Introduction
The development of ovarian follicles begins with the
proliferation of granulosa cells (GCs), which change shape from
flat to cubical and from single- to multi-layered cells (1,2). At the
late stage of preantral follicle development, follicle-stimulating
hormone receptor (FSHR) appears in GC membranes. The activity of
GCs is regulated by follicle-stimulating hormone (FSH) and
luteinizing hormone (LH) (3). The
preovulatory follicle comprises follicular fluid, an oocyte,
hundreds to thousands of GCs and two layers of surrounding theca
cells. GCs of the preovulatory ovarian follicle represent a
predominant somatic cell type of the ovarian follicle. GCs are
responsible for the communication between the oocyte and the theca
cells, which are regulated by the gonadotropins FSH and LH, produce
estrogen and nurse the oocyte. Following ovulation, GCs become
luteinized GCs, which produce large amounts of progesterone to
support the corpus luteum and subsequent pregnancy (4).
Luteinized GCs have long been considered as
terminally differentiated cells with a limited life span (5). Previous studies demonstrated that GCs
present certain characteristics of stem cells (6–8).
Lavranos et al (6) reported
that bovine luteinized GCs are able to grow in colonies and retain
the ultrastructural features of follicular GCs in an
anchorage-independent culture system. Van Deerlin et al
(7) revealed that luteinized GCs
from a follicle are derived from a small number of stem cells.
Furthermore, Lavranos et al (8) demonstrated that the telomerase activity
in the ovary originated mainly from preovulatory GCs and not the
ovarian follicle oocyte, which supported the hypothesis that GCs
could originate from a stem cell population. Additional studies
demonstrated that GCs possess a multipotent differentiation
capacity. Bukovsky et al (9)
reported that porcine preovulatory GCs can convert into neural stem
cells and differentiate into neurons. In addition,
Kossowska-Tomaszczuk et al (3) demonstrated that luteinized GCs cultured
in vitro can differentiate into neurons, chondrocytes and
osteoblasts. These studies confirmed the presence of granulosa stem
cells in the ovary (10–13).
Culture and expanding granulosa stem cells in
vitro may be useful for basic research and clinical
applications; however, cultivating human GCs in vitro
remains a major challenge. Several attempts have been made to
prolong the lifespan of GCs in culture, although these methods had
limited success. These attempts included creating a
three-dimensional culture system (14), supplementing follicular fluid into
culture media (15), or adding
growth factors into culture media (3). Mouse embryonic fibroblasts (MEFs)
usually serve as a feeder cell layer for mouse and human embryonic
stem cell cultures and can provide a suitable environment
containing growth factors, cytokines and allowing cell-cell
interactions, which maintain cells in an undifferentiated state
(16–18). MEF-conditioned culture medium may
therefore be helpful to support the growth of GCs in vitro.
To test this hypothesis, follicular fluid from infertile women who
underwent oocyte retrieval during in vitro fertilization
(IVF) was collected. Cells from the follicular fluid were cultured
in the presence of MEF-conditioned medium. Immortalized ovarian GCs
were purified following >1 year culture. In addition, the
phenotypic and functional features of the GCs were
characterized.
Materials and methods
Collection of follicular fluid
cells
Follicular fluid cells were collected from 109
infertile women who underwent IVF at the Department of Assisted
Reproduction of the Ninth People's Hospital of Shanghai Jiaotong
University School of Medicine between March 2013 and December 2016.
The study included women aged between 22 and 32 years with tubal,
unexplained or male factors of infertility, and excluded women who
had ovarian cyst or tumor. Patients were first treated with
controlled ovarian stimulation drugs such as 150–225 IU human
menotropin (Anhui Fengyuan Pharmaceutical Co., Ltd.), 10 mg
medroxyprogesterone acetate (Zhejiang Xianju Pharmaceutical Co.,
Ltd.) or 0.1 mg triptorelin (Decapeptyl®; Ferring
Pharmaceuticals) per day for 7 to 10 days. Next, ovulation was
triggered with 0.1 mg triptorelin and/or 2,000–5,000 IU human
chorionic gonadotropin (Lizhu Pharmaceutical Trading Co., Ltd.).
After 34–38 h of trigger administration, all oocytes in follicles
with diameters >10 mm were retrieved by transvaginal
ultrasound-guided aspiration (19).
Following the removal of the oocyte-corona-cumulus
complexes, the fresh follicular fluid was centrifuged for 5 min at
524 × g and 4°C. Next, the cell pellet was collected and washed
three times with PBS. Following this step, cells were isolated by
density gradient centrifugation with 2 ml Percol (Santa Cruz
Biotechnology, Inc.) for 30 min at 2,095 × g and 4°C. Cells in the
interphase layer were collected and washed three times with
Dulbecco's-modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were placed on 100-mm2 tissue
culture dishes in the presence of MEF-conditioned medium as
described below. To obtain enough cells, follicular fluid from 5 to
10 patients was pooled into one primary cell culture.
The ovarian tissue sample was obtained from one
female patient who underwent laparoscopic excision of dermoid cysts
at the Department of Gynecology, Shanghai Ninth People's Hospital.
The patient was 22 years old and the recruitment/collection date
was February 2016.
The BMSCs were obtained from one male patient who
suffered from femoral head necrosis and underwent BMSCs
implantation procedures at the Department of Orthopedics, Shanghai
Ninth People's Hospital. He was 23 years old and the
recruitment/collection date was March 2016.
Human chondrocytes were obtained from a patient who
underwent rhinoplasty procedures at the Department of Plastic and
Reconstructive Surgery, Shanghai Ninth People's Hospital (20). She was a 27-year old female and the
recruitment/collection date was April 2016.
Fresh follicle cells (FCs) were obtained from female
patients who underwent IVF procedures at the Department of Assisted
Reproduction, Shanghai Ninth People's Hospital. Their age
distribution was between 24 and 32 years old (the median age was 29
years old). The date of recruitment/sample collection was between
December 2015 and April 2016. The exclusion criteria were women
with ovarian cysts or tumors.
The study was approved by the local Ethics Committee
of the Ninth People's Hospital of Shanghai. All participants,
including patients undergoing IVF, and patients from whom ovarian
tissue samples, bone marrow mesenchymal stem cells (BMSCs),
chondrocytes and fresh FCs were obtained, provided written informed
consent.
Cell culture
Follicular fluid cells were cultured in a mixed
culture medium that consisted of 70% basic culture medium and 30%
MEF-conditioned medium. The basic culture medium comprised DMEM
containing 4.5 g/l glucose (Gibco; Thermo Fisher Scientific, Inc.)
and supplemented with 10% fetal calf serum (FCS; Gibco; Thermo
Fisher Scientific, Inc.), 50 g/ml penicillin/streptomycin (Gibco;
Thermo Fisher Scientific, Inc.), 3 mmol/l L-glutamine
(Sigma-Aldrich; Merck KGaA) and 10 mM β-mercaptoethanol
(Sigma-Aldrich; Merck KGaA). Cells were seeded at a density of
1.5×106 cells/100 mm2 bacterial-culture dish
and incubated at 37°C and 5% CO2. Medium was exchanged
after 24 h and cells were passaged every 3–4 days. Images of
primary passages 1, 2, 4, 50 and 99 were captured under an inverted
light microscope (magnification, ×100; Vert.A1; Zeiss GmbH).
The BMSCs were suspended in α-MEM (alpha-Minimum
Essential Medium) containing 10% fetal bovine serum (Hyclone; GE
Healthcare Life Sciences), 50 mg/ml sodium ascorbate
(Sigma-Aldrich; Merck KGaA), antibiotic/antimycotic and
10−8 M dexamethasone (Sigma-Aldrich; Merck KGaA) and
then seeded at a density of 1.5×106 cells/100
mm2 bacterial-culture dish and incubated at 37°C and 5%
CO2. Medium was exchanged every 2–3 days. BMSCs were
passaged every 5–7 days.
Fresh FCs were cultured in DMEM containing 4.5 g/l
glucose (Gibco; Thermo Fisher Scientific, Inc.) and supplemented
with 10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific,
Inc.), 50 g/ml penicillin/streptomycin (Gibco; Thermo Fisher
Scientific, Inc.). Medium was exchanged after 24 h and changed
every 2 days.
MEFs were prepared in our laboratory as previously
described (21). Briefly, MEFs were
seeded at a density of 1×106 cells/100 mm2
bacterial-culture dish and then cultured with DMEM medium
supplemented with 10% FBS and 50 g/ml penicillin/streptomycin at
37°C and 5% CO2. The MEFs were passaged every 3–4 days.
From passages 2–5, the supernatant of MEFs was collected and
centrifuged at 524 × g at 37°C for 5 min every 48 h to produce
conditioned medium. The conditioned medium was then sterilized with
0.22-µm filters and frozen at −20°C until further use. Cells,
including follicular fluid cells, BMSCs and MEFs, were passaged
using 0.1% trypsin/EDTA (Invitrogen; Thermo Fisher Scientific,
Inc.) once they reached 80% confluence.
Growth curve of human GCs
Cell counting was performed from passage 4 up to
passage 34 with a hemocytometer. The population doubling time (DT)
was determined according to the following formula: DT= t ×
[lg2/(lgNt-lgN0)] (15) where ‘t’ is
the time between cell seeding and harvesting, ‘N0’ is the number of
cells seeded and ‘Nt’ is the number of cells following culture.
This determination was performed in three dishes for each
experiment.
Colony formation assay
Colony formation assays were performed as previously
described (22). Briefly, cells at
passage 8 were seeded at the density of 100 single cells per plate
in triplicate. Following 20 days culture, cells were stained with
crystal violet and the number of clones was counted by naked eye.
The images of a large colony derived from a single cell was
observed on days 1, 2, 3, 6, 12 and 15 under an inverted light
microscope (magnification, ×40; Vert.A1; Zeiss GmbH). The colony
formation efficiency was calculated from three repeated
experiments. The efficiency was calculated as mean ± SD.
Flow cytometric analysis
Cells at passages 10 and 30 were harvested for flow
cytometric analysis. Prior to staining with the fluorescence
conjugated antibodies, cells were incubated with 2.5 µg/test of
Human BD Fc BlockTM at 4°C for 10 min (cat. no. 564219). Cells were
incubated with phycoerythrin-conjugated CD13 (cat. no. 560998;
1:200 dilution), CD29 (cat. no. 556049; 1:200 dilution), CD31 (cat.
no. 554061; 1:200 dilution), CD34 (cat. no. 550761; 1:200
dilution), CD45 (cat. no. 557055; 1:200 dilution), CD49f (cat. no.
555736; 1:200 dilution), CD90 (cat. no. 555596; 1:200 dilution),
CD105 (cat. no. 560839; 1:200 dilution), CD166 (cat. no. 560903;
1:200 dilution) and human leukocyte antigen-ABC (HLA-ABC)
antibodies (cat. no. 560168; 1:200 dilution) for 30 min at 4°C
(antibodies were all from BD Biosciences). Cells were washed three
times with PBS and analyzed with a flow cytometer (Epics Altra;
Beckman Coulter, Inc.). Flow cytometry data were analyzed with CXP
software (EXPO32 v1.2; Beckman Coulter, Inc.).
Immunofluorescence staining
FCs and BMSc at passages 10 and 60 were fixed with
4% cold paraformaldehyde (Sigma-Aldrich; Merck KGaA) in PBS for 15
min at 4°C and permeabilized with 0.25% Triton X-100
(Sigma-Aldrich; Merck KGaA) in PBS for 10 min. Fixed cells were
blocked for 30 min at 37°C with PBS containing 1% bovine serum
albumin (BSA; Sigma-Aldrich; Merck KGaA) and 10% goat serum
(Sigma-Aldrich; Merck KGaA), and incubated with goat anti-human
FSHR (cat. no. sc7798; 1:100;), rabbit anti-human luteinizing
hormone receptor (LHR; cat. no. sc25828; 1:100;) or mouse
anti-human cytochrome P450 aromatase (CYP19A; cat. no. sc374176;
1:100; all from Santa Cruz Biotechnology, Inc.) antibodies at 4°C
overnight. Cells were then incubated with secondary antibodies
labelled with Alexa Fluor 555 for 30 min at 37°C. Specifically, the
donkey anti-goat IgG (1:1,000; cat no: A21432) was used for binding
the primary antibody goat anti-human FSHR, goat anti-rabbit IgG
(1:1,000; cat no: A21428) was used for binding the primary antibody
rabbit anti-human luteinizing hormone receptor and goat anti-mouse
IgG (1:1,000; cat no: A21422) were used for binding the primary
antibody mouse anti-human cytochrome P450 aromatase CYP19A. All
secondary antibodies were purchased form Invitrogen (Thermo Fisher
Scientific, Inc.), Nuclei were counterstained with DAPI for 5 min
and 25°C (Invitrogen; Thermo Fisher Scientific, Inc.). Human BMSCs,
which were isolated and expanded as previously described (23), served as a negative control.
Reverse transcription
(RT)-semi-quantitative PCR
Human ovarian tissue and FCs served as positive
controls while chondrocytes served as negative control. Total RNA
was extracted from ovarian tissue, FCs, GCs at passage 10 or
passage 60 and chondrocytes using TRIzol® (Invitrogen;
Thermo Fisher Scientific, Inc.). Ovarian tissue was dissected into
5×5×5 mm samples and quickly placed into the sterile culture tube
containing TRIzol® (1.5 ml) for 15 min then RNA was
extracted from the homogenized solution. RT-PCR was performed using
an RT-PCR kit (TaKaRa Taq™ and Reverse Transcriptase XL (AMV);
Takara Bio, Inc.) according to the manufacturer's instructions. The
temperature protocol for RT was as follow: 30°C for 10 min, 42°C
for 60 min, 99°C for 5 min and 5°C for 5 min. The thermocycling
conditions for PCR was as follow: 95°C for 3 min, 95°C for 30 sec,
60–62°C for 30 sec, 72°C for 30 sec and 29–34 cycles, 72°C for 10
min. The sequences of the analyzed genes were searched in the
GenBank from the National Centre for Biotechnology Information
(www.ncbi.nlm.nih.gov/genbank).
Primers were synthesized by Sangon Biotech Co., Ltd. The sequences
of the primers are listed in Table
I. DNA polymerase used was TaKaRa Taq™ (Takara Bio, Inc.) and
reverse transcriptase used was Reverse Transcriptase XL (AMV)
(Takara Bio, Inc.). In the procedure of semi-quantitative PCR, the
thermocycling conditions were as following: FSHR at 60°C for 34
cycles; LHR at 60°C for 34 cycles; CYP19A at 62°C for 34 cycles;
estrogen receptor-α (ER-α), 60°C, 29 cycles; estrogen receptor-β
(ER-β), 60°C, 29 cycles; progesterone receptor (PR) at 60°C for 29
cycles and androgen receptor (AR) at 60°C for 29 cycles. The
amplified products were separated on 1.2% agarose gels and
visualized with ethidium bromide (Sigma-Aldrich; Merck KGaA).
 | Table I.Primer sequences used for
reverse-transcription-semi-quantitative PCR. |
Table I.
Primer sequences used for
reverse-transcription-semi-quantitative PCR.
| Name | Primers
(5′-3′) | Size (bp) |
|---|
| FSHR |
| 248 |
|
Forward |
GCGGAACCCCAACATCGTGTC |
|
|
Reverse |
TGAAGAAATCTCTGCGAAAGT |
|
| LHR |
| 256 |
|
Forward |
TCAATGTGGTGGCCTTCTTCATA |
|
|
Reverse |
TTGGCACAAGAATTGATGATGGGATA |
|
| CYP19A |
| 396 |
|
Forward |
CCATAAAGACCCGATTCCACCA |
|
|
Reverse |
GCTGAGGCATAAATCGACAGAC |
|
| ER-α |
| 207 |
|
Forward |
GAGAGGTCATTGGTTATAGAGA |
|
|
Reverse |
CCGAGTCACATCAGTAATAGT |
|
| ER-β |
| 257 |
|
Forward |
TTCTCCTTCCTCCTACAACT |
|
|
Reverse |
ATGTGATAACTGGCGATGG |
|
| PR |
| 248 |
|
Forward |
GGGATGAAGCATCAGGCTGT |
|
|
Reverse |
AGCATCCAGTGCTCTCACAA |
|
| AR |
| 246 |
|
Forward |
GTGCTGGACAGCACAACAAC |
|
|
Reverse |
GATCAGGGGCGAAGTAGAGC |
|
| β-actin |
| 185 |
|
Forward |
GGACGACATGGAGGAAAT |
|
|
Reverse |
GATAGCACAGCCTGGATA |
|
Measurement of intracellular cyclic
adenosine-monophosphate (cAMP)
Intracellular cAMP following stimulation with
recombinant human FSH (rhFSH; Gonal-f®; Merck KGaA) and
human chorionic gonadotropin (HCG, Lizhu Pharmaceutical Trading
Co., Ltd.) was measured with an ELISA kit (Deco; DECO0254;
http://www.seranachina.com/sh_product.asp?id=826).
The biological potency of rhFSH was 900 IU/1.5 ml (100 ng/ml).
Cells at passage 13 were serum starved 12 h prior to the
experiments and cells that reached confluence in six-well plates
(Falcon™; Thermo Fisher Scientific, Inc.) were transferred into
medium (it was called a cAMP basic medium, in order to distinguish
with medium containing rhFSH or HCG.) containing 1.0% (w/v) BSA
(Sigma-Aldrich; Merck KGaA), 0.5 mM 3-isobutyl-1-methylxanthine
(Sigma-Aldrich; Merck KGaA) and 10−5 M forskolin
(Sigma-Aldrich; Merck KGaA). The cells were further cultured for 3
h in the presence or absence of 5 IU/ml rhFSH, 100 IU/ml HCG or 5
IU/ml rhFSH+100 IU/ml HCG. For the measurement of intracellular
cAMP, cells were washed three times with 200 µl PBS. Freeze-thaw
cycles (−80°C for 30 min then 37°C for 20 min) were repeated six
times and cells were lysed and released their intracellular
components. Following centrifugation at 931 × g and 4°C for 5 min,
cAMP in the supernatant was measured by ELISA according to the
manufacturer's protocol. All experiments were conducted in
triplicate.
Measurement of the secreted steroid
content in cell medium
To measure the concentrations of estradiol and
progesterone in the medium, cells were cultured in DMEM containing
5% charcoal-stripped FCS (GeneTex, Inc.) for 24 h at 37°C.
Subsequently, cells were treated with 10 µM androstenedione
(4-androstene-3,17-dione; A2; Jinchun Biochem Inc.,
Shanghai, China) and stimulated with 5 IU/ml rhFSH, 100 IU/ml HCG,
10−4 M dibutyryl cAMP [Bu2cAMP; Enzo Life
Sciences] or 10−5 M forskolin for 48 h. The estradiol
and progesterone levels in the culture medium were determined with
the chemiluminescent immunoassay i2000SR (ARCHITECT®,
Abbott Pharmaceutical Co. Ltd.). All experiments were conducted in
triplicate.
Cell labeling
Cells at passage 8 were labelled by transfection
with an enhanced green fluorescent protein (eGFP) sequence using a
lentivirus vector (pNL-EGFP/CMV/WPREdU3; SunBio, Inc.; http://www.sbo-bio.com.cn) according to the
manufacturer's protocol. The cells at passages 8 were seeded into a
24-well plate at a density of 3.6×104 cells/ml. After
incubation for 24 h, the culture medium was removed, and the
virus-containing medium were added into the cells at a multiplicity
of infection (MOI) of 10. A total of 1.8 µl of 2X 105
TU/µl lentiviral particles for transfecting 3.6×104
cells, so the MOI was calculated as 10. Following 48-h of
transfection, puromycin selection was performed. The viral solution
was replaced with 1 mL DMEM medium with 1 µl Puromycin (2 mg/ml,
SunBio, Inc.). Following incubation for 24 h, puromycin-containing
medium was replaced with DMEM medium. The labeling efficiency was
directly observed under a fluorescence inverted microscope
(Vert.A1; Zeiss GmbH).
Intra-ovarian transplantation
eGFP-labelled cells were suspended in DMEM at the
density of 1×107 cells/ml. A total of 1×105
cells were injected into the right ovary of 22 7-week-old female
severe combined immunodeficiency (SCID) mice (Shanghai Sipuer-Bik
Laboratory Animal Co., Ltd.) using a microinjector (Microsyringe).
Mice were about 15–20 g and were housed at 21±2°C in a humidified
atmosphere (40–70%) with 12-h light/dark cycles with free access to
food and water. Meanwhile, 1×105 cells were
subcutaneously injected into two sides of the dorsal region of 4
SCID mice and 1×105 cells were peritoneal cavity of 4
SCID mice. Animals were sacrificed by cervical dislocation. Ovarian
tissues were harvested at 8 weeks post-transplantation. Ovarian
tissues were fixed in 4% paraformaldehyde for 24 h at room
temperature, then placed in freezing microtome, embedded at −25°C
with an OCT embedding agent, the frozen section thickness was 6 µm.
Fresh-frozen sections were used. The sections were observed under
the fluorescence inverted microscope (Vert.A1; Zeiss GmbH). For
H&E staining, fresh-frozen sections were first fixed with 10%
neutral buffered formalin for 1 min at room temperature and then
dehydrated in an alcohol series, which was as follow: 100% alcohol
for 5 min, 95% alcohol for 5 min, 85% alcohol for 3 min and 75%
alcohol for 2 min. Next, the sections were stained with Harris
hematoxylin solution for 8 min and washed under running tap water
for 1 min. Following that, the sections were counter-stained with
DAPI for 5 min at room temperature. Finally, sections were observed
under a light microscope (magnification, ×200; Nikon, ECLIPSE
Ni-E).
Statistical analysis
SPSS software (ver. 23.0, IBM Corp.) was used to
analyze data. Data are presented as the mean ± standard deviation.
One-way ANOVA was used to compare data among different groups, and
Dunnett's and Tukey's post hoc tests were used to perform multiple
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Human GCs cultured in vitro
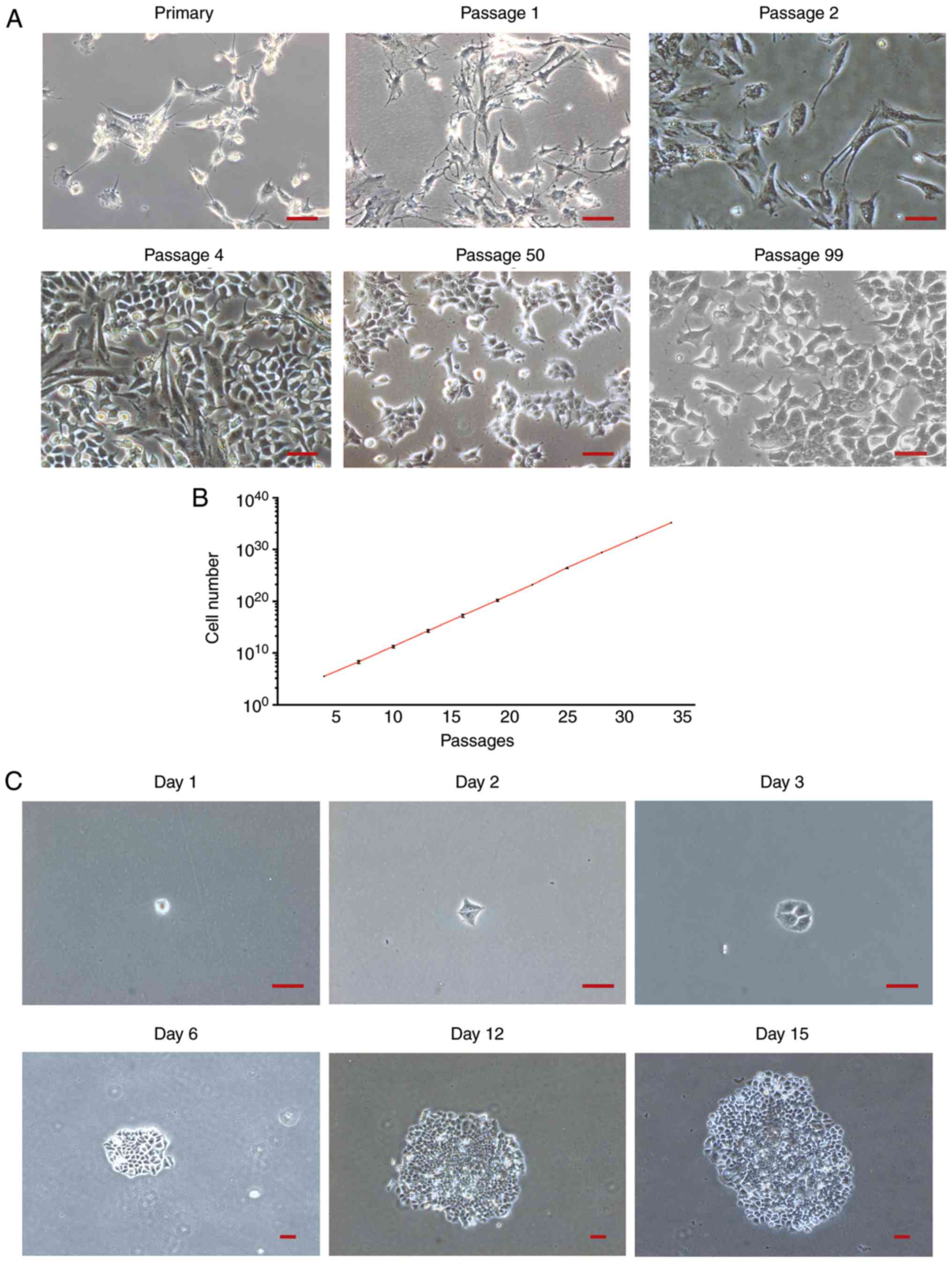
The morphological changes of the GCs in culture are
presented in Fig. 1A. Cells with
different morphologies were observed during early passages. From
passage 4, a small group of epithelial-like cells surrounded by
spindle-shaped fibroblastic cells was observed to proliferate
rapidly. The number of epithelial-like cells increased in the
following passages along with a decrease in fibroblastic cells.
From passage 8, a uniform epithelial-like cell population was
observed (data not shown). Cells were passaged every three days and
presented a cobblestone-like appearance when they reached
confluence (data not shown). No notable morphological changes were
observed during the following passages. Images of primary, passages
1, 2, 4, 50 and 99 were captured under an inverted light microscope
(magnification, ×100). Out of 20 experiments, cells died eventually
in 19 experiments. Only within one culture, a group of cells were
able to proliferate and continue to grow for up to 130 passages.
Images of primary, passages 1, 2, 4, 50 and 99 were captured under
an inverted light microscope. The GCs growth curve between passages
4 and 34 is presented in Fig. 1B.
Cell proliferation was stable and the average doubling time was ~22
h. To further test the cell proliferation capacity, colony
formation assays were performed at passage 8. The formation of a
large colony derived from a single cell was observed during the
first 15 days of culture (Fig. 1C).
Following seeding of 100 cells, 57, 53 and 59 clones were observed
after 20 days of culturing in three repeated experiments (data not
shown). The colony formation efficiency was calculated as
56.3±1.8%.
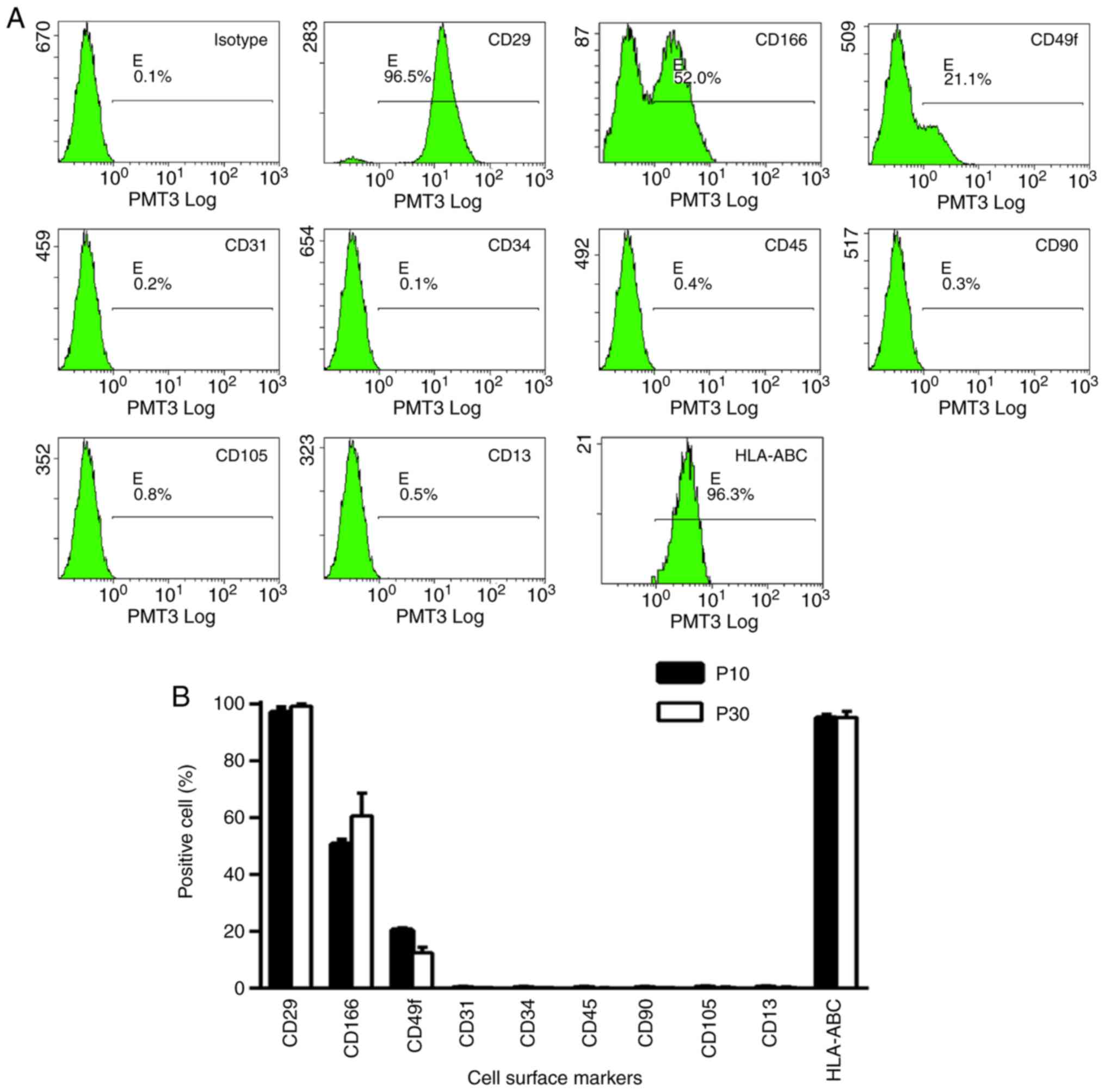
Surface marker expression profile
Expression profile of cell surface markers was
determined at passages 10 and 30 by flow cytometry (Fig. 2). Mouse IgG1 was used as an isotype.
Cells expressed mesenchymal cell markers, including CD29, CD166,
CD49f and HLA-ABC but did not express hematopoietic cell markers,
including CD13, CD31, CD34, CD45, CD90 and CD105. The expression
profile was stable and no significant difference was observed
between cells at passages 10 and 30.
Expression of FSHR, LHR, CYP19A,
estrogen receptor (ER)-α, ER-β, progesterone receptor (PR) and
androgen receptor (AR)
To further confirm cell identity, the expression of
FSHR, LHR and CYP19A were analyzed at passages 10 and 60 by
immunofluorescence staining. As presented in Fig. 3A, GCs in P10, 60 and FCs were
positive for FSHR, LHR, and CYP19A whilst BMSCs were negative for
FSHR, LHR, and CYP19A. Furthermore, the expression of FSHR, LHR,
CYP19A, ER-α, ER-β, PR and AR was confirmed by RT-PCR analysis
(Fig. 3B). FSHR, LHR and CYP19A were
expressed in GCs P10, 60, FCs and ovary tissue but not in mock and
chondrocytes. ER-α, ER-β, PR and AR were expressed in GCs P10, 60,
FCs, ovary tissue and chondrocytes but not in mock.
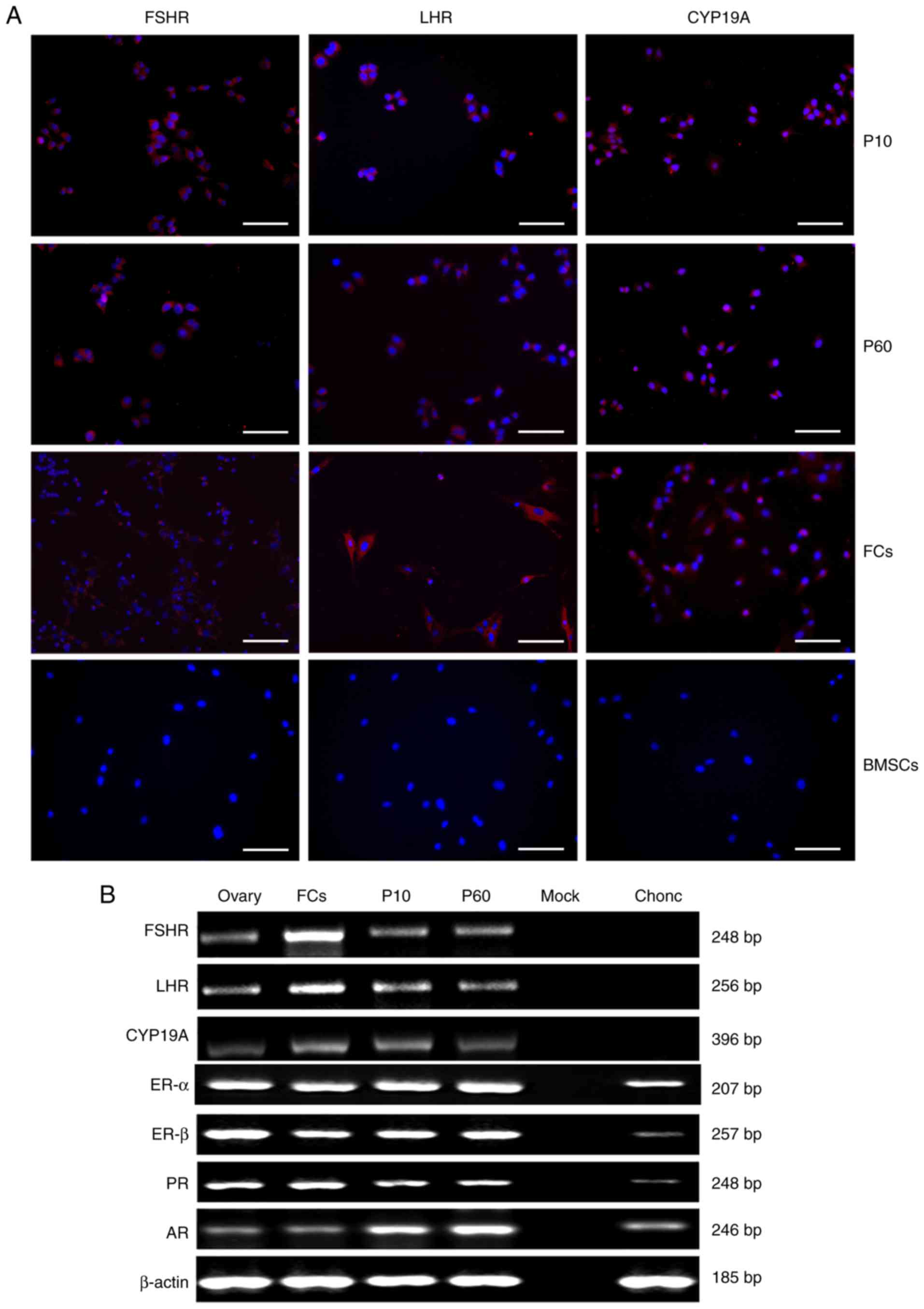 | Figure 3.Expression of FSHR, LHR, CYP19A,
ER-α, ER-β, PR and AR. (A) Immunofluorescence staining for FSHR
(red), LHR (red) and CYP19A (red) in follicular fluid-derived human
GCs at P10, follicular fluid-derived human GCs at P60, human FCs
and human BMSCs. Cell nuclei (blue) were counterstained with DAPI.
Scale bars, 200 µm. (B) Reverse transcription-semi-quantitative
polymerase chain reaction analyses of reproduction-associated
receptor genes in GCs at P10 and P60, ovary tissue, FCs and
chondrocytes. β-actin was used as the internal control. AR,
androgen receptor; BMSCs, bone marrow mesenchymal stem cells;
Chonc, human chondrocytes; CYP19A, cytochrome P450 aromatase; ER-α,
estrogen receptor-α; ER-β, estrogen receptor-β; FCs, human follicle
cells; FSHR, follicle-stimulating hormone receptor; GCs, granulosa
cells; LHR, luteinizing hormone receptor; Mock, control; Ovary,
human ovarian tissue; P10, follicular fluid-derived human GCs at
passage 10; P60, follicular fluid-derived human GCs at passage 60;
PR, progesterone receptor. |
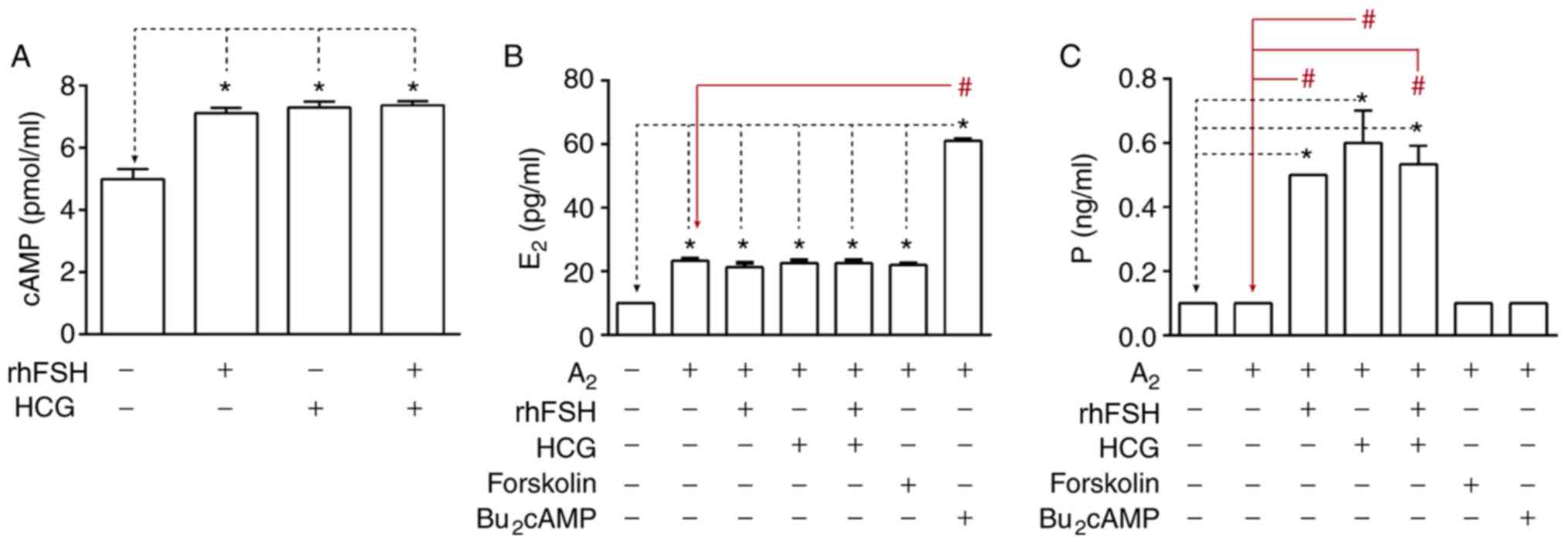
Intracellular cAMP accumulation
following rhFSH and HCG stimulation
Intracellular cAMP accumulation was measured
following 3-h stimulation with 5 IU/ml rhFSH, 100 IU/ml HCG or 5
IU/ml rhFSH+ 100 IU/ml HCG. Intracellular cAMP levels were
significantly increased following rhFSH and/or HCG stimulation
compared with untreated cells (Fig.
4A).
Steroidogenic activities of GCs in
vitro
The steroidogenic activities of GCs were evaluated
by measuring the estradiol and progesterone concentrations in the
culture medium. The results revealed detectable levels of basal
secretion of estradiol and progesterone in the medium. Following 48
h of treatment with A2, estradiol level in the medium
was significantly increased, No additional changes were observed
when cells were co-stimulated with rhFSH/HCG/forskolin. However,
estradiol level was significantly increased following cell
co-stimulation with A2 and Bu2cAMP (Fig. 4B). Furthermore, after 48 h of
culturing, the levels of progesterone in medium significantly
increased following the treatment with rhFSH and/or HCG in the
presence of A2. But there was no difference when cells were treated
with forskolin, Bu2cAMP combined with A2 or sole A2 administration
(Fig. 4C).
Cell survival in SCID mice
Prior to cell transplantation, cells were
transfected with an eGFP sequence using a lentivirus vector. Almost
all cells expressed eGFP following eGFP transfection and puromycin
selection (Fig. 5A). Cells were
subsequently injected into the right ovaries of the SCID mice.
Ovarian tissues were harvested at 8 weeks post-transplantation, 12
tumors were formed in the injected ovaries of 22 SCID mice with no
tumors formed in the uninjected ovaries of 22 SCID mice (three
tumors and three control ovaries are presented in Fig. 5B). In addition, fluorescence
microscopy of frozen tissue sections demonstrated that almost all
tumors comprised eGFP-positive cells and that a few of
follicle-like structures were observed (Fig. 5C). The presence of follicle-like
structures was confirmed by histological analysis following
hematoxylin and eosin staining (Fig.
5D). In addition, numerous adipocytes-like cells and some
granulosa-like cells were identified in the tumors (Fig. 5D). No mass or tumor was detected in
the SCID mice which were injected with cells into subcutaneous back
or abdominal cavity (data not shown).
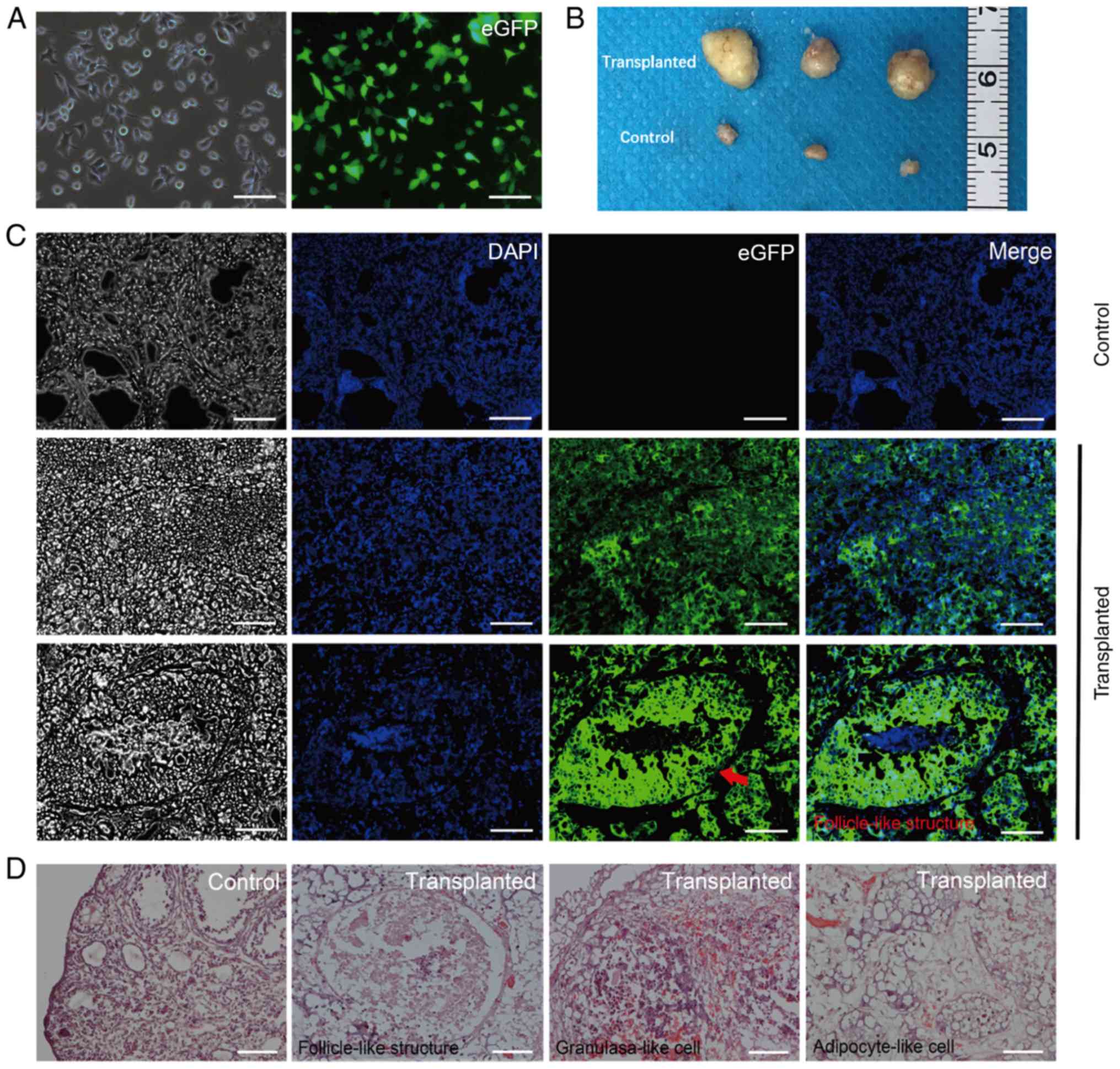 | Figure 5.Ovaries of SCID mice 8 weeks
following transplantation with eGFP-labelled cells. (A) Follicular
fluid-derived human GCs were transfected with eGFP. Left image
demonstrates GCs transfected with eGFP under white light, the right
image was GCs transfected with eGFP under fluorescence. Almost all
cells expressed eGFP following transfection with eGFP. Scale bars,
200 µm. (B) Murine ovaries 8 weeks following cell transplantation,
the untransplanted ovaries were used as the control group. (C)
Transplanted ovaries under fluorescence inverted microscope. The
left black/white images were captured under white light. Red arrow,
follicle-like structure in the transplanted ovary. Scale bars, 200
µm. (D) Follicle-like structures, granulosa-like cells and
adipocyte-like cells in the transplanted ovaries observed following
hematoxylin and eosin staining. The control group was ovaries which
were not transplanted with eGFP-labeled GCs, so different stages of
follicles can be observed in the ovaries. Scale bars, 100 µm. eGFP,
enhanced GFP; GCs, granulosa cells; SCID, severe combined
immunodeficiency. |
Discussion
The present study aimed to develop an improved
culture medium to support GC proliferation in vitro.
MEF-conditioned medium, which had commonly been used in mouse and
human embryonic stem cell cultures (24,25), was
tested. Cells from follicular fluid could be maintained up to 14
weeks in culture (data not shown), which was similar to findings
previously reported (12,15). Although we tried to establish an
immortal GCs cell line 20 times, only one clone was able to grow up
to 130 passages without morphological changes. Because the cultured
cells usually stopped proliferating eventually in the present
study, a group of epithelial-like cells at passage 4 was discovered
and was found to rapidly proliferate. At passage 8, a uniform cell
population was obtained, which kept growing up to 130 passages
without morphological changes. Cells proliferated in a stable
manner, the average DT was ~22 h and the colony formation ability
was ~56.3%. These results indicated the presence of stable GCs
cultured in vitro for over one year.
Numerous GCs, theca cells and blood cells are
present in the follicular fluid of patients included in IVF
programs (26). Furthermore,
contamination with vaginal and ovarian surface epithelial cells
commonly occurs due to transvaginal follicular aspiration (27). To confirm the identity of the cell
culture established in the current study, flow cytometric analysis,
immunofluorescence staining and RT-qPCR analysis were performed.
The results demonstrated that GCs expressed HLA-ABC, the most
important human histocompatibility antigen (28), and also mesenchymal lineage markers
including CD29, CD166 and CD49f (25) but were negative for hematopoietic
cell markers including CD13, CD31 and CD45 (12), which indicated that cells were of
mesodermal origin (29). In
addition, cells were positive for FSHR, LHR and CYP19A. For the
immunofluorescence staining experiment, BMSCs were used as the
negative control. It has been commonly accepted that
pituitary-derived hormones, including FSH and LH, act exclusively
on the gonads and are only expressed on ovary and testis gonad
cells (30); however, in the last
decade, whether FSHR and LHR are expressed in nongonadal
tissues/cells remains a controversy. Previous studies reported
extremely low levels of FSH, LH receptors in nongonadal
tissues/cells (31,32), including human umbilical cord and
osteoclasts. Kumar (33) therefore
highlighted the need for novel genetic models to investigate the
extragonadal actions of FSH. Previous studies revealed no
expression of FSH and LH receptors in umbilical cord and bone cells
(34–36). In the present study, FSHR and LHR
were not detected in BMSCs. In addition, experiments were performed
where mock cells were only incubated with secondary antibodies and
fresh GCs were used as a positive control. GCs are the only cell
type in the female body possessing the FSHR (37). The expression of FSHR and LHR were
neither observed on BMSCs nor on mock cells, which suggested that
cells may be GCs. Kossowska-Tomaszczuk et al (3) revealed that long-term cultivated human
GCs expressed human mesenchymal stem cell markers, including CD29,
CD44, CD90, CD105, CD117 and CD166 but not CD73. Bruckova et
al (15) reported that when
follicular fluid was added to the culture medium, long-term
cultured human GCs highly expressed human mesenchymal cell markers,
including CD29, CD44, CD73, CD90 and CD166, and FSHR; however, they
did not express CD31, CD34, CD49d, CD49e, CD106, CD184, CD197 or
HLA-II. Furthermore, the immortalized GC line COV434 highly
expresses HLA-I, slightly expresses FSHR, LHR and CD166, and does
not express CD29, CD31, CD34, CD44, CD45, CD49d, CD49e, CD73, CD90,
CD105, CD106 and HLA-II (15). The
phenotype of the cells established in the present study was
therefore similar to the GC line COV434 and the GC lines that have
been previously reported.
In humans, immortalized GC lines can be established
using ovarian tumor samples (COV434, KGN and HSOGT cell lines) or
via gene transfection (SVOG-4o, HGL5, HO-23, GC1a, HGP53 and HGrC1
cell lines) (38). The functions of
immortalized GCs, including their role in hormone secretion and
their response to hormones, vary from cell to cell. For example,
KGN, HGL5 and HGrC1 have been reported to secrete estrogen and
progesterone (39–41); however, COV434 and HSOGT only produce
estrogen (42–44), and HO-23, HGP53 and SVOG-4o only
synthesize progesterone (45–47).
Furthermore, COV434, KGN, HGP53 and HGrC1 cells respond to FSH
stimulation (41–43,47,48)
whereas SVOG-4o cells are sensitive to LH/HCG stimulation (45). The cell lines HGL5 (39), HO-23 (46) and GC1a (49) do not respond to either FSH or LH.
Functional analyses of the immortalized GCs established in the
present study demonstrated that intracellular cAMP accumulation
could be upregulated by FSH/HCG stimulation. In addition,
A2 treatment induced estradiol secretion, combined
A2 and Bu2cAMP treatments significantly
improved estradiol secretion, whereas A2 treatment
combined with FSH/HCG did not stimulate estradiol secretion. In
addition, the present study demonstrated that progesterone
secretion was induced by FSH and/or HCG treatment, but not by the
PKA pathway activator forskolin (50) or by Bu2cAMP. These
results, and the underlying mechanisms, should be further
investigated in the future. FSH/HCG binding to their receptors
activates the protein kinase A (PKA) pathway that stimulates cAMP
accumulation (51,52). Cells established in the present study
may act directly on FSHR and LH/HCG receptors which may activate
the PKA pathway in order for FSH/HCG to exert their roles. However,
certain other crucial factors are involved in the steroid synthesis
process such as certain hormone glycosylation variants,
potentiating antibodies and small molecule ligands (52), which could be further investigated in
future studies. The long-term cultivated cells from the present
study may provide a model to study the physiological mechanisms of
steroidogenesis and folliculogenesis.
To determine whether the cells established in the
present study could survive in SCID mice, cells were injected into
SCID mice ovaries. Tumors were observed at 8 weeks post-injection.
However, no tumor was observed when the same number of cells was
injected subcutaneously or intraperitoneally in SCID mice (data not
shown). This could be due to the blood supply in the ovaries being
better compared with the other two locations or due to ovaries
providing a unique environment for cell survival. In particular,
this study reported that cells formed follicle-like structures.
However, whether the surviving cells could respond to FSH/HCG
stimulation in vivo was not investigated. In addition, the
hormone levels of the tumor-bearing animals should be determined in
the future.
Previous studies reported that GCs possess a
multipotent differentiation capacity (9,11,13,53).
In the current study, cells spontaneously differentiated into
adipocyte-like structures following intra-ovarian injection. The
multipotent differentiation capacity of this cell line is currently
being investigated.
Acknowledgements
The authors would like to thank Dr QinQjn Hong, Dr
Rengfei Cai, Dr Meiting Qiu and Wei Jing of the Department of
Assisted Reproduction at the Shanghai Ninth People's Hospital for
their contributions to data collection and statistical
analysis.
Funding
This work was supported by the National Key Research
and Development Program of China (grant no. 2016YFC1101400) and the
National Natural Science Foundation of China (grant no.
81771993).
Availability of data and materials
The datasets used and/or analyzed during the present
study available from the corresponding author on reasonable
request.
Authors' contributions
AA and WZ designed the study, interpreted the
results, wrote the manuscript and approved the final form of the
manuscript. AA, ZT and YL conducted the experiments. SY performed
the statistical analysis. BL, XW and HH participated in the data
collection. YC participated in study design, data interpretation,
critical revision and approved the final form of the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Shanghai Ninth People's Hospital affiliated with Shanghai Jiao
Tong University School of Medicine. Written informed consent was
provided by all participants prior to the study. Animal studies
were approved by the Animal Experimentation Ethics Committee of the
Shanghai Ninth People's Hospital affiliated with Shanghai Jiao Tong
University School of Medicine.
Patient consent for publication
All patients provided informed consent for the
publication of their clinical data.
Competing interest
The authors declare that no conflicts of interest to
declare.
Glossary
Abbreviations
Abbreviations:
|
AR
|
androgen receptor
|
|
CYP19A
|
cytochrome P450 aromatase
|
|
ER-α
|
estrogen receptor-α
|
|
ER-β
|
estrogen receptor-β
|
|
FSHR
|
follicle-stimulating hormone
receptor
|
|
LHR
|
luteinizing hormone receptor
|
|
PR
|
progesterone receptor
|
References
|
1
|
Senbon S, Hirao Y and Miyano T:
Interactions between the oocyte and surrounding somatic cells in
follicular development: Lessons from in vitro culture. J Reprod
Dev. 49:259–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dzafic E, Stimpfel M and Virant-Klun I:
Plasticity of granulosa cells: On the crossroad of stemness and
transdifferentiation potential. J Assist Reprod Genet.
30:1255–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kossowska-Tomaszczuk K, De Geyter C, De
Geyter M, Martin I, Holzgreve W, Scherberich A and Zhang H: The
multipotency of luteinizing granulosa cells collected from mature
ovarian follicles. Stem Cells. 27:210–219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gougeon A: Regulation of ovarian
follicular development in primates: Facts and hypotheses. Endocr
Rev. 17:121–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niswender GD, Juengel JL, Silva PJ,
Rollyson MK and McIntush EW: Mechanisms controlling the function
and the life span of the corpus luteum. Physiol Rev. 80:1–29. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lavranos TC, Rodgers HF, Bertoncello I and
Rodgers RJ: Anchorage-independent culture of bovine granulosa
cells: The effects of basic fibroblast growth factor and dibutyryl
cAMP on cell division and differentiation. Exp Cell Res.
211:245–251. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Deerlin PG, Cekleniak N, Coutifaris C,
Boyd J and Strauss JF 3rd: Evidence for the oligoclonal origin of
the granulosa cell population of the mature human follicle. J Clin
Endocrinol Metab. 82:3019–3024. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavranos TC, Mathis JM, Latham SE,
Kalionis B, Shay JW and Rodgers RJ: Evidence for ovarian granulosa
stem cells: Telomerase activity and localization of the telomerase
ribonucleic acid component in bovine ovarian follicles. Biol
Reprod. 61:358–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bukovsky A, Caudle MR and Svetlikova M:
Steroid-mediated differentiation of neural/neuronal cells from
epithelial ovarian precursors in vitro. Cell Cycle. 7:3577–3583.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bukovsky A, Gupta SK, Virant-Klun I,
Upadhyaya NB, Copas P, Van Meter SE, Svetlikova M, Ayala ME and
Dominguez R: Study origin of germ cells and formation of new
primary follicles in adult human and rat ovaries. Methods Mol Biol.
450:233–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virant-Klun I, Zech N, Rozman P, Vogler A,
Cvjeticanin B, Klemenc P, Malicev E and Meden-Vrtovec H: Putative
stem cells with an embryonic character isolated from the ovarian
surface epithelium of women with no naturally present follicles and
oocytes. Differentiation. 76:843–856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Varras M, Griva T, Kalles V, Akrivis C and
Paparisteidis N: Markers of stem cells in human ovarian granulosa
cells: Is there a clinical significance in ART? J Ovarian Res.
5:362012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kossowska-Tomaszczuk K and De Geyter C:
Cells with stem cell characteristics in somatic compartments of the
ovary. Biomed Res Int. 2013:3108592013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kossowska-Tomaszczuk K, Pelczar P, Güven
S, Kowalski J, Volpi E, De Geyter C and Scherberich A: A novel
three-dimensional culture system allows prolonged culture of
functional human granulosa cells and mimics the ovarian
environment. Tissue Eng Part A. 16:2063–2073. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruckova L, Soukup T, Visek B, Moos J,
Moosova M, Pavelkova J, Rezabek K, Kucerova L, Micuda S, Brcakova E
and Mokry J: Proliferative potential and phenotypic analysis of
long-term cultivated human granulosa cells initiated by addition of
follicular fluid. J Assist Reprod Genet. 28:939–950. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Evans MJ and Kaufman MH: Establishment in
culture of pluripotential cells from mouse embryos. Nature.
292:154–156. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim JW and Bodnar A: Proteome analysis of
conditioned medium from mouse embryonic fibroblast feeder layers
which support the growth of human embryonic stem cells. Proteomics.
2:1187–1203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi YT, Huang YZ, Tang F and Chu JX: Mouse
embryonic stem cell-derived feeder cells support the growth of
their own mouse embryonic stem cells. Cell Biol Int. 30:1041–1047.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu
Q, Ai A and Shoham Z: Medroxyprogesterone acetate is an effective
oral alternative for preventing premature luteinizing hormone
surges in women undergoing controlled ovarian hyperstimulation for
in vitro fertilization. Fertil Steril. 104:62–70 e63. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, He A, Yin Z, Yu Z, Luo X, Liu W,
Zhang W, Cao Y, Liu Y and Zhou G: Regeneration of human-ear-shaped
cartilage by co-culturing human microtia chondrocytes with BMSCs.
Biomaterials. 35:4878–4887. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Ai A, Tang ZY, Zhou GD, Liu W, Cao
Y and Zhang WJ: Mesenchymal-like stem cells derived from human
parthenogenetic embryonic stem cells. Stem Cells Dev. 21:143–151.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baroffio A, Dupin E and Le Douarin NM:
Clone-forming ability and differentiation potential of migratory
neural crest cells. Proc Natl Acad Sci USA. 85:5325–5329. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gan Y, Dai K, Zhang P, Tang T, Zhu Z and
Lu J: The clinical use of enriched bone marrow stem cells combined
with porous beta-tricalcium phosphate in posterior spinal fusion.
Biomaterials. 29:3973–3982. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu C, Inokuma MS, Denham J, Golds K, Kundu
P, Gold JD and Carpenter MK: Feeder-free growth of undifferentiated
human embryonic stem cells. Nat Biotechnol. 19:971–974. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Peng Z, Xu Z, Huang H and Wei X:
Mouse embryonic fibroblast (MEF)/BMP4-conditioned medium enhanced
multipotency of human dental pulp cells. J Mol Histol. 49:17–26.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrero H, Delgado-Rosas F, Garcia-Pascual
CM, Monterde M, Zimmermann RC, Simón C, Pellicer A and Gómez R:
Efficiency and purity provided by the existing methods for the
isolation of luteinized granulosa cells: A comparative study. Hum
Reprod. 27:1781–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beckmann MW, Polacek D, Seung L and
Schreiber JR: Human ovarian granulosa cell culture: Determination
of blood cell contamination and evaluation of possible culture
purification steps. Fertil Steril. 56:881–887. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thorsby E: A short history of HLA. Tissue
Antigens. 74:101–116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dzafic E, Stimpfel M, Novakovic S,
Cerkovnik P and Virant-Klun I: Expression of mesenchymal stem
cells-related genes and plasticity of aspirated follicular cells
obtained from infertile women. Biomed Res Int. 2014:5082162014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rice S, Elia A, Jawad Z, Pellatt L and
Mason HD: Metformin inhibits follicle-stimulating hormone (FSH)
action in human granulosa cells: Relevance to polycystic ovary
syndrome. J Clin Endocrinol Metab. 98:E1491–E1500. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
James K, Bhartiya D, Ganguly R, Kaushik A,
Gala K, Singh P and Metkari SM: Gonadotropin and steroid hormones
regulate pluripotent very small embryonic-like stem cells in adult
mouse uterine endometrium. J Ovarian Res. 11:832018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar TR: Extragonadal FSH receptor: Is it
real? Biol Reprod. 91:992014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar TR: Extragonadal actions of FSH: A
critical need for novel genetic models. Endocrinology. 159:2–8.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Allan CM, Kalak R, Dunstan CR, McTavish
KJ, Zhou H, Handelsman DJ and Seibel MJ: Follicle-stimulating
hormone increases bone mass in female mice. Proc Natl Acad Sci USA.
107:22629–22634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stelmaszewska J, Chrusciel M, Doroszko M,
Akerfelt M, Ponikwicka-Tyszko D, Nees M, Frentsch M, Li X, Kero J,
Huhtaniemi I, et al: Revisiting the expression and function of
follicle-stimulation hormone receptor in human umbilical vein
endothelial cells. Sci Rep. 6:370952016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Davis MR and Summers KM: Structure and
function of the mammalian fibrillin gene family: Implications for
human connective tissue diseases. Mol Genet Metab. 107:635–647.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Méduri G, Charnaux N, Driancourt MA,
Combettes L, Granet P, Vannier B, Loosfelt H and Milgrom E:
Follicle-stimulating hormone receptors in oocytes. J Clin
Endocrinol Metab. 87:2266–2276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Havelock JC, Rainey WE and Carr BR:
Ovarian granulosa cell lines. Mol Cell Endocrinol. 228:67–78. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rainey WH, Sawetawan C, Shay JW, Michael
MD, Mathis JM, Kutteh W, Byrd W and Carr BR: Transformation of
human granulosa cells with the E6 and E7 regions of human
papillomavirus. J Clin Endocrinol Metab. 78:705–710. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tsutsumi R, Hiroi H, Momoeda M, Hosokawa
Y, Nakazawa F, Koizumi M, Yano T, Tsutsumi O and Taketani Y:
Inhibitory effects of cholesterol sulfate on progesterone
production in human granulosa-like tumor cell line, KGN. Endocr J.
55:575–581. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bayasula, Iwase A, Kiyono T, Takikawa S,
Goto M, Nakamura T, Nagatomo Y, Nakahara T, Kotani T, Kobayashi H,
et al: Establishment of a human nonluteinized granulosa cell line
that transitions from the gonadotropin-independent to the
gonadotropin-dependent status. Endocrinology. 153:2851–2860. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van den Berg-Bakker CA, Hagemeijer A,
Franken-Postma EM, Smit VT, Kuppen PJ, van Ravenswaay Claasen HH,
Cornelisse CJ and Schrier PI: Establishment and characterization of
7 ovarian carcinoma cell lines and one granulosa tumor cell line:
Growth features and cytogenetics. Int J Cancer. 53:613–620. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Vollmer M, De Geyter M,
Litzistorf Y, Ladewig A, Dürrenberger M, Guggenheim R, Miny P,
Holzgreve W and De Geyter C: Characterization of an immortalized
human granulosa cell line (COV434). Mol Hum Reprod. 6:146–153.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kondo H, Kiguchi K, Okamura A, Okuma Y,
Iida T, Kobayashi Y, Takagi M, Ishizuka B and Ishiwata I:
Establishment and characterization of a human ovarian granulosa
tumor cell line (HSOGT). Hum Cell. 16:123–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lie BL, Leung E, Leung PC and Auersperg N:
Long-term growth and steroidogenic potential of human
granulosa-lutein cells immortalized with SV40 large T antigen. Mol
Cell Endocrinol. 120:169–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hosokawa K, Dantes A, Schere-Levy C,
Barash A, Yoshida Y, Kotsuji F, Vlodavsky I and Amsterdam A:
Induction of Ad4BP/SF-1, steroidogenic acute regulatory protein,
and cytochrome P450scc enzyme system expression in newly
established human granulosa cell lines. Endocrinology.
139:4679–4687. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tajima K, Hosokawa K, Yoshida Y, Dantes A,
Sasson R, Kotsuji F and Amsterdam A: Establishment of
FSH-responsive cell lines by transfection of pre-ovulatory human
granulosa cells with mutated p53 (p53val135) and Ha-ras genes. Mol
Hum Reprod. 8:48–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nishi Y, Yanase T, Mu Y, Oba K, Ichino I,
Saito M, Nomura M, Mukasa C, Okabe T, Goto K, et al: Establishment
and characterization of a steroidogenic human granulosa-like tumor
cell line, KGN, that expresses functional follicle-stimulating
hormone receptor. Endocrinology. 142:437–445. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Okamura H, Katabuchi H and Ohba T: What we
have learned from isolated cells from human ovary? Mol Cell
Endocrinol. 202:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Nguyen TD, Filliatreau L, Klett D and
Combarnous Y: Comparative effects of sub-stimulating concentrations
of non-human versus human Luteinizing Hormones (LH) or chorionic
gonadotropins (CG) on adenylate cyclase activation by forskolin in
MLTC cells. Gen Comp Endocrinol. 261:23–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Reed BG and Carr BR: The Normal Menstrual
Cycle and the Control of Ovulation. Endotext [Internet]. Feingold
KR, Anawalt B, Boyce A, et al: MDText.com; South Dartmouth, MA:
2000
|
|
52
|
Gloaguen P, Crépieux P, Heitzler D, Poupon
A and Reiter E: Mapping the follicle-stimulating hormone-induced
signaling networks. Front Endocrinol (Lausanne). 2:452011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Virant-Klun I, Rozman P, Cvjeticanin B,
Vrtacnik-Bokal E, Novakovic S, Rülicke T, Dovc P and Meden-Vrtovec
H: Parthenogenetic embryo-like structures in the human ovarian
surface epithelium cell culture in postmenopausal women with no
naturally present follicles and oocytes. Stem Cells Dev.
18:137–149. 2009. View Article : Google Scholar : PubMed/NCBI
|