Introduction
The increasing use of cardiac resynchronization
therapy device/defibrillators (CRT/CRTDs) has been paralleled by a
rise in the requirement for CRT/CRTD extraction and reimplantation
due to device infection or dysfunction. The CRT/CRTD system, which
is notably used for left ventricular lead (LVL) removal, appears to
be associated with a higher risk than conventional pacemakers due
to the thin wall of the coronary sinus (CS) and superior vena cava
system. CRT/CRTD devices are usually implanted via the left
subclavian vein. The right subclavian vein is used to reimplant the
CRT/CRTD system following the removal of infected devices. However,
the stenosis/occlusion of the original CS branch and the anatomical
complexity of the right-side pathway render reimplantation
considerably difficult. To date, studies on LVL removal and
reimplantation have been limited to small sample size studies
(1–4), whereas no previous studies have
examined these parameters in Chinese patients, to the best of our
knowledge. The present study was performed in the largest removal
and reimplantation center for CRT/CRTD in China and its aim was to
provide information on the success rate and incidence of
complications in patients with LVL removal and reimplantation.
Materials and methods
Patients
All patients with CRT/CRTD who underwent device
removal due to infection or dysfunction between January 2012 and
October 2018 were enrolled. The present study was approved by the
Ethics Committee of Peking University People's Hospital (Beijing,
China). All patients enrolled provided written informed consent for
their participation in the study and for publication of associated
images. The patients' characteristics, as well as laboratory blood
test and imaging results, were analyzed. The indications of lead
extraction were determined based on the 2017 Heart Rhythm Society
(HRS) expert consensus statement on cardiovascular implantable
electronic device lead management and extraction (5). High-risk patients included a duration
of passive fixed lead implantation of >10 years, a duration of
defibrillation lead implantation of >3 years, a duration of lead
perforation of >1 month and a total number of electrodes of
>5.
Electrode extraction
The lead extraction methods were performed as
previously described (4,6). The lead was removed under general
anesthesia and transesophageal ultrasound monitoring in high-risk
patients, and the remaining patients were subjected to local
anesthesia. Pacemaker-dependent patients were implanted with a
right ventricular (RV) pacing lead through the left femoral vein.
Bilateral elbow venography was performed prior to extraction. The
lead devices were removed using a manual or locking stylet traction
in case where were implanted 12 months prior to enrolment (Cook
Medical).
The femoral vein approach (Cook Medical) was used by
an evolution mechanical sheath (Cook Medical) and/or a laser sheath
(CVX-300). These methods were applied alone or in combination in
those patients with an implantation duration of >12 months.
Endocardial active fixation ventricular electrodes were implanted
in the pacemaker-dependent patients as a transition to permanent
pacemaker reimplantation. The definitions of successful removal or
clinical success of removal in the presence or absence of
complications were according to the 2017 HRS expert consensus
(5).
Reimplantation of LV electrode
The reimplantations were performed on the
contralateral side in infective patients (all via the right
subclavian vein). The LV lead dysfunction was reimplanted at the
ipsilateral side. Antimicrobial therapy according to the 2017 HRS
expert consensus recommendations was used for the management of the
suspected electronic device-associated infection. The antimicrobial
therapy used for endocarditis has a minimum duration of 4–6 weeks,
whereas for bacteremia, it has a lower duration (at least 2 weeks)
(5). A new implantation may be
reasonably postponed until blood cultures are negative for 72 h in
patients with bacteremia and endocarditis (5). In subjects with pocket site infection,
the reimplantation procedure was performed with a treatment
duration of 48–72 h following device removal (5). Optional balloon dilatation was used if
the target vein exhibited apparent stenosis. RV double site or
bundle pacing were performed for patients with LVL reimplantation
failure.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation for parametric variables. Dichotomous variables
were presented as an absolute number with percentage. All analyses
were performed using SPSS software (version 18.0; SPSS Inc.).
Results
Case characteristics
A total of 54 patients with infection/dysfunction
(n=51/3) were enrolled (CRT/CRTD, 34/20). The general information
of these patients is presented in Table
I. The average age of patients was 65.33±7.38 (male, 46;
female, 8). As presented in Table
II, a total of 41 patients (76%) exhibited pocket infection, 7
(13%) presented with bacteremia and 3 (6%) with infective
endocarditis. The dimensions of the vegetation were 1.3×1.5 cm,
1.5×2.1 cm and 1.6×1.7 cm (transverse diameter × vertical diameter
cm), respectively. Following infection, 29 (54%) of the patients
had failed debridement at local hospitals. In the 54 patients, 156
electrode leads were removed and the average implantation time was
53.5 months. Among these patients, a Medtronic 4195 Starfix LV
active lead was used in 3 cases (Table
II). In addition, 3 non-infectious patients underwent electrode
extraction and reimplantation due to elevated LV pacing threshold
and severe diaphragmatic stimulation.
 | Table I.Baseline characteristics of the
patients (n=54). |
Table I.
Baseline characteristics of the
patients (n=54).
| Parameter | Value |
|---|
| Age (years) | 65.33±7.38 |
| Gender (male) | 46 (85) |
| CRT/CRTD device | 34/20 |
| Ischemic
cardiomyopathy | 11 (20) |
| Atrial
fibrillation | 6 (11) |
| Serum white blood
cell count/l |
8.72±3.07×109 |
| Serum hemoglobin
g/l | 123.39±13.17 |
| Serum creatinine
µmol/l | 86.73±30.85 |
| Hypertension | 22 (41) |
| Hyperlipidemia | 8 (15) |
| History of CVA or
TIA | 12 (22) |
| History of CABG | 3 (6) |
| Diabetes
mellitus | 8 (15) |
| Left ventricular
ejection fraction (%) | 33.17±9.86 |
| Left ventricular
end-diastolic diameter (mm) | 67.77±9.81 |
| Left ventricular
end-systolic diameter (mm) | 46.75±10.39 |
 | Table II.Types of pacemaker and electrode
infection and implantation details in the total cohort (n =54). |
Table II.
Types of pacemaker and electrode
infection and implantation details in the total cohort (n =54).
| Feature | Value |
|---|
| Pocket infection | 41 (76) |
| Bacteremia | 7 (13) |
| Infective
endocarditis | 3 (6) |
| Vegetation
(transverse diameter × vertical diameter cm) |
|
| Left ventricular lead
dysfunction | 1.3×1.5, 1.5×2.1,
1.6×1.7 |
|
| 3 (6) |
| Total number of leads
removed | 156 |
| Average number of
leads removed per case | 2.95 |
| Average time of lead
implantation (months) | 53.5 |
| Special left
ventricular leads | 3a |
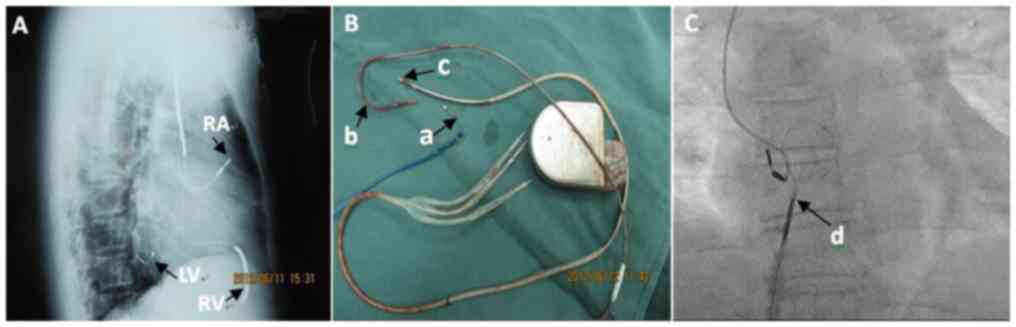
Electrode wire removal and associated
complications
The success rate of complete and clinical removal of
the LV electrodes was 94 and 6%, respectively. Manual traction was
used in 6 patients (11%), whereas removal with locking of steel
wire was performed in 23 patients (43%). In one patient (2%), a
lock wire combined with evolution sheath was used. In a total of 24
cases (44%) the electrodes were removed by Snares via the femoral
vein (Table III; Fig. 1C). The success rate of complete
removal of the right atrial and RV electrodes was 96.1%, whereas
the success rate of clinical removal was 3.9% (Table IV). Following LVL extraction, one
patient did not survive (mortality rate, 2%) due to severe heart
failure. No minor complications were observed in the cohort.
 | Table III.Extraction of LVL and associated
complications in the cohort (n=54). |
Table III.
Extraction of LVL and associated
complications in the cohort (n=54).
| Extraction method or
complication | N (%) |
|---|
| LVL removal complete
success | 51 (94) |
| LVL removal clinical
success | 3 (6) |
| Bare-handed
extraction | 6 (11) |
| Locking steel wire
extraction | 23 (43) |
| Locking steel wire
combined with evolution sheath extraction | 1 (2) |
| Snare catcher
extraction | 24 (44) |
| Patient death after
LVL extraction | 1 (2) |
| Major
complications | 1 (2) |
|
Pericardium tamponade | 0 (0) |
|
Respiratory failure | 1 (2) |
| In-cath
lab deaths | 0 (0) |
| Minor
complications | 0 (0) |
| Hematoma
requiring evacuation | 0 (0) |
|
Significant blood loss
requiring blood transfusion | 0 (0) |
 | Table IV.Details regarding removal and
reimplantation of RA and RV in patients (n=51a). |
Table IV.
Details regarding removal and
reimplantation of RA and RV in patients (n=51a).
| Item | N (%) |
|---|
| RA and RV lead
removal complete success | 49 (96.1) |
| RA and RV lead
removal clinical success | 2 (3.9) |
| Total number of RA
leads extracted | 51 |
| Total number of RV
defibrillation leads extracted | 17 |
| Total number of RV
general leads extracted | 34 |
| Reimplantation CRTD
to the right after | 8 |
| original
extraction |
|
| Reimplantation CRT to
the right after original | 7 |
| CRTD extraction |
|
| Cases requiring laser
extraction of RV defibrillation leads | 3 (5.9) |
| Cases requiring Snare
extraction | 31 (57.4) |
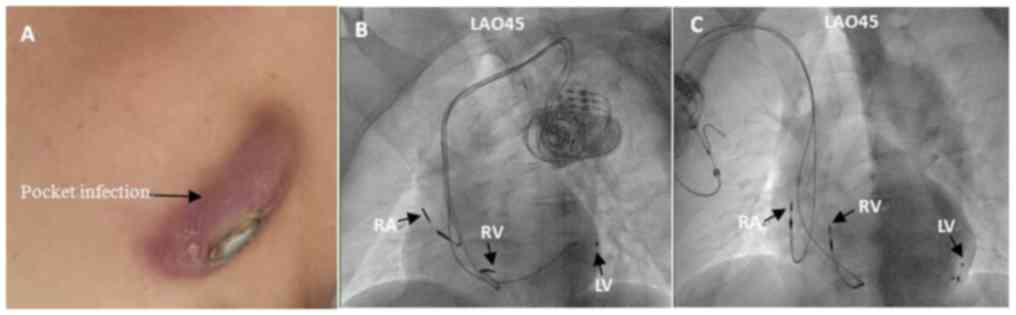
Reimplantation of LV electrode and
CRT/CRTD
A total of 3 out of 39 cases underwent LVL
reimplantation (right/left side, 36/3). In a total of 31 cases,
right LVL reimplantation was successful, and all 3 left LVL
reimplantations were successful. The total success rate of LVL
reimplantation was 87.2%. This percentage was decreased to 86.1%
for the right-side approach (Fig. 2;
Table V). After LVL extraction,
stenosis of the original lateral vein was >50% in 16 cases
(41.0%) and occlusion of the original lateral vein occurred in 3
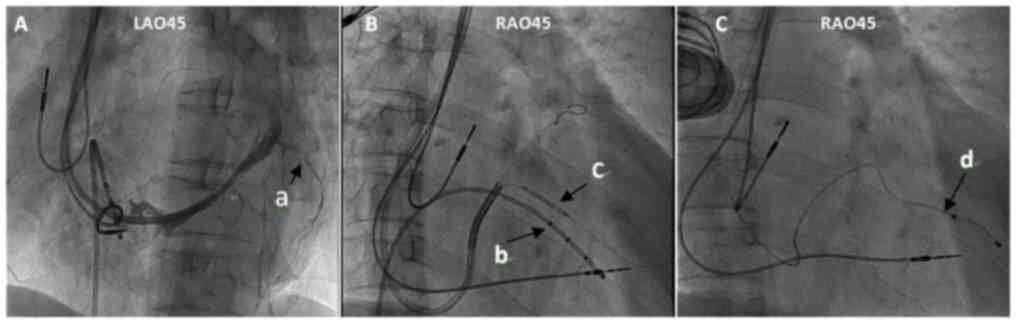
cases (7.7%; Table VI). Severe
stenosis of the original posterior vein was observed in one patient
on coronary venography, and the LV lead was successfully implanted
following dilatation with a 2.0×20 mm balloon at 6 or 8 atm twice
(Fig. 3).
 | Table V.Details regarding reimplantation of
LVL. |
Table V.
Details regarding reimplantation of
LVL.
| Item | Cases, n (%) | Success rate (%) |
|---|
| Reimplant on right
side of LVL | 36 |
|
| Successful
reimplantation of LVL on the right side | 31 | 86.1 |
| Successful
reimplantation of LV dysfunction lead on left side | 3 |
|
| Total cases of
successful reimplantation after extraction | 34 | 87.2 |
| Patient refused
reimplantation | 7 (12.9) |
|
| Biventricular pacing
deemed to be no longer indicated | 3 (6) |
|
| Deemed to be high
risk for reimplantation | 4 (7.4) |
|
| Patient death prior
to reimplantation | 1 (2) |
|
 | Table VI.Details regarding coronary venography
and reimplantation site of LVL in the relevant cases
(n=39a). |
Table VI.
Details regarding coronary venography
and reimplantation site of LVL in the relevant cases
(n=39a).
| Item | N (%) |
|---|
| Original lateral vein
stenosis >50% after LVL extraction | 16 (41.0) |
| Original lateral vein
occluded after LVL extraction | 3 (7.7) |
| Reimplantation into
original lateral vein after LVL extraction | 25 (64.1) |
| Reimplantation in
other lateral vein due to original stenosis or occlusion | 10 (25.6) |
| Balloon used to
dilate original lateral vein stenosis | 1 (2.6) |
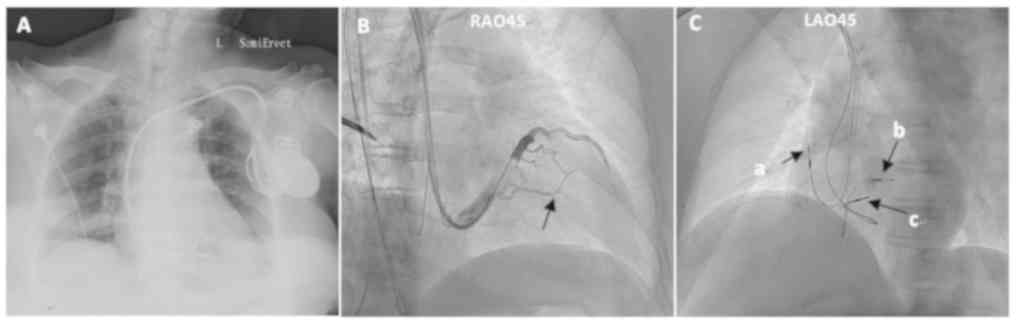
A total of 5 cases did not receive successful
treatment due to severe stenosis or occlusion of the target vessel
in the branch of the coronary vein. A total of 2 out of 5 patients
received RV double site pacing (Fig.
4) and the other 3 patients were treated with His bundle
pacing. A total of 3 non-infectious patients with LVL dysfunction
were successfully reimplanted with LVL at the same stage and at the
ipsilateral region following removal of the LV electrodes. A total
of 7 patients refused to receive reimplantation therapy following
removal of the electrodes. Following removal of the electrodes, one
patient recovered to normal cardiac function and 3 patients
exhibited no indication for biventricular pacing. A total of two
patients exhibited atrial fibrillation with a QRS wave duration of
<120 msec and one patient presented with persistent atrial
fibrillation with a normal ejection fraction. A total of 4 patients
did not receive CRT reimplantation due to their critical clinical
condition (severe infection, cardiac dysfunction with severe
hypotension, systemic infection and lower extremity venous
thrombosis requiring anticoagulant therapy).
Discussion
The difficulties and risks of CRT/CRTD lead removal
are mainly attributed to the rupture of coronary sinus and/or
superior vena cava. The incidence of minor complications in this
group was considerably lower than that reported in previous studies
(1,2). The incidence of major complications of
LVL removal was 2.0% in the present study, which was consistent
with that reported previously (1).
The LV lead was removed successfully and safely, without the
rupture of CS and of the superior vena cava due to the snare
sheath's cutting effect localized to the surrounding tissue and
LVL.
In the present study, the success rate of LVL
reimplantation was 87.2%, which was similar to that reported by a
previous study (7). The majority of
reimplantations was via the right subclavian vein, which is more
difficult than the implantation from the left side. From the right
subclavian vein to the coronary sinus, two physical curves were
present, which resulted in difficulties of LVL implantation. An
Amplatz L1/L2 sheath and super smooth guide wire or adjustable
curve catheter may be used to guide the LVL sheath entering the
coronary sinus.
The other major difficulty encountered in the
reimplantion of the LVL following removal was attributed to
occlusion or stenosis of the originally implanted branches. Burke
et al (8) reported that the
occlusion rate of the original coronary vein following removal of
the LV electrode was 50%. In the present study, this parameter was
estimated to be 48.7%. The LVL was successfully implanted in one
patient with stenosis following balloon dilatation. Balloon
dilatation is usually effective when the target vessel stenosis
occurs during the first implantation of LVL, whereas vascular
stenosis following removal frequently occurs due to fibrous tissue
hyperplasia and/or thrombosis. Therefore, balloon dilatation is not
recommended for routine use during reimplantation.
A total of 3 out of 5 patients who experienced
unsuccessful LVL reimplantation received His bundle pacing. The
clinical application of His bundle pacing has increased in recent
years (9). Long-term follow-up of
these cases has indicated similar results to those observed for CRT
in patients with heart failure (7).
This treatment may be considered in patients with CS branch
occlusion following removal. However, the clinical outcome should
be evaluated in a long-term follow-up period.
A total of 8 of these patients were reimplanted with
CRT/CRTD on the right side following removal. Several studies have
suggested that although the defibrillation threshold may be
elevated in the right implantable cardiac defibrillator (ICD)
compared with that noted in the left ICD, it is still considered
safe and effective in clinical applications (10–12).
Taken collectively, the results of the present study
suggested that CRTD/CRTD removal and reimplantation was feasible
and had a relatively high success rate. However, the present study
has certain limitations, including the single-center study design,
the small sample size and the relatively short follow-up time
period. Patients with better cardiac function and fewer
complications benefit more from reimplantation within a short
period of time after LVL removal. While the number of cases is
limited by the low incidence, a larger cohort would provide more
comprehensive and reliable information, but as our institution is
the center with the largest number of cases of LVL removal in
China, only these cases are available at present. According to
common practice at our institution, after the LVL is removed, the
patient's cardiac function and indications for CRT/CRTD are
reassessed and if there is no indication, the CRT/CRTD is not
reimplanted.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ and DL performed the study and wrote the
manuscript. WZ and DL contributed to data analysis and
interpretation. WZ, DL, XL, FZ, LW, JD and CY contributed to
performing the operations.
Ethics approval and consent to
participate
All experiments were performed with the approval of
the Ethics Committee of Peking University People's Hospital
(Beijing, China). All patients provided written informed consent
regarding their participation in the study.
Patient consent for publication
Patients provided written informed consent for
publication of associated images.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Williams SE, Arujuna A, Whitaker J, Shetty
AK, Bostock J, Patel N, Mobb M, Cooklin M, Gill J, Blauth C, et al:
Percutaneous lead and system extraction in patients with cardiac
resynchronization therapy (CRT) devices and coronary sinus leads.
Pacing Clin Electrophysiol. 34:1209–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamid S, Arujuna A, Khan S, Ladwiniec A,
McPhail M, Bostock J, Mobb M, Patel N, Bucknall C and Rinaldi CA:
Extraction of chronic pacemaker and defibrillator leads from the
coronary sinus: Laser infrequently used but required. Europace.
11:213–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Martino G, Orazi S, Bisignani G,
Toscano S, Messano L, Parisi Q, Santamaria M, Pelargonio G, Dello
Russo A, Bellocci F, et al: Safety and feasibility of coronary
sinus left ventricular leads extraction: A preliminary report. J
Interv Card Electrophysiol. 13:35–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bongiorni MG, Zucchelli G, Soldati E,
Arena G, Giannola G, Di Cori A, Lapira F, Bartoli C, Segreti L, De
Lucia R and Barsotti A: Usefulness of mechanical transvenous
dilation and location of areas of adherence in patients undergoing
coronary sinus lead extraction. Europace. 9:69–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kusumoto FM, Schoenfeld MH, Wilkoff BL,
Berul CI, Birgersdotter-Green UM, Carrillo R, Cha YM, Clancy J,
Deharo JC, Ellenbogen KA, et al: 2017 HRS Expert Consensus
Statement on Cardiovascular Implantable Electronic Device Lead
Management and Extraction. Heart Rhythm. 14:e503–e551. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bongiorni MG, Soldati E, Zucchelli G, Di
Cori A, Segreti L, De Lucia R, Solarino G, Balbarini A, Marzilli M
and Mariani M: Transvenous removal of pacing and implantable
cardiac defibrillating leads using single sheath mechanical
dilatation and multiple venous approaches: High success rate and
safety in more than 2000 leads. Eur Heart J. 29:2886–2893. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rickard J, Tarakji K, Cronin E, Brunner
MP, Jackson G, Baranowski B, Borek PP, Martin DO, Wazni O and
Wilkoff BL: Cardiac venous left ventricular lead removal and
reimplantation following device infection: A large single-center
experience. J Cardiovasc Electrophysiol. 23:1213–1216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burke MC, Morton J, Lin AC, Tierney S,
Desai A, Hong T, Kim S, Salem Y, Alberts M and Knight BP:
Implications and outcome of permanent coronary sinus lead
extraction and reimplantation. J Cardiovasc Electrophysiol.
16:830–837. 2010. View Article : Google Scholar
|
|
9
|
Vijayaraman P, Subzposh FA and Naperkowski
A: Extraction of the permanent His bundle pacing lead: Safety
outcomes and feasibility of reimplantation. Heart Rhythm.
2019.(Epub ahead of print). View Article : Google Scholar
|
|
10
|
Keyser A, Hilker MK, Ucer E, Wittmann S,
Schmid C and Diez C: Significance of intraoperative testing in
right-sided implantable;cardioverter-defibrillators. J Cardiothorac
Surg. 8:772013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nery PB, Fernandes R, Nair GM, Sumner GL,
Ribas CS, Menon SM, Wang X, Krahn AD, Morillo CA, Connolly SJ and
Healey JS: Device-related infection among patients with pacemakers
and implantable defibrillators: Incidence, risk factors, and
consequences. J Cardiovasc Electrophysiol. 21:786–790.
2010.PubMed/NCBI
|
|
12
|
Hamid S, Arujuna A, Ginks M, McPhail M,
Patel N, Bucknall C and Rinaldi C: Pacemaker and defibrillator lead
extraction: Predictors of mortality during follow-up. Pacing Clin
Electrophysiol. 33:209–216. 2010. View Article : Google Scholar : PubMed/NCBI
|