies were obtained
from 28 cases to match the age and sex distributions of patient
group and to serve as control group. The present study was approved
by the Ethics Committee of The Affiliated Second Hospital Xinjiang
Medical University. All participants signed informed consent.
Laryngeal biopsies were obtained from the specimen library of the
Affiliated Second Hospital Xinjiang Medical University. All
specimens were stored in liquid nitrogen (−195.79°C) prior to
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used to extract total RNA.
All operations were performed in accordance with manufacturer's
protocol. cDNA was synthesized through reverse transcription using
SuperScript III Reverse Transcriptase (Thermo Fisher Scientific,
Inc.) under the following conditions: 50°C for 30 min and 75°C for
15 min. qPCR was performed using SYBR® Green Real-Time
PCR Master Mix (Thermo Fisher Scientific, Inc.) with following
primers: lncRNA loc285194 forward, 5′-TGTGCCTGTTTGACCTCTGA-3′ and
reverse, 5′-AGGAAGGATAAAAGACCGACCA-3′; HK-2 mRNA forward,
5′-AAGGCTTCAAGGCATCTG-3′ and reverse, 5′-CCACAGGTCATCATAGTTCC-3′;
β-actin forward, 5′-GACCTCTATGCCAACACAGT-3′ and reverse,
5′-AGTACTTGCGCTCAGGAGGA-3′. The qPCR reaction conditions were as
follows: 50 sec at 95°C, followed by 40 cycles of 20 sec at 95°C
and 40 sec at 59°C. The 2−ΔΔCq method was used for data
normalization (13).
Cell lines, cell culture and cell
transfection
Human LSCC cell line UM-SCC-17A (EMD Millipore,
Billerica, MA, USA) and normal larynx cell line HuLa-PC (American
Type Culture Collection, Manassas, VA, USA) were used in this
study. Cells of each cell line were cultured with DMEM containing
10% fetal bovine serum (FBS; Sangon Biotech Co., Ltd. Shanghai,
China) at 37°C with 5% CO2. Full-length loc285194 and
HK-2 cDNAs were amplified through PCR using primers with
NheI cutting site at the 5′ end. Loc285194 and HK-2 cDNAs
were inserted into pIRSE2 vector (Clontech Laboratories, Inc.,
Mountainview, CA, USA) to construct loc285194 and HK-2 expression
vectors. Loc285194 and HK-2 expression vectors were transfected
into 5×105 cells at a dose of 10 nM using Lipofectamine
2000 reagent (Thermo Fisher Scientific, Inc.). Cells without
transfection were used as control cells. Cells transfected with
empty vector were used as negative control cells. The expression of
loc285194 and HK-2 was confirmed by RT-qPCR 12 h after
transfection. Subsequent experiments were performed only in cases
where an overexpression rate of >200% compared with control
cells and negative control cells was reached.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was detected using a CCK-8 assay.
Briefly, cell suspensions (1% FBS) with a cell density of
5×104 cells/ml were prepared. Subsequently,
5×103 cells in 0.1 ml cell suspension were added into
each well of a 96-well plate. Cells were cultured at 37°C in a 5%
CO2 incubator, followed by addition of 10 µl CCK-8
solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) 24, 48, 72
and 96 h later. Cells were cultured for additional 4 h and
Fisherbrand™ accuSkan™ GO UV/Vis microplate spectrophotometer
(Thermo Fisher Scientific, Inc.) was used to measure the optical
density values at 450 nM.
Western blot analysis
RIPA solution (Thermo Fisher Scientific, Inc.) was
mixed with cultured cells to extract total protein. Subsequently, a
bicinchoninic acid assay was performed to measure protein
concentration. Protein samples were denatured and subjected to 10%
SDS-PAGE gel electrophoresis with 45 µg protein/lane. Subsequently,
PVDF membranes were blocked in 5% skimmed milk at room temperature
for 1 h, followed by incubation with rabbit anti-human primary
antibodies against HK-2 (1:1,200; cat. no. ab37593; Abcam,
Cambridge, UK) and GAPDH (1:2,000; cat. no. ab8245; Abcam) at 4°C
overnight. The following day, a goat anti-rabbit IgG-horseradish
peroxidase secondary antibody (1:1,000; cat. no. MBS435036;
MyBioSource, San Diego, CA, USA) was used to further incubate with
membranes at 25°C for 2 h. Then, ECL (Sigma-Aldrich, Merck KGaA)
was added to develop the signals. Signals were normalized using
ImageJ v1.46 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Data analyses were performed using GraphPad Prism 6
software (GraphPad Software, Inc., La Jolla, CA, USA). Gene
expression and cell proliferation data are presented as the mean ±
standard deviation. One-way analysis of variance followed by the
least significant difference test among multiple groups or unpaired
t-test between two groups was used. Associations between loc285194
expression and clinicopathological data of LSCC patients were
analyzed using the Chi-square test. Correlations between expression
of loc285194 and HK-2 mRNA were analyzed using the Pearson
correlation coefficient. The diagnostic value of loc285194 and HK-2
mRNA for LSCC was evaluated using receiver operating characteristic
curve analysis (ROC). Origin Pro 8.0 (OriginLab, Northampton, MA,
USA) was used to calculate statistical power. Statistical power
>90% was reached in all cases. All data provided sufficient
statistical power. P<0.05 was considered to indicate a
statistically significant difference.
Results
Loc285194 is downregulated and HK-2
mRNA is upregulated in patients with LSCC compared with healthy
controls
The expression of loc285194 and HK-2 mRNA in
laryngeal biopsies of patients with LSCC or healthy controls was
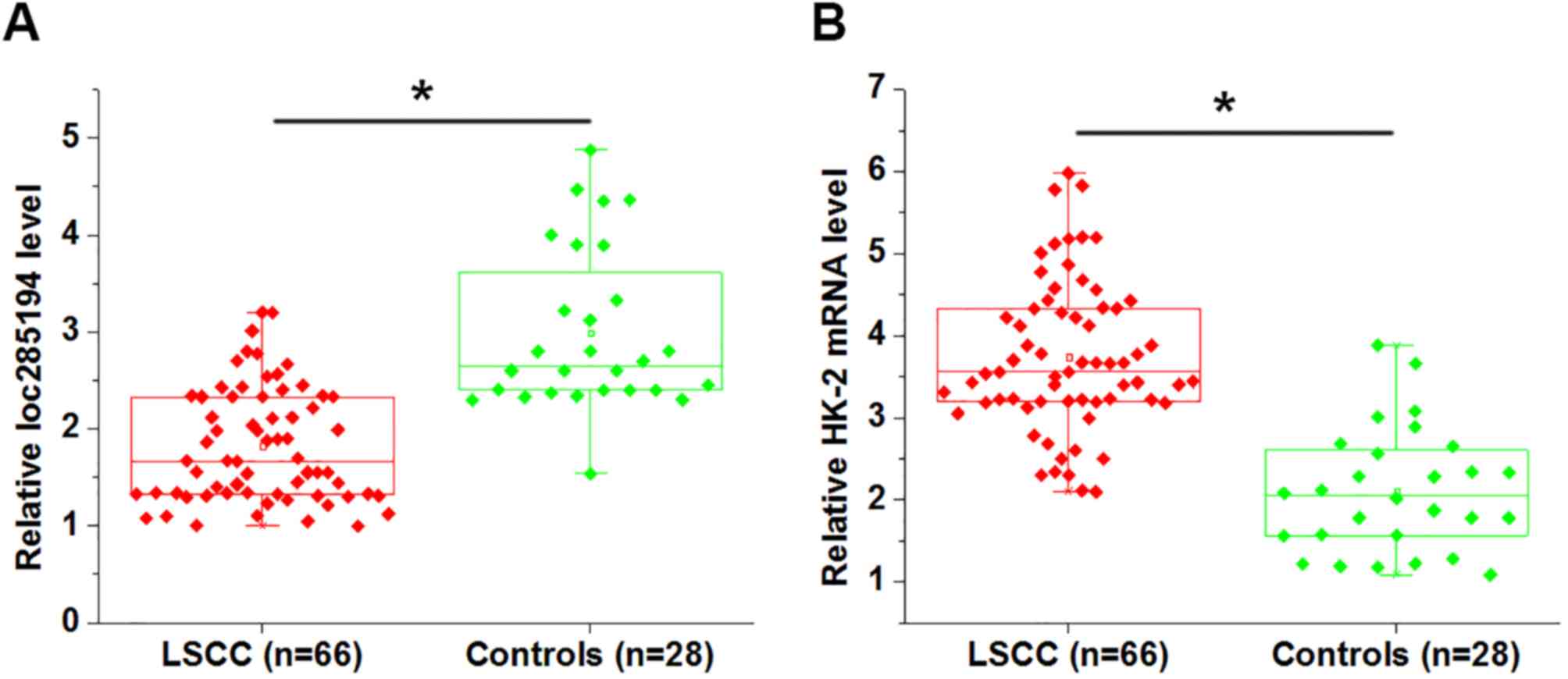
detected using RT-qPCR. As shown in Fig.
1A, loc285194 expression was significantly downregulated in
patients with LSCC compared with that in healthy controls
(P<0.05). In contrast, HK-2 mRNA was significantly upregulated
in LSCC patients compared with that in healthy controls (Fig. 1B; P<0.05).
Loc285194 and HK-2 mRNA are potential
diagnostic biomarkers for LSCC
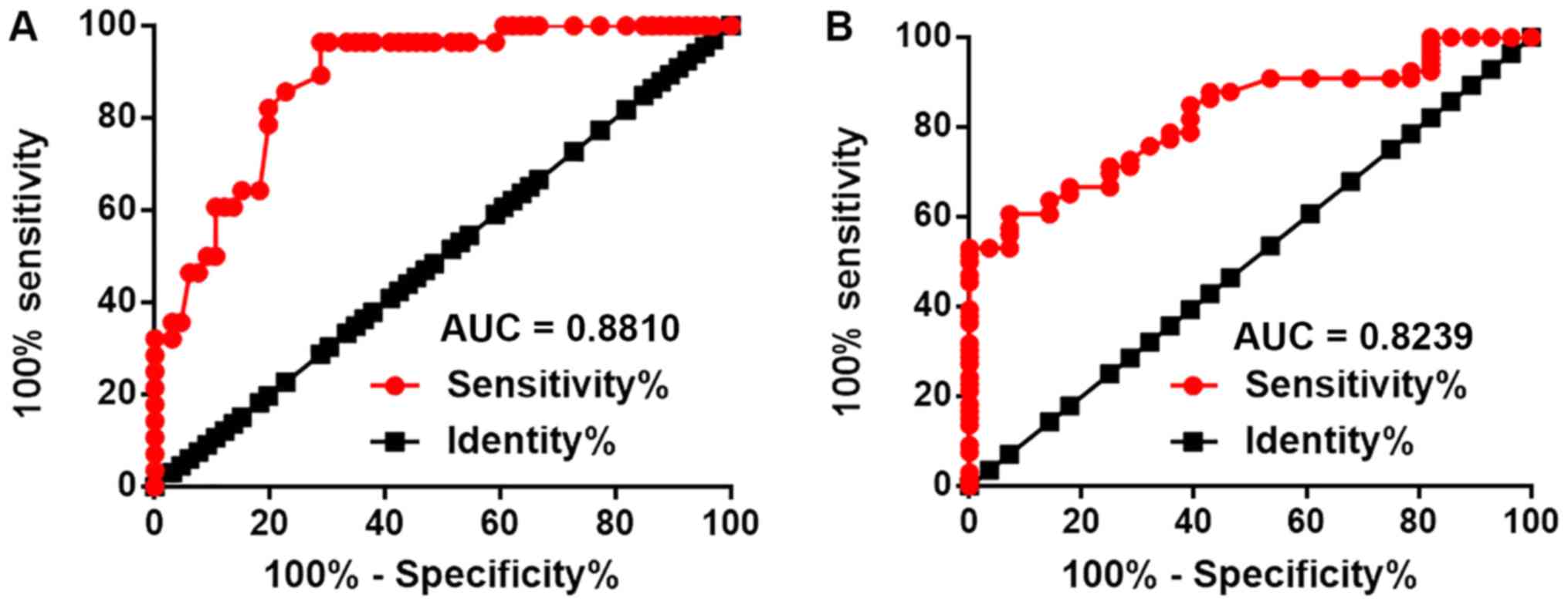
ROC curve analysis was performed to evaluate the
diagnostic values of loc285194 and HK-2 mRNA for LSCC (Fig. 2). For loc285194, the area under the
curve (AUC) was 0.8810 [standard error (SE), 0.03499; 95%
confidence interval (CI), 0.8124–0.9405; P<0.0001]. For HK-2
mRNA, the AUC was 0.8239 (SE, 0.04211; 95% CI, 0.7413–0.9064;
P<0.0001).
loc285194 and HK-2 mRNA expression
levels are inversely correlated in patients with LSCC, but not in
healthy controls
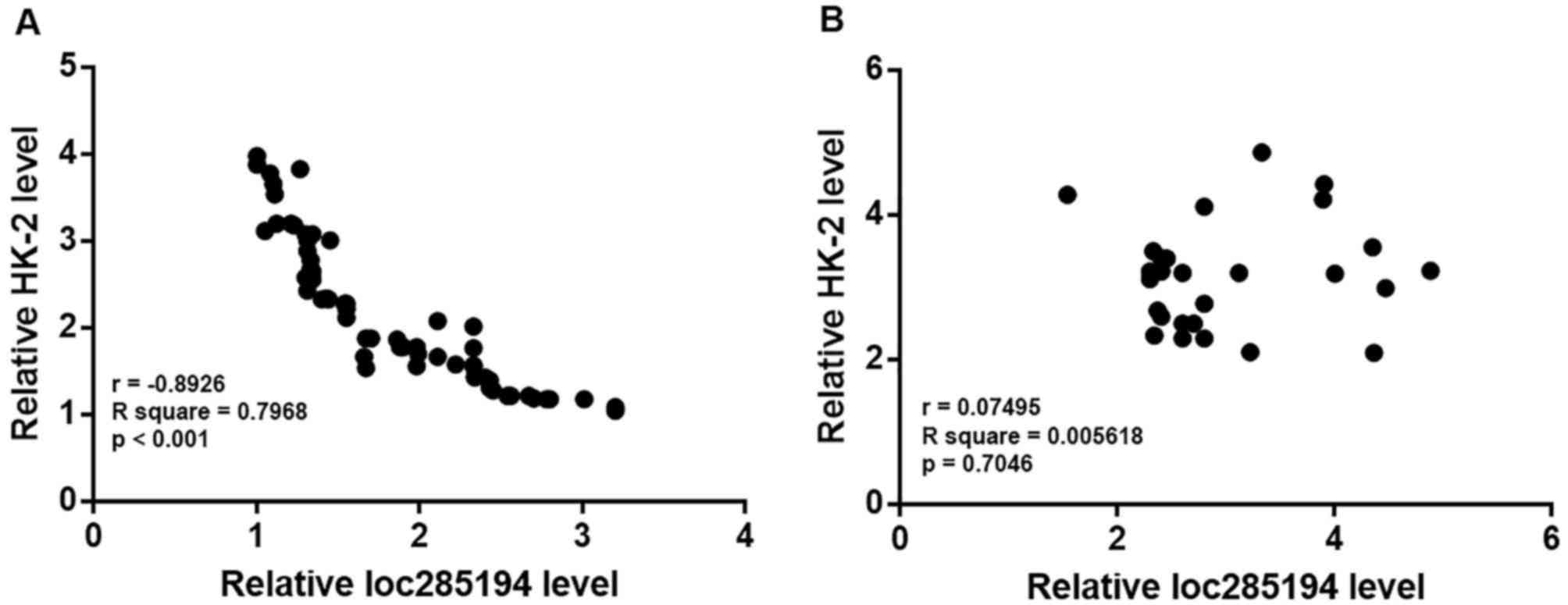
The correlation between the expression levels of
loc285194 and HK-2 mRNA was analyzed using the Pearson correlation
coefficient. A significantly inverse correlation was identified
between the expression levels of loc285194 and HK-2 mRNA in
patients with LSCC (Fig. 3A). In
contrast, no significant correlation between loc285194 and HK-2
mRNA expression levels was observed in healthy controls (Fig. 3B).
Expression of loc285194 is
significantly associated with tumor size, but not distant tumor
metastasis
Patients were divided into high and low expression
groups according to the median expression level of loc285194
(1.72). The association between loc285194 and clinicopathological
data of patients with LSCC was analyzed using the Chi-square test.
As shown in Table I, loc285194
expression was not significantly associated with the age, sex,
smoking and drinking habits of patients as well as the presence of
tumor metastasis. In contrast, a significant association was
observed between loc285194 expression and primary tumor size.
 | Table I.Associations between loc285194
expression and clinicopathological data of LSCC patients. |
Table I.
Associations between loc285194
expression and clinicopathological data of LSCC patients.
|
|
| Expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Cases | High | Low | Chi-square | P-value |
|---|
| Sex |
| Male | 36 | 16 | 20 | 0.98 | 0.32 |
|
Female | 30 | 17 | 13 |
|
|
| Age, years |
|
>45 | 32 | 14 | 18 | 0.97 | 0.32 |
| ≤45 | 34 | 19 | 15 |
|
|
| Smoking |
| Yes | 28 | 12 | 16 | 0.99 | 0.32 |
| No | 38 | 21 | 17 |
|
|
| Drinking |
| Yes | 33 | 15 | 18 | 0.55 | 0.46 |
| No | 33 | 18 | 15 |
|
|
| Tumor size, cm |
| ≥6 | 19 | 5 | 14 | 6.02 | 0.049 |
| 3–6 | 23 | 14 | 9 |
|
|
| ≤3 | 24 | 14 | 10 |
|
|
| Distant
metastasis |
| Yes | 43 | 19 | 24 | 1.67 | 0.196 |
| No | 23 | 14 | 9 |
|
|
Loc285194 is an upstream activator of
HK-2 in LSCC cells
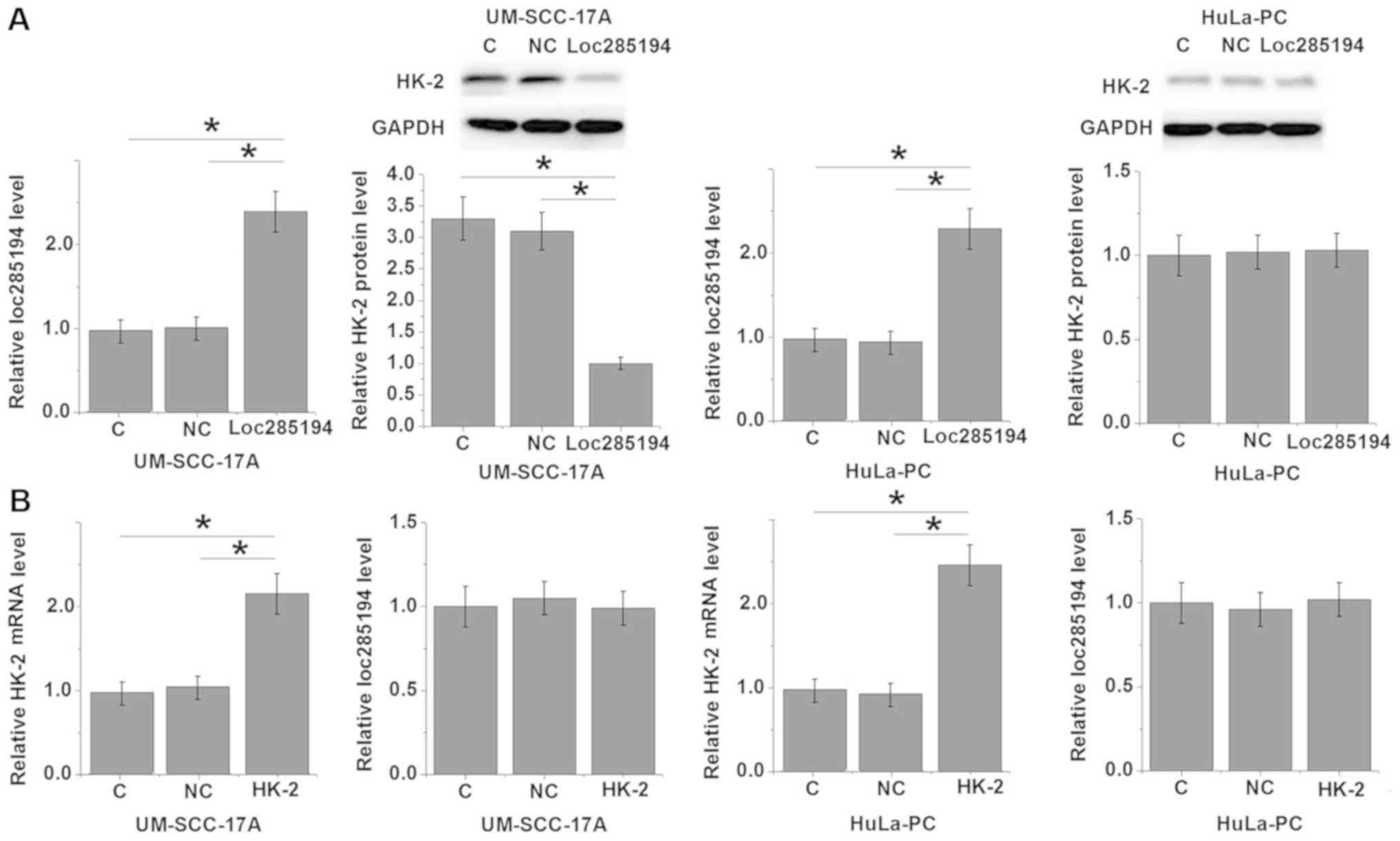
Cells with transient loc285194 and HK-2 were
produced, and the expression of loc285194 and HK-2 was detected by
RT-qPCR and western blot analysis. As shown in Fig. 4A, compared with the control cells and
negative control cells, loc285194 overexpression significantly
inhibited the expression of HK-2 in the human LSCC UM-SCC-17A cell
line, but not in the normal HuLa-PC larynx cell line (P<0.05).
In contrast, HK-2 overexpression demonstrated no significant
effects on loc285194 expression in the UM-SCC-17A and HuLa-PC cell
lines (Fig. 4B).
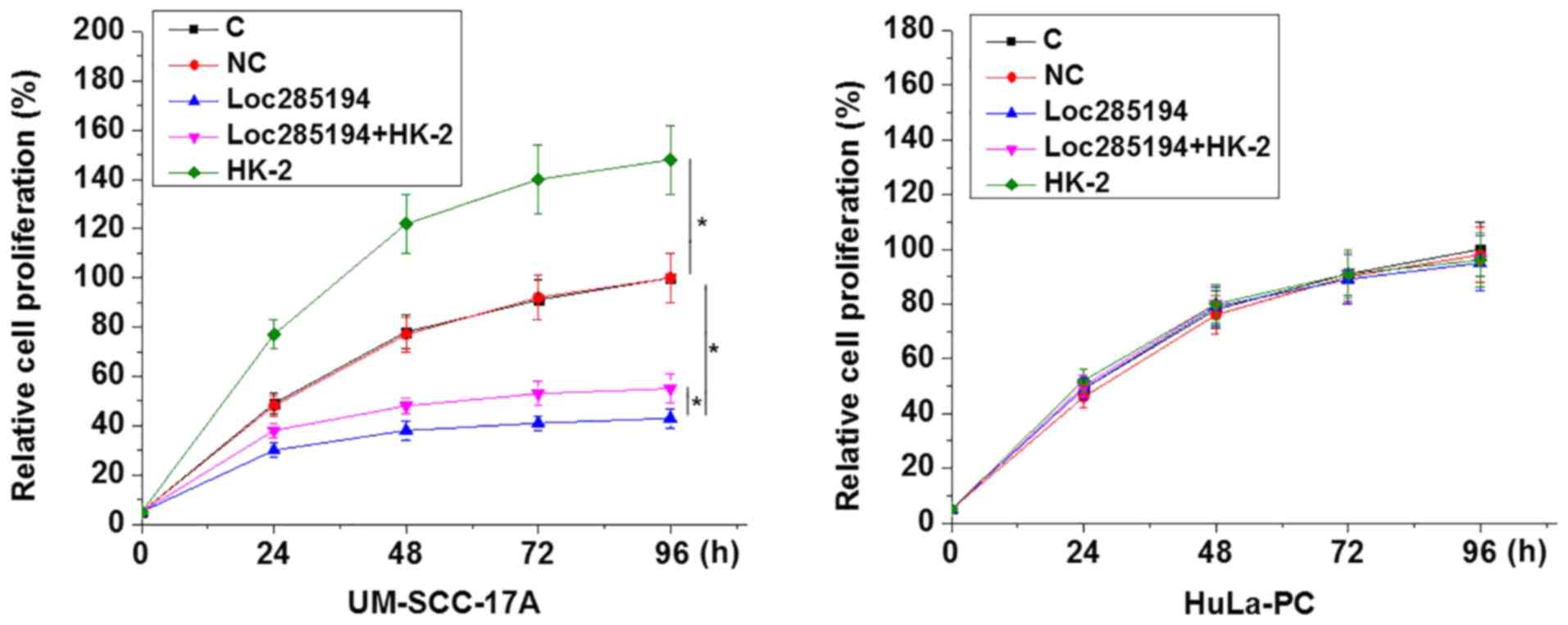
Loc285194 overexpression inhibits HK-2
expression and LSCC cell proliferation
A CCK-8 assay was performed to detect cell
proliferation following loc285194 and HK-2 overexpression. As shown
in Fig. 5, loc285194 overexpression
led to significantly inhibited HK-2 expression and LSCC cell
proliferation compared with the control and negative control groups
(P<0.05). In addition, HK-2 overexpression attenuated the
inhibitory effects of loc285194 overexpression on LSCC cell
proliferation (P<0.05). Loc285194 and HK-2 overexpression showed
no significant effects on the proliferation of normal HuLa-PC
cells.
Discussion
One of the key findings of the present study
demonstrated that loc285194, a well-studied tumor suppressor lncRNA
in several types of human malignancies, may also participate in the
regulation of tumor growth, but not tumor metastasis in patients
with LSCC. The action of loc285194 in LSCC is likely to be achieved
though interactions with HK-2.
LncRNA loc285194 is a tumor suppressor gene that is
downregulated in several types of cancer, including esophageal
squamous cell carcinoma (14),
pancreatic ductal adenocarcinoma (15) and colorectal cancer (16). In the present study, significantly
downregulated expression of lncRNA loc285194 was observed in
patients with LSCC comparing with healthy controls. The
downregulation of lncRNA loc285194 effectively distinguished
patients with LSCC from healthy controls, indicating the potential
role of lncRNA loc285194 as a tumor suppressor in LSCC.
Accelerated glucose metabolism distinguishes cancer
cells from normal cells (17). HK-2
is a key player in glycolysis and is usually upregulated in the
development of cancer (18). In a
study on LSCC, Chen et al (9)
reported that HK-2 is overexpressed in patients with LSCC and the
overexpression of HK-2 promotes tumor growth. Consistent with this
study, the results of the current study reported that HK-2 was
significantly upregulated in patients with LSCC compared with in
healthy controls and HK-2 overexpression significantly promoted the
proliferation of LSCC cells cultured in vitro. In addition,
the overexpression of HK-2 effectively distinguished patients with
LSCC from healthy controls.
In the present study, lncRNA loc285194 expression
was demonstrated to be significantly associated with tumor size,
but not the existing of tumor metastasis, indicating the
involvement of lncRNA loc285194 in the regulation of tumor growth.
It has been reported that the expression of HK-2 can be regulated
by certain lncRNAs (11,19). The results of the current study
suggest that lncRNA loc285194 serves as an upstream inhibitor of
HK-2 in LSCC. This conclusion may be made based on following
observations: i) lncRNA loc285194 overexpression led to a
significantly upregulation of HK-2 expression in LSCC cells; ii)
HK-2 overexpression showed no significant effects on lncRNA
loc285194 expression; iii) the overexpression of HK-2 significantly
attenuated the inhibitory effects of lncRNA loc285194 on LSCC cell
proliferation. However, the regulatory role of lncRNA loc285194 on
HK-2 is unlikely to be direct due to the lack of significant
correlation between lncRNA loc285194 on HK-2 in healthy controls
and the absence of inhibitory effects of loc285194 expression on
HK-2 expression.
Notably, lncRNA loc285194 overexpression
demonstrated no significant effects on biological behaviors of
normal larynx cells. Therefore, lncRNA loc285194 may serve as a
potential therapeutic target for LSCC. However, a previous study
has reported that smaller LSCC tumor size is more significantly
correlated with an unfavorable prognosis of patients with LSCC
(5). Therefore, the correlation
between lncRNA loc285194 expression and the prognosis of LSCC
patients remains to be further investigated. Overexpression of
lncRNA loc285194 also failed to affect the expression of other
players in glucose metabolism, such as glucose transporter protein
type 1–3 (data not shown). Therefore, lncRNA loc285194 may
specifically regulate HK-2. The present study failed to perform
HK-2 and loc285194 knockdown assays due to the unsatisfactory
knockdown rate. Future studies intend to perform knockdown
assays.
In conclusion, the results of the current study
demonstrated that lncRNA loc285194 is significantly downregulated
in patients with LSCC. LncRNA loc285194 may participate in the
regulation of tumor growth in LSCC through the inhibition of HK-2
expression.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ guaranteed the integrity of the entire study. YZ,
YG and ZW provided the study concepts. YG and ZW were responsible
for the study design, the definition of intellectual content, and
for literature research. YG, ZW and JT were responsible for
clinical and experimental studies. JT was responsible for data
acquisition. YG and ZW were responsible for data analysis,
statistical analysis, manuscript preparation, manuscript editing
and manuscript review.
Ethics approval and consent to
participate
This study got approval from the ethics committee of
the Affiliated Second Hospital Xinjiang Medical University. All
participants signed informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sulciner ML, Serhan CN, Gilligan MM, Mudge
DK, Chang J, Gartung A, Lehner KA, Bielenberg DR, Schmidt B, Dalli
J, et al: Resolvins suppress tumor growth and enhance cancer
therapy. J Exp Med. 215:115–140. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ragin CC and Taioli E: Survival of
squamous cell carcinoma of the head and neck in relation to human
papillomavirus infection: Review and meta-analysis. Int J Cancer.
121:1813–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang SY, Lu ZM, Luo XN, Chen LS, Ge PJ,
Song XH, Chen SH and Wu YL: Retrospective analysis of prognostic
factors in 205 patients with laryngeal squamous cell carcinoma who
underwent surgical treatment. PLoS One. 8:e601572013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Markou K, Goudakos J, Triaridis S,
Konstantinidis J, Vital V and Nikolaou A: The role of tumor size
and patient's age as prognostic factors in laryngeal cancer.
Hippokratia. 15:75–80. 2011.PubMed/NCBI
|
|
6
|
Moreno F, Vega M and Herrero P: The
nuclear Hexokinase 2 acts as a glucose sensor in Saccharomyces
cerevisiae. J Biol Chem. 291:164782016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson M, Marayati R, Moffitt R and Yeh
JJ: Hexokinase 2 promotes tumor growth and metastasis by regulating
lactate production in pancreatic cancer. Oncotarget. 8:56081–56094.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Xiong H, Wu F, Zhang Y, Wang J,
Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, et al: Hexokinase
2-mediated Warburg effect is required for PTEN-and
p53-deficiency-driven prostate cancer growth. Cell Rep.
8:1461–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Zhang S, Li Y, Tang Z and Kong W:
Hexokinase 2 overexpression promotes the proliferation and survival
of laryngeal squamous cell carcinoma. Tumour Biol. 35:3743–3753.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
‘Warburg Effect’ and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Li X, Wu S, Xue M and Chen W: Long
non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase
2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci.
105:951–955. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Q, Huang J, Zhou N, Zhang Z, Zhang A,
Lu Z, Wu F and Mo YY: LncRNA loc285194 is a p53-regulated tumor
suppressor. Nucleic Acids Res. 41:4976–4987. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong YS, Zhou XL, Wang XW, Wu QQ, Yang TX,
Lv J, Yang JS, Zhu B and Cao XF: Association of decreased
expression of long non-coding RNA LOC285194 with chemoradiotherapy
resistance and poor prognosis in esophageal squamous cell
carcinoma. J Transl Med. 12:2332014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ding YC, Yu W, Ma C, Wang Q, Huang CS and
Huang T: Expression of long non-coding RNA LOC285194 and its
prognostic significance in human pancreatic ductal adenocarcinoma.
Int J Clin Exp Pathol. 7:8065–8070. 2014.PubMed/NCBI
|
|
16
|
Qi P, Xu MD, Ni SJ, Huang D, Wei P, Tan C,
Zhou XY and Du X: Low expression of LOC285194 is associated with
poor prognosis in colorectal cancer. J Transl Med. 11:1222013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shaw RJ: Glucose metabolism and cancer.
Curr Opin Cell Biol. 18:598–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolf A, Agnihotri S, Micallef J, Mukherjee
J, Sabha N, Cairns R, Hawkins C and Guha A: Hexokinase 2 is a key
mediator of aerobic glycolysis and promotes tumor growth in human
glioblastoma multiforme. J Exp Med. 208:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Liu Y and Xu X: Knockdown of
LncRNA-UCA1 suppresses chemoresistance of pediatric AML by
inhibiting glycolysis through the microRNA-125a/hexokinase 2
pathway. J Cell Biochem. 119:6296–6308. 2018. View Article : Google Scholar : PubMed/NCBI
|