Introduction
Sepsis is a major cause of mortality and critical
illness in intensive care units and is defined as a
life-threatening organ dysfunction caused by a dysregulated host
response against infection that triggers systemic inflammatory
response syndrome (1,2). Among the organ failures involved in
sepsis, hepatic dysfunction is a prominent complication that
contributes to an increased risk of development of multiple organ
failure and is a strong independent predictor of mortality
(3,4).
Clinically, the survival outcome of patients with
sepsis remains unimproved although several clinical trials employ
novel agents to modulate the hyperreactive inflammatory response,
and a wide spectrum of effective antibiotics are available against
sepsis (5,6). Previous studies have demonstrated a
close interaction between cecal ligation and puncture (CLP)-induced
septic organ failure and cell dysfunction due to autophagy
(7–9). Autophagy is a well-conserved
intracellular lysosomal degradation process in which specific
enzymes degrade the damaged or abnormal cellular components and
recycle the redundant or inefficient components in cells (3,10).
Autophagy exhibits an inducible response towards stress, including
tissue injury, oxidative stress, infection and protein aggregation
(3). Several studies have indicated
that autophagy is activated after performing CLP in animal models
of sepsis (7,11). Furthermore, multiple studies revealed
that autophagy serves an important role in liver physiology and
pathology following CLP (12,13).
Various clinical experiments have suggested that novel strategies
that focus on cell survival, in which an important role is served
by autophagy, may improve the survival outcome in patients with
septic shock (14,15). Therefore, further investigations are
required to develop novel therapeutic interventions against
sepsis.
Several studies investigated herbal medicines with
an increasing interest in pre-clinical and clinical studies
(16–18). Multiple studies support the high
efficiency of medicinal plants in the prevention and cure of a wide
range of diseases (16,19). Thymoquinone
(2-isopropyl-5-methylbenzo-1,4-quinone; TQ), an agent extracted
from the Nigella sativa (black cumin) plant, is used in
Middle and Far Eastern countries as a traditional medicine to treat
a wide range of pathological conditions (20). According to traditional medicine, TQ
exhibits various types of therapeutic properties, including
antioxidative, anti-inflammatory, immunomodulatory and
antimicrobial activities (21).
However, the effect of TQ on CLP-induced septic liver injury
remains unclear. Therefore, in the present study, a murine model
that simulates human peritonitis was established by performing CLP
in order to understand the effect of TQ on CLP-induced septic liver
injury.
Materials and methods
Maintenance of animal models
All animal experiments were approved by the Animal
Studies Committee of the Affiliated Zhongshan Hospital of Dalian
University. Male BALB/c mice (n=48; weight, 18.8±1.3 g; age, 8
weeks) were purchased from Beijing Vital River Lab Animal
Technology Co., Ltd. All mice were housed under constant
conditions, including 23–25°C temperature, 40–60% humidity and a
12-h light/dark cycle with ad libitum access to food and
water.
Development of a murine model of
sepsis
To induce polymicrobial sepsis, a murine model was
established by performing CLP according to a previously described
method (22). The mice were
anesthetized with sodium pentobarbital (50 mg/kg; intraperitoneal
injection). After the peritoneum was surgically opened and the
bowel was exposed, a section (two-thirds) of the cecum was tied and
punctured once with a 21-gauge needle. A gentle pressure was
applied at the perforation site to extrude a small amount of feces,
which were placed in the peritoneal cavity. Finally, the laparotomy
site was stitched. Sham-operated (control and TQ group) mice
underwent the same procedure that involved surgical opening of the
peritoneum and exposing the bowel; however, the ligation and needle
perforation of cecum were not performed. Male BALB/c mice (n=48;
age, 8 weeks) were randomly divided into four groups (n=12/group),
namely, the control, TQ (50 mg/kg/day; Sigma-Aldrich; Merck KGaA),
CLP and TQ + CLP groups. In the TQ + CLP group, CLP was performed
following gavage of TQ for 2 weeks. Mice exhibiting signs of humane
endpoints were immediately sacrificed. Before the sacrifice of
animals at 48 h post-CLP, the mortality rates of CLP group and TQ +
CLP group were 16.67 and 8.33% respectively, and no mice died in
the control and TQ groups. At 48 h post-CLP, all mice that survived
were sacrificed, and blood samples were obtained from their
inferior vena cava, collected in serum tubes and stored at −80°C
until further use. The coronal sections of liver tissues (LTs) were
fixed in 10% formalin for 30 min at room temperature, dehydrated in
75% ethanol overnight then embedded in paraffin for histological
evaluation. The remnants of LTs were snap-frozen (−80°C) and used
to perform mRNA or immunoblotting analyses. All animal experiments
were performed according to the Guide for the Care and Use of
Laboratory Animals (23). The
present study was approved by the Animal Studies Committee of the
Affiliated Zhongshan Hospital of Dalian University.
Serum analysis
The concentrations of alanine aminotransferase (ALT;
cat. no. G0050), aspartate aminotransferase (AST; cat. no. G0073)
and alkaline phosphatase (ALP; cat. no. G0040) in the serum were
estimated using ELISA kits according to the manufacturer's
protocols (Shanghai Westang Bio-Tech Co., Ltd).
Hematoxylin and eosin (H&E)
staining
Sections (4-µm thickness) were serially cut to
perform the morphometric analysis of LTs. These sections were
stained with hematoxylin for 15 min and eosin for 5 min at room
temperature to perform histological analysis.
Morphological and IHC analyses
IHC analysis was performed using a histone simple
stain kit (cat. no. 414341F; Nichirei Corporation) according to the
manufacturer's protocol. Paraffin-embedded sections were
deparaffinized using xylene and rehydrated in the descending
ethanol series. These sections (4-µm thickness) were blocked with
3% H2O2 in methanol for 15 min at room
temperature to inactivate the endogenous peroxidases then incubated
at room temperature for 1 h with primary antibodies against
sequestosome-1 (cat. no. 18420-1-AP; p62; rabbit anti-p62 antibody;
1:200 dilution; Wuhan Sanying Biotechnology) and
microtubule-associated protein light chain 3 (cat. no. 14600-1-AP;
LC3; rabbit anti-LC3 antibody; 1:200 dilution; Wuhan Sanying
Biotechnology). The secondary antibody from Histone Simple Stain
kit (cat. no. 414341F; Nichirei Corporation) was placed on the
tissue for 30 min at room temperature. Histological examination was
performed under a light microscope (magnification, ×400; Olympus
BX43; Olympus Corporation).
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the LTs using ISOGEN
reagent (Nippon Gene Co., Ltd.) according to the manufacturer's
protocol. Complementary DNA (cDNA) was synthesized from total RNA
using a first-strand cDNA synthesis kit (SuperScript VILO cDNA
synthesis kit; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The specific gene expression levels were
quantitatively analyzed by performing qPCR using fluorescent SYBR
Green technology (Light Cycler; Roche Molecular Diagnostics).
β-actin cDNA was amplified and quantified in each cDNA preparation
to normalize the relative expression of target genes. The
thermocycling conditions were as follows: 95°C for 3 min; 95°C for
15 sec; 60°C for 15 sec; 72°C for 1 min (35 cycles); and 72°C for
10 min. The relative expression of genes was determined using the
2−ΔΔCq method (24) with
normalization to 36B4 expression. The primer sequences used in the
present study are listed in Table
I.
 | Table I.Primer oligonucleotide sequences. |
Table I.
Primer oligonucleotide sequences.
| Gene | Primer sequences
(5′-3′) |
|---|
| TNF-α | F:
TCTCATGCACCACCATCAAGGACT |
|
| R:
ACTTGCAGAACTCA |
| IL-6 | F:
TACCAGTTGCCTTCTTGGGACTGA |
|
| R:
TAAGCCTCCGACTTGTGAAGTGGT |
| IL-1β | F:
TGCCCCTTTTGACAGTGAT |
|
| R:
TGTGCTGCTGCGAGATTTGA |
| MCP-1 | F:
ACTGAAGCCAGCTCTCTCTTCCTC |
|
| R:
TTCCTTCTTGGGTCAGCACAGAC |
| IL-10 | F:
GACAACATACTGCTAACCGACT |
|
| R:
ATCACTCTTCACCTGCTCCAC |
| β-actin | F:
CGATGCCCTGAGGGTCTTT |
|
| R:
TGGATGCCACAGGATTCCAT |
Western blot analysis (WBA)
Proteins were extracted from the LT using
radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime
Institute of Biotechnology). Protein concentration was detected
with a bicinchoninic acid Protein Assay kit (Beyotime Institute of
Biotechnology). Protein samples (20 µg per lane) were separated by
SDS-PAGE (10% gel), and proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (Immobilon; EMD
Millipore). PVDF membranes were blocked in TBS with 0.1% Tween-20
containing 5% skimmed milk for 2 h at room temperature, incubated
in primary antibody diluent (cat. no. P0023A; Beyotime Institute of
Biotechnology) and gently agitated overnight at 4°C. Primary
antibodies against p62 (cat. no. 18420-1-AP; 1:1,000 dilution), LC3
(cat. no. 14600-1-AP; 1:1,000 dilution), beclin 1 (cat. no.
11306-1-AP; 1:1,000 dilution), PI3K (cat. no. 20584-1-AP; 1:1,000
dilution) all from Wuhan Sanying Biotechnology, and anti-β-actin
(cat. no. 4970; 1:1,000 dilution; Cell Signaling Technology, Inc.)
were utilized in the present study. Membranes were then further
incubated with horseradish peroxidase-conjugated goat anti-rabbit
Immunoglobulin G (cat. no. 7074; 1:1,000 dilution; Cell Signaling
Technology, Inc.) for 1 h at room temperature. WBA was performed as
three independent experiments. The protein levels were expressed as
a specific protein/β-actin ratio to minimize the loading
variations. Protein bands were visualized by Enhanced
Chemiluminescence kit (Beyotime Institute of Biotechnology). The
relative chemiluminescent signal intensity was semi-quantified
using ImageJ software version 1.48 (National Institutes of
Health).
Statistical analysis
All experiments were repeated three times
independently. All data are presented as the mean ± standard error
of the mean. Statistical analysis was performed using SPSS software
version 23.0 (IBM Corp.). Inter-group variation was assessed by
performing one-way analysis of variance and subsequent Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Metabolic characterization
The metabolic characteristics of the four groups of
BALB/c mice subjected to various treatments are summarized in
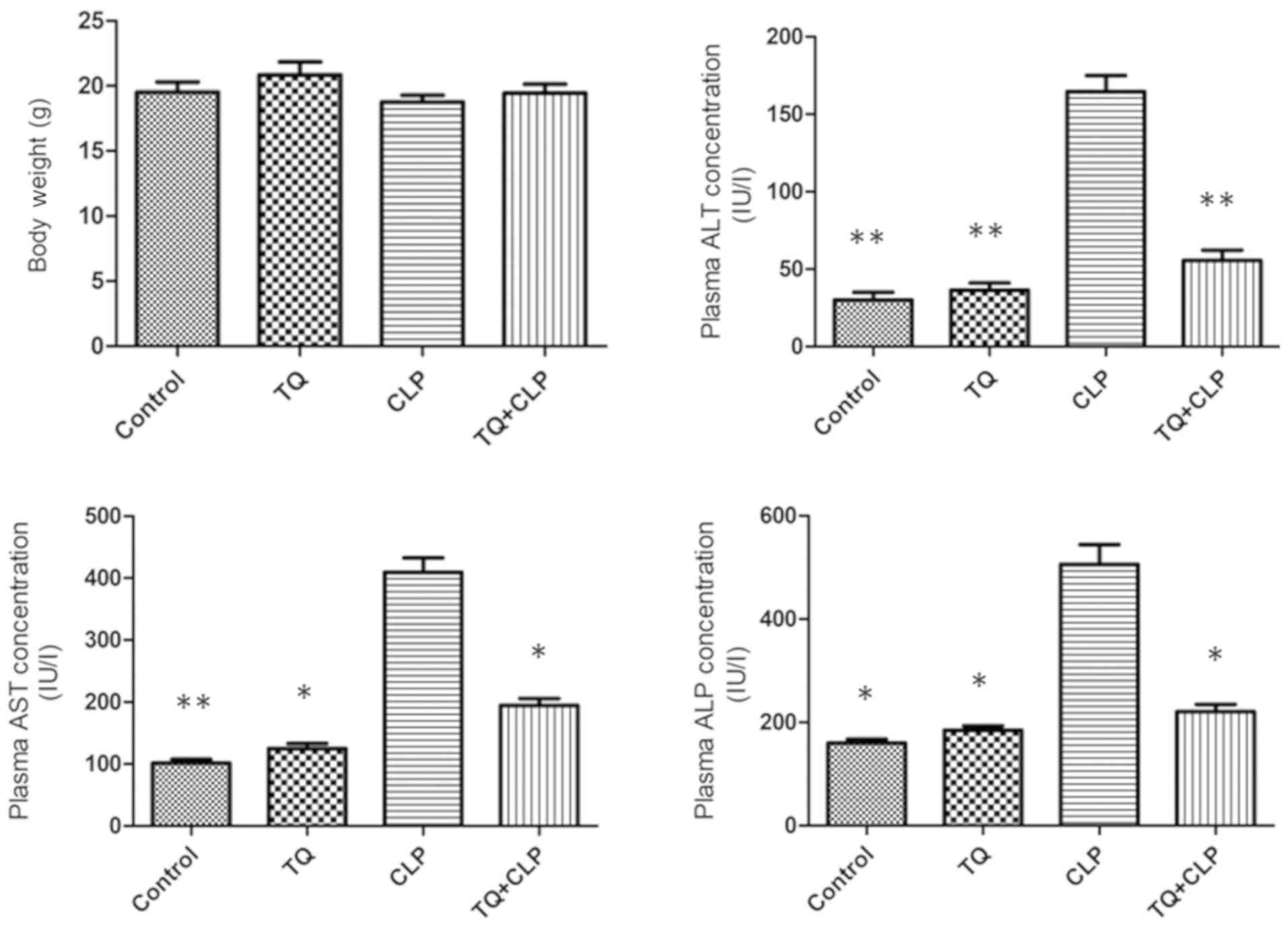
Fig. 1. The body weight was
recorded 48 h post-operation did not vary significantly
among the four groups. The CLP group exhibited a significant
increase in the ALT, AST and ALP levels compared with all
other groups; however, these levels were significantly decreased in
the TQ + CLP group. There was no significant difference with
respect to the aforementioned parameters between the control and TQ
groups.
TQ reduces histopathological damage in
LT
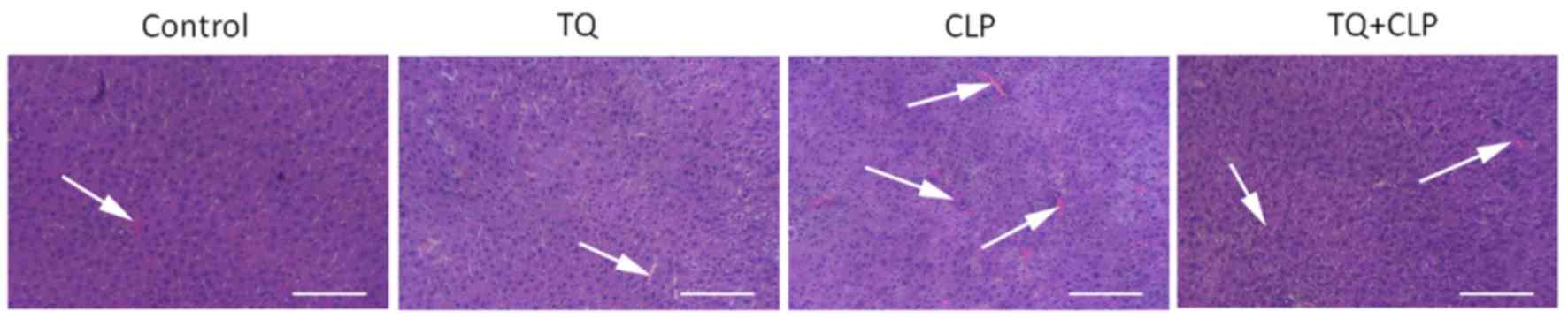
H&E staining was performed to evaluate the
histopathological damage in LT (Fig.
2) and the results for the control and TQ groups revealed that
hepatocytes were in regular shape and contained a large spheroidal
nucleus. Contrastingly, the CLP group exhibited a marked liver
injury demonstrated by hepatic strand disorganization, zonal
necrosis, mononuclear cell infiltrations, centrilobular swelling,
and sinusoidal and centrilobular congestions compared with the
other groups. However, the administration of TQ to mice prevented
the degenerative alterations in the hepatic structure induced by
CLP.
TQ increases LC3 and beclin 1
expression and decreases p62 expression in LT
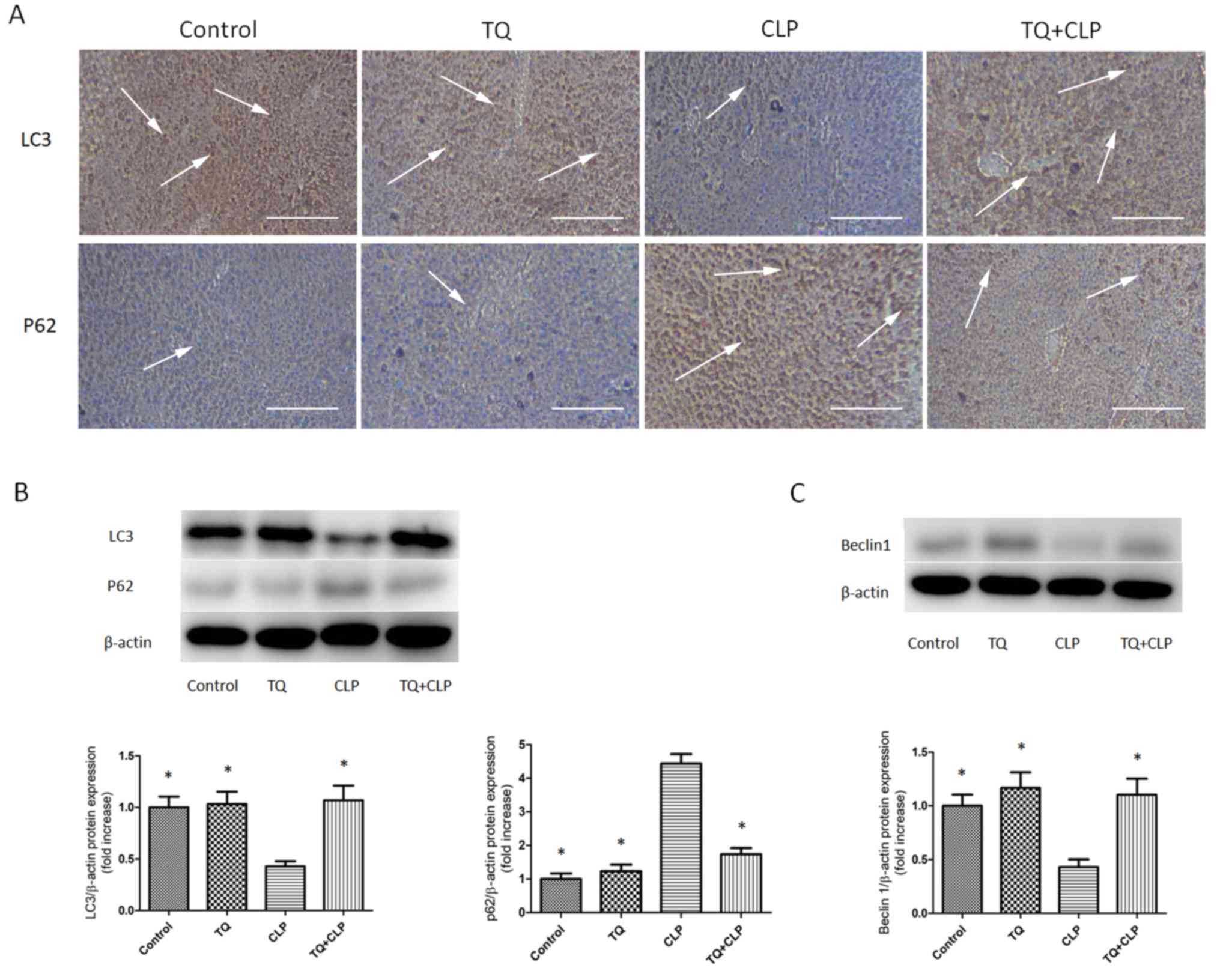
IHC staining was performed to evaluate the
expression levels of LC3 and p62 in LTs (Fig. 3A). The TQ + CLP group exhibited a
marked increase in LC3 expression and a decrease in p62 expression
in the LT compared with the CLP group. WBA was performed to
semi-quantify LC3, beclin 1 and p62 levels (Fig. 3B and C). The present study revealed
that the expression levels of LC3 and beclin 1 were significantly
increased and the expression levels of p62 were significantly
decreased in the TQ + CLP group compared with the CLP group
(Fig. 3B and C).
TQ reduces PI3K expression in the
damaged LT
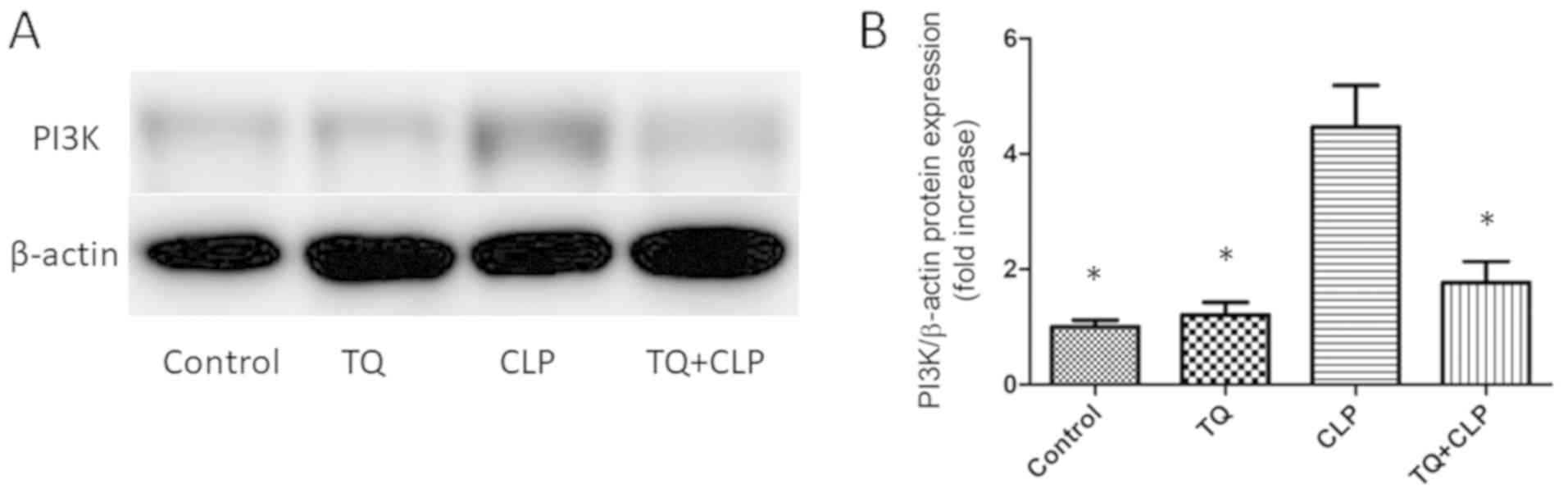
To investigate the effect of TQ on the regulation of
the PI3K signaling pathway, the PI3K level was assessed in the
treatment groups by performing WBA (Fig.
4A). The present study revealed that PI3K expression was higher
in the CLP group compared with the control group. Additionally, the
TQ + CLP group exhibited significantly decreased PI3K expression
compared with the CLP group (Fig.
4B).
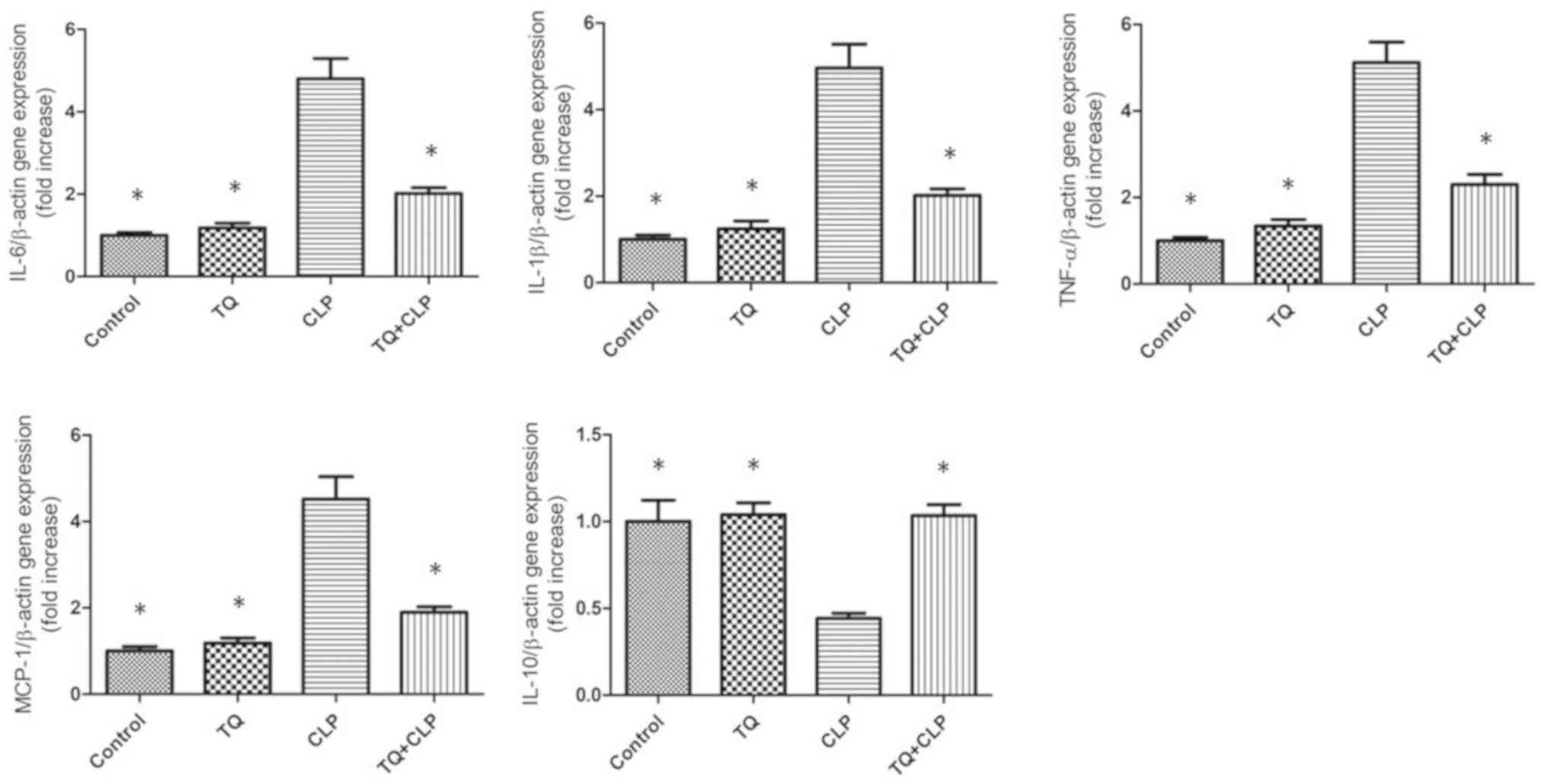
TQ reduces the expression levels of
interleukin (IL)-6, IL-1β, monocyte chemoattractant protein-1
(MCP-1) and tumor necrosis factor α (TNF-α) but increases IL-10
expression in the LTs of the CLP group
To examine the involvement of inflammatory factors
at the gene expression level in the LTs obtained from mice, the
expression levels of IL-6, IL-1β, MCP-1, IL-10 and TNF-α were
quantified using RT-qPCR (Fig. 5).
IL-6, IL-1β, MCP-1 and TNF-α expression were upregulated in the CLP
group; however, this upregulation was attenuated in the TQ + CLP
group. By contrast, IL-10 expression was downregulated in the CLP
group; however, this was upregulated in the TQ + CLP group.
Discussion
To the best of our knowledge, TQ affects autophagy
in myocardial ischemia-reperfusion (I/R) injury in Langendorff-
perfused rat hearts and exhibits anti-inflammatory activities in
rheumatoid arthritis rat model (25,26).
However, limited information is available regarding the effect of
TQ on CLP-induced septic liver injury. Therefore, the present study
investigated the effect of TQ on CLP-induced septic liver injury
using a murine model of CLP-induced polymicrobial sepsis. The
present study demonstrated that TQ ameliorated septic liver injury
as indicated by the regulation of hepatic enzymes (AST, ALT and
ALP), autophagy indicators (LC3, p62 and beclin 1), the PI3K
signaling pathway and inflammatory factors (IL-6, IL-1β, IL-10,
MCP-1 and TNF-α).
In the present study, no significant variation was
observed regarding the increment in body weight among the four
groups of mice. The sepsis-induced liver damage could further be
investigated by assessment of liver cell integrity markers (AST and
ALT) and cholestasis parameters (ALP) (27). The serum levels of hepatic enzymes
primarily mirror the degree of liver injury (28) and have been used in the present study
as a diagnostic marker for hepatotoxicity. The present study
indicated that the serum levels of AST, ALT and ALP were
significantly increased in the CLP group compared with in the
control group. The TQ group exhibited a prominent reduction in
these hepatic biochemical parameters compared with the CLP group.
These hepatic biochemical parameters clearly reflected the damage
of hepatotoxicity (ALT and AST) and cholestasis (ALP) (27) that was alleviated by TQ. Previous
studies indicated that treatment with TQ led to similar results
regarding the serum levels of AST, ALT and ALP during lead-induced
hepatic toxicity and alcoholic liver injury induced by
chronic-plus-binge ethanol feeding in murine models (29,30).
Additionally, according to the histological evidence of liver
injury, as assessed by performing H&E staining, treatment of
murine models with TQ protected mice against the liver damage
caused by CLP-induced sepsis.
Several studies demonstrated a close interaction
between CLP-induced sepsis-associated organ failure and cell
dysfunction caused by autophagy (3,31).
Additionally, a previous study indicated that prominent
autophagosomal accumulation was observed in the liver of patients
who died of sepsis as well as in the early stages of
experimentally-induced sepsis in mouse model (3). In the present study, autophagosome
formation was determined by performing IHC analysis and verified
using WBA. The present study revealed that the expression levels of
autophagy-specific proteins in the liver were altered in the CLP
group compared with in the control group.
LC3, p62 and beclin 1 are three major proteins
involved in autophagy (32). The
levels of LC3 have been used to assess autophagosome formation
(33). The results of the present
study indicated that low expression levels of LC3 aggregation in
the LT were observed at 48 h in the CLP group compared with in the
control group. Additionally, several studies have indicated a
time-course-dependent alteration in LC3 aggregation in CLP-induced
sepsis. LC3 aggregation was significantly increased at 6–8 h and
gradually decreased 12–24 h after CLP (34–36). In
the present study, LC3 expression in the TQ + CLP group was higher
compared with that in the CLP group at 48 h post-CLP. This
indicated that pretreatment with TQ may increase the autophagosome
formation in CLP-induced sepsis. As a traditional Chinese medicine
that has been used for centuries, multiple studies support the
positive effect of TQ in the prevention and cure of various
diseases. A previous study reported that TQ dose-dependently
increased intracellular LC3 expression in camptothecin-11-resistant
LoVo colon cancer cells (37).
Additionally, Chu et al (38)
reported that TQ treatment increased the accumulation of
autophagosomes in human squamous carcinoma cells. As for beclin 1,
the present study indicated that pretreatment with TQ may increase
expression of beclin 1 in CLP-induced sepsis. Wan et al
(36) demonstrated that the
expression of autophagy-associated proteins, including beclin 1,
declined to basal levels by 12 h post-CLP. Liu et al
(39) revealed that TQ increased
beclin 1 gene and protein expression levels; this result was in
accordance with the results of the present study. However, high p62
aggregation in the LT was observed in the CLP group compared with
in the control group. Pretreatment with TQ after performing CLP
reduced the p62 level compared with that in the CLP group. Previous
studies have established that p62 is a multidomain adaptor protein
that transports ubiquitinated proteins during autophagy. Consistent
with the results of the present study, other studies suggest that
p62 concentration is generally inversely associated with
autophagosomal activity (40–42).
Generally, autophagy is a stringently regulated
machinery that degrades and recycles the unnecessary or damaged
components of a cell to maintain cellular homeostasis (43). In the present study, TQ was used to
prevent CLP-induced septic liver injury in vivo.
In the present study, to investigate the effect of
TQ on the regulation of the PI3K signaling pathway, PI3K expression
was initially assessed by performing WBA. PI3K represents a key
signaling molecule that regulates several cellular functions,
including proliferation, survival, adhesion and migration in the
liver (44,45). Previous studies suggested that the
PI3K pathway is closely associated with autophagy. Li et al
(46) reported that several
microbial virulent factors induced autophagy by inactivating the
PI3K/Akt/mTOR pathway, and Li et al (47) suggested that spironolactone promoted
autophagy by inhibiting the PI3K/AKT/mTOR signaling pathway.
A recent study indicated that TQ significantly
augmented cisplatin-induced antitumor effects in gastric cancer
in vitro as well as in vivo through the inhibition of
the PI3K/Akt signaling pathway (48). Furthermore, another study suggested
that TQ inhibited PI3K phosphorylation in thioacetamide-induced
hepatic fibrosis and inflammation (49). Upon lipopolysaccharide stimulation,
the PI3K pathway activation serves to balance between
proinflammatory and anti-inflammatory responses (50). Recknagel et al (51) demonstrated that PI3K signaling was
spontaneously induced in a rat model with long-term fecal
peritonitis. A significantly increased PI3K expression was also
observed in the murine liver following aluminum overload (52). In the present study, high PI3K
expression was observed in the CLP group compared with in the
control group. Conversely, the TQ + CLP group exhibited a reduced
PI3K level compared with the CLP group. Collectively, TQ may
inhibit PI3K signaling to protect against CLP-induced septic liver
injury.
The present study demonstrated that the mRNA
expression levels of IL-6, IL-1β, MCP-1 and TNF-α in the liver were
significantly upregulated in the CLP group compared with those in
the control group. In the TQ pretreatment group, the expression
levels of IL-6, IL-1β, MCP-1 and TNF-α in the liver were reduced as
a response against CLP. Inversely, IL-10 expression was
downregulated in the CLP group; however, its expression levels were
upregulated in the TQ + CLP group. Experimental studies
indicated that TQ reduced the production of IL-6, IL-1β, MCP-1 and
TNF-α in the blood and other tissues (53). Furthermore, a previous study reported
that oral administration of TQ results in significantly increased
expression levels of IL-10 in the collagen-induced arthritis in
Wistar rats model (54).
Additionally, Hsiao et al (55) reported that treatment using an
inducer of autophagy diminished TNF-α-induced DNA fragmentation.
Additionally, Giegerich et al (56) illustrated that autophagy-dependent
pellino E3 ubiquitin protein ligase family member 3 degradation
inhibited proinflammatory IL-1β expression.
In conclusion, the present study demonstrated that
TQ assisted the mitigation of septic liver injury, as indicated by
the upregulation of LC3, beclin 1 and IL-10 expression as well as
the suppression of p62, PI3K, IL-6, IL-1β, MCP-1 and TNF-α
expression. These findings provided novel insights into the role of
TQ in sepsis-induced liver injury, and improve the possibility of
developing novel therapeutic interventions to treat liver
injury.
Acknowledgements
Not applicable.
Funding
The present study was finally supported by the
Postdoctoral Foundation of Liaoning Province, China (grant no.
194008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZP designed this study. FW and XL performed
experiments. ZP, HZ, YZ and QY analyzed the data, interpreted the
results of the experiments and prepared the figures. QL analyzed
the data, interpreted the results of the experiments and prepared
the figures. XL drafted the manuscript. ZP, QL and YZ contributed
to the revision of the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Studies Committee of the Affiliated Zhongshan Hospital of Dalian
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ALP
|
alkaline phosphatase
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
CLP
|
cecal ligation and puncture
|
|
IHC
|
immunohistochemical
|
|
IL
|
interleukin
|
|
LC3
|
microtubule-associated protein light
chain 3
|
|
LT
|
liver tissue
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
p62
|
sequestosome-1
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TQ
|
thymoquinone
|
|
WBA
|
western blot analysis
|
References
|
1
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alkharfy KM, Ahmad A, Jan BL and Raish M:
Thymoquinone reduces mortality and suppresses early acute
inflammatory markers of sepsis in a mouse model. Biomed
Pharmacother. 98:801–805. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin CW, Lo S, Perng DS, Wu DB, Lee PH,
Chang YF, Kuo PL, Yu ML, Yuan SS and Hsieh YC: Complete activation
of autophagic process attenuates liver injury and improves survival
in septic mice. Shock. 41:241–249. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiong X, Ren Y, Cui Y, Li R, Wang C and
Zhang Y: Obeticholic acid protects mice against
lipopolysaccharide-induced liver injury and inflammation. Biomed
Pharmacother. 96:1292–1298. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marshall JC: Why have clinical trials in
sepsis failed? Trends Mol Med. 20:195–203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lalazar G, Ilyas G, Malik SA, Liu K, Zhao
E, Amir M, Lin Y, Tanaka KE and Czaja MJ: Autophagy confers
resistance to lipopolysaccharide-induced mouse hepatocyte injury.
Am J Physiol Gastrointest Liver Physiol. 311:G377–G386. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh CH, Pai PY, Hsueh HW, Yuan SS and
Hsieh YC: Complete induction of autophagy is essential for
cardioprotection in sepsis. Ann Surg. 253:1190–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Zhao P, Quan N, Wang L, Chen X,
Cates C, Rousselle T and Li J: The endotoxemia cardiac dysfunction
is attenuated by AMPK/mTOR signaling pathway regulating autophagy.
Biochem Biophys Res Commun. 492:520–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li T, Zhao J, Miao S, Xu Y, Xiao X and Liu
Y: Dynamic expression and roles of sequestome1/p62 in LPSinduced
acute kidney injury in mice. Mol Med Rep. 17:7618–7626.
2018.PubMed/NCBI
|
|
10
|
Watanabe E, Muenzer JT, Hawkins WG, Davis
CG, Dixon DJ, McDunn JE, Brackett DJ, Lerner MR, Swanson PE and
Hotchkiss RS: Sepsis induces extensive autophagic vacuolization in
hepatocytes: A clinical and laboratory-based study. Lab Invest.
89:549–561. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF,
Hsueh HW, Lee PH and Hsieh YC: Lc3 over-expression improves
survival and attenuates lung injury through increasing
autophagosomal clearance in septic mice. Ann Surg. 257:352–363.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni HM, Jaeschke H and Ding WX: Targeting
autophagy for drug-induced hepatotoxicity. Autophagy. 8:709–710.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oami T, Watanabe E, Hatano M, Teratake Y,
Fujimura L, Sakamoto A, Ito C, Toshimori K, Swanson PE and Oda S:
Blocking liver autophagy accelerates apoptosis and mitochondrial
injury in hepatocytes and reduces time to mortality in a murine
sepsis model. Shock. 50:427–434. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oami T, Watanabe E, Hatano M, Sunahara S,
Fujimura L, Sakamoto A, Ito C, Toshimori K and Oda S: Suppression
of t cell autophagy results in decreased viability and function of
t cells through accelerated apoptosis in a murine sepsis model.
Crit Care Med. 45:e77–e85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Daba MH and Abdel-Rahman MS:
Hepatoprotective activity of thymoquinone in isolated rat
hepatocytes. Toxicol Lett. 95:23–29. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan MA, Ashfaq MK, Zuberi HS, Mahmood MS
and Gilani AH: The in vivo antifungal activity of the aqueous
extract from Nigella sativa seeds. Phytother Res. 17:183–186. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Houghton PJ, Zarka R, de las Heras B and
Hoult JR: Fixed oil of Nigella sativa and derived thymoquinone
inhibit eicosanoid generation in leukocytes and membrane lipid
peroxidation. Planta Med. 61:33–36. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reddy L, Odhav B and Bhoola KD: Natural
products for cancer prevention: A global perspective. Pharmacol
Ther. 99:1–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Salem ML: Immunomodulatory and therapeutic
properties of the Nigella sativa L. seed. Int Immunopharmacol.
5:1749–1770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Darakhshan S, Bidmeshki Pour A,
Hosseinzadeh Colagar A and Sisakhtnezhad S: Thymoquinone and its
therapeutic potentials. Pharmacol Res. 95-96:138–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. (8th).
National Academies Press (US). (Washington, D.C.). 2011.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tekeoglu I, Dogan A and Demiralp L:
Effects of thymoquinone (volatile oil of black cumin) on rheumatoid
arthritis in rat models. Phytother Res. 20:869–871. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao J, Ke ZP, Shi Y, Zeng Q and Cao Z:
The cardioprotective effect of thymoquinone on ischemia-reperfusion
injury in isolated rat heart via regulation of apoptosis and
autophagy. J Cell Biochem. 119:7212–7217. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Penndorf V, Saner F, Gerken G and Canbay
A: Liver parameters in intensive care medicine. Zentralbl Chir.
138:636–642. 2013.(In German). PubMed/NCBI
|
|
28
|
Hsu DZ, Chien SP, Li YH and Liu MY: Sesame
oil does not show accumulatively enhanced protection against
oxidative stress-associated hepatic injury in septic rats. JPEN J
Parenter Enteral Nutr. 32:276–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mabrouk A, Bel Hadj Salah I, Chaieb W and
Ben Cheikh H: Protective effect of thymoquinone against
lead-induced hepatic toxicity in rats. Environ Sci Pollut Res Int.
23:12206–12215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Bai T, Yao YL, Zhang DQ, Wu YL,
Lian LH and Nan JX: Upregulation of SIRT1-AMPK by thymoquinone in
hepatic stellate cells ameliorates liver injury. Toxicol Lett.
262:80–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carchman EH, Whelan S, Loughran P, Mollen
K, Stratamirovic S, Shiva S, Rosengart MR and Zuckerbraun BS:
Experimental sepsis-induced mitochondrial biogenesis is dependent
on autophagy, TLR4, and TLR9 signaling in liver. FASEB J.
27:4703–4711. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmitz KJ, Ademi C, Bertram S, Schmid KW
and Baba HA: Prognostic relevance of autophagy-related markers LC3,
p62/sequestosome 1, Beclin-1 and ULK1 in colorectal cancer patients
with respect to KRAS mutational status. World J Surg Oncol.
14:1892016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escobar DA, Botero-Quintero AM, Kautza BC,
Luciano J, Loughran P, Darwiche S, Rosengart MR, Zuckerbraun BS and
Gomez H: Adenosine monophosphate-activated protein kinase
activation protects against sepsis-induced organ injury and
inflammation. J Surg Res. 194:262–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takahashi W, Watanabe E, Fujimura L,
Watanabe-Takano H, Yoshidome H, Swanson PE, Tokuhisa T, Oda S and
Hatano M: Kinetics and protective role of autophagy in a mouse
cecal ligation and puncture-induced sepsis. Crit Care. 17:R1602013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sunahara S, Watanabe E, Hatano M, Swanson
PE, Oami T, Fujimura L, Teratake Y, Shimazui T, Lee C and Oda S:
Influence of autophagy on acute kidney injury in a murine cecal
ligation and puncture sepsis model. Sci Rep. 8:10502018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wan SX, Shi B, Lou XL, Liu JQ, Ma GG,
Liang DY and Ma S: Ghrelin protects small intestinal epithelium
against sepsis-induced injury by enhancing the autophagy of
intestinal epithelial cells. Biomed Pharmacother. 83:1315–1320.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen MC, Lee NH, Hsu HH, Ho TJ, Tu CC,
Hsieh DJ, Lin YM, Chen LM, Kuo WW and Huang CY: Thymoquinone
induces caspase-independent, autophagic cell death in
CPT-11-resistant lovo colon cancer via mitochondrial dysfunction
and activation of JNK and p38. J Agric Food Chem. 63:1540–1546.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chu SC, Hsieh YS, Yu CC, Lai YY and Chen
PN: Thymoquinone induces cell death in human squamous carcinoma
cells via caspase activation-dependent apoptosis and LC3-II
activation-dependent autophagy. PLoS One. 9:e1015792014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu H, Sun Y, Zhang Y, Yang G, Guo L, Zhao
Y and Pei Z: Role of thymoquinone in cardiac damage caused by
sepsis from BALB/c mice. Inflammation. 42:516–525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao P, Kuai J, Gao J, Sun L, Wang Y and
Yao L: Delta opioid receptor agonist attenuates
lipopolysaccharide-induced myocardial injury by regulating
autophagy. Biochem Biophys Res Commun. 492:140–146. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang GQ, Tang T, Wang ZS, Liu YY, Wang L,
Luo PF and Xia ZF: Overexpression of hypo-phosphorylated IκBβ at
ser313 protects the heart against sepsis. PLoS One.
11:e01608602016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang H, Cui N, Han W, Su LX, Long Y and
Liu DW: Accelerated autophagy of cecal ligation and
puncture-induced myocardial dysfunction and its correlation with
mammalian target of rapamycin pathway in rats. Chin Med J (Engl).
131:1185–1190. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chung KW, Kim KM, Choi YJ, An HJ, Lee B,
Kim DH, Lee EK, Im E, Lee J, Im DS, et al: The critical role played
by endotoxin-induced liver autophagy in the maintenance of lipid
metabolism during sepsis. Autophagy. 13:1113–1129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miao B and Degterev A: Targeting
phospshatidylinositol 3-kinase signaling with novel
phosphatidylinositol 3,4,5-triphosphate antagonists. Autophagy.
7:650–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Reif S, Lang A, Lindquist JN, Yata Y,
Gabele E, Scanga A, Brenner DA and Rippe RA: The role of focal
adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in
hepatic stellate cell proliferation and type I collagen expression.
J Biol Chem. 278:8083–8090. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li P, Shi J, He Q, Hu Q, Wang YY, Zhang
LJ, Chan WT and Chen WX: Streptococcus pneumoniae induces autophagy
through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS
hypergeneration in A549 cells. PLoS One. 10:e01227532015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li D, Lu Z, Xu Z, Ji J, Zheng Z, Lin S and
Yan T: Spironolactone promotes autophagy via inhibiting
PI3K/AKT/mTOR signalling pathway and reduce adhesive capacity
damage in podocytes under mechanical stress. Biosci Rep.
36:e003552016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ma J, Hu X, Li J, Wu D, Lan Q, Wang Q,
Tian S and Dong W: Enhancing conventional chemotherapy drug
cisplatin-induced anti-tumor effects on human gastric cancer cells
both in vitro and in vivo by thymoquinone targeting PTEN gene.
Oncotarget. 8:85926–85939. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bai T, Yang Y, Wu YL, Jiang S, Lee JJ,
Lian LH and Nan JX: Thymoquinone alleviates thioacetamide-induced
hepatic fibrosis and inflammation by activating LKB1-AMPK signaling
pathway in mice. Int Immunopharmacol. 19:351–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang JC, Wu SC, Rau CS, Lu TH, Wu YC, Chen
YC, Lin MW, Tzeng SL, Wu CJ and Hsieh CH: Inhibition of the
phosphoinositide 3-kinase pathway decreases innate resistance to
lipopolysaccharide toxicity in TLR4 deficient mice. J Biomed Sci.
21:202014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Recknagel P, Gonnert FA, Westermann M,
Lambeck S, Lupp A, Rudiger A, Dyson A, Carre JE, Kortgen A, Krafft
C, et al: Liver dysfunction and phosphatidylinositol-3-kinase
signalling in early sepsis: Experimental studies in rodent models
of peritonitis. PLoS Med. 9:e10013382012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hu C, Yang J, He Q, Luo Y, Chen Z, Yang L,
Yi H, Li H, Xia H, Ran D, et al: CysLTR1 blockage ameliorates liver
injury caused by aluminum-overload via PI3K/AKT/mTOR-mediated
autophagy activation in vivo and in vitro. Mol Pharm. 15:1996–2006.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ghazwani M, Zhang Y, Gao X, Fan J, Li J
and Li S: Anti-fibrotic effect of thymoquinone on hepatic stellate
cells. Phytomedicine. 21:254–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Umar S, Zargan J, Umar K, Ahmad S, Katiyar
CK and Khan HA: Modulation of the oxidative stress and inflammatory
cytokine response by thymoquinone in the collagen induced arthritis
in Wistar rats. Chem Biol Interact. 197:40–46. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hsiao HW, Tsai KL, Wang LF, Chen YH,
Chiang PC, Chuang SM and Hsu C: The decline of autophagy
contributes to proximal tubular dysfunction during sepsis. Shock.
37:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Giegerich AK, Kuchler L, Sha LK, Knape T,
Heide H, Wittig I, Behrends C, Brüne B and von Knethen A:
Autophagy-dependent PELI3 degradation inhibits proinflammatory IL1B
expression. Autophagy. 10:1937–1952. 2014. View Article : Google Scholar : PubMed/NCBI
|