Introduction
Hypertrophic cardiomyopathy (HCM) is an inherited
disorder of the cardiac muscle caused by a genetic defect and
affects approximately one in 500 individuals (1). Patients with HCM present with multiple
morphologic variants. Obstructive hypertrophic cardiomyopathy (OHC)
is a classic pathophysiological feature of HCM, characterized by
unexplained left ventricular (LV) hypertrophy in association with
non-dilated ventricular chambers (1). OHC is associated with an increased risk
of clinical deterioration and cardiovascular mortality (1). Obstruction can cause an increase in LV
systolic pressure, which leads to prolonged ventricular relaxation,
elevated LV diastolic pressure, mitral regurgitation, myocardial
ischemia and decreased cardiac output (2–4). The
presence of an aneurysmal apex in association with mid-ventricular
obstruction is a rare morphological subgroup within the
heterogeneous cardiomyopathy phenotypic spectrum (5). Mid-ventricular OHC (MVOHC) with apical
aneurysm portends a wide spectrum of adverse outcomes, including
ventricular arrhythmias, thromboembolism, heart failure and sudden
cardiac death (6). The present study
presents a rare case of MVOHC combined with apical aneurysm and its
management.
Case presentation
A 61-year-old Chinese male was admitted to his local
cardiology department in June 2016 due to episodes of syncope after
palpitation at rest. The patient had no family history of heart
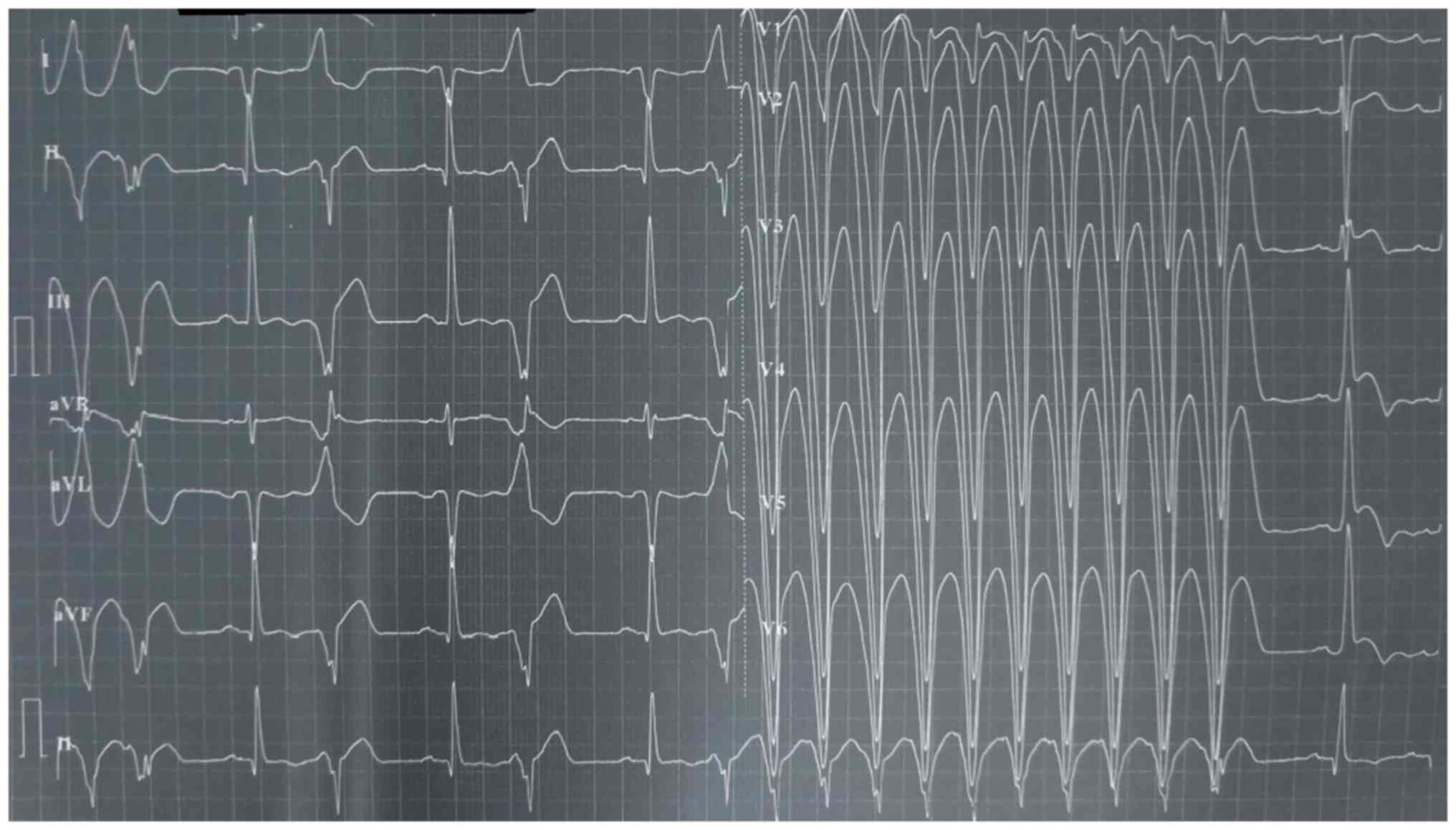
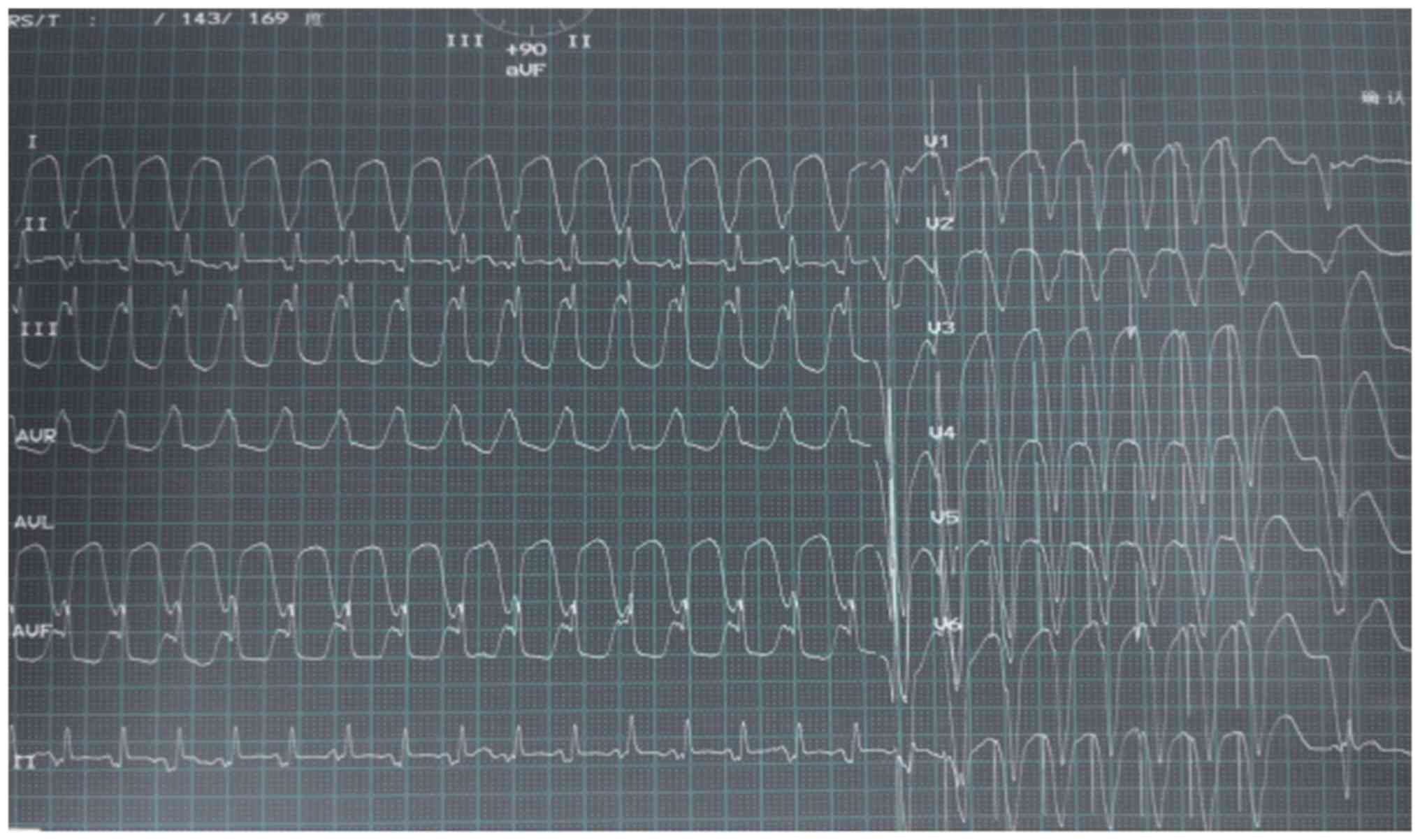
disease and his electrocardiogram indicated monomorphic ventricular
tachycardia (Fig. 1). The initial
troponin I level was 42.96 ng/ml and the patient was diagnosed with
ST-segment elevation myocardial infarction. The abnormal
electrocardiogram prompted invasive coronary angiography, which
provided no evidence of obstructive coronary artery disease. The
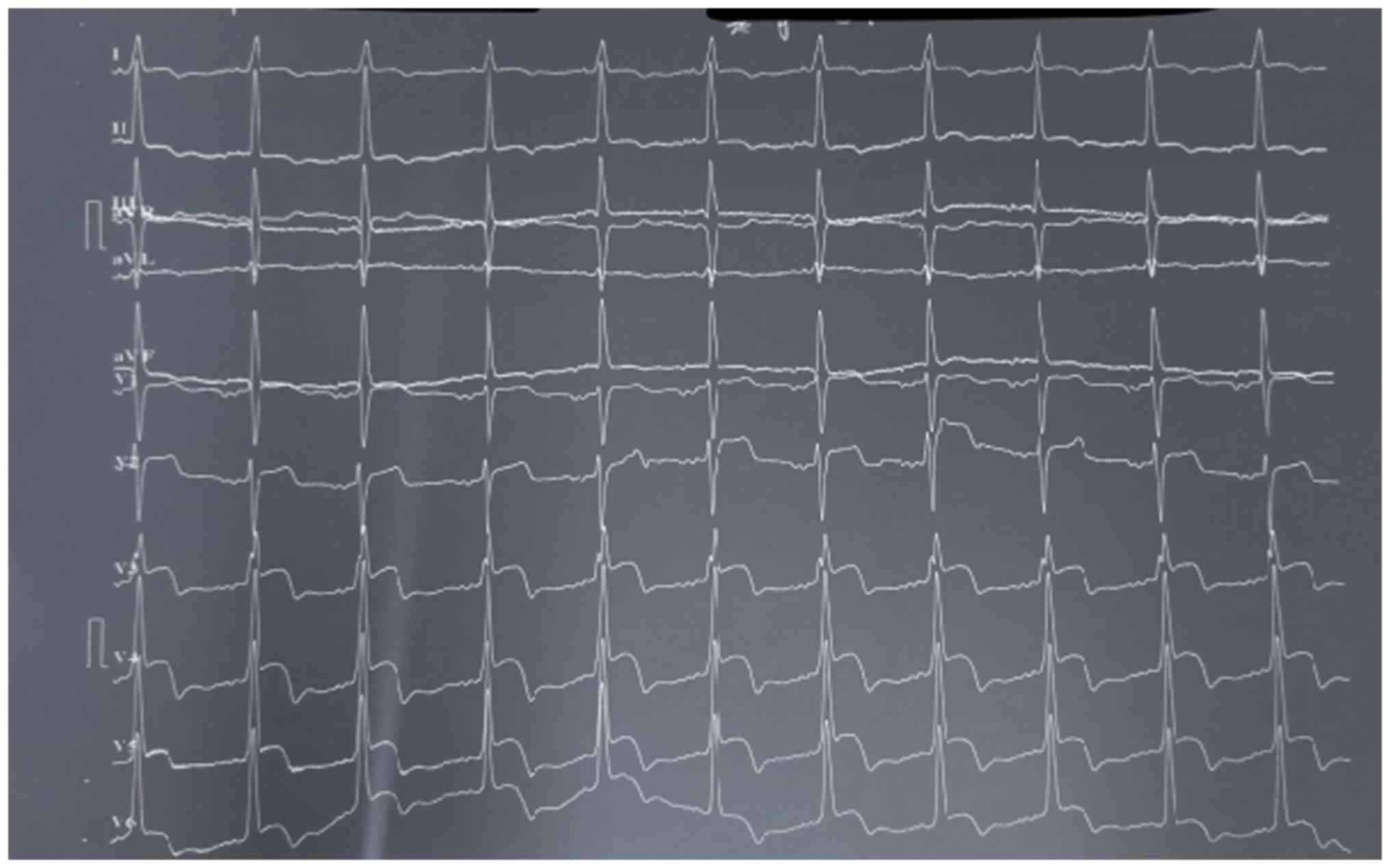
electrocardiogram that was then performed exhibited sinus rhythm,
ST elevation and T wave abnormality (Fig. 2). The sinus rhythm was quickly
restored but could not be maintained.
After 10 days, the patient was transferred to the
Department of Cardiology of West China Hospital (Chengdu, China).
The electrocardiogram revealed sinus rhythm, ST elevation (≥1 mm)
and T wave abnormality in V2 through V6 (negative T waves).
Stabilization of the patient was obtained after electric
cardioversion and concomitant use of β-blockers and amiodarone.
Coronary angiograms indicated no narrowing of any major epicardial
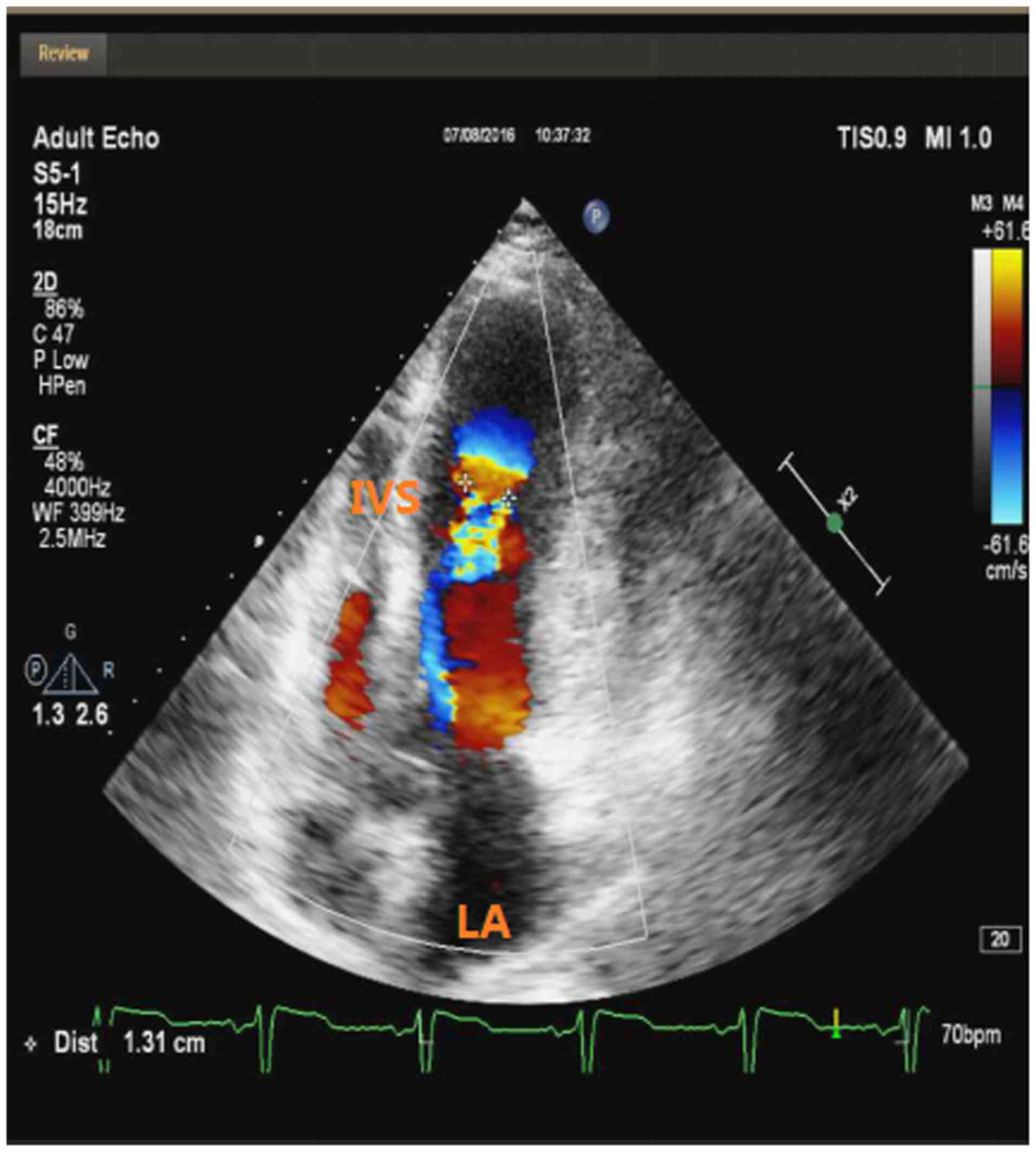
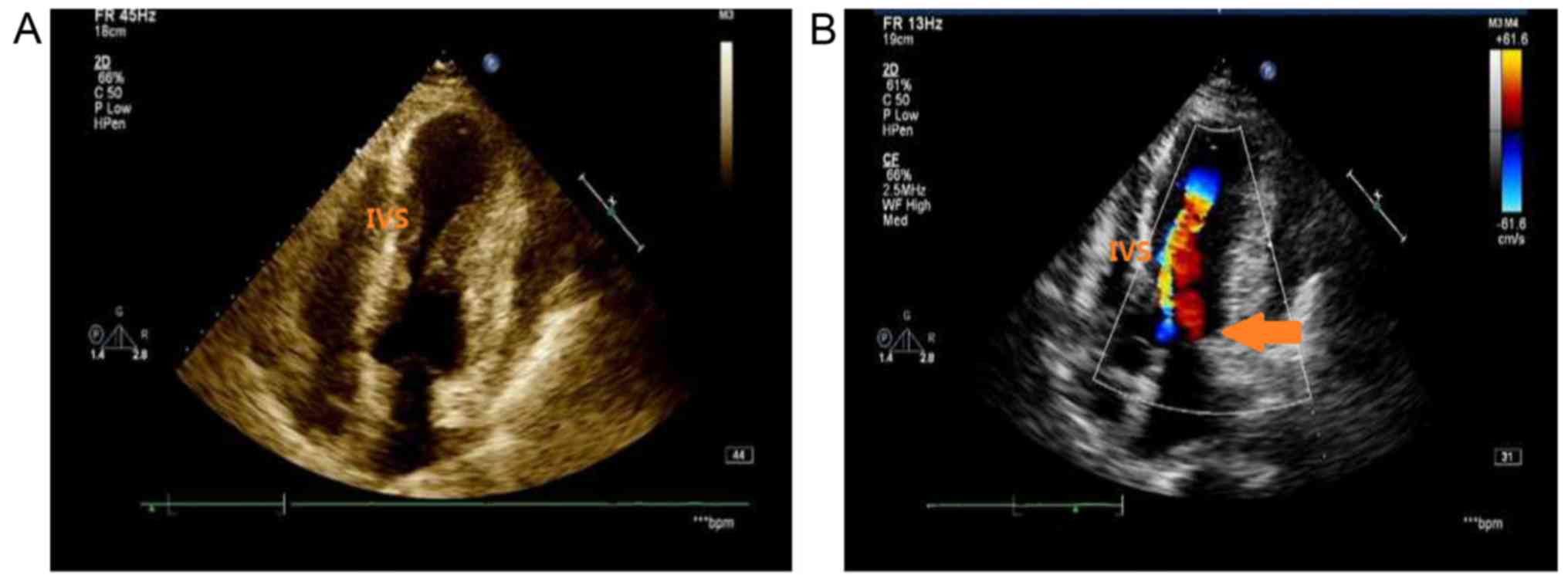
coronary arteries. Transthoracic echocardiography revealed normal
dimensions of the LV (48 mm) with mid-cavity hypertrophy (maximal
wall thickness in the interventricular septum, 25 mm), left atrial
dilatation (39 mm), a measured ejection fraction of 62% and an
aneurysmal apex (Fig. 3). A
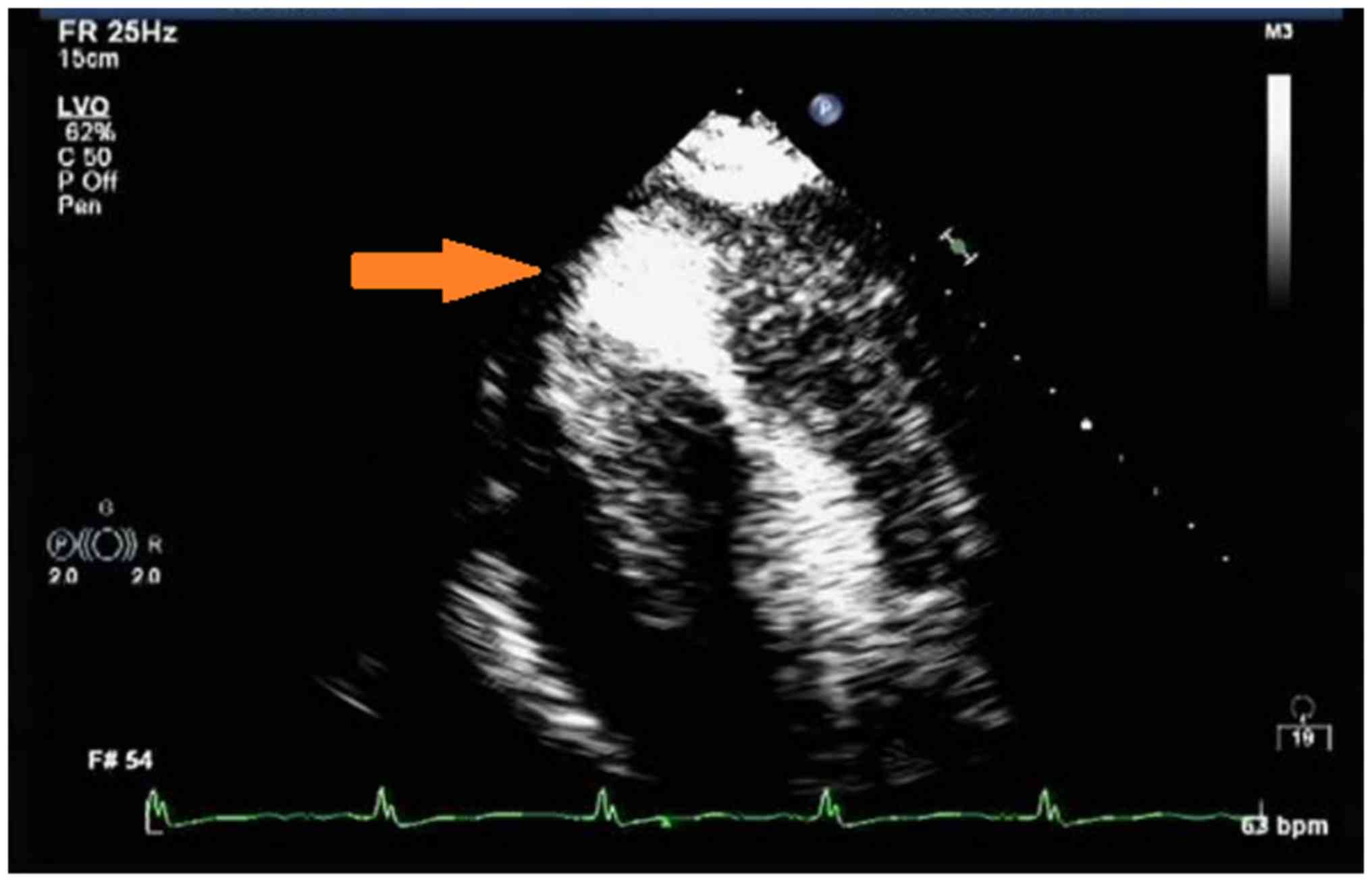
subsequent cardiac acoustic contrast demonstrated clear evidence of
HCM with an hourglass shape of the LV and a 35×32 mm apical
aneurysm (Fig. 4). The patient then
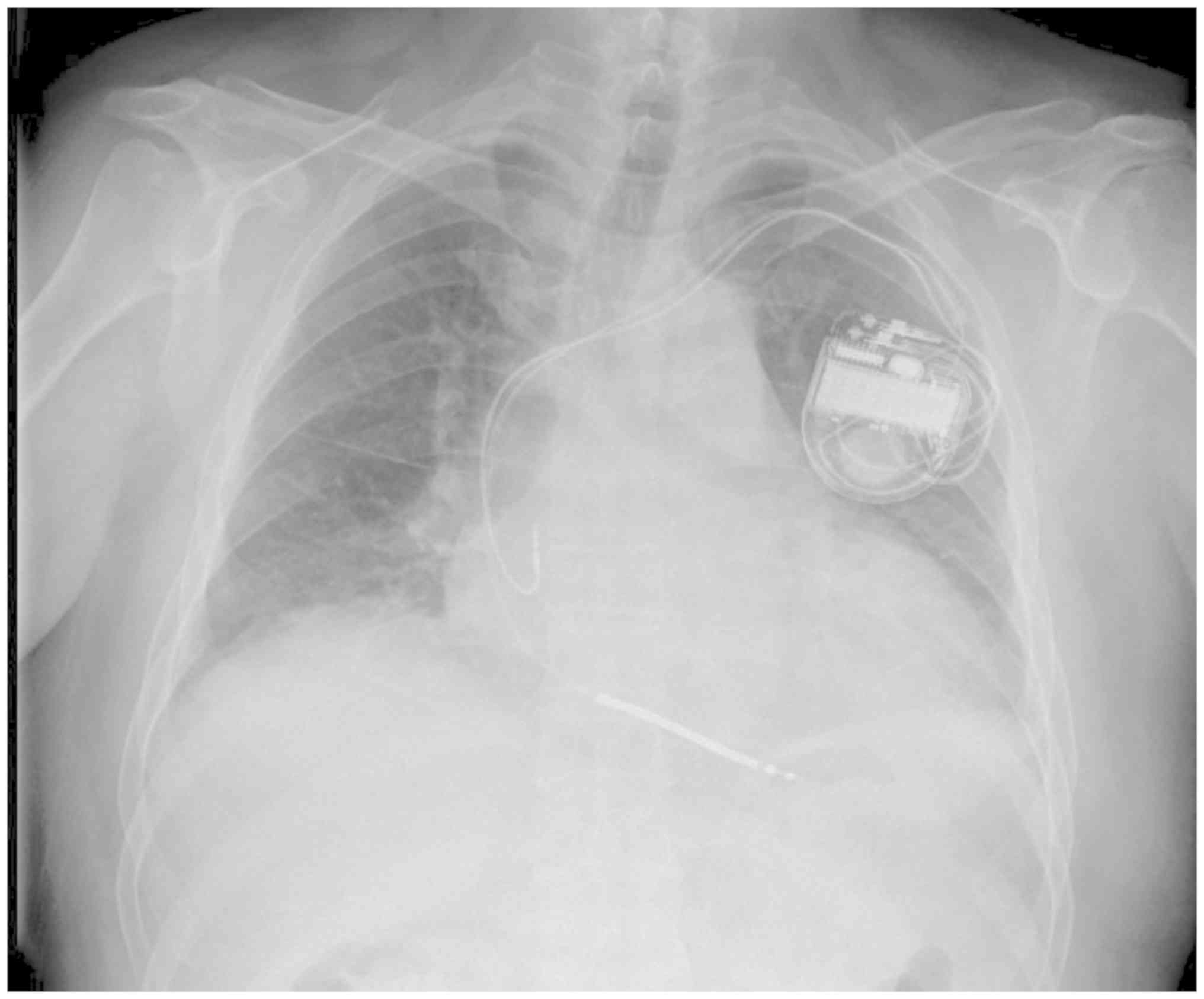
underwent dual chamber implantable cardioverter defibrillator (ICD)
implantation for prevention of fatal ventricular arrhythmias
(Fig. 5). Subsequently, the patient
remained clinically and hemodynamically stable.
One year later, the patient experienced an
electrical storm with 100 episodes of sustained ventricular
tachycardia (VT) and multiple ICD shock (Fig. 6). Recurrent sustained monomorphic VT
was terminated by increased doses of amiodarone and β-blocker.
Furthermore, the patient experienced dyspnea on exertion. On
transthoracic echocardiography, an apical aneurysm and a provoked
mid-ventricular gradient of 64 mmHg were noted. The patient refused
surgical treatment and but provided written informed consent to
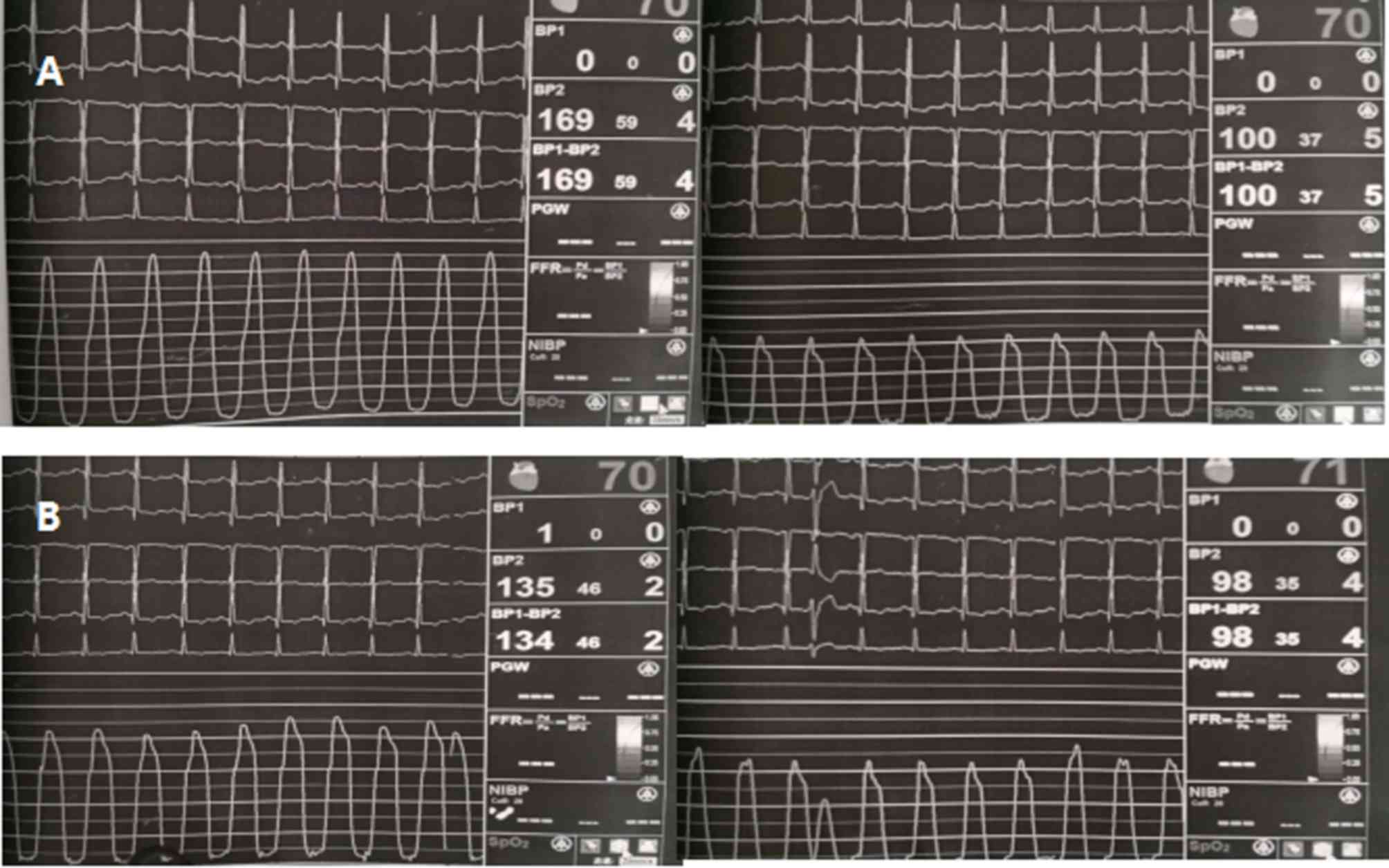
undergo alcohol septal ablation. Hemodynamic assessment revealed a
significant intra-ventricular pressure gradient of 69 mmHg (LV
apex, 169 mmHg; LV outflow, 100 mmHg; Fig. 7A). Repeated hemodynamic measurements
after the procedure revealed a decrease of the post-extrasystolic
gradient to 37 mmHg (LV apex, 135 mmHg; LV outflow, 98 mmHg) at the
mid-ventricular level (Fig. 7B). The
fourth and the fifth septal branches were dominant and echocontrast
injection into target vessels resulted in the largest echocontrast
view in the hypertrophic muscle segment. Therefore, the fourth and
the fifth septal branches were assumed as target vessels and
occlusion of these vessels resulted in a significant decrease in
the intraventricular pressure gradient. The post-procedural
echocardiographic examination indicated reductions in the left
atrial dimension to 30 mm, intra-ventricular septum thickness to 20
mm and a mid-ventricular peak systolic gradient of 19 mmHg. There
were no further VT episodes during treatment with amiodarone (200
mg, once a day) and metoprolol (47.5 mg, twice per day).
At the 6-month follow-up, the patient had developed
dyspnea on exertion (New York Heart Association functional class
III (7). Echocardiography indicated
marked intracavitary LV obstruction at the mid-ventricular level,
with a peak gradient of 80 mmHg. The LV ejection fraction was 45%
(Fig. 8).
Discussion
According to the 2011 American College of Cardiology
Foundation (ACCF)/American Heart Association (AHA) guidelines, the
clinical entity HCM is defined as a disease state characterized by
unexplained LV hypertrophy associated with nondilated ventricular
chambers in the absence of any other cardiac or systemic disease
(8). Based on the electrocardiogram
at the onset of the disease, indicating persistent ST-segment
elevation associated with T-wave inversion in the left precordial
leads, the absence of obstructive coronary artery disease and clear
evidence of HCM with an apical aneurysm on Doppler
echocardiography, the patient of the present study was diagnosed
with MVOHC and apical aneurysm.
Development of an apical aneurysm from MVO was
observed in a single-center patient cohort during the 4-year
follow-up period (9). Maron et
al (5) hypothesized that apical
aneurysm formation and the decrease in cardiac output may be
secondary to increased LV wall stress as a result of mid-cavity LV
obstruction and elevated intracavitary systolic pressure. Increased
wall stress imposes an increased pressure load on the apical
myocardium, increasing the oxygen demand, while simultaneously
reducing the coronary flow through extravascular compression of the
coronary artery, leading to chronic myocardial ischemia and
aneurysm formation (5). MVOHC with
apical aneurysm was reported to have a poor long-term outcome, with
a high risk of sudden death and potentially lethal arrhythmic
events (6,10). The conventional primary prevention
risk markers in individual HCM patients are the following (1): i) Family history of ≥1 HCM-associated
sudden cardiac death; ii) ≥1 episode of unexplained, recent
syncope; iii) massive LV hypertrophy (thickness, ≥30 mm); iv)
unsustained VT on serial ambulatory 24-h (Holter)
electrocardiograms; and v) hypotensive or attenuated blood pressure
response to exercise. ICDs following cardiac arrest or sustained VT
in HCM patients for secondary prevention have achieved widespread
acceptance (8,11). A previous study also reported that
the presence of an apical aneurysm may be a novel risk factor
necessitating ICD implantation (5).
Considering the clinical context associated with the patient's
hemodynamic instability, the patient of the present study received
an ICD for prevention of fatal ventricular arrhythmias.
One year later, the patient experienced an
electrical storm with 100 episodes of sustained VT and multiple ICD
shock. Recurrent sustained monomorphic VT was terminated by
pharmacologic therapy with increased doses of amiodarone and
β-blocker. Catheter ablation is a controversial method for patients
with MVOHC and apical aneurysm. VT recurrence in patients with
non-ischaemic cardiomyopathy attributed to the myocardial tissue
scarring of pathogenetic processes (12). A previous study reported on
monomorphic VT in a case of MVOHC with apical aneurysm, and VT
recurred on the next day after ablation (13). In addition, acute ablation failure is
frequently associated with inability to identify the VT focus and
control VT by catheter ablation (14). In the patient of the present study,
ICD was implanted to prevent sudden death due to ventricular
arrhythmias. Furthermore, amiodarone and β-blocker were
administered to prevent arrhythmic recurrences. Therefore, the VT
ablation was not performed.
According to the 2011 ACCF/AHA Guidelines for the
Diagnosis and Treatment of HC, patients with marked LV outflow
tract obstruction (peak instantaneous gradient at rest or with
exercise, ≥50 mmHg) whose severe symptoms are refractory to maximum
tolerate medical therapy and invasive relief of outflow tract
obstruction should be considered (8). For HCM with subaortic obstruction and
severe drug-refractory symptoms, surgical septal myectomy should be
the gold standard for septal reduction therapy in centers with
comprehensive experience (15).
Alcohol septal ablation (ASA) has been proposed as a less invasive
alternative to surgical myectomy in patients with advanced age,
those that had previously received a pacemaker or defibrillator, as
well as those with significant comorbidities and a strong aversion
to surgery (16,17). Seggewiss and Faber (18) have reported on a case of
mid-ventricular obstruction, which responded favorably to alcohol
ablation. However, another previous study compared the
effectiveness of ASA and transaortic extended myectomy (TEM) in HCM
with MVO. The results indicated that TEM may provide a more
reliable reduction in gradients compared to ASA, while serious
ventricular arrhythmias were uncommon after either of the two
therapies (19). Furthermore, a
recent clinical study reported that surgical myectomy appeared to
be protective against further malignant arrhythmias (20). Considering the high risk of surgery,
the patient refused surgical treatment and underwent ASA for relief
of intracavitary obstruction.
At the 6-month follow-up, rebound of the gradient
following alcohol ablation was observed with an LV ejection
fraction of 45% determined by echocardiography. Previous studies
have reported that deterioration in LV function may due to aneurysm
formation, leading to primary regional systolic impairment and
cavity dilatation as a compulsory remodeling mechanism (5,21).
Therefore, surgical management may be a consideration in certain
patients with advanced heart failure to enlarge the LV cavity.
In summary, timely recognition of MVOHC with apical
aneurysm may prompt prophylactic defibrillator implantation in
clinical practice for further malignant arrhythmias. Surgical
management should be considered in symptomatic patients with MVOHC
and apical aneurysm.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL performed the operation and perioperative medical
treatment. XL performed pre-operative examination and diagnosis. XZ
collected the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
participating patient.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veselka J, Anavekar NS and Charron P:
Hypertrophic obstructive cardiomyopathy. Lancet. 389:1253–1267.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Criley JM and Siegel RJ: Has ‘obstruction’
hindered our understanding of hypertrophic cardiomyopathy?
Circulation. 72:1148–1154. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maron BJ: Hypertrophic cardiomyopathy: A
systematic review. JAMA. 287:1308–1320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wigle ED, Sasson Z, Henderson MA, Ruddy
TD, Fulop J, Rakowski H and Williams WG: Hypertrophic
cardiomyopathy. The importance of the site and the extent of
hypertrophy. A review. Prog Cardiovasc Dis. 28:1–83. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maron MS, Finley JJ, Bos JM, Hauser TH,
Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ and Maron
BJ: Prevalence, clinical significance, and natural history of left
ventricular apical aneurysms in hypertrophic cardiomyopathy.
Circulation. 118:1541–1549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rowin EJ, Maron BJ, Haas TS, Garberich RF,
Wang W, Link MS and Maron MS: Hypertrophic cardiomyopathy with left
ventricular apical aneurysm: Implications for risk stratification
and management. J Am Coll Cardiol. 69:761–773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yancy CW, Jessup M, Bozkurt B, Butler J,
Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi
JL, et al: 2013 ACCF/AHA guideline for the management of heart
failure: A report of the American College of Cardiology
Foundation/American Heart Association Task Force on Practice
Guidelines. J Am Coll Cardiol. 62:e147–e239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gersh BJ, Maron BJ, Bonow RO, Dearani JA,
Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et
al: 2011 ACCF/AHA guideline for the diagnosis and treatment of
hypertrophic cardiomyopathy: A report of the American College of
Cardiology Foundation/American Heart Association Task Force on
Practice Guidelines. Circulation. 124:e783–e831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minami Y, Kajimoto K, Terajima Y, Yashiro
B, Okayama D, Haruki S, Nakajima T, Kawashiro N, Kawana M and
Hagiwara N: Clinical implications of midventricular obstruction in
patients with hypertrophic cardiomyopathy. J Am Coll Cardiol.
57:2346–2355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Efthimiadis GK, Pagourelias ED,
Parcharidou D, Gossios T, Kamperidis V, Theofilogiannakos EK, Pappa
Z, Meditskou S, Hadjimiltiades S, Pliakos C, et al: Clinical
characteristics and natural history of hypertrophic cardiomyopathy
with midventricular obstruction. Circ J. 77:2366–2374. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Authors/Task Force members, ; Elliott PM,
Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege
AA, Lafont A, Limongelli G, et al: 2014 ESC Guidelines on diagnosis
and management of hypertrophic cardiomyopathy: The task force for
the diagnosis and management of hypertrophic cardiomyopathy of the
European Society of Cardiology (ESC). Eur Heart J. 35:2733–2779.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang JJ, Santangeli P and Callans DJ:
Long-term outcomes of ventricular tachycardia ablation in different
types of structural heart disease. Arrhythm Electrophysiol Rev.
4:177–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mantica M, Della Bella P and Arena V:
Hypertrophic cardiomyopathy with apical aneurysm: A case of
catheter and surgical therapy of sustained monomorphic ventricular
tachycardia. Heart. 77:481–483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokuda M, Kojodjojo P, Tung S, Tedrow UB,
Nof E, Inada K, Koplan BA, Michaud GF, John RM, Epstein LM and
Stevenson WG: Acute failure of catheter ablation for ventricular
tachycardia due to structural heart disease: Causes and
significance. J Am Heart Assoc. 2:e0000722013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maron BJ, Maron MS, Wigle ED and Braunwald
E: The 50-year history, controversy, and clinical implications of
left ventricular outflow tract obstruction in hypertrophic
cardiomyopathy from idiopathic hypertrophic subaortic stenosis to
hypertrophic cardiomyopathy: From idiopathic hypertrophic subaortic
stenosis to hypertrophic cardiomyopathy. J Am Coll Cardiol.
54:191–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tengiz I, Ercan E, Alioglu E and Turk UO:
Percutaneous septal ablation for left mid-ventricular obstructive
hypertrophic cardiomyopathy: A case report. BMC Cardiovasc Disord.
6:152006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Spirito P, Rossi J and Maron BJ: Alcohol
septal ablation: In which patients and why? Ann Cardiothorac Surg.
6:369–375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seggewiss H and Faber L: Percutaneous
septal ablation for hypertrophic cardiomyopathy and mid-ventricular
obstruction. Eur J Echocardiogr. 1:277–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang YJ, Fan CM, Yuan JQ, Wang SY, Song
YH, Qiao SB, You SJ, Wang ZM, Duan FJ and Li YS: Effectiveness of
alcohol septal ablation versus transaortic extended myectomy in
hypertrophic cardiomyopathy with midventricular obstruction. J
Interv Cardiol. 29:619–627. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nguyen A, Schaff HV, Nishimura RA, Dearani
JA and Ommen SR: Early outcomes of repair of left ventricular
apical aneurysms in patients with hypertrophic cardiomyopathy.
Circulation. 136:1979–1981. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Opie LH, Commerford PJ, Gersh BJ and
Pfeffer MA: Controversies in ventricular remodelling. Lancet.
367:356–367. 2006. View Article : Google Scholar : PubMed/NCBI
|