Introduction
Mycobacterium tuberculosis (TB) infection
remains a large global health problem. In 2017, an estimated 10.0
million individuals developed TB. TB is now the 10th leading cause
of death worldwide and the leading cause of mortality from a single
infectious agent. China is one of 30 high TB-burden countries
(1). To reach the goal of TB
elimination, individuals with active TB require rapid
identification and treatment. Microscopy, growth in culture and
molecular tests are the gold standards for the diagnosis of active
TB, as they directly indicate the presence of actual TB bacilli or
their DNA (1,2). However, not all cases of TB infection
may be bacteriologically confirmed. For patients with a negative
acid-resistant bacillus sputum-smear test, diagnosis and treatment
decisions may be challenging.
The tuberculin skin test (TST) has been widely used
for detecting latent TB infection (LTBI) and active TB for almost a
century. The important advantages of the TST include its low cost
and convenience. However, the TST result may be influenced by prior
Bacillus Calmette-Guerin (BCG) vaccination and infection with
non-tuberculous mycobacteria (NTM) (3). In recent years, several commercially
available interferon-γ release assays (IGRAs) have been developed
as an alternative screening approach for TB infection. These tests,
including the T-SPOT.TB test, QuantiFERON-TB Gold or QuantiFERON-TB
Gold In-Tube target unique and specific M. tuberculosis
proteins that are not present in BCG or in most environmental
mycobacteria (3). Several
meta-analyses have indicated a relatively enhanced sensitivity and
specificity of IGRAs over the TST in identifying TB infection.
However, neither the IGRAs nor the TST exhibited ideal stability
(4–6).
To assess the value of these two methods, the
present retrospective analysis was performed. The performance of
the T-SPOT.TB in detecting active TB was compared with that of the
TST and the comparison between these two detection methods was
determined.
Materials and methods
Participants and data collection
A retrospective analysis was performed on patients
diagnosed at the Respiratory Department of Ningbo First Hospital
(Ningbo, China) between October 2016 and 2017. A total of 118
patients who were suspected of active TB infection on admission
were included in the analysis. Each patient was subjected to the
TST as well as the T-SPOT.TB test. The patients' demographics and
clinical information, including previous history of TB, were
collected.
Definitions and diagnoses
Final diagnoses were made considering all clinical,
radiological, microbiological and pathological information.
Patients who had clinical, bacteriological and/or radiographic
evidence of active TB infection were defined as active TB cases of
the following varieties: i) Pulmonary TB: M. tuberculosis
was cultured from sputum, bronchial specimens or patients whose
data met the definition of a clinical case of TB (7). For clinical cases of TB, chest
radiographic findings were defined as the presence of cavities,
branching linear lesions, multiple centrilobular nodules or lobular
consolidation upon high resolution CT (8,9). Lesions
that mostly appeared as calcified nodules or fibrotic scars were
not considered to be indicative of active TB infection. For
patients with lesions that suggested active TB but who had a
negative bacteriologic status, broad-spectrum antibiotics were
given for one week. If the lesions significantly improved, a
diagnosis of active TB was ruled out. All clinical cases were
followed up for at least 3 months for further confirmation. ii)
Pleural TB: M. tuberculosis was detected in the pleural
fluid or a tissue biopsy, or exudative pleural effusion exhibited
predominant lymphocytosis, high protein, low carcinoembryonic
antigen (<5 ng/ml) and high adenosine deaminase (≥40 IU/l)
(7,10). iii) Lymph node TB: M.
tuberculosis was detected in lymph node tissue.
TST and T-SPOT.TB
A TST was performed following standard procedures. A
total of 0.1 ml of purified protein derivate (Chengdu Institute of
Biological Products Co., Ltd.) was injected intradermally into the
inner side of the forearm, and the transverse induration was
measured in mm after 48–72 h by trained nurses. An induration of
≥10 mm (or ≥5 mm in immunosuppressed individuals) was classified as
a positive result. The T-SPOT.TB assays (Beijing Wan Tai
Bio-Pharmaceutical Co., Ltd) were performed and interpreted
according to the manufacturer's specifications (11).
Statistical analysis
Continuous variables are expressed as the mean and
standard deviation. Frequencies were calculated for demographic and
clinical data. Comparisons between different groups were performed
using a Student's t-test or least-significant difference (LSD)
test. The LSD test was performed following one-way analysis of
variance. Fisher's exact or χ2 tests were used for
univariate analyses. In each analysis, P<0.05 was considered to
indicate a statistically significant difference. Statistical
analyses were performed using SPSS for Windows, version 22.0 (IBM
Corp.) and GraphPad Prism 5.0 (GraphPad Software, Inc.).
Results
Characteristics of the study
population
A total of 118 patients were included in the
analysis and 70 (59.3%) of them were female. The median age was
56.5 years (range, 18–95 years). One patient (0.8%) was confirmed
as HIV-positive and seven patients (5.9%) were currently receiving
immunosuppressive treatment. A total of 10 patients (8.5%) had
previously been diagnosed with TB and had received anti-TB
treatment. BCG scars were present in 88 patients (74.6%). Active TB
infection was diagnosed in 30 patients (25.4%) and 15 of them had
pulmonary TB. A total of 88 patients (74.6%) were diagnosed with
non-TB conditions. Pneumonia was the most common disease among
those non-TB cases. Concerning the laboratory data, TB patients had
a lower lymphocyte ratio than that in the non-TB group. There were
no significant differences between the CD4+ lymphocyte
ratio and the CD8+ lymphocyte ratio between the two
groups (Table I).
 | Table I.Baseline characteristics of the
subjects. |
Table I.
Baseline characteristics of the
subjects.
| Characteristic | TB (n=30) | Non-TB (n=88) | P-value |
|---|
| Male sex | 14 (47.7) | 56 (63.7) | 0.102 |
| Age, years | 50.0±19.5 | 58.7±17.4 | 0.024 |
| BMI | 20.7±3.8 | 21.2±3.6 | 0.485 |
| Smoking index | 198.5±350.3 | 283.8±503.8 | 0.393 |
| Prior treatment of
TB | 3 (10.0) | 7 (8.0) | 0.728 |
| Current
immunosuppressive treatment | 4 (13.3) | 3 (3.4) | 0.047 |
| BCG scar present | 18 (60.0) | 70 (79.5) | 0.100 |
| WBC
(109/l) | 6.7±1.9 | 7.3±4.0 | 0.404 |
| Lymphocytes (%) | 19.0±6.4 | 23.3±9.7 | 0.007 |
| CRP (mg/dl) | 20.0±22.1 | 27.2±48.4 | 0.273 |
| CD4+ T
lymphocytes (%) | 41.4±10.5 | 40.3±8.8 | 0.736 |
| CD8+ T
lymphocytes (%) | 24.1±11.1 | 24.6±10.3 | 0.900 |
| Final diagnosis |
| Pulmonary
TB | 14 (46.7) |
|
|
|
Endobronchial TB | 1 (3.3) |
|
|
| Pleural
TB | 13 (43.3) |
|
|
| Lymph
node TB | 2 (6.7) |
|
|
|
Pneumonia |
| 65 (73.9) |
|
| Pulmonary
fungal infection |
| 4 (4.5) |
|
|
Sarcoidosis |
| 2 (2.3) |
|
| Lung
tumor |
| 6 (6.8) |
|
|
Others |
| 11 (12.5) |
|
Performance of T-SPOT.TB and TST in
active TB
Of all of the 118 patients, the TST results were
positive for 43 patients (36%), 23 of whom were diagnosed with
active TB; the TST results were negative for 75 patients (64%), 7
of whom were diagnosed with active TB. The overall sensitivity and
specificity of the TST were 76.7 and 77.3%, respectively. In the
T-SPOT.TB test, 53 patients (45%) had positive results, 25 of whom
were diagnosed with active TB; 65 patients (55%) had negative
results, 5 of whom were diagnosed with active TB. The overall
sensitivity and specificity of the T-SPOT.TB test were 88.3 and
68.1%, respectively. However, no significant difference was
observed between T-SPOT.TB and TST (Table II).
 | Table II.Performance of T-SPOT.TB and TST in
active TB. |
Table II.
Performance of T-SPOT.TB and TST in
active TB.
| Parameter (%) | T-SPOT.TB | TST | P-value |
|---|
| Overall
sensitivity | 83.3 (25/30) | 76.7 (23/30) | 0.51 |
| Overall
specificity | 68.1 (60/88) | 77.3 (68/88) | 0.17 |
| Pulmonary TB |
|
Sensitivity | 80.0 (12/15) | 80.0 (12/15) | 1 |
|
Specificity | 60.2 (62/103) | 70.0 (72/103) | 0.14 |
|
PPV | 23.6 (12/53) | 27.9 (12/43) | 0.55 |
|
NPV | 95.4 (62/65) | 96.0 (72/75) | 0.86 |
| Extrapulmonary
TB |
|
Sensitivity | 86.7 (13/15) | 73.3 (11/15) | 0.41 |
|
Specificity | 61.2 (63/103) | 68.9 (71/103) | 0.24 |
|
PPV | 24.5 (13/53) | 25.6 (11/43) | 0.91 |
|
NPV | 96.9 (63/65) | 94.7 (71/75) | 0.51 |
The accuracy of the TST and T-SPOT.TB test for
pulmonary and extrapulmonary TB (EPTB) was calculated separately.
For pulmonary TB, the sensitivity of the TST was 80.0%, and the
specificity was 70.0%. The sensitivity and specificity of the
T-SPOT.TB test was 80.0 and 60.2%, respectively. For the EPTB, the
TST sensitivity was 73.3% and the specificity was 68.9%, while the
sensitivity and specificity of the T-SPOT.TB were 86.7 and 61.2%,
respectively. No significant difference was noted in the above
results between the TST and the T-SPOT.TB test. The negative
predictive value (NPVs) of the TST and T-SPOT.TB test was higher
than the respective positive predictive value (PPVs) (Table II).
Association between the TST spot size
and T-SPOT.TB results
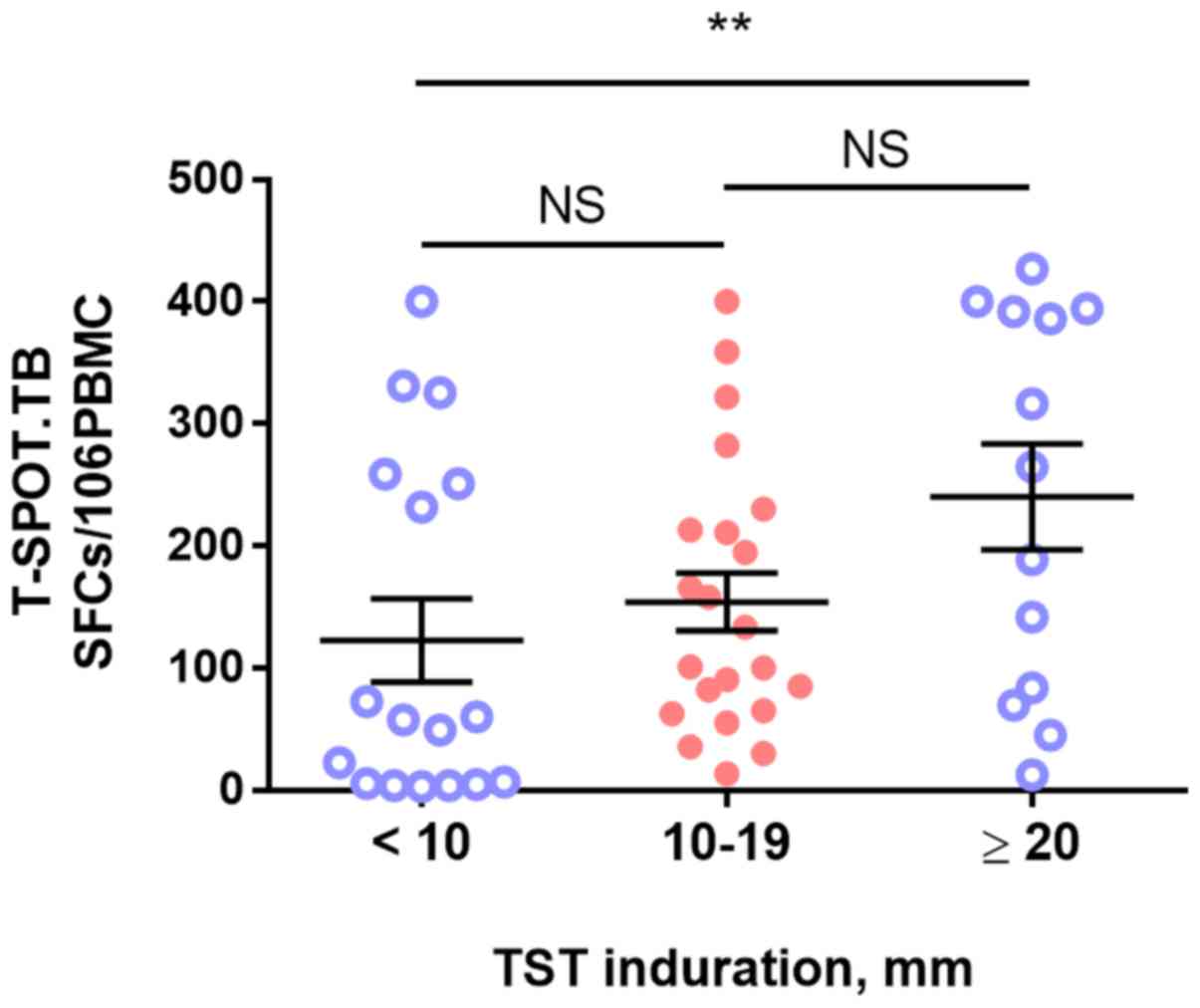
There was a trend toward an increased likelihood of
T-SPOT.TB positivity with increased TST spot size. Patients with a
large TST size (>20 mm) had a higher number of gamma
interferon-producing T cells among their peripheral blood
mononuclear cells (PBMCs) than that of TST-negative patients (spot
size, <10 mm; 240.2±155.6 vs. 101.4±129.1; spot-forming
cells/106 PBMCs, P=0.008). However, no such differences
were observed among other stratified data based on the TST size
(Table III and Fig. 1).
 | Table III.Comparison between size of TST
induration and T-SPOT.TB result. |
Table III.
Comparison between size of TST
induration and T-SPOT.TB result.
|
|
| T-SPOT.TB |
|---|
|
|
|
|
|---|
| TST induration
(mm) | N | Positive n (%) | SFCs/106
PBMC, mean ± SD |
|---|
| <10 | 75 | 18 (24.0) | 101.4±129.1 |
| ≥10 | 43 | 34 (79.1) | 196.7±134.9 |
|
|
|
| aP=0.017 |
| 10–14 | 22 | 16 (72.7) | 168.9±112.0 |
| 15–19 | 6 | 5 (83.3) | 172.8±143.1 |
| ≥20 | 15 | 13 (86.7) | 240.2±155.6 |
|
|
|
| bP=0.297 |
Discussion
The major results of the present study were as
follows: i) The T-SPOT.TB test had a higher sensitivity than the
TST, but the specificity and PPV were comparatively lower than
those of the TST. However, none of the above results were
statistically significant; ii) the NPVs of the TST and the
T-SPOT.TB test were much higher than the PPVs; and iii) increased
TST spot size is associated with a trend toward increased rates of
T-SPOT.TB positivity.
The overall sensitivity of the TST and T-SPOT.TB
test were 76.7 and 88.3%, respectively, in the present study. The
T-SPOT.TB test had a comparatively higher sensitivity than that of
the TST, which was consistent with the results of certain previous
meta-analyses (6,12). However, compared to most data for
cohorts from developed countries (3,12), a
lower specificity (68.1%) and PPV (47.2%) of the T-SPOT.TB was
determined in the present study, which is more consistent with the
result of one large-scale retrospective multicenter study from
China (13). The accuracy of the TST
and T-SPOT.TB test for EPTB was also evaluated. The specificity of
the T-SPOT.TB test did not exhibit any advantage over that of the
TST (61.2 vs. 68.9%), which may indicate a relatively high
prevalence of LTBI in the region of residence of the present
cohort. The T-SPOT.TB test and the TST are based on cellular immune
responses, and they are unable to distinguish between latent TB
infection and active TB (14).
Therefore, the T-SPOT.TB may have limited value in detecting active
TB, particularly in high TB burden settings (15,16).
It was found that the NPVs were much higher than the
PPVs for the TST (91 vs. 53%) and for the T-SPOT.TB test (92 vs.
47%). This indicated that the T-SPOT.TB test and the TST may be
more appropriate for ruling out active TB than for ruling it in. A
previous study suggested that the combination of negative results
obtained by IGRAs with the TST may enable the rapid exclusion of TB
(17,18). Further studies are required to
identify the optimal combined strategy for targeted screening.
However, considering the low PPV of the two methods, active TB
should not simply be excluded for high-risk individuals without a
thorough microbiological examination of M. tuberculosis.
In the present study, a trend toward an increased
likelihood of a positive T-SPOT.TB result with increased TST spot
size was observed. Several studies have evaluated the association
between the TST size and IGRAs result in LTBI, and the results
indicated that the TST size may help identify those subjects with
the highest risk of LTBI (19–21). All
of these studies lack the gold standard testing for determining
LTBI. The present study identified a relative concordance of a
positive T-SPOT.TB result and the TST size in detecting active TB.
Patients with a TST size of >20 mm had a higher number of gamma
interferon-producing T cells than those with a negative TST result,
which implied that the body had a strong immune response to the
exposure to TB bacilli. However, when the TST size was <20 mm,
the correlation between the number of T cells producing gamma
interferon among PBMCs and the size of the TST spot was not high.
One possible explanation is that a positive TST test cannot
differentiate between M. tuberculosis infection, prior BCG
vaccination and exposure to NTM (22), particularly when the TST induration
is <15 mm (23,24). In addition, the TST and the T-SPOT.TB
test are designed to detect the presence of M.
tuberculosis-specific T-cell responses (25) and represent indirect evidence for
past or present exposure to TB bacilli. Neither a positive TST size
nor T-SPOT.TB result may discriminate active TB infection from
LTBI. In light of the high TB burden in China, even if the TST
result is strongly positive, a diagnosis of active TB still
requires further examination and comprehensive consideration.
There are certain limitations of the present study.
First, as a retrospective study, the decision to perform the
T-SPOT.TB test and TST depended on the physician's judgment at that
time, possibly introducing a selection bias. Second, the number of
cases included is limited. On the one hand, as a commercial test,
the T-SPOT.TB test has been introduced at our hospital only
recently, so the number of cases is not high. On the other hand,
the number of patients diagnosed with active TB infection is also
not large (pulmonary TB, 15 cases and extrapulmonaryTB, 15 cases).
Considering these limitations, the results of the present study
should be interpreted with caution. A population-based study with a
sufficient sample size and follow-up is required to fully compare
the performance of IGRAs and the TST in high-risk TB populations
(26).
In conclusion, the T-SPOT.TB test had a higher
sensitivity than the TST. An increased TST spot size was associated
with a trend toward an increased rate of T-SPOT.TB positivity.
However, neither the T-SPOT.TB test nor the TST was sufficiently
accurate to be used for detecting active TB disease. Given the
comparable performance, the selection of TST or T-SPOT.TB should
rather depend on other considerations, including cost, benefits and
resources.
Acknowledgements
The authors wish to thank Professor Chao Cao
(Department of Respiratory Medicine, Ningbo First Hospital, Ningbo,
China) for his constant support during the course of this work.
Funding
No funding was received.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JY, WK and NX were responsible for the acquisition,
analysis and interpretation of the data and contributed to the
drafting of the manuscript. XC provided statistical support and
data interpretation. XH and XC made critical revisions to the
manuscript for important intellectual content and performed the
final proofing. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The present study was performed with the informed
consent of each subject and with the approval of the local Ethics
Committee of Ningbo First Hospital (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Organization WH: Global tuberculosis
report 2018. http://www.who.int/tb/publications/global_report/en/December
26–2018
|
|
2
|
Dinnes J, Deeks J, Kunst H, Gibson A,
Cummins E, Waugh N, Drobniewski F and Lalvani A: A systematic
review of rapid diagnostic tests for the detection of tuberculosis
infection. Health Technol Assess. 11:1–196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pai M, Zwerling A and Menzies D:
Systematic review: T-cell-based assays for the diagnosis of latent
tuberculosis infection: An update. Ann Intern Med. 149:177–184.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun L, Xiao J, Miao Q, Feng WX, Wu XR, Yin
QQ, Jiao WW, Shen C, Liu F, Shen D and Shen AD: Interferon gamma
release assay in diagnosis of pediatric tuberculosis: A
meta-analysis-gamma. FEMS Immunol Med Microbiol. 63:165–173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sester M, Sotgiu G, Lange C, Giehl C,
Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, et
al: Interferon-γ release assays for the diagnosis of active
tuberculosis: A systematic review and meta-analysis-γA. Eur Respir
J. 37:100–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu P, Chen X, Zhu LM and Yang HT:
Interferon-gamma release assays for the diagnosis of tuberculosis:
A systematic review and meta-analysis. Lung. 194:447–458. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diagnostic standards and classification of
tuberculosis in adults and children, . This official statement of
the American thoracic society and the centers for disease control
and prevention was adopted by the ats board of directors, July
1999. This statement was endorsed by the council of the infectious
disease society of America, September 1999. Am J Respir Crit Care
Med. 161:1376–1395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han
MC and Noma S: Pulmonary tuberculosis: CT findings-early active
disease and sequential change with antituberculous therapy.
Radiology. 186:653–660. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Im JG, Webb WR, Han MC and Park JH: Apical
opacity associated with pulmonary tuberculosis: High-resolution CT
findings. Radiology. 178:727–731. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang QL, Shi HZ, Wang K, Qin SM and Qin
XJ: Diagnostic accuracy of adenosine deaminase in tuberculous
pleurisy: A meta-analysis. Respir Med. 102:744–754. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pai M, Riley LW and Colford JM Jr:
Interferon-gamma assays in the immunodiagnosis of tuberculosis: A
systematic review. Lancet Infect Dis. 4:761–776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diel R, Loddenkemper R and Nienhaus A:
Evidence-based comparison of commercial interferon-gamma release
assays for detecting active TB: A metaanalysis. Chest. 137:952–968.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang WL, Wang GR, Wu MY, Yang KY, Er-Tai
A, Wu SC, Geng SJ, Li ZH, Li MW, Li L and Tang SJ: Interferon-gamma
release assay is not appropriate for the diagnosis of active
tuberculosis in high-burden tuberculosis settings: A retrospective
multicenter investigation. Chin Med J (Engl). 131:268–275. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pai M and Menzies D: Interferon-gamma
release assays: What is their role in the diagnosis of active
tuberculosis? Clin Infect Dis. 44:74–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Metcalfe JZ, Everett CK, Steingart KR,
Cattamanchi A, Huang L, Hopewell PC and Pai M: Interferon-γ release
assays for active pulmonary tuberculosis diagnosis in adults in
low- and middle-income countriesγ: Systematic review and
meta-analysis. J Infect Dis. 204 (Suppl 4):S1120–S1129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan L, Chen Z, Hao XH, Hu ZY and Xiao HP:
Interferon-gamma release assays for the diagnosis of extrapulmonary
tuberculosis: A systematic review and meta-analysis. FEMS Immunol
Med Microbiol. 65:456–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goletti D, Carrara S, Butera O, Amicosante
M, Ernst M, Sauzullo I, Vullo V, Cirillo D, Borroni E, Markova R,
et al: Accuracy of immunodiagnostic tests for active tuberculosis
using single and combined results: A multicenter TBNET-study. PLoS
One. 3:e34172008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dosanjh DP, Hinks TS, Innes JA, Deeks JJ,
Pasvol G, Hackforth S, Varia H, Millington KA, Gunatheesan R,
Guyot-Revol V and Lalvani A: Improved diagnostic evaluation of
suspected tuberculosis. Ann Intern Med. 148:325–336. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cruz AT and Starke JR: Relationship
between tuberculin skin test (TST) size and interferon gamma
release assay (IGRA) result: When should clinicians obtain IGRAs in
children with positive TSTs? Clin Pediatr (Phila). 53:1196–1199.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leung CC, Yam WC, Yew WW, Ho PL, Tam CM,
Law WS, Wong MY, Leung M and Tsui D: Comparison of T-Spot.TB and
tuberculin skin test among silicotic patients. Eur Respir J.
31:266–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahan CS, Johnson DF, Curley C and van der
Kuyp F: Concordance of a positive tuberculin skin test and an
interferon gamma release assay in bacille Calmette-Guérin
vaccinated persons. Int J Tuberc Lung Dis. 15174–178.
(i)2011.PubMed/NCBI
|
|
22
|
Gualano G, Mencarini P, Lauria FN,
Palmieri F, Mfinanga S, Mwaba P, Chakaya J, Zumla A and Ippolito G:
Tuberculin skin test-Outdated or still useful for Latent TB
infection screening? Int J Infect Dis 80S. S20–S22. 2019.
View Article : Google Scholar
|
|
23
|
Tissot F, Zanetti G, Francioli P,
Zellweger JP and Zysset F: Influence of bacille Calmette-Guérin
vaccination on size of tuberculin skin test reaction: To what size?
Clin Infect Dis. 40:211–217. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mazurek GH, Lobue PA, Daley CL, Bernardo
J, Lardizabal AA, Bishai WR, Iademarco MF and Rothel JS: Comparison
of a whole-blood interferon gamma assay with tuberculin skin
testing for detecting latent Mycobacterium tuberculosis
infection. JAMA. 286:1740–1747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mack U, Migliori GB, Sester M, Rieder HL,
Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B,
et al: LTBI: latent tuberculosis infection or lasting immune
responses to M. tuberculosis? A TBNET consensus statement.
Eur Respir J. 33:956–973. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Auguste P, Tsertsvadze A, Pink J, Court R,
McCarthy N, Sutcliffe P and Clarke A: Comparing interferon-gamma
release assays with tuberculin skin test for identifying latent
tuberculosis infection that progresses to active tuberculosis:
Systematic review and meta-analysis. BMC Infect Dis. 17:2002017.
View Article : Google Scholar : PubMed/NCBI
|