Introduction
Diabetes is associated with chronic complications
that affect almost every system in the body. In particular, the
risk of diabetic encephalopathy has been increasingly recognized
(1). Previous studies have reported
that a high incidence of cognitive deficits, including Alzheimer's
disease and vascular dementia, in addition to other types of
dementia, is observed among patients with type 1 or 2 diabetes
(1,2). Diabetic mouse models have also been
reported to be associated with decreased hippocampal cell
proliferation and survival, in line with reduced performance in
learning and memory tests (3,4).
Multiple pathogenic mechanisms appear to be involved in the
development of diabetic encephalopathy, including vascular
dysfunction, hyperglycemia or hypoglycemia and the deficiency of or
resistance to insulin (5). Studies
in different experimental models have established that
hyperglycemia reduces antioxidant levels and concomitantly
increases the production of free radicals, which may contribute to
neuronal dysfunction (6,7). Therefore, developing novel antioxidants
to antagonize oxidative stress is crucial for reducing
diabetes-associated morbidity.
Accumulating evidence suggests that nuclear factor
erythroid 2-related factor 2 (Nrf2) serves an important function in
reducing oxidative stress in neurodegenerative disorders,
demonstrated in in vitro and in vivo studies
(8). Nrf2 exerts antioxidant effects
by increasing the expression of endogenous antioxidant enzymes,
including heme oxygenase-1 (HO-1), which may protect cells by
catalyzing the degradation of heme to carbon monoxide, catalytic
iron and bilirubin (9).
Nrf2-deficienct mice exhibit more severe neurological disorders,
along with higher levels of β-amyloid and tau protein (10), whilst the overexpression of Nrf2
resulted in the damage being reversed (11). It was reported that the activation of
Nrf2 by sulforaphane, a pharmacological activator, observably
improved cognitive functions in streptozotocin-induced type 1
diabetic rats, in addition to db/db mice, by reducing
hyperglycemia-induced neuronal apoptosis in the hippocampus
(12,13).
Ferulic acid (FA) belongs to the family of phenolic
acids and is present in a wide variety of fruits, vegetables and
grains (14,15). FA has anti-inflammatory and
antioxidant properties, and has been demonstrated to exert
neuroprotective effects against cerebral ischemia-reperfusion
injury (14) and Alzheimer's disease
(15). Sodium ferulate (SF) is a
sodium salt of FA, which is more stable in air and more easily
dissolved in water (16). One
previous study demonstrated that SF may increase antioxidant enzyme
activity, thereby exerting protective effects in diabetic
cardiomyopathy and other chronic complications of diabetes
(16). However, to the best of our
knowledge, no study to date has clearly demonstrated the function
of SF in neuronal functions under high-glucose conditions. It was
hypothesized that the protective effects of SF may be associated
with the activation of the Nrf2/HO-1 pathway. The aim of the
present study was to investigate the protective function of SF in
high-glucose cultured HT22 hippocampal cells and elucidate the
underlying mechanisms.
Materials and methods
Cell culture
The HT22 mouse hippocampal cell line was obtained
from Jennio Biotech Co., Ltd. (Guangzhou, China). Cells were
cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with normal (25 mM) or
high (50 mM) glucose concentrations, supplemented with 10% fetal
bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel).
SF (Shandong XiYa Chemical Industry Co., Ltd., Shandong, China) was
added to the high-glucose group at various concentrations (50, 100,
250, 500 µM), followed by incubation at 37°C with 5% CO2
in a humidified atmosphere.
Cell Counting Kit-8 (CCK-8) assay
HT22 cells in the logarithmic growth phase were
plated onto 96-well plates at a density of 4×104 cells
per well. Cell viability was estimated using a CCK-8 assay,
according to the manufacturer's protocol (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). CCK-8 was added into each
well at 0, 48 and 72 h following culturing, and then incubated for
3 h at 37°C prior to measurement. Absorbance at 450 nm was detected
using a microplate reader (Multiskan™ FC; Thermo Fisher Scientific,
Inc.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the cells using TRIzol
reagent (Takara Bio, Inc., Otsu, Japan). cDNA was synthesized from
total RNA using a two-temperature cycle at 37°C for 15 min and 85°C
for 5 sec using Prime-Script™ RT reagent kits with gDNA eraser
(Takara Bio, Inc.), according to the manufacturer's protocol. mRNA
expression levels were measured using RT-qPCR on Biosystems 7500
(Applied Biosystems, Inc., Carlsbad, Cal, USA). Reaction mixtures
(10 µl) contained SYBR Select Master Mix (Takara Bio, Inc.), (5 µl)
cDNA samples (1 µl) and forward or reverse primers (0.5 µl). A
two-temperature cycle at 95°C for 10 sec and 60°C for 30 sec was
run and repeated for 40 cycles. Relative quantities of sample
transcripts were calculated using the 2−ΔΔCq method
(17) with GAPDH used as a reference
gene. All samples were expressed relative to the mean. The primer
sequences used are listed in Table
I.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primers | Reverse primers |
|---|
| Nuclear factor
erythroid 2-related factor 2 |
5′-GAAATGATGTCCAAGGAGCAA-3′ |
5′-AAGACTTCAAGATACAAGGTGCTG-3′ |
| Nuclear
factor-κB |
5′-ACCCTGAAATCAAAGACAAAGAG-3′ |
5′-GAAATCCGTAGTTCGAGTAGCC-3′ |
| Heme oxygenase-1 |
5′-TGACAGAAGAGGCTAAGACCG-3′ |
5′-GTGAGGACCCACTGGAGGA-3′ |
| GAPDH |
5′-ATTCAACGGCACAGTCAAGG-3′ |
5′-CACCAGTGGATGCAGGGAT-3′ |
Gel electrophoresis and western
blotting
Cell lysates were prepared using
radioimmunoprecipitation assay lysis buffer (CW Biotech) in the
presence of a protease inhibitor cocktail (Thermo Fisher
Scientific, Inc.). Protein concentrations of cell lysates were
quantified using a Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturers protocol. Total
protein (10 ug) were loaded in each well of 12% sodium dodecyl
sulfate polyacrylamide gel and subjected to electrophoresis. The
proteins were then transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat milk for 1 h at room temperature and
incubated with primary antibodies against GAPDH (1:5,000; cat. no.
10494-1-AP; ProteinTech Group, Inc.), Nrf2 (1:1,000; cat. no.
ab62352; Abcam), HO-1 (1:1,000; cat. no. ab13243; Abcam) and NF-κB
(1:10,000; cat. no. ab16502; Abcam) at 4°C overnight, followed by
incubation with a horseradish peroxidase-conjugated secondary
antibody (1:5,000; cat. no. SA00001-2; ProteinTech Group, Inc.) for
1 h at room temperature. Detection was performed using ECL Plus
western blotting detection reagents (EMD Millipore) and the blots
were semi-quantified using ImageJ 2 (National Institute of
Health).
Measurement of reactive oxygen species
(ROS) generation
Cells were cultured in 6-well plates for 0 or 72 h,
then washed with phosphate-buffered saline (HyClone; GE Healthcare,
Logan, UT, USA) and incubated with 5 µM CellROX® Deep
Red Reagent (Molecular Probes; Thermo Fisher Scientific, Inc.) in
completed medium for 30 min at 37°C. Subsequently, cells were
examined using a flow cytometer (BD FACScanto II; BD Biosciences)
and data was analyzed using FlowJo (V10.0; BD Biosciences).
Statistical analysis
The data were expressed as the mean ± standard
deviation. One-way analysis of variance followed by a least
significant difference post-hoc test was used to compare the mean
values amongst control and treatment groups using SPSS17.0 software
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
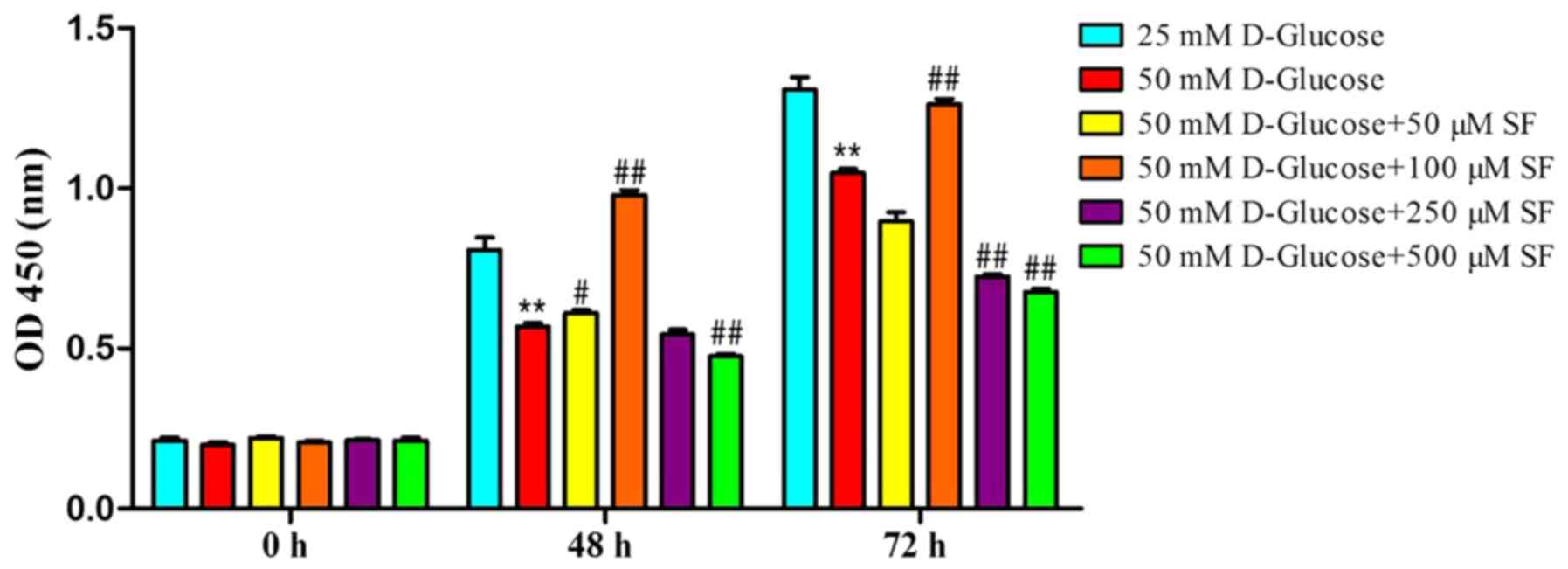
SF preserves HT22 cell viability under
high-glucose conditions
The present study established an in vitro
model of hippocampal neuron cells exposed to a high glucose
concentration (50 mM), as previously reported (18). To verify the effects of SF, HT22
cells were exposed to high glucose (50 mM) with various
concentrations of SF (50, 100, 250 and 500 µM) for 0, 48 and 72 h.
Cell viability was determined using a CCK-8 assay. Compared with
the normal-glucose group, the high-glucose group without SF
exhibited a significant decrease (P<0.01) in cell viability
subsequent to culturing for 48 or 72 h. The addition of 50 µM SF to
the high-glucose group did not significantly affect cell viability
at 72 h. However, when 100 µM SF was added to the high-glucose
group, the cell viability increased significantly compared with the
high-glucose alone group (P<0.01), to levels comparable with
those in the normal-glucose group. However, cell viability did not
increase further with higher (250 and 500 µM) concentrations of SF
(Fig. 1).
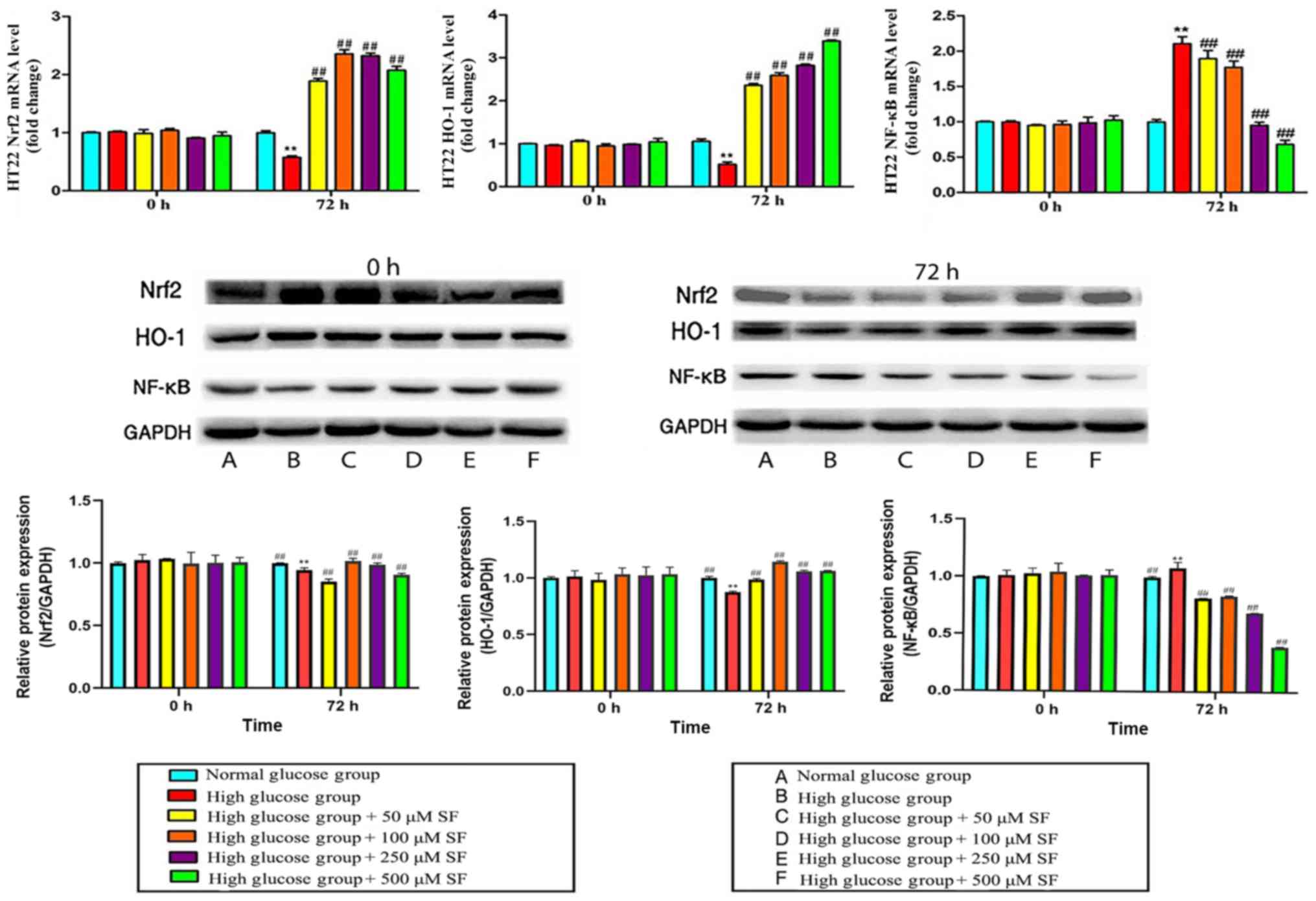
SF upregulates Nrf2-1 expression
levels in HT22 cells
The Nrf2 mRNA levels in HT22 cells cultured with a
high glucose concentration were significantly downregulated
compared with those in the normal-glucose group at 72 h
(P<0.01). Following the addition of different concentrations of
SF (50, 100, 250 and 500 µM) to each group for 72 h, the Nrf2 mRNA
levels were significantly increased compared with those in the
high-glucose group without SF (P<0.01), to levels even higher
compared with those in the normal-glucose group. Subsequently,
western blotting was performed to determine the expression levels
of the Nrf2 protein. However, upon increasing the expression of the
Nrf2 protein with different concentrations of SF (50, 100, 250 and
500 µM) was significantly upregulated compared to the high glucose
group. (P<0.01; Fig. 2).
SF upregulates HO-1 expression levels
in HT22 cells
HO-1 mRNA and protein expression levels were
significantly decreased when HT22 cells were exposed to a high
glucose concentration for 72 h compared with the normal glucose
group (P<0.01). Subsequent to culturing with SF (50, 100, 250
and 500 µM), the expression levels of HO-1 were significantly
increased compared with that in the high-glucose and normal-glucose
groups (P<0.01; Fig. 2).
SF downregulates NF-κB expression
levels in HT22 cells
The expression of NF-κB at the mRNA and protein
levels was determined using RT-qPCR and western blotting,
respectively. The results demonstrated that the mRNA and protein
levels of NF-κB in HT22 cells in the high-glucose group were
significantly upregulated compared with the normal-glucose group at
72 h (P<0.01). High-glucose group with SF at 50, 100, 250 or 500
µM significantly downregulated the expression of NF-κB mRNA in a
concentration-dependent manner compared with the high glucose alone
group (P<0.01; Fig. 2).
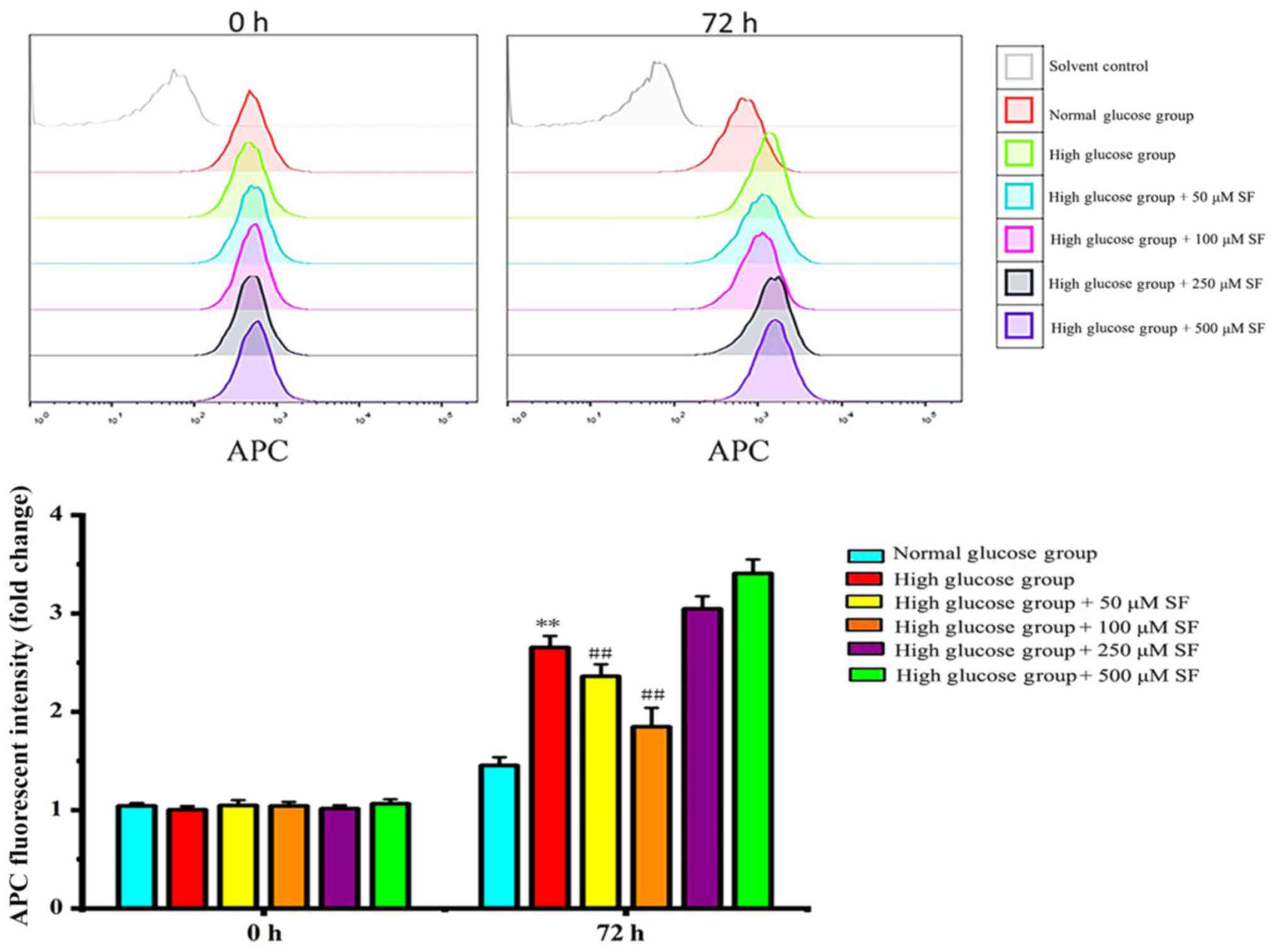
SF inhibits the production of ROS
The intracellular ROS levels were measured using
flow cytometry. HT22 cells subjected to a high glucose
concentration exhibited a significant increase in fluorogenic
intensity compared with the normal glucose group (P<0.01), which
meant an increase in cellular ROS. This effect was inhibited by SF.
Therefore, high glucose increased ROS production in HT22 cells,
whereas SF (50, 100 µM) significantly attenuated this
high-glucose-induced ROS generation compared with the high glucose
group (P<0.01; Fig. 3).
Discussion
HT22 is a hippocampal neuronal cell line that has
been widely used in in vitro models to study the mechanisms
underlying neurodegenerative diseases. In the present study, an
in vitro model mimicking hyperglycemia was designed to
investigate the protective effect of SF on HT22 cells. It was
observed that high glucose levels increased ROS production and
decreased the viability of HT22 cells, which was induced by
downregulating Nrf2/HO-1 pathway activation and upregulating
NF-κB.
Hyperglycemia is associated with increased oxidative
stress. Enhanced ROS production and oxidative injury serve a key
function in the progression of diabetic encephalopathy (19). It has been reported that FA exerts
protective effects against amyloid-β-induced neurodegeneration
(20). FA has been demonstrated to
protect cortical synaptosomal membranes by reducing protein
oxidation, lipid peroxidation and cell death induced by oxidative
radicals (21). The present study
demonstrated the cytoprotective effects of SF at concentrations of
50 and 100 µM by decreasing ROS production; however, higher
concentrations of SF (250 and 500 µM) decreased cell viability,
consistent with the results of previous studies (20,21).
Therefore, a more precise safe dose of SF must be determined in
future studies.
SF functions as a direct scavenger of ROS. This
characteristic certainly contributes to its neuroprotective effects
(22) In addition to their
antioxidant properties, a number of polyphenols, including SF,
appear to exert pleiotropic effects on cells and tissues (22). The Nrf2/HO-1 pathway serves an
important function in the regulation of cellular redox status. When
cells are exposed to ROS, Nrf2 translocates to the nucleus and
binds to antioxidant response elements, inducing the production of
endogenous antioxidant enzymes to restore cellular homeostasis
(23). FA has been revealed to
induce HO-1 expression via activating extracellular
signal-regulated kinase, thus protecting lymphocytes from
radiation-induced injury (24). It
was demonstrated that FA protects neuroblastoma cells from
oxidative injury through upregulating HO-1 expression and the
biliverdin reductase system by fostering the nuclear translocation
of the transcriptional activator Nrf2 (25). Ethyl ferulate, a naturally occurring
ester of FA, was also proven to protect rat neurons against
oxidative stress by promoting the expression of HO-1 at the mRNA
and protein levels (26). The
present study demonstrated that SF upregulated Nrf2/HO-1 mRNA and
protein expression levels in HT22 cells. Although Nrf2/HO-1 was
revealed to be implicated in the neuroprotective effect of SF, the
causal association remains unclear. To further confirm this
association, a Nrf2 knockout model is required to verify whether
this protective effect is attenuated when Nrf2 expression is
downregulated.
NF-κB is a transcription factor that regulates the
expression of multiple cytokines, including tumor necrosis
factor-α, interleukin (IL)-1β and IL-8, and serves a key function
in oxidative stress and inflammation (27). One previous study suggests that the
activation of NF-κB and its downstream genes are implicated in the
pathobiology of diabetes and its complications (28). The persistent activation of NF-κB was
demonstrated in the hippocampi of streptozotocin-induced diabetic
rats (29). SF was reported to
protect hippocampal neurons against sodium nitroprusside-induced
toxicity and decrease the expression of NF-κB P65 (30). Furthermore, SF may markedly prevent
amyloid β-induced IL-1β increase and inhibit neuronal apoptotic
death in a rat hippocampus (31).
The results of the present study suggest that SF may prevent the
high-glucose-induced activation of NF-κB.
In conclusion, SF increases the resistance of HT22
cells to glucose toxicity by activating the Nrf2/HO-1 pathway and
inhibiting the expression of NF-κB, thereby attenuating
high-glucose-induced neuronal death and indicating potential novel
strategies for neuroprotection in diabetic encephalopathy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Project of Guangdong Province, China (grant no.
2014A020212534), the Science and Technology Planning Project of
Guangzhou, China (grant no. 201604020119) and the Medical
Scientific Research Foundation of Zhejiang Province, China (grant
no. 2016KYA0005).
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ contributed to the conception of the study and
was a major contributor in writing the manuscript. TZ designed the
present study, interpreted the results, wrote and revised the
manuscript and gave final approval of the version to be published.
LL performed the experiments. LZ contributed to statistics and data
analysis, drafting and revising the manuscript, and making the
figures. JL, XG and XL helped to perform the experiments.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu WL, von Strauss E, Qiu CX, Winblad B
and Fratiglioni L: Uncontrolled diabetes increases the risk of
Alzheimer's disease: A population-based cohort study. Diabetologia.
52:1031–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Harten B, Oosterman J, Muslimovic D,
van Loon BJ, Scheltens P and Weinstein HC: Cognitive impairment and
MRI correlates in the elderly patients with type 2 diabetes
mellitus. Age Ageing. 36:164–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li ZG, Zhang W and Sima AA: Alzheimer-like
changes in rat models of spontaneous diabetes. Diabetes.
56:1817–1824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alvarez EO, Beauquis J, Revsin Y, Banzan
AM, Roig P, De Nicola AF and Saravia F: Cognitive dysfunction and
hippocampal changes in experimental type 1 diabetes. Behav Brain
Res. 198:224–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sima AA: Encephalopathies: The emerging
diabetic complications. Acta Diabetol. 47:279–293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Russell JW, Golovoy D, Vincent AM,
Mahendru P, Olzmann JA, Mentzer A and Feldman EL: High
glucose-induced oxidative stress and mitochondrial dysfunction in
neurons. FASEB J. 16:1738–1748. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamboj SS and Sandhir R: Protective effect
of N-acetylcysteine supplementation on mitochondrial oxidative
stress and mitochondrial enzymes in cerebral cortex of
streptozotocin-treated diabetic rats. Mitochondrion. 11:214–222.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karkkainen V, Pomeshchik Y Savchenko E,
Dhungana H, Kurronen A, Lehtonen S, Naumenko N, Tavi P, Levonen AL,
Yamamoto M, et al: Nrf2 regulates neurogenesis and protects neural
progenitor cells against Aβ toxicity. Stem Cells. 32:1904–1916.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rojo AI, Pajares M, Rada P, Nuñez A,
Nevado-Holgado AJ, Killik R, Van Leuven F, Ribe E, Lovestone S,
Yamamoto M and Cuadrado A: NRF2 deficiency replicates
transcriptomic changes in Alzheimer's patients and worsens APP and
TAU pathology. Redox Biol. 13:444–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanninen K, Malm TM, Jyrkkänen HK,
Goldsteins G, Keksa-Goldsteine V, Tanila H, Yamamoto M,
Yia-Herttuala S, Levonen AL and Koistinaho J: Nuclear factor
erythroid 2-related factor 2 protects against beta amyloid. Mol
Cell Neurosci. 39:302–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang G, Fang H, Zhen Y, Xu G, Tian J,
Zhang Y, Zhang D, Zhang G, Xu J, Zhang Z, et al: Sulforaphane
prevents neuronal apoptosis and memory impairment in diabetic rats.
Cell Physiol Biochem. 39:901–907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pu D, Zhao Y, Chen J, Sun Y, Lv A, Zhu S,
Luo C, Zhao K and Xiao Q: Protective effects of sulforaphane on
cognitive impairments and AD-like lesions in diabetic mice are
associated with the upregulation of Nrf2 transcription activity.
Neuroscience. 381:35–45. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren Z, Zhang R, Li Y, Li Y, Yang Z and
Yang H: Ferulic acid exerts neuroprotective effects against
cerebral ischemia/reperfusion-induced injury via antioxidant and
anti-apoptotic mechanisms in vitro and in vivo. Int J
Mol Med. 40:1444–1456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mancuso C and Santangelo R: Ferulic acid:
Pharmacological and toxicological aspects. Food Chem Toxicol.
65:185–195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu X, Xiao H, Zhao J and Zhao T:
Cardioprotective effect of sodium ferulate in diabetic rats. Int J
Med Sci. 9:291–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ward R and Ergul A: Relationship of
endothelin-1 and NLRP3 inflammasome activation in HT22 hippocampal
cells in diabetes. Life Sci. 159:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rolo AP and Palmeira CM: Diabetes and
mitochondrial function: Role of hyperglycemia and oxidative stress.
Toxicol Appl Pharm. 212:167–178. 2006. View Article : Google Scholar
|
|
20
|
Kikugawa M, Tsutsuki H, Ida T, Nakajima H,
Ihara H and Sakamoto T: Water-soluble ferulic acid derivatives
improve amyloid-beta-induced neuronal cell death and dysmnesia
through inhibition of amyloid-β aggregation. Biosci Biotechnol
Biochem. 80:547–553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanski J, Aksenova M, Stoyanova A and
Butterfield DA: Ferulic acid antioxidant protection against
hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal
cell culture systems in vitro: Structure-activity studies. J Nutr
Biochem. 13:273–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X, Lin Q, He G, Hou X, Shan Z and Du
Y: Therapeutic effect of QQL prescription on type 2 diabetic rats.
Chin J Pathophysiol. 33:1794–1800. 2017.
|
|
23
|
de Vries HE, Witte M, Hondius D,
Rozemuller AJ, Drukarch B, Hoozemans J and van Horssen J:
Nrf2-induced antioxidant protection: A promising target to
counteract ROS-mediated damage in neurodegenerative disease? Free
Radic Biol Med. 45:1375–1383. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma ZC, Hong Q, Wang YG, Liang QD, Tan HL,
Xiao CR, Tang XL, Shao S, Zhou SS and Gao Y: Ferulic acid induces
heme oxygenase-1 via activation of ERK and Nrf2. Drug Discov Ther.
5:299–305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Catino S, Paciello F, Miceli F, Rolesi R,
Troiani D, Calabrese V, Santangelo R and Mancuso C: Ferulic acid
regulates the Nrf2/Heme oxygenase-1 system and counteracts
trimethyltin-induced neuronal damage in the human neuroblastoma
cell line SH-SY5Y. Front Pharmacol. 6:3052015.PubMed/NCBI
|
|
26
|
Scapagnini G, Butterfield DA, Colombrita
C, Sultana R, Pascale A and Calabrese V: Ethyl ferulate, a
lipophilic polyphenol, induces HO-1 and protects rat neurons
against oxidative stress. Antioxid Redox Signal. 6:811–818. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mezzasoma L, Antognelli C and Talesa VN: A
novel role for brain natriuretic peptide: Inhibition of IL-1β
secretion via downregulation of NF-kB/Erk 1/2 and
NALP3/ASC/Caspase-1 activation in human THP-1 monocyte. Mediators
Inflamm. 2017:58583152017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang B, Miao Y, Zhao Z and Zhong Y:
Inflammatory macrophages promotes development of diabetic
encephalopathy. Cell Physiol Biochem. 36:1142–1150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aragno M, Mastrocola R, Brignardello E,
Catalano M, Robino G, Manti R, Parola M, Danni O and Boccuzzi G:
Dehydroepiandrosterone modulates nuclear factor-kappaB activation
in hippocampus of diabetic rats. Endocrinology. 143:3250–3258.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Li Y and An Y: Effect of sodium
ferulate on NF-κB P65 and Par-4 expression of apoptotic hippocampal
neurons induced by SNP. Chin J Gerontol. 28:1361–1364. 2008.(In
Chinese).
|
|
31
|
Jin Y, Yan EZ, Li XM, Fan Y, Zhao YJ, Liu
Z and Liu WZ: Neuroprotective effect of sodium ferulate and signal
transduction mechanisms in the aged rat hippocampus. Acta Pharmacol
Sin. 29:1399–1408. 2008. View Article : Google Scholar : PubMed/NCBI
|