Introduction
Sepsis is a systemic inflammatory syndrome, which
results from infection. Due to septic shock and its deleterious
effects on cardiac function, sepsis is the leading cause of death
in critically ill patients (1).
Cardiac dysfunction aggravates septic syndrome, influences its
prognosis and is very common in patients with sepsis (2). A significantly higher mortality rate is
observed in patients with sepsis and cardiac dysfunction (70–90%)
compared with patients exhibiting sepsis alone, implying that
sepsis-associated mortality may be associated with cardiac
dysfunction. Pro-inflammatory factors, mitochondrial structural
dysfunction, dysfunction of the autonomic nervous system,
myocardial cell injury and apoptosis have all been associated with
the pathogenesis of sepsis (3–5). In
particular, pro-inflammatory factors including cytokines, may serve
a critical role in the pathogenesis of sepsis (5). Due to the fact that heart failure
associated mortality in patients with sepsis is high, the presence
of heart failure may be used as a predictor of survival.
Serotonin (5-HT) neurons regulate the function of
the visceral sensory system, motor nervous system and autonomic
nervous system and are widely distributed in these areas (6). 5-HT and its receptors are widely
distributed in the cardiovascular system and serve an important
role in the regulation of cardiovascular function (1). The abnormal regulation of 5-HT can
result in the development of cardiovascular disease (7,8). This is
due to the involvement of 5-HT in cardiac remodeling, a process
that includes myocardial cell hypertrophy, apoptosis and necrosis,
which can lead to heart failure (9).
High 5-HT concentrations are associated with an increased severity
of heart failure symptoms and systolic dysfunction (10).
Mitochondria are an important site for cellular
oxidation and provide ~95% of the energy a cell requires (11). Mitochondrial dysfunction in the
myocardium is a major cause of myocardial impairment in sepsis
(11). A previous study demonstrated
that stress may result in myocardial injury (12) with another study identifying that
stress leads to the impairment of myocardial ultrastructure,
particularly in mitochondria, resulting in the apoptosis of
myocardial cells (13). Mitochondria
are important for the maintenance of normal function in myocardial
cells (14,15). In the current study, the effects of
sepsis on 5-HT responses and cardiac action potentials were
investigated using a rat model.
Materials and methods
Animals
A total of 20 healthy adult male Sprague-Dawley rats
(weight, 180–250 g; age, 2–3 months) were provided by the
Experimental Animal Center of Southern Medical University
(qualified certificate number: 44002100014706). Rats were housed in
a specific pathogen-free facility at 21±2°C and a humidity of
50±10%, under a 12-h light/dark cycle (lights on 07:00-19:00), with
free access to food and drinking water. After a 1-week adaption
period in the Animal Feeding Center of Qingyuan People's Hospital,
The Sixth Affiliated Hospital of Guangzhou Medical University, rats
were randomly divided into a sepsis and a control group (each,
n=10). The experiments of the current study conformed to the Guide
for the Care and Use of Laboratory Animals, published by the
National Institutes of Health (publication no. 85-23) and were
approved by the Animal Ethics Committee of the People's Hospital of
Xinjiang (Xinjiang, China). All efforts were made to minimize the
suffering and number of rats used in the current study.
Induction of sepsis
After anesthesia the sterile intraperitoneal
administration of 10% chloral hydrate (300 mg/kg), a 3 cm median
abdominal incision was made. The cecum was ligated with a 4-0 silk
suture 5 cm away from the tip. Two cecal perforations were
performed using a 14-gauge needle at the free cecal end, following
which the cecum was replaced and the abdominal incision closed.
Penicillin (100,000–150,000 U/250 g) was intraperitoneally
administered prior to and following surgery. Normal saline (5
ml/100 g) was subcutaneously administered to replace body fluid
lost during surgery. There were no signs of peritonitis following
anesthesia. In the normal group, a sham procedure without cecal
ligation was performed (16,17). The rats were monitored every 6-h for
18-h of continuous behavior observation.
Action potential duration in ex vivo
atria and ventricles
At 18 h post surgery, all rats were deeply
anesthetized with an intraperitoneal injection of 10% chloral
hydrate (300 mg/kg) and subsequently sacrificed by cervical
dislocation. Rat hearts were extracted rapidly after opening the
chest and perfused using the Langendorff method (18) with Krebs-Henseleit buffer (NaCl,
118.5 mM; KCl, 4.7 mM; CaCl2, 2.5 mM; NaHCO3,
25 mM; MgSO4, 1.2 mM; KH2PO4, 1.2
mM; glucose, 11 g; pH=7.4) at a temperature of 36°C, with 95%
O2 and 5% CO2 (flow rate, 1.5 ml/min). Atrial
and ventricular action potential duration was measured 30 min after
perfusion using a multichannel electrophysiology instrument
(Lead-2000; Sichuan Jinjiang Electronic Science and Technology Co.,
Ltd.).
Sample collection
Two pieces of left ventricular apical tissue were
fixed at room temperature overnight in 10% formaldehyde solution,
embedded in paraffin, cut into 4 µm sections and stained with
hematoxylin and eosin at 37°C for 24-h or subjected to 5-HT
immunohistochemistry. The remaining apical tissue was sectioned,
fixed with 2.5% glutaraldehyde at 37°C for 8 h, dehydrated with an
acetone series (a gradient dehydration of 85% alcohol-95%
alcohol-anhydrous alcohol), embedded with neutral resin, stained
with uranyl acetate and lead citrate at 37°C for 8 h and cut into
ultrathin sections (0.5 mm thick). The ultrastructure of the
myocardial cells was then evaluated using transmission electron
microscopy.
Immunohistochemistry
Paraffin-embedded sections were dewaxed, treated
with 3% H2O2 at room temperature for 10 min
to remove endogenous peroxidase activity then washed with PBS three
times. Samples were then microwave irradiated at 92–98°C for
antigen retrieval and dehydrated with an acetone series (a gradient
dehydration of 85% alcohol-95% alcohol-anhydrous alcohol). Samples
were then incubated with rabbit primary antibody against serotonin
(1:100; cat. no. PB0442; Wuhan Boster Biological Technology, Ltd.)
overnight at 4°C. Samples were incubated with goat anti-rabbit
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:1,000; cat. no. SV-002; Wuhan Boster Biological
Technology, Ltd.) at 37°C for 30 min then washed with PBS three
times. Samples were stained with 3,3′-diaminobenzidine (cat. no.
AR1022; Boster Biological Technology, Ltd.) at 37°C for 30 min.
Distilled water was used to dilute the kit reagents (cat. no.
AR1022; Wuhan Boster Biological Technology, Ltd.), tissue sections
were treated with a mixture of kit reagents A, B and C, developed
at room temperature then exposed to running water to terminate the
reaction. Finally, sections were counterstained with hematoxylin at
37°C for 30 min, cleared with ethanol and hydrochloric acid then
mounted in resin. Three sections from each tissue were observed
using an Olympus optical microscope (Olympus Corporation). Five
fields (magnification, ×200) were randomly selected in each section
using the Kontron IBAS computerized image analysis system (Carl
Zeiss AG). The positive reaction area/total area and positively
stained cells/total number of cells in the same area was
measured.
Statistical analysis
Data are expressed as the mean ± standard deviation
and were analyzed using SPSS 16.0 software (SPSS, Inc.). A
Student's t-test was used for comparison between the two groups and
P<0.05 was considered to indicate a statistically significant
result.
Results
Rat behavior and pathologic changes in
myocardial cells
In the sepsis group, 6–8 h after surgery, rats
exhibited bradykinesia, tachypnea, a hunched posture and reacted
slowly to stimuli. At 18 h after surgery, conjunctival hyperemia,
watery stool, intermittent trembling and coughing was observed. In
contrast, the rats in the control group exhibited no adverse
effects subsequent to surgery. In the sepsis group, the dissected
heart was a dark color, soft and friable, whereas the control group
heart was bright red and hard. There were no differences in the
mass of the whole heart or the mass of ventricles between the
control group (0.00267±0.000205) and the sepsis group
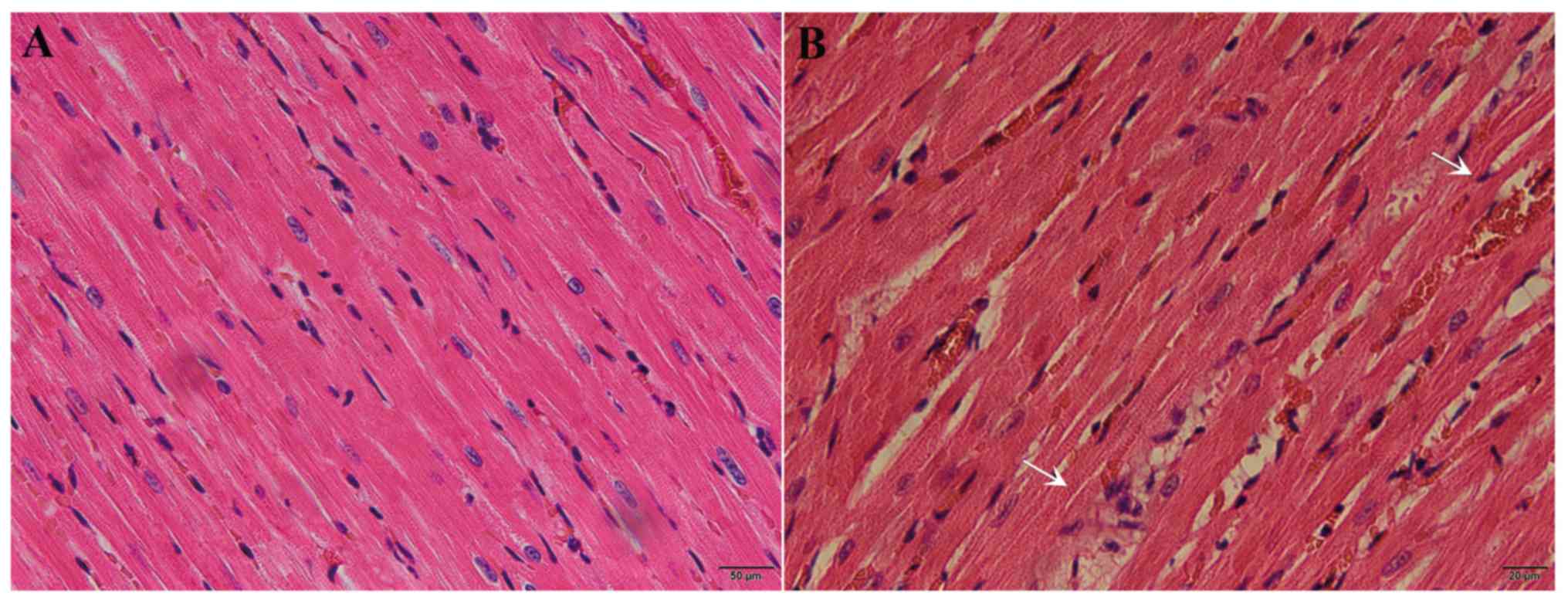
(0.00273±0.000192). Following hematoxylin and eosin staining,
interstitial congestion, edema and inflammatory cell infiltration
were observed in the myocardial tissue of septic rats, but not in
necrotic myocardial cells (Fig. 1A and
B). Transmission electron microscopy revealed disordered
myocardial myofibers and mitochondria, mitochondrial enlargement,
swelling and vacuolar degeneration of the mitochondrial cristae.
Myocardial fibers were arranged irregularly, some were distended,
muscle bundles were separated, Z lines and intercalated disks were
distorted. The distance between Z lines was also shortened and the
nuclear membranes were irregular. Furthermore, the mitochondrial
membranes were fragmented, mitochondrial cristae were absent and
mitochondria were disordered and vacuolated (Fig. 2B). However, normal histology and
ultrastructure was present in the hearts of the control group
(Fig. 2A).
Heart rate and duration of action
potentials
At 18-h post-surgery, the atrium action potentials
in cardiac tissue were significantly longer (P<0.01) and heart
rate was significantly higher (P<0.05) in the sepsis group
compared with the control group. There was no significant
difference in ventricular action potential between the sepsis and
control groups (P>0.05; Table
I).
 | Table I.Rat heart rate, atrial/ventricular
action potentials and heart/body mass following cecal ligation and
puncture/sham surgery. |
Table I.
Rat heart rate, atrial/ventricular
action potentials and heart/body mass following cecal ligation and
puncture/sham surgery.
| Group | Number | Heart rate (bpm) | Atrium (ms) | Ventricles (ms) | Heart/body mass |
|---|
| Sepsis | 10 |
361.10±12.39a |
106.40±2.95b | 155.80±3.15 | 0.00273±0.000192 |
| Control | 10 | 348.60±12.38 | 86.60±4.12 | 153.10±3.84 | 0.00267±0.000205 |
| t-value |
| −2.15 | −12.364 | −1.717 | 1.116 |
| P-value |
| <0.045 | <0.001 | 0.1104 | 0.293 |
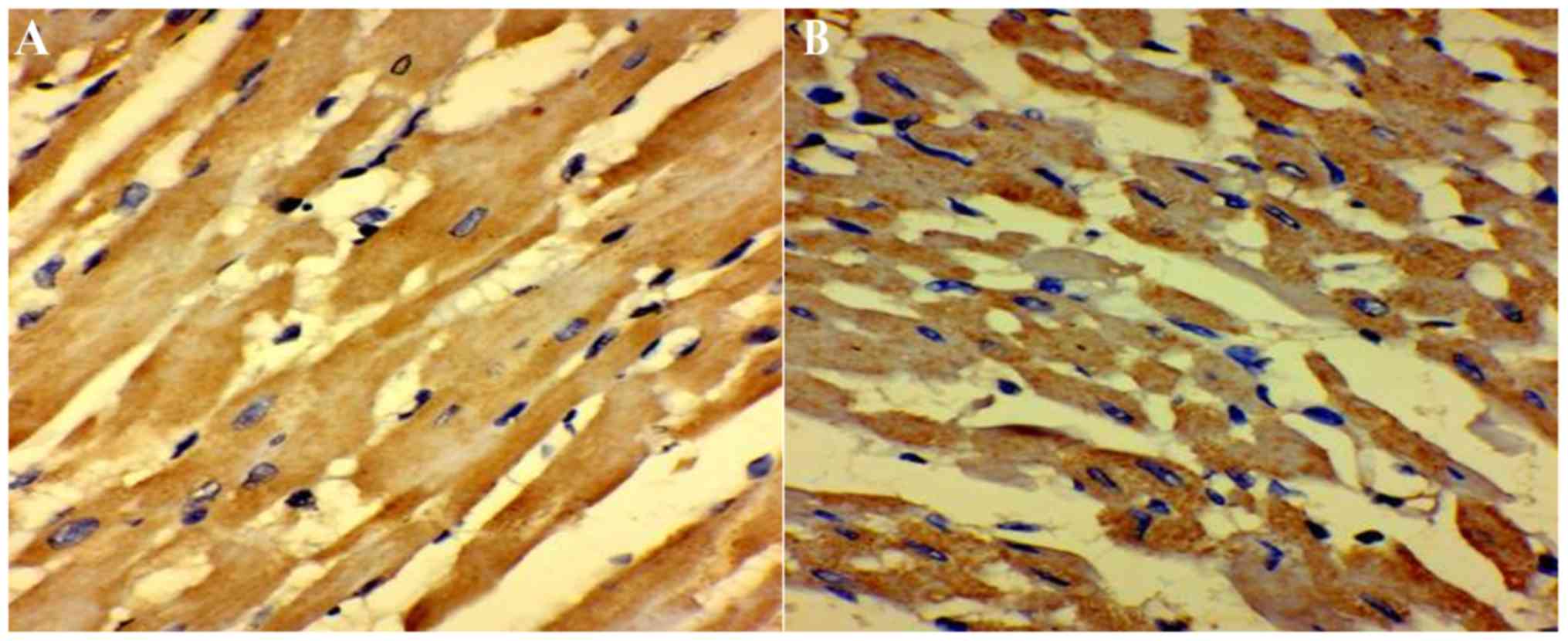
Immunohistochemistry of cardiac
tissue
As presented in Table
II, 5-HT-positive nerve fibers were observed in the septic and
control groups. In the control group, myocardial fibers were
arranged in an orderly manner with less interstitial matrix, and
with 5-HT-positive nerve fibers projecting along the muscle
bundles. However, in the sepsis group, myocardial fibers were more
diffuse with more interstitial matrix. The area of 5-HT-positive
myocardial cells and staining intensity was also significantly
higher (P<0.01; Fig. 3A and B;
Table II).
 | Table II.Serotonin protein expression in the
left ventricular apex following cecal ligation and puncture/sham
surgery. |
Table II.
Serotonin protein expression in the
left ventricular apex following cecal ligation and puncture/sham
surgery.
| Group | Number of rats | Area of positive
staining (µm2) | Number of positive
cells (cells/field) |
|---|
| Control | 10 | 0.39±0.05. | 0.46±0.01 |
| Sepsis | 10 |
0.62±0.06a |
0.92±0.02b |
| t-value |
| 0.477 | 0.589 |
| P-value |
| <0.05 | <0.001 |
Discussion
Sepsis results in heart failure in ~50% of patients,
accounting for the higher mortality rate in patients with heart
failure compared with those who have sepsis alone (1). Patients with sepsis-induced cardiac
injury often demonstrate changes in electrocardiograms. Makara
et al (19) demonstrated that
within 48 h of Francisella novicida infection, R waves
became more prominent, QRS intervals were prolonged and heart rate
was higher in a rat sepsis model. In the aforementioned study, the
changes in electrocardiogram became more pronounced at 96 h,
indicating severe electrical conduction defects. The results of the
rat model used in the current study demonstrated a higher heart
rate and longer atrial action potentials, which are indicative of
altered ion channel function, an atrial electrophysiologic disorder
and/or altered atrial structure (20). Atrial disease has a marked effect on
cardiac function and is associated with a high risk of arrhythmia
and stroke (21,22). The results of the current study
indicated that cardiac injury begins at the time of bacterial
infection, with atrial cells responding quickly and significantly
to a pathologic stimulus. This is consistent with the hypothesis
that electrophysiological changes in the heart caused by bacterial
sepsis increase mortality (23).
Inflammatory mediators and myocarditis are
considered to be contributors to the high incidence of sudden
death, severe arrhythmia and heart failure associated with sepsis
(24). 5-HT is an auto-active
substance that is not only associated with inflammation and the
immune response, but also serves roles in a number of other
pathophysiologic processes. Humoral immunity and/or cellular
responses occur in response to tissue damage, inducing 5-HT
release, which binds to its specific cell receptors and results in
an inflammatory response (25). When
the physiologic state of the body changes, 5-HT directly or
indirectly alters the physiology or structure of target organs
(7). However, 5-HT also increases
calcium conductance, triggering spontaneous pulsatile activity in
resting cells (26). In sepsis, a
high concentration of 5-HT increases myocardial free
Ca2+ concentration, which leads to calcium overload in
myocardial cells. Sepsis also activates the 5-HT4 receptor and
enhances Ca2+ influx via the cAMP-dependent pathway,
triggering cardiac L-type Ca2+ channels and leading to
diastolic depolarization (27). This
results in atrial action potentials being prolonged, atrial
electrophysiologic disorder and subsequently, contractile
dysfunction (28).
Mitochondria are important in the maintenance of
cardiac function. Accumulating evidence has indicated that
mitochondria contribute to the pathophysiology of cardiac
dysfunction associated with infection (29). 5-HT-induced myocardial
Ca2+ overload is likely the result of inflammatory
cytokines, including tumor necrosis factor-α, interleukin (IL)-1
and IL-6, inducing apoptotic signaling in myocardial mitochondria
(25). Most cardiovascular diseases,
such as hypertension, atherosclerosis, coronary artery disease, and
myocardial infarction are associated with the opening of
mitochondrial membrane pores (30).
This is followed by a series of events including the loss of
mitochondrial transmembrane potential, the uncoupling of the
respiratory chain, leakage of mitochondrial Ca2+,
excessive production of reactive oxygen species (15) and the release of resident
mitochondrial proteins, which include apoptosis-inducing factor
cytochrome c and second mitochondrial-derived activator caspase
(31). These events lead to
mitochondrial dysfunction, which involves the reduction of ATP
production, an increase in oxidation products, imbalances in the
regulation of mitochondrial proteins and ultimately cell apoptosis,
all of which are associated with inadequate energy supply to the
heart (11). The results of the
aforementioned studies are consistent with the contention that
abnormal mitochondrial energy metabolism is the underlying
mechanism of sepsis-associated cardiac mortality.
5-HT activates the cell apoptosis pathway and
induces the production of toxic oxygen free radicals, which
aggravate cardiac dysfunction (32).
Continuous inflammation and cardiac exposure to cytokines may lead
to left ventricular dysfunction, which results in poor muscle
strength and altered myocardial metabolism (5). This contributes to myocardial
remodeling and therefore promotes the progression of heart failure
(32). Oxidative stress is also a
cause of cardiac injury. All these factors change the polarization
and contractility of myocardial cells, resulting in cardiac
dysfunction (15). The pathologic
changes identified in the present study, which include larger
myocardial mitochondria, swollen cristae and vacuolar degeneration,
are consistent with oxidative stress. An insufficient energy supply
to the heart may delay the recovery of myocardial function, lead to
cardiac electrical disorders, activate the sympathetic nervous
system, induce 4-phase depolarization, increase heart rate and
eventually result in heart failure, shock and death (33).
The heart is a vulnerable target organ in sepsis
(1,4). Cardiac dysfunction is very common in
sepsis/septic shock and is associated with a high mortality rate.
However, the molecular mechanisms underlying the pathophysiologic
changes in myocardial cells are yet to be elucidated (34). 5-HT is a widely distributed,
endogenous monoamine that serves as a pro-inflammatory factor.
However, its role in cardiovascular regulation depends on the
distribution of its receptor (35).
In the current study, a preliminarily investigation of the
pathophysiologic role of 5-HT in the septic heart was undertaken.
During sepsis, the regulation of 5-HT is abnormal, but whether 5-HT
receptor antagonists reverse the pathologic effects of
sepsis-induced endogenous 5-HT elevation and the dose-response
associations of 5-HT receptor antagonists in the heart, was not
assessed in the present study and therefore warrants further
investigation. Furthermore, the most appropriate parameters for the
assessment of cardiac dysfunction during sepsis, including markers
of oxidative stress, cytochrome c activity, mitochondrial ATP,
oxidation products and mitochondrial Ca2+, should be
investigated in future studies.
Acknowledgements
Not applicable.
Funding
This work was supported by Hospital Fund of Qingyuan
People's Hospital, The Sixth Affiliated Hospital of Guangzhou
Medical University (grant no. 2017-1014106).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
ZJL, HL, CDW and KDX performed the experiments. HL
and KDX collected and analyzed the data. ZJL and CDW conceived and
designed the study. ZJL wrote the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
This study was approved by the Animal Research
Ethics Committee of The Sixth Affiliated Hospital of Guangzhou
Medical University. All procedures performed in studies involving
animals were in accordance with the ethical standards of the
institution or practice at which the studies were conducted.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zaky A, Deem S, Bendjelid K and Treggiari
MM: Characterization of cardiac dysfunction in sepsis: An ongoing
challenge. Shock. 41:12–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Annane D, Bellissant E and Cavaillon JM:
Septic shock. Lancet. 365:63–78. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: Analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merx MW and Weber C: Sepsis and the heart.
Circulation. 116:793–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alvarez S, Vico T and Vanasco V: Cardiac
dysfunction, mitochondrial architecture, energy production, and
inflammatory pathways: Interrelated aspects in endotoxemia and
sepsis. Int J Biochem Cell Biol. 81:307–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vikenes K, Farstad M and Nordrehaug JE:
Serotonin is associated with coronary artery disease and cardiac
events. Circulation. 100:483–489. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu MY, Ren YP, Zhang LJ and Ding JY:
Pretreatment with Ginseng Fruit Saponins affects serotonin
expression in an experimental comorbidity model of myocardial
infarction and depression. Aging Dis. 7:680–686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cowen P: Serotonin depression and
antidepressant action. S Afr J Psychiatr. 19:942013.
|
|
9
|
Selim AM, Sarswat N, Kelesidis I, Iqbal M,
Chandra R and Zolty R: Plasma aerotonin in heart failure: Possible
marker and potential treatment target. Heart Lung Circ. 26:442–449.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Villeneuve C, Guilbeau-Frugier C, Sicard
P, Lairez O, Ordener C, Duparc T, De Paulis D, Couderc B,
Spreux-Varoquaux O, Tortosa F, et al: p53-PGC-1α pathway mediates
oxidative mitochondrial damage and cardiomyocyte necrosis induced
by monoamine oxidase-A upregulation: Role in chronic left
ventricular dysfunction in mice. Antioxid Redox Signal. 18:5–18.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu AL, Zhu HD, Yu XZ, Liu J, Ma S, Liu ZY
and Guo SB: The mechanisms of LPS-induced cardiac dysfunction in
septic mice. Chin J Emerg Med. 24:825–829. 2015.
|
|
13
|
Walley KR: Deeper understanding of
mechanisms contributing to sepsis-induced myocardial dysfunction.
Crit Care. 18:1372014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong HL, Li ZQ, Zhao YJ, Zhao SM, Zhu L,
Li T, Fu Y and Li HJ: Ginsenoside Rb1 protects cardiomyocytes
against CoCl2-induced apoptosis in neonatal rats by inhibiting
mitochondria permeability transition pore opening. Acta Pharmacol
Sin. 31:687–695. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Passarelli C, Tozzi G, Pastore A, Bertini
E and Piemonte F: GSSG-mediated complex I defect in isolated
cardiac mitochondria. Int J Mol Med. 26:95–99. 2010.PubMed/NCBI
|
|
16
|
Williams DL, Ha T, Li C, Kalbfleisch JH,
Schweitzer J, Vogt W and Browder IW: Modulation of tissue Toll-like
receptor 2 and 4 during the early phases of polymicrobial sepsis
correlates with mortality. Crit Care Med. 31:1808–1818. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ha T, Hua F, Grant D, Xia Y, Ma J, Gao X,
Kelley J, Williams DL, Kalbfleisch J, Browder IW, et al: Glucan
phosphate attenuates cardiac dysfunction and inhibits cardiac MIF
expression and apoptosis in septic mice. Am J Physiol Heart Circ
Physiol. 291:H1910–H1918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Liu H and Zeng ZH: Chronic
unpredictable mild stress causing cardiac and thoracic spinal cord
electrophysiological abnormalities may be associated with increased
cardiac expression of serotonin and growth-associated protein-43 in
rats. Biomed Res Int. 2018:86979132018.PubMed/NCBI
|
|
19
|
Makara MA, Hoang KV, Ganesan LP, Crouser
ED, Gunn JS, Turner J, Schlesinger LS, Mohler PJ and Rajaram MV:
Cardiac electrical and structural changes during bacterial
infection: An instructive model to study cardiac dysfunction in
sepsis. J Am Heart Assoc. 5(pii): e0038202016.PubMed/NCBI
|
|
20
|
Christ T, Rozmaritsa N, Engel A, Berk E,
Knaut M, Metzner K, Canteras M, Ravens U and Kaumann A:
Arrhythmias, elicited by catecholamines and serotonin, vanish in
human chronic atrial fibrillation. Proc Natl Acad Sci USA.
111:11193–11198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goette A, Kalman JM, Aguinaga L, Akar J,
Cabrera JA, Chen SA, Chugh SS, Corradi D, D'Avila A, Dobrev D, et
al: EHRA/HRS/APHRS/SOLAECE expert consensus on Atrial
cardiomyopathies: Definition, characterisation, and clinical
implication. J Arrhythm. 32:247–278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goette A, Bukowska A, Dobrev D,
Pfeiffenberger J, Morawietz H, Strugala D, Wiswedel I, Röhl FW,
Wolke C, Bergmann S, et al: Acute atrial tachyarrhythmia induces
angiotensin II type 1 receptor-mediated oxidative stress and
microvascular flow abnormalities in the ventricles. Eur Heart J.
30:1411–1420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tucker NR and Ellinor PT: Emerging
directions in the genetics of atrial fibrillation. Circ Res.
114:1469–1482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grün S, Schumm J, Greulich S, Wagner A,
Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, et al:
Long-term follow-up of biopsy-proven viral myocarditis: Predictors
of mortality and incomplete recovery. J Am Coll Cardiol.
59:1604–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Zeng Z, Wu C and Liu H: Tropisetron
inhibits sepsis by repressing hyper-inflammation and regulating the
cardiac action potential in rat models. Biomed Pharmacother.
110:380–388. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pennec JP, Talarmin H, Droguet M,
Giroux-Metgès MA, Gioux M and Dorange G: Characterization of the
voltage-activated currents in cultured atrial myocytes isolated
from the heart of the common oyster Crassostrea gigas. J Exp
Biol. 207:3935–3944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahme MM, Cotter B, Leistad E, Wadhwa MK,
Mohabir R, Ford AP, Eglen RM and Feld GK: Electrophysiological and
antiarrhythmic effects of the atrial selective 5-HT(4) receptor
antagonist RS-100302 in experimental atrial flutter and
fibrillation. Circulation. 100:2010–2017. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galindo-Tovar A, Vargas ML, Escudero E and
Kaumann AJ: Ontogenic changes of the control by phosphodiesterase-3
and −4 of 5-HT responses in porcine heart and relevance to human
atrial 5-HT(4) receptors. Br J Pharmacol. 156:237–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singer M: Mitochondrial function in
sepsis: Acute phase versus multiple organ failure. Crit Care Med 35
(9 Suppl). S441–S448. 2007. View Article : Google Scholar
|
|
30
|
Wang X, Liu X, Kong R, Zhan R, Wang X,
Leng X, Gong J, Duan M, Wang L, Wu L and Qian L: NGFI-B targets
mitochondria and induces cardiomyocyte apoptosis in
restraint-stressed rats by mediating energy metabolism disorder.
Cell Stress Chaperones. 14:639–648. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xinxing W, Hong F, Rui Z, Yun Z, Jingbo G
and Lingjia Q: Phosphorylated nerve growth factor-induced clone B
(NGFI-B) translocates from the nucleus to mitochondria of stressed
rat cardiomyocytes and induces apoptosis. Stress. 15:545–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Briasoulis A, Androulakis E, Christophides
T and Tousoulis D: The role of inflammation and cell death in the
pathogenesis, progression and treatment of heart failure. Heart
Fail Rev. 21:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu ZJ, Liu H, Xue KD and Wu S:
Experimental research of heart serotonin and myocardial action
potential change in the model of sepsis rats. Chin J Clinicians.
10:72–75. 2016.
|
|
34
|
Chung HY, Kollmey AS, Schrepper A, Kohl M,
Bläss MF, Stehr SN, Lupp A, Gräler MH and Claus RA: Adjustment of
dysregulated ceramide metabolism in a murine model of
sepsis-induced cardiac dysfunction. Int J Mol Sci. 18(pii):
E8392017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mössner R and Lesch KP: Role of serotonin
in the immune system and in neuroimmune interactions. Brain Behav
Immun. 12:249–271. 1998. View Article : Google Scholar : PubMed/NCBI
|