Introduction
Retinoblastoma (RB), the most prevalent intraocular
cancer, is a childhood malignant tumor derived from immature cells
in the retina (1). RB accounts for
~2–4% of all childhood malignancies, in which the morbidity is
~1:15,000–1:20,000 (2). The typical
clinical symptoms of RB include strabismus, nystagmus, red eyes and
blindness, which are attributed to the position of the tumor
(3). Multiple factors, including
genetic and epigenetic mutation, inactivation of tumor suppressors
and the activation of oncogenes, have been revealed to be closely
associated with the pathogenesis of RB (4,5).
However, the detailed mechanisms responsible for RB occurrence and
development remain undetermined. Despite significant developments
in RB diagnostic and treatment methods, the therapeutic outcomes
for patients remains poor (6–8).
Therefore, assessing the molecular mechanisms of RB formation and
progression may provide the basis for their identification as
promising therapeutic targets for the treatment of this aggressive
disease.
Recently, microRNAs (miRNAs or miRs) have been
revealed to be important in cancer research (9). These endogenous, non-coding and short
RNAs are able to regulate gene expression through the binding of
miRNA ‘seed’ regions to complementary sequences of the
3′-untranslational region (3′-UTR) of target genes, ultimately
causing mRNA degradation and/or translation reduction (10). In total, 4,469 different miRNAs,
including 1,881 precursor and 2,588 mature miRNAs, have been
identified in the human genome (11). A growing body of evidence has
revealed that miRNAs serve a crucial role within carcinogenesis and
cancer progression through their effect on numerous physiological
and pathological processes including cell proliferation, cycle,
apoptosis, angiogenesis, differentiation, metabolism, invasion and
metastasis (12–14). The aberrant expression of miRNAs has
been observed in nearly all types of human cancer, including RB
(15), lung cancer (16), gastric cancer (17) and thyroid cancer (18). Depending on the characteristics of
their targets, miRNAs may serve an oncogenic or tumor suppressor
role within the progression and development of RB (19,20).
However, further investigation into the detailed roles and
mechanisms underlying the effects of dysregulated miRNAs in RB may
reveal effective targets for use in patient therapy.
miR-503 has been reported to be dysregulated,
serving crucial roles in many types of human cancer, including
non-small cell lung cancer (21),
hepatocellular carcinoma (22),
endometrial cancer (23) and
cervical cancer (24). However, the
biological roles and expression patterns of miR-503 in RB have not
yet been fully elucidated. In the current study, miR-503 expression
in RB tissues and cell lines was detected, and the role of miR-503
in the development of RB was also determined. In addition, the
mechanisms underlying the activity of miR-503 in RB were assessed.
The results of the current study indicate that miR-503 may
represent a potential therapeutic target for patients with RB.
Materials and methods
Tissue specimens
The current study was approved by the Ethics
Committee of Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology (Hubei, China). All
participants provided written informed consent for the use of their
clinical tissues. RB specimens were collected from 26 patients with
RB (17 males; 9 females; age range, 15–43 years) who received
surgery at Union Hospital, Tongji Medical College, Huazhong
University of Science and Technology (Hubei, China) between August
2015 and July 2017. A total of 8 normal retinal tissues were
obtained from patients (5 males; 3 females; age range, 28–61 years)
suffering from globe rupture. All patients who received
radiotherapy or chemotherapy were excluded from the current study.
Fresh tissues were frozen in liquid nitrogen followed by transfer
to a −80°C cryogenic refrigerator until further use.
Cell culture
A total of three RB cell lines (SO-RB50, Y79 and
Weri-RB1) and a normal retinal pigmented epithelial cell line,
ARPE-19, were purchased from the American Type Culture Collection.
Cells were cultured at 37°C in a humidified incubator supplied with
5% CO2. DMEM containing 10% v/v heat-inactivated FBS,
100 U/ml penicillin and 100 mg/ml streptomycin (all, Gibco; Thermo
Fisher Scientific, Inc.) was used to culture all cell lines.
Transfection assay
A miR-503 inhibitor and the corresponding negative
control miRNA inhibitor (NC inhibitor) were purchased from Shanghai
GenePharma Co., Ltd. The miR-503 inhibitor sequence was
5′-CUGCAGAACUGUUCCCGCUGCUA-3′ and the NC inhibitor sequence was
5′-ACUACUGAGUGACAGUAGA-3′. For protein tyrosine phosphatase
nonreceptor type 12 (PTPN12) silencing, small interfering RNA
(siRNA) targeting PTPN12 (cat. no. siB0729143707-1-5; PTPN12 siRNA)
or negative control siRNA (cat. no. siN0000002-1-5; NC siRNA) were
purchased from Guangzhou Ribobio Co., Ltd. To restore PTPN12
expression, the PTPN12 overexpression plasmid pcDNA3.1-PTPN12
(pc-PTPN12) and an empty pcDNA3.1 plasmid were constructed by the
Chinese Academy of Sciences. Cells were inoculated into six-well
plates with a density of 8×105 cells per well one night
prior to transfection at 37°C. Transfection of 100 pmol
oligonucleotide, 100 pmol siRNA or 4 µg plasmid was performed using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), in
accordance with manufacturers protocol. The reverse
transcription-quantitative (RT-q) PCR analysis was carried out at
48 h post-transfection. Cell Counting Kit-8 (CCK-8) and in
vitro invasion assays were performed after 24- and 48-h
incubations at 37°C, respectively.
RT-qPCR
Total RNA was extracted from clinical samples and
cells using a Trizol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), in accordance with manufacturer's protocol. The
concentration and purity of total RNA was evaluated using
NanoDrop-2000 (Thermo Fisher Scientific, Inc.).
To detect miR-503, total RNA was reverse transcribed
into cDNA using a TaqMan MicroRNA Reverse Transcription kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions for reverse transcription were as follows:
16°C for 30 min, 42°C for 30 min and 85°C for 5 min. qPCR was
subsequently performed using the Bio-Rad CFX96TM Real-Time PCR
System (Bio-Rad Laboratories, Inc.) with a TaqMan MicroRNA PCR kit
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The
temperature protocol for qPCR was as follows: 50°C for 2 min, 95°C
for 10 min, and 40 cycles of denaturation at 95°C for 15 sec and
annealing/extension at 60°C for 60 sec.
For the quantification of PTPN12 expression, a
PrimeScript RT Reagent kit was used for reverse transcription and
synthesized cDNA was subjected to quantitative PCR using a SYBR
Premix Ex Taq™ (both, Takara Biotechnology Co., Ltd.). The
temperature protocol for reverse transcription was as follows: 37°C
for 15 min and 85°C for 5 sec. The thermocycling conditions for
qPCR were as follows: 5 min at 95°C, followed by 40 cycles of 95°C
for 30 sec and 65°C for 45 sec.
U6 small nuclear RNA and GAPDH were employed as
internal controls to normalize the relative expression of miR-503
and PTPN12, respectively. All data were analyzed using the
2−ΔΔCq method (25). The
primer sequences were as follows: miR-503 forward,
5′-GCGTAGCAGCGGGAACAGT-3′ and reverse, 5′-CCAGTGCGTGTCGTGGAGT-3′;
U6 forward, 5′-GCTTCGGCAGCACATATACTA-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTC-3′; PTPN12 forward,
5′-GCAGGAACAACACATTCAGG-3′ and reverse,
5′-TCCATTCCGATCTTACAGGTG-3′; GAPDH forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′.
CCK-8 assay
Transfected cells were harvested 24 h after
incubation at 37°C and resuspended in DMEM containing 10% FBS. A
total of 3,000 transfected cells in 100 µl culture medium were
inoculated per well into 96-well plates. Cells were incubated at
37°C in an atmosphere supplied with 5% CO2 for 0, 24, 48
and 72 h. A CCK-8 assay was then performed to assess cellular
proliferation at these time points. CCK-8 solution (Dojindo
Molecular Technologies, Inc.; 10 µl) was added into each well
followed by incubation at 37°C for a further 2 h. Absorbance at 450
nm was measured using a microplate reader (Bio-Rad Laboratories,
Inc.).
In vitro invasion assay
The invasive ability of RB cells was determined
using transwell apparatus pre-coated with Matrigel (each, BD
Biosciences). Cells with appropriate transfection treatments were
collected after 48 h of incubation at 37°C and resuspended in
FBS-free DMEM. In total, 5×104 transfected cells were
resuspended in FBS-free DMEM were plated into the upper compartment
of transwell apparatus and bottom compartments were covered with
500 µl DMEM containing 20% FBS. After a 24-h incubation at 37°C,
the non-invasive cells were removed with a cotton swab, while the
invasive cells were fixed with 4% paraformaldehyde at room
temperature for 30 min, stained with 0.5% crystal violet at room
temperature for 30 min and air-dried. Invasive ability was assessed
by counting the number of invasive cells in five random fields of
view using a light microscope (magnification, ×200; CKX41; Olympus
Corporation).
Bioinformatics prediction
The putative genes of miR-503 were predicted using
the miRNA target prediction software, TargetScan (http://www.targetscan.org) and miRDB (http://www.mirdb.org/).
Luciferase reporter assay
The 3′-UTR of PTPN12 containing the wild-type (wt)
miR-503 binding site and its mutant (mut) 3′-UTR were chemically
synthesized by Shanghai GenePharma Co., Ltd. and inserted into the
pMIR-REPORT miRNA Expression Reporter vector (Ambion; Thermo Fisher
Scientific, Inc.) to generate pMIR-wt-PTPN12-3′-UTR and
pMIR-mut-PTPN12-3′-UTR, respectively. One night prior to
transfection, cells were plated into 24-well plates with an initial
density of 1.0×105 cells/well. Co-transfection of a
miR-503 inhibitor or NC inhibitor and pMIR-wt-PTPN12-3′-UTR or
pMIR-mut-PTPN12-3′-UTR was performed using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturers protocol. The Dual-Luciferase® Reporter
Assay system (cat. no. E1910; Promega Corporation) was used to
detect luciferase activity 48 h after transfection. Relative
luciferase activity was normalized to that of Renilla
luciferase activity.
Western blot analysis
A Total Protein Extraction kit (Nanjing KeyGen
Biotech Co., Ltd.) was used to isolate total cellular protein from
cultured cells according to the manufacturer's protocol. The
concentration of total protein was detected using a BCA Protein
Quantification kit (Beyotime Institute of Biotechnology).
Equivalent proteins (30 µg/lane) were resolved on 10% sodium
dodecyl sulfate-polyacrylamide gels, transferred to PVDF membranes
and then blocked with 5% fat-free milk at room temperature for 2 h.
The following primary antibodies were then added and incubated
overnight at 4°C: Rabbit anti-human PTPN12 (1:1,000; cat. no.
ab154892) and rabbit anti-human GAPDH (1:1,000; cat. no. ab181603;
both, Abcam). The membranes were subsequently probed with the goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:5,000; cat. no. ab6721; Abcam) for 1 h at room temperature.
Specific protein bands were developed by an enhanced
chemiluminescence system (EMD Millipore). GAPDH was used as a
loading control. Quantity One software version 4.62 (Bio-Rad
Laboratories, Inc.) was used for the quantification of protein
bands.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS, Inc.). Differences between groups were assessed
using a Student's t-tests or one-way ANOVA. A Student-Newman-Keuls
test was used as a post-hoc test in multiple group analyses. All
data were expressed as the mean ± standard deviation and P<0.05
was considered to indicate a statistically significant result.
Results
Expression of miR-503 is upregulated
in RB tissues and cell lines
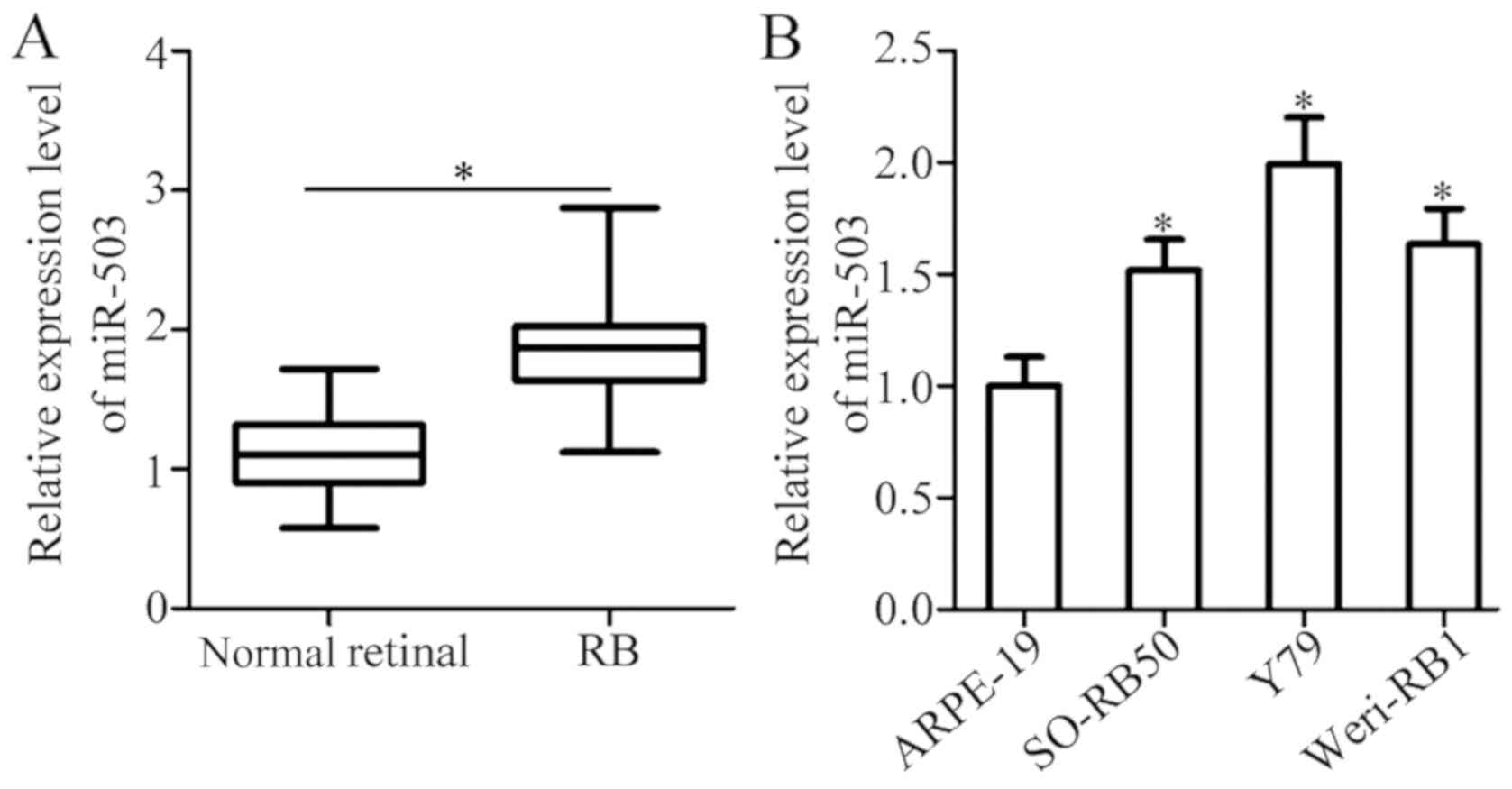
To determine the expression status of miR-503 in RB,
RT-qPCR was performed in 26 RB and 8 normal retinal tissues. The
results reveal that the expression of miR-503 in RB tissues was
significantly higher compared with normal retinal tissues (Fig. 1A; P<0.05). miR-503 expression in
three RB cell lines (SO-RB50, Y79 and Weri-RB1) and a normal
retinal pigmented epithelial cell line (ARPE-19) were subsequently
determined using RT-qPCR. The significant upregulation of miR-503
was observed in all three RB cell lines compared with ARPE-19 cells
(Fig. 1B; P<0.05). The results
demonstrate that miR-503 is highly expressed in RB and that the
upregulation of miR-503 may be associated with RB progression.
miR-503 knockdown suppresses the
proliferation and invasion of RB cells
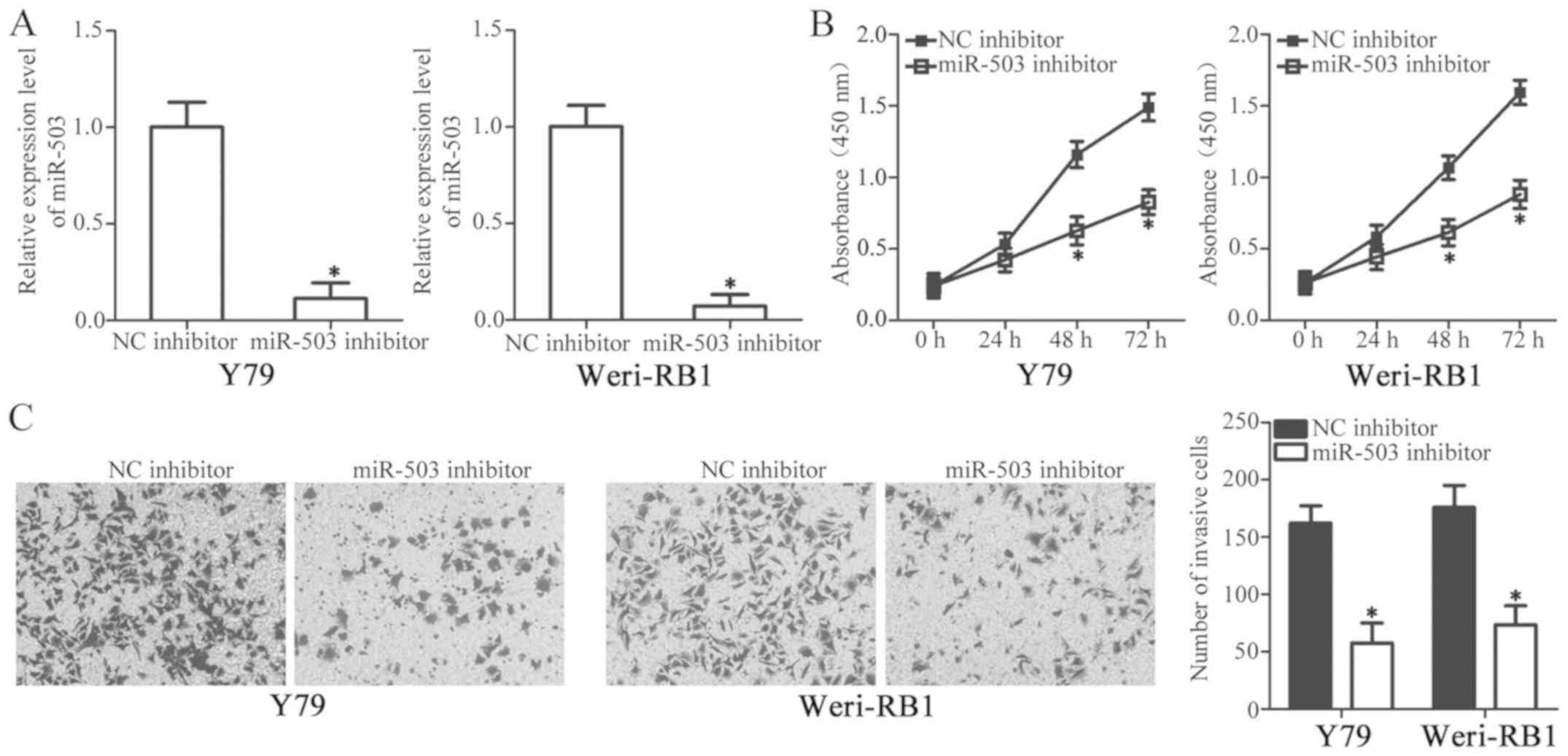
To identify the specific roles of miR-503 in the
development of RB, Y79 and Weri-RB1 cells with the highest relative
miR-503 level among the three RB cell lines were selected for
further study and transfected with a miR-503 inhibitor or NC
inhibitor. RT-qPCR analysis revealed that the transfection of
miR-503 inhibitor significantly decreased miR-503 expression in Y79
and Weri-RB1 cells (Fig. 2A;
P<0.05). Subsequently, a CCK-8 assay was utilized to detect the
proliferative ability of RB cells, which were transfected with the
miR-503 inhibitor or NC inhibitor. The results demonstrated that
the downregulation of miR-503 significantly decreased the
proliferation of Y79 and Weri-RB1 cells compared with NC inhibitor
treated cells after 48 and 72 h (Fig.
2B; P<0.05). Furthermore, an in vitro invasion assay
was used to investigate the effect of miR-503 downregulation on RB
cell invasion. As indicated in Fig.
2C, the inhibition of miR-503 led to a marked decrease in the
invasive capacity of Y79 and Weri-RB1 cells (P<0.05). The
results of the present study indicate that miR-503 may serve an
oncogenic role in RB.
PTPN12 is a direct target gene of
miR-503 in RB cells
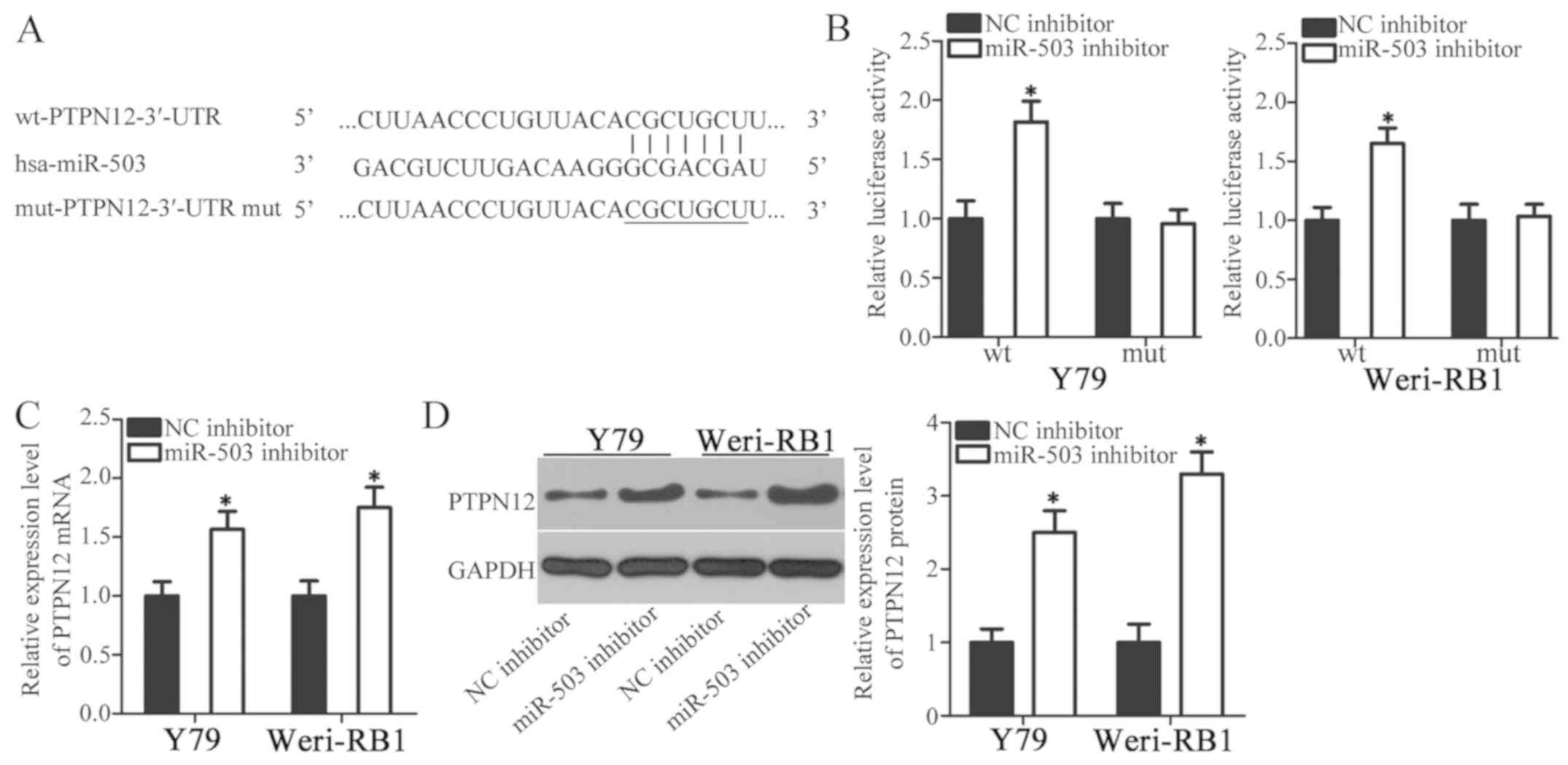
To assess the underlying mechanisms that may be
responsible for the action of miR-503 in RB cells, bioinformatics
analysis was performed to predict the potential targets of miR-503.
PTPN12 possessed miR-503 binding sequences in its 3′-UTR regions
(Fig. 3A). PTPN12 has previously
been demonstrated to serve as a tumor-suppressant in multiple types
of human malignancy, so was selected for additional analysis
(26–29). A luciferase reporter assay was used
to verify the prediction that PTPN12 served a role in the
expression of miR-503. Y79 and Weri-RB1 cells were co-transfected
with a miR-503 inhibitor or an NC inhibitor and
pMIR-wt-PTPN12-3′-UTR or pMIR-mut-PTPN12-3′-UTR. Luciferase
activity detection at 48 h post-transfection revealed that the
downregulation of miR-503 significantly increased the luciferase
activity of the plasmid carrying wild-type miR-503 binding site in
Y79 and Weri-RB1 cells (Fig. 3B;
P<0.05). However, the luciferase activity in Y79 and Weri-RB1
cells co-transfected with the miR-503 inhibitor and
pMIR-mut-PTPN12-3′-UTR was not altered significantly (Fig. 3B). To assess the roles of miR-503 in
the regulation of PTPN12 expression, RT-qPCR and western blot
analysis were performed in order to measure PTPN12 expression in
Y79 and Weri-RB1 cells in response to miR-503 downregulation. The
mRNA (Fig. 3C; P<0.05) and
protein (Fig. 3D; P<0.05)
expression of PTPN12 were significantly upregulated in Y79 and
Weri-RB1 cells treated with the miR-503 inhibitor. Collectively,
these results indicate that PTPN12 may be a direct target gene of
miR-503 in RB cells.
PTPN12 restoration phenocopies the
effects of miR-503 downregulation in RB cells
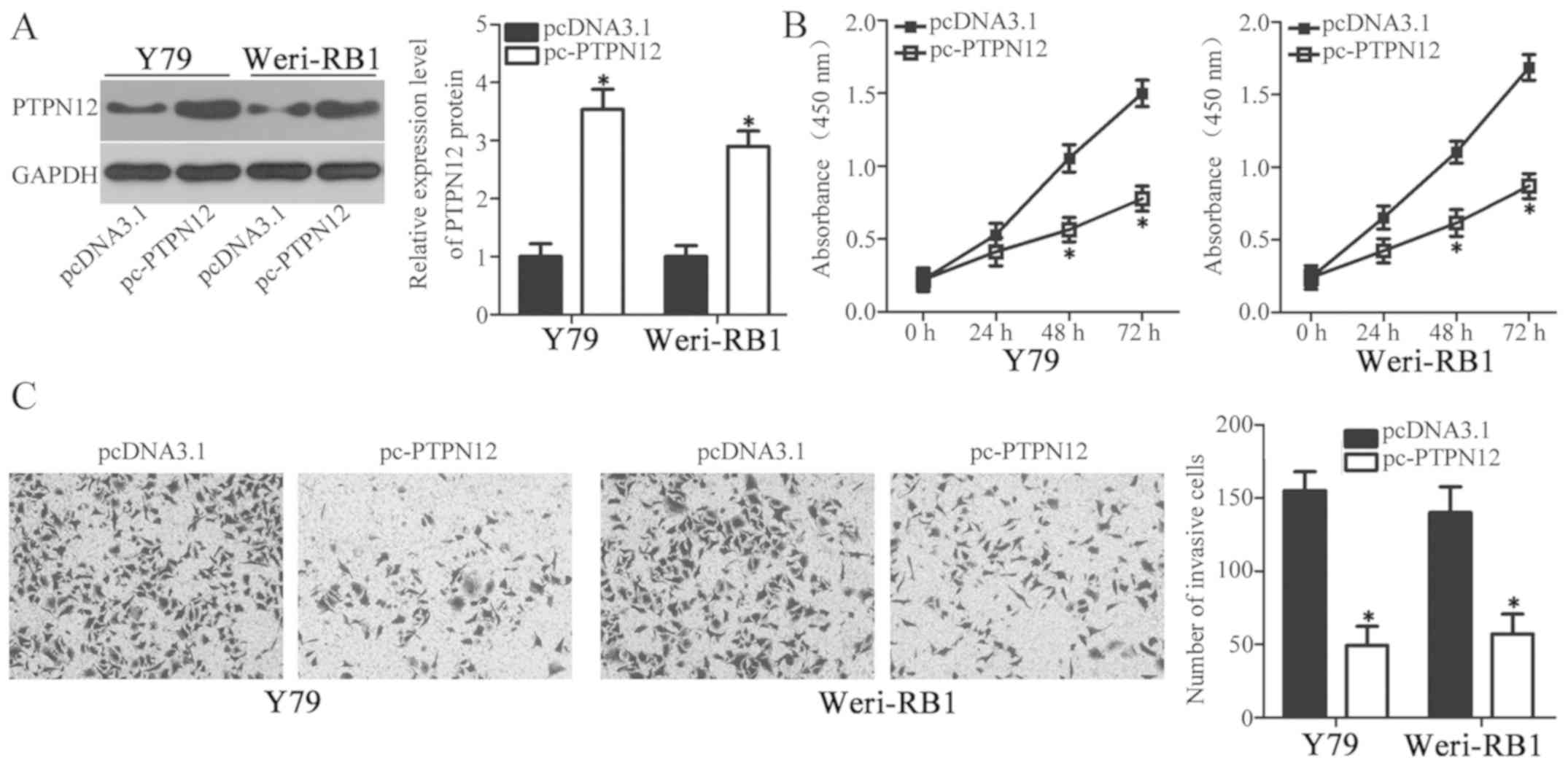
To further assess whether PTPN12 is a direct
functional downstream target of RB cell miR-503, a series of
functional assays were performed to investigate whether the effects
of miR-503 downregulation in RB cells could be achieved by PTPN12
upregulation. Y79 and Weri-RB1 cells were transfected with the
PTPN12 overexpression plasmid pcDNA3.1-PTPN12 (pc-PTPN12) to
significantly enhance PTPN12 expression (Fig. 4A; P<0.05). CCK-8 and in
vitro invasion assays revealed that resumption of PTPN12
expression significantly restricted the proliferation (Fig. 4B; P<0.05) and invasion (Fig. 4C; P<0.05) of Y79 and Weri-RB1
cells, which were similar with those induced by miR-503
downregulation. These results further demonstrate that PTPN12 is a
direct target gene of miR-503 in RB cells.
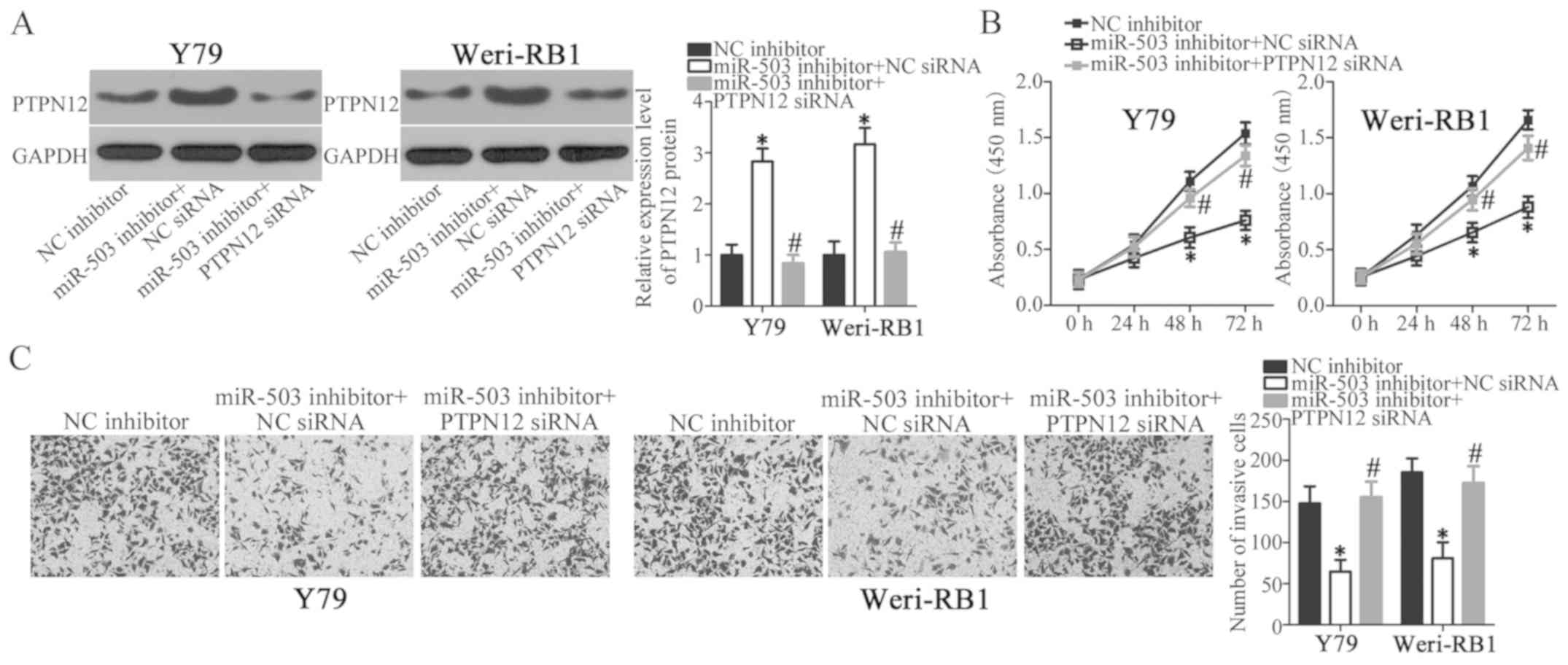
PTPN12 silencing abolishes the effects
of miR-503 knockdown in RB cells
Rescue experiments were performed within the current
study in order to investigate whether PTPN12 was required to
regulate the proliferation and invasion of RB cells mediated by
miR-503 downregulation. Y79 and Weri-RB1 cells were co-transfected
with a miR-503 inhibitor and a specific siRNA targeting PTPN12
(PTPN12 siRNA) or NC siRNA. Western blot analysis revealed that
miR-503 knockdown significantly increased PTPN12 protein expression
in Y79 and Weri-RB1 cells; however, the protein level of PTPN12 was
recovered in Y79 and Weri-RB1 cells after co-transfection with
PTPN12 siRNA (Fig. 5A; P<0.05).
Similarly, PTPN12 silencing abrogated the effects of miR-503
downregulation in Y79 and Weri-RB1 cell proliferation (Fig. 5B; P<0.05) and invasion (Fig. 5C; P<0.05), as determined by CCK-8
and in vitro invasion assays, respectively. Data from the
current study demonstrates that miR-503 downregulation prohibits
the proliferation and invasion of RB cells, at least partly, by
negatively modulating PTPN12 expression.
Discussion
A number of studies have demonstrated that miRNAs
including miR-137 (30), miR-448
(31) and miR-506 (15) are abnormally expressed in RB. It is
now widely accepted that miRNAs may serve roles in tumor-suppressor
or oncogene activity in RB development by modulating various
biological behaviors, including cell proliferation, apoptosis,
angiogenesis and metastasis (32).
miRNAs therefore, may be effective therapeutic targets for
miRNA-based therapy in patients with RB. In the present study,
miR-503 expression in RB tissues and cell lines was detected. In
addition, the detailed roles and underlying mechanisms of miR-503
in RB progression was investigated. The current study may provide
novel insight into RB pathogenesis and offer a promising
therapeutic target for patients with this disease.
miR-503 is downregulated in non-small cell lung
cancer, and this downregulation is significantly correlated with
lymphatic invasion, distant metastasis, TNM stage and tumor grade
(21). Patients with non-small cell
lung cancer and low miR-503 expression possess poorer clinical
outcomes than patients with high miR-503 expression (21). Multivariate analysis identifies
miR-503 as an independent prognostic factor for assessing prognosis
in patients with non-small cell lung cancer (21). Low expression of miR-503 is exhibited
in hepatocellular carcinoma (22),
endometrial cancer (23), cervical
cancer (24), osteosarcoma (33), gastric cancer (34,35),
breast cancer (36) and prostate
cancer (37,38). By contrast, miR-503 is overexpressed
in colorectal (39,40) and oesophageal (41) cancer. However, the expression of
miR-503 in RB remains unclear. In the current study therefore,
RT-qPCR analysis was used for the detection of miR-503 expression
in RB tissues and cell lines. The results of the present study
demonstrated that miR-503 is significantly upregulated in RB
tissues and cell lines. miR-503 may be an attractive biomarker for
the diagnosis of patients with these specific cancer types.
miR-503 serves tumor-suppressive roles in
hepatocarcinogenesis and progression by affecting cell
angiogenesis, cell cycle, growth, metastasis and chemotherapy
sensitivity (22,42–45). In
non-small cell lung cancer, restoration of miR-503 expression
inhibits cell proliferation and metastasis in vitro and
in vivo and improves chemosensitivity to cisplatin (46,47). In
endometrial cancer, miR-503 upregulation attenuates cell viability,
colon formation ability and cell-cycle status in vitro
(23). In gastric cancer, miR-503
re-expression restricts cell proliferation, migration, invasion,
epithelial-to-mesenchymal transition and cisplatin resistance
(34,35). In prostate cancer, resumption of
miR-503 expression suppresses cell colony formation in
vitro; decreases tumor growth and metastasis in vitro
and in vivo (37,38). In contrast, miR-503 serves as an
oncogene in colorectal (39,40) and oesophageal (41) cancer and participates in the
regulation of biological behaviors associated with tumorigenesis
and tumor development. However, the specific roles of miR-503 in RB
development remain largely unknown. In the current study, CCK-8 and
in vitro invasion assays were used to investigate the
effects of miR-503 underexpression in RB cell proliferation and
invasion, respectively. The results of the present study
demonstrated that the downregulation of miR-503 impedes the
proliferative and invasive abilities of RB cells, indicating that
miR-503 may be a potential therapeutic target for the anticancer
therapy of patients with these human malignancies types.
Various genes have previously been identified as
direct targets of miR-503, including fibroblast growth factor 2
(22), vascular endothelial growth
factor A (22), cyclin D3 (42), transcription factor E2F3 (42) and protein arginine
N-methyltransferase 1 (43). In the
current study, PTPN12 was demonstrated to be a direct target gene
of miR-503 in RB. PTPN12, is a member of the Protein tyrosine
phosphatases family (48) and is
frequently downregulated in several types of human cancer,
including nasopharyngeal carcinoma (28), ovarian cancer (49), breast cancer (50) and hepatocellular carcinoma (51). PTPN12 serves as a tumor-suppressor in
cancer initiation and progression by regulating a wide range of
biological processes, including cell proliferation, apoptosis,
migration, invasion, metastasis and chemotherapeutic resistance
(26–29). The current study demonstrated that
PTPN12 upregulation inhibits the proliferation and invasion of RB
cells and that the inhibition of miR-503 directly targets PTPN12,
suppressing the progression of RB. Therefore, miR-503 knockdown or
the restoration of PTPN12 expression may be potential therapeutic
techniques for patients with RB.
In conclusion, the results of the current study
revealed that miR-503 was significantly upregulated in human RB
tissues and cell lines. The downregulation of miR-503 inhibited the
proliferation and invasion of RB cells by directly binding to the
3′-UTR of PTPN12 and negatively regulating its expression. Due to
this, miR-503 may provide further insight into RB development and
offer a valuable therapeutic target for improving the outcomes of
patients with RB. The current study included two limitations.
Firstly, the correlation between clinical factors and miR-503
expression in patients with RB was not investigated. Secondly, the
effects miR-503 overexpression RB cells were not examined. These
limitations should be resolved in future study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC and WL designed the research. YC performed
RT-qPCR, CCK-8 and in vitro invasion assays. WL performed
western blot analysis, luciferase reporter assay and statistical
analysis. All authors read and approved the final draft.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Union Hospital, Tongji Medical College, Huazhong of
Science and Technology (Hubei, China), and was performed in
accordance with the Declaration of Helsinki and the guidelines of
the Ethics Committee of Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology. Written informed
consent was obtained from all patients for the use of their
clinical tissues.
Patient consent for publication
Patient consent for publication was obtained.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Broaddus E, Topham A and Singh AD:
Incidence of retinoblastoma in the USA: 1975–2004. Br J Ophthalmol.
93:21–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramson DH, Beaverson K, Sangani P, Vora
RA, Lee TC, Hochberg HM, Kirszrot J and Ranjithan M: Screening for
retinoblastoma: Presenting signs as prognosticators of patient and
ocular survival. Pediatrics. 112:1248–1255. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beta M, Venkatesan N, Vasudevan M,
Vetrivel U, Khetan V and Krishnakumar S: Identification and
insilico analysis of retinoblastoma serum microRNA profile and gene
targets towards prediction of novel serum biomarkers. Bioinform
Biol Insights. 7:21–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Wang X, Wu G, Hou D and Hu Q:
MiR-365b-3p, down-regulated in retinoblastoma, regulates cell cycle
progression and apoptosis of human retinoblastoma cells by
targeting PAX6. FEBS Lett. 587:1779–1786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jabbour P, Chalouhi N, Tjoumakaris S,
Gonzalez LF, Dumont AS, Chitale R, Rosenwasser R, Bianciotto CG and
Shields C: Pearls and pitfalls of intraarterial chemotherapy for
retinoblastoma. J Neurosurg Pediatr. 10:175–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaliki S, Shields CL, Rojanaporn D,
Al-Dahmash S, McLaughlin JP, Shields JA and Eagle RC Jr: High-risk
retinoblastoma based on international classification of
retinoblastoma: Analysis of 519 enucleated eyes. Ophthalmology.
120:997–1003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Canturk S, Qaddoumi I, Khetan V, Ma Z,
Furmanchuk A, Antoneli CB, Sultan I, Kebudi R, Sharma T,
Rodriguez-Galindo C, et al: Survival of retinoblastoma in
less-developed countries impact of socioeconomic and health-related
indicators. Br J Ophthalmol. 94:1432–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin S and Gregory RI: MicroRNA biogenesis
pathways in cancer. Nat Rev Cancer. 15:321–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagnyukova TV, Pogribny IP and Chekhun VF:
MicroRNAs in normal and cancer cells: A new class of gene
expression regulators. Exp Oncol. 28:263–269. 2006.PubMed/NCBI
|
|
11
|
D'Angelo B, Benedetti E, Cimini A and
Giordano A: MicroRNAs: A puzzling tool in cancer diagnostics and
therapy. Anticancer Res. 36:5571–5575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu L, Chen Z and Xing Y: MiR-506-3p
inhibits cell proliferation, induces cell cycle arrest and
apoptosis in retinoblastoma by directly targeting NEK6. Cell Biol
Int. 2018. View Article : Google Scholar
|
|
16
|
Jin RH, Yu DJ and Zhong M: MiR-1269a acts
as an onco-miRNA in non-small cell lung cancer via down-regulating
SOX6. Eur Rev Med Pharmacol Sci. 22:4888–4897. 2018.PubMed/NCBI
|
|
17
|
Liu F, Hu H, Zhao J, Zhang Z, Ai X, Tang L
and Xie L: miR-124-3p acts as a potential marker and suppresses
tumor growth in gastric cancer. Biomed Rep. 9:147–155.
2018.PubMed/NCBI
|
|
18
|
Huang Y, Yu S, Cao S, Yin Y, Hong S, Guan
H, Li Y and Xiao H: MicroRNA-222 promotes invasion and metastasis
of papillary thyroid cancer through targeting protein phosphatase 2
regulatory subunit B alpha expression. Thyroid. 28:1162–1173. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J and You X: MicroRNA-758 inhibits
malignant progression of retinoblastoma by directly targeting PAX6.
Oncol Rep. 40:1777–1786. 2018.PubMed/NCBI
|
|
20
|
Yang L, Wei N, Wang L, Wang X and Liu QH:
miR-498 promotes cell proliferation and inhibits cell apoptosis in
retinoblastoma by directly targeting CCPG1. Childs Nerv Syst.
34:417–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu L, Qu W and Zhong Z: Down-regulation
of miR-503 expression predicate advanced mythological features and
poor prognosis in patients with NSCLC. Int J Clin Exp Pathol.
8:5609–5613. 2015.PubMed/NCBI
|
|
22
|
Zhou B, Ma R, Si W, Li S, Xu Y, Tu X and
Wang Q: MicroRNA-503 targets FGF2 and VEGFA and inhibits tumor
angiogenesis and growth. Cancer Lett. 333:159–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu YY, Wu HJ, Ma HD, Xu LP, Huo Y and Yin
LR: MicroRNA-503 suppresses proliferation and cell-cycle
progression of endometrioid endometrial cancer by negatively
regulating cyclin D1. FEBS J. 280:3768–3779. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yin ZL, Wang YL, Ge SF, Guo TT, Wang L,
Zheng XM and Liu J: Reduced expression of miR-503 is associated
with poor prognosis in cervical cancer. Eur Rev Med Pharmacol Sci.
19:4081–4085. 2015.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su Z, Tian H, Song HQ, Zhang R, Deng AM
and Liu HW: PTPN12 inhibits oral squamous epithelial carcinoma cell
proliferation and invasion and can be used as a prognostic marker.
Med Oncol. 30:6182013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Villa-Moruzzi E: PTPN12 controls PTEN and
the AKT signalling to FAK and HER2 in migrating ovarian cancer
cells. Mol Cell Biochem. 375:151–157. 2013.PubMed/NCBI
|
|
28
|
Lin Q, Wang H, Lin X, Zhang W, Huang S and
Zheng Y: PTPN12 affects nasopharyngeal carcinoma cell proliferation
and migration through regulating EGFR. Cancer Biother Radiopharm.
33:60–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang YY, Liu H, Mao XY, Jin F, Ma B, Jiang
JY and Cao Y: Identifying the role of PTPN12 expression in
predicting the efficacy of capecitabine to neoadjuvant chemotherapy
in breast cancer treatment. Eur Rev Med Pharmacol Sci.
20:3400–3409. 2016.PubMed/NCBI
|
|
30
|
Zhang J, He J and Zhang L: The
down-regulation of microRNA-137 contributes to the up-regulation of
retinoblastoma cell proliferation and invasion by regulating
COX-2/PGE2 signaling. Biomed Pharmacother. 106:35–42. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu S, Ai N, Liu Q and Zhang J: MicroRNA448
inhibits the progression of retinoblastoma by directly targeting
ROCK1 and regulating PI3K/AKT signalling pathway. Oncol Rep.
39:2402–2412. 2018.PubMed/NCBI
|
|
32
|
Golabchi K, Soleimani-Jelodar R, Aghadoost
N, Momeni F, Moridikia A, Nahand JS, Masoudifar A, Razmjoo H and
Mirzaei H: MicroRNAs in retinoblastoma: Potential diagnostic and
therapeutic biomarkers. J Cell Physiol. 233:3016–3023. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chong Y, Zhang J, Guo X, Li G, Zhang S, Li
C, Jiao Z and Shao M: MicroRNA-503 acts as a tumor suppressor in
osteosarcoma by targeting L1CAM. PLoS One. 9:e1145852014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Peng Y, Liu YM, Li LC, Wang LL and Wu XL:
microRNA-503 inhibits gastric cancer cell growth and
epithelial-to-mesenchymal transition. Oncol Lett. 7:1233–1238.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang T, Ge G, Ding Y, Zhou X, Huang Z, Zhu
W, Shu Y and Liu P: MiR-503 regulates cisplatin resistance of human
gastric cancer cell lines by targeting IGF1R and BCL2. Chin Med J
(Engl). 127:2357–2362. 2014.PubMed/NCBI
|
|
36
|
Long J, Ou C, Xia H, Zhu Y and Liu D:
MiR-503 inhibited cell proliferation of human breast cancer cells
by suppressing CCND1 expression. Tumour Biol. 36:8697–8702. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo J, Liu X and Wang M: miR-503
suppresses tumor cell proliferation and metastasis by directly
targeting RNF31 in prostate cancer. Biochem Biophys Res Commun.
464:1302–1308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chi Y, Ding F, Zhang W and Du L:
microRNA-503 suppresses the migration, proliferation and colony
formation of prostate cancer cells by targeting tumor protein D52
like 2. Exp Ther Med. 15:473–478. 2018.PubMed/NCBI
|
|
39
|
Noguchi T, Toiyama Y, Kitajima T, Imaoka
H, Hiro J, Saigusa S, Tanaka K, Inoue Y, Mohri Y, Toden S and
Kusunoki M: miRNA-503 promotes tumor progression and is associated
with early recurrence and poor prognosis in human colorectal
cancer. Oncology. 90:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li L, Zhang X, Yi Z, Liang X, Yin W and Li
S: MiR-503 promotes the migration and invasion of colorectal cancer
cells by regulating PDCD4. J BUON. 23:579–586. 2018.PubMed/NCBI
|
|
41
|
Ide S, Toiyama Y, Shimura T, Kawamura M,
Yasuda H, Saigusa S, Ohi M, Tanaka K, Mohri Y and Kusunoki M:
MicroRNA-503 promotes tumor progression and acts as a novel
biomarker for prognosis in oesophageal cancer. Anticancer Res.
35:1447–1451. 2015.PubMed/NCBI
|
|
42
|
Xiao F, Zhang W, Chen L, Chen F, Xie H,
Xing C, Yu X, Ding S, Chen K, Guo H, et al: MicroRNA-503 inhibits
the G1/S transition by downregulating cyclin D3 and E2F3 in
hepatocellular carcinoma. J Transl Med. 11:1952013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li B, Liu L, Li X and Wu L: miR-503
suppresses metastasis of hepatocellular carcinoma cell by targeting
PRMT1. Biochem Biophys Res Commun. 464:982–987. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xiao Y, Tian Q, He J, Huang M, Yang C and
Gong L: MiR-503 inhibits hepatocellular carcinoma cell growth via
inhibition of insulin-like growth factor 1 receptor. Onco Targets
Ther. 9:3535–3544. 2016.PubMed/NCBI
|
|
45
|
Yang X, Zang J, Pan X, Yin J, Xiang Q, Yu
J, Gan R and Lei X: miR-503 inhibits proliferation making human
hepatocellular carcinoma cells susceptible to 5-fluorouracil by
targeting EIF4E. Oncol Rep. 37:563–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang Y, Liu L, Zhang Y, Guan H, Wu J, Zhu
X, Yuan J and Li M: MiR-503 targets PI3K p85 and IKK-β and
suppresses progression of non-small cell lung cancer. Int J Cancer.
135:1531–1542. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qiu T, Zhou L, Wang T, Xu J, Wang J, Chen
W, Zhou X, Huang Z, Zhu W, Shu Y and Liu P: miR-503 regulates the
resistance of non-small cell lung cancer cells to cisplatin by
targeting Bcl-2. Int J Mol Med. 32:593–598. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tonks NK: Protein tyrosine phosphatases:
From genes, to function, to disease. Nat Rev Mol Cell Biol.
7:833–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang T, Li L, Cheng Y, Ren C and Zhang G:
MicroRNA-194 promotes the growth, migration and invasion of ovarian
carcinoma cells by targeting protein tyrosine phosphatase
nonreceptor type 12. Onco Targets Ther. 9:4307–4315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li J, Davidson D, Martins Souza C, Zhong
MC, Wu N, Park M, Muller WJ and Veillette A: Loss of PTPN12
stimulates progression of ErbB2-dependent breast cancer by
enhancing cell survival, migration and epithelial-to-mesenchymal
transition. Mol Cell Biol. 35:4069–4082. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Luo RZ, Cai PQ, Li M, Fu J, Zhang ZY, Chen
JW, Cao Y, Yun JP, Xie D and Cai MY: Decreased expression of PTPN12
correlates with tumor recurrence and poor survival of patients with
hepatocellular carcinoma. PLoS One. 9:e855922014. View Article : Google Scholar : PubMed/NCBI
|