Introduction
Immune thrombocytopenia (ITP) is an autoimmune
disorder characterized by a low platelet count with mucocutaneous
or other types of bleeding. The pathogenesis of ITP remains poorly
understood, while immune disorders are thought to have an important
implication. The presence of anti-platelet glycoprotein (GP)
antibodies is also considered to be critically involved in ITP
(1). In addition, a complex
dysregulation of cellular mechanisms has been reported, including
the imbalance of the type 1 T-helper cell (Th1)/Th2 ratio (2), increased number of Th17 cells (3), decreased number or functional deficit
of regulatory T cells (4,5) and increased cytotoxic T
lymphocyte-mediated cytotoxicity (6).
Interleukin (IL)-37, a novel anti-inflammatory
cytokine previously known as interleukin-1 family member 7 before
it was renamed, has a pivotal role in the suppression of immune
responses (7–10). Alternative splicing of the human gene
for IL-37 gives rise to 5 different isoforms: IL-37a, b, c, d and
e, among which IL-37b is the most likely one to be biologically
functional. IL-37 is widely expressed in several types of cells,
tissues and organs, including peripheral blood mononuclear cells
(PBMCs) (7). The major role of IL-37
is to decrease excessive inflammation in innate and adaptive immune
diseases, mainly by inhibiting the expression, production and
function of pro-inflammatory cytokines, including IL-1α, IL-6,
tumor necrosis factor (TNF) and macrophage inflammatory protein-2.
The abundance of these cytokines has been reported to increase with
the silencing of endogenous IL-37 in human blood cells (7,11). In
vivo, IL-37-transgenic mice exhibited markedly reduced
manifestations of endotoxemia, dextran sulphate sodium colitis,
ischemia-reperfusion injury and obesity-induced inflammation
(12–14). Furthermore, treatment with
recombinant IL-37 in wild-type mice has been demonstrated to exert
protective effects in several models of inflammation and injury
(15–17).
Aberrant expression of IL-37 has been observed in
several inflammatory and autoimmune diseases, including rheumatoid
arthritis (RA) (18–21), systemic lupus erythematosus (SLE)
(22,23), inflammatory bowel disease (IBD)
(24,25), ankylosing spondylitis (AS) (26) and Graves' disease (GD) (27). However, the role of IL-37 in ITP has
remained elusive. To investigate the expression of IL-37 and its
potential role in the pathogenesis of ITP, the levels of IL-37 in
ITP patients were measured and their correlation to disease
activity was determined.
Materials and methods
Patients and controls
A total of 34 patients with ITP were enrolled in the
present study (Table I), consisting
of 18 newly diagnosed ITP patients with active disease (11 females
and 7 males; age range, 19–62 years; median age, 39.89 years) and
16 patients in remission (10 females and 6 males; age range, 19–62
years; median age, 37.25 years). The platelet count of patients
with active ITP was 1–30×109/l (median,
12.28×109/l), which was significantly lower than that of
patients in remission (101–305×109/l; median,
163×109/l; P<0.001). Out of the 34 patients with ITP,
12 were treated with glucocorticoids, including high-dose
dexamethasone (HD-DEX) 40 mg daily for 4 day severy 4 weeks and
prednisone 1.0 mg/kg daily, which was then tapered.
 | Table I.Clinical characteristics of ITP
patients. |
Table I.
Clinical characteristics of ITP
patients.
| Case no. | Sex/age (years) | Plt count
(×109/l) |
Anti-GPIIb/IIIa/anti-GPIb/IX | Bleeding
symptoms | Medications |
|---|
| 1 | F/38 | 5 | −/− | PT | NONE |
| 2 | F/25 | 12 | +/+ | PT | NONE |
| 3 | M/48 | 9 | +/− | EC, GH | NONE |
| 4 | F/27 | 18 | −/− | PT, EC | NONE |
| 5 | F/24 | 10 | −/− | PT | NONE |
| 6 | F/35 | 21 | −/+ | EC | NONE |
| 7 | M/23 | 3 | −/+ | PT, EC | NONE |
| 8 | M/29 | 15 | −/− | PT, GH | NONE |
| 9 | F/38 | 30 | +/+ | EC | NONE |
| 10 | F/59 | 13 | +/− | PT, GUH | NONE |
| 11 | M/42 | 4 | −/+ | EC | NONE |
| 12 | F/62 | 1 | −/− | PT, EC, GUH | Plt |
| 13 | F/58 | 17 | −/+ | PT, EC | NONE |
| 14 | M/47 | 2 | +/+ | PT, GH | NONE |
| 15 | F/37 | 8 | −/+ | PT, EP | NONE |
| 16 | F/48 | 11 | −/− | PT | NONE |
| 17 | M/59 | 19 | +/− | GUH | NONE |
| 18 | M/19 | 23 | −/− | PT | NONE |
| 19 | F/49 | 162 | −/− | NONE | Pred, R |
| 20 | M/26 | 104 | −/− | NONE | IVIG, Plt |
| 21 | F/29 | 203 | +/+ | NONE | HD-DEX |
| 22 | F/62 | 113 | −/− | NONE | Plt, R |
| 23 | M/50 | 134 | +/− | NONE | Plt, HD-DEX |
| 24 | F/48 | 224 | −/− | NONE | IVIG, HD-DEX |
| 25 | F/25 | 180 | +/− | NONE | HD-DEX |
| 26 | F/31 | 158 | −/+ | NONE | HD-DEX |
| 27 | M/49 | 123 | −/− | NONE | Pred, HD-DEX,
R |
| 28 | F/24 | 305 | −/− | NONE | HD-DEX |
| 29 | M/55 | 150 | +/− | NONE | Plt, R |
| 30 | F/29 | 107 | −/− | NONE | IVIG, HD-DEX |
| 31 | F/51 | 158 | +/− | NONE | HD-DEX,R |
| 32 | M/23 | 137 | −/− | NONE | HD-DEX |
| 33 | F/19 | 101 | −/− | NONE | Plt, R |
| 34 | M/26 | 249 | −/− | NONE | IVIG, HD-DEX |
The control group consisted of 15 healthy volunteers
admitted to the hospital for routine physical examination (9
females and 6 males; age range, 16–66 years; median age, 39.67
years). The platelet count was 128–325×109/l (median,
231×109/l).
The enrollment of patients and healthy volunteers
took place between November 2016 and January 2018 at the Department
of Hematology of the Shandong Provincial Hospital affiliated to
Shandong University (Jinan, China). All cases met the diagnostic
criteria of ITP, as previously described (28). Patients complicated with diabetes,
hypertension, cardiovascular diseases, pregnancy, active or chronic
infection or connective tissue diseases were excluded from the
study.
PBMC preparation
Plasma samples were separated by centrifugation and
stored at −20°C to be used for the determination of IL-37 and
anti-platelet autoantibodies.
PBMCs were isolated from heparinized blood samples
by Ficoll-Hypaque density gradient centrifugation at 780 × g for 20
min at 20°C, and stored at −80°C for future use.
ELISA of IL-37
The plasma concentration of IL-37 was measured by a
commercial ELISA kit, according to the manufacturer's protocols
(AdipoGen). The lower detection limit of this assay was 16
pg/ml.
Determination of the mRNA expression
of IL-37
For reverse transcription (RT), total RNA was
extracted from PBMCs using TRIzol reagent (Thermo Fisher
Scientific, Inc.). The RNA was then reverse transcribed to
complementary DNA using the PrimeScript™ RT Reagent kit (Takara
Bio, Inc.), according to the manufacturer's protocol. Quantitative
(q)PCR for IL-37 was performed on an ABI PRISM_7500 Sequence
Detection System (Thermo Fisher Scientific, Inc.) using SYBR Green
(Toyobo Life Science) according to the manufacturer's protocol.
β-Actin was used as the endogenous control. The sequences of
specific primers were as follows: IL-37 forward,
5′-AAGACCTACGCCATGGGACATC-3′ and reverse,
5′-TCTTGGTATTGCAAGTTGGAGTTCA-3′; β-actin forward,
5′-TTGCCGACAGGATGCAGAA-3′ and reverse,5′-GCCGATCCACACGGAGTACT-3′.
The relative expression levels between IL-37 and β-actin were
compared using the 2−ΔΔCq method (29).
Anti-platelet autoantibody
determination
The specific anti-platelet autoantibodies to
GPIIb/IIIa and/or GPIb/IX were analyzed by modified monoclonal
antibody-specific immobilization of platelet antigens, as
previously described (30).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical significance between two groups was
determined using an unpaired Student's t-test. For multiple
comparisons, one-way analysis of variance followed by Bonferroni's
post-hoc test was used. The correlation analysis was performed
using Pearson's correlation. All statistical analyses were
performed using SPSS 13.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
IL-37 expression in ITP patients and
controls
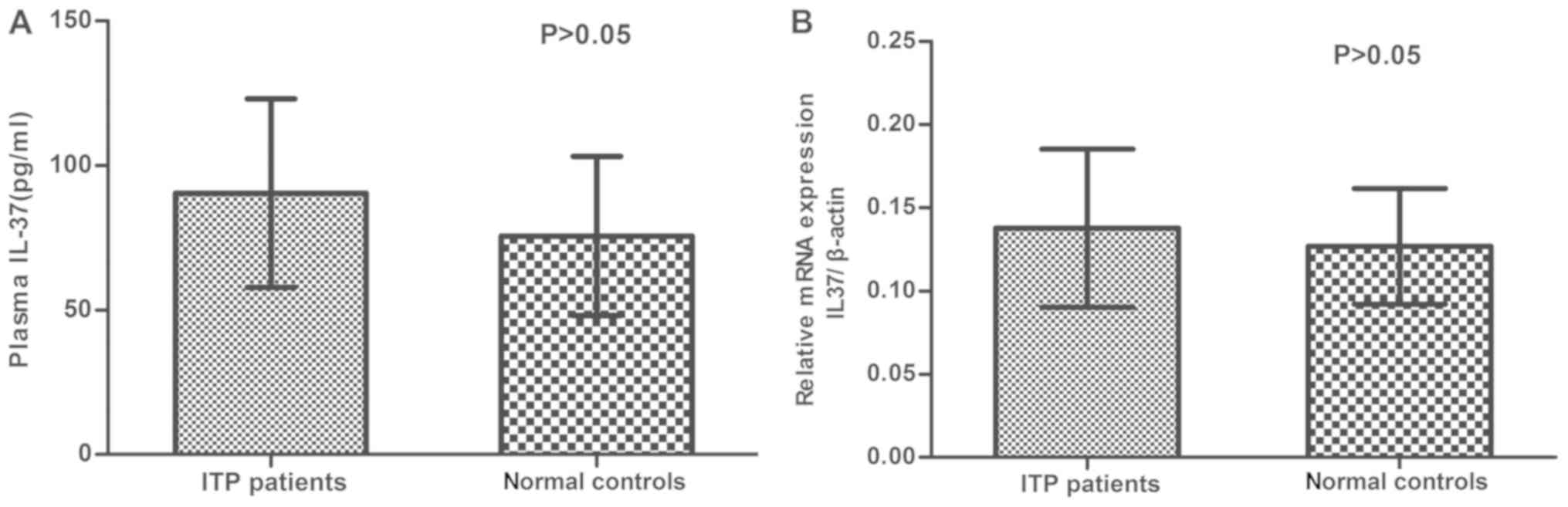
Fig. 1A presents the
plasma concentration of IL-37 in the ITP and control groups. The
mean plasma level of IL-37 in the ITP patients (90.41±32.56 pg/ml)
was higher than that in normal controls (75.62±27.52 pg/ml), but
this was not statistically significant (P>0.05). In Fig. 1B, the mRNA levels of IL-37 in PBMCs
determined using the 2−ΔΔCq method are presented as the
fold change in gene expression normalized to an endogenous
reference gene (β-actin) and relative to normal controls. The
relative mRNA expression of IL-37 in untreated patients was 1.07
times that in the normal controls, with no statistically
significant difference observed (P>0.05; Fig. 1B).
IL-37 expression in patients with
active ITP and in remission
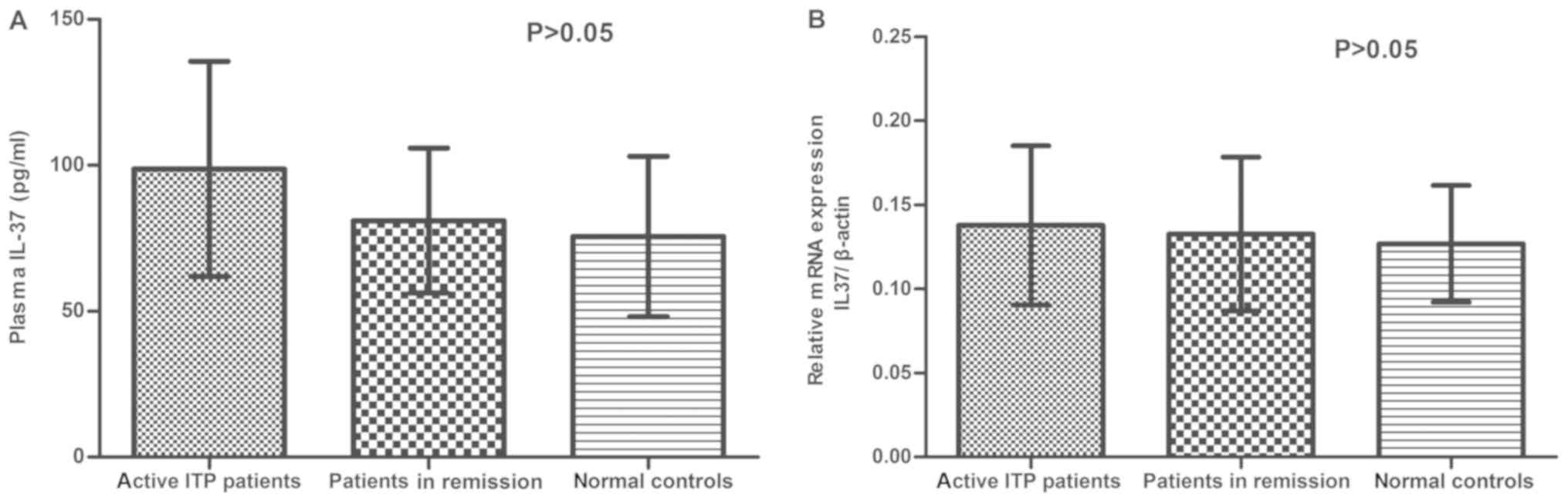
To investigate any potential correlation between
IL-37 and disease activity, the concentration of plasma IL-37 and
IL-37 mRNA were further analyzed in patients with active ITP,
patients in remission and healthy controls. The concentration of
plasma IL-37 in the different groups was as follows: 98.75±36.85
pg/ml (active ITP patients), 81.04±24.83 pg/ml (ITP patients in
remission) and 75.62±27.52 pg/ml (normal controls); no significant
differences were observed among active ITP patients, patients in
remission and healthy controls (P>0.05; Fig. 2A). Despite not reaching statistical
significance, the P-value for the comparison of plasma levels of
IL-37 between active ITP patients and controls was 0.107, as
determined by Bonferroni's post-hoc test. Similarly, no significant
difference in the IL-37 mRNA expression was observed among the
three groups (P>0.05; Fig.
2B).
Plasma IL-37 concentration in
anti-platelet autoantibody positive/negative patients
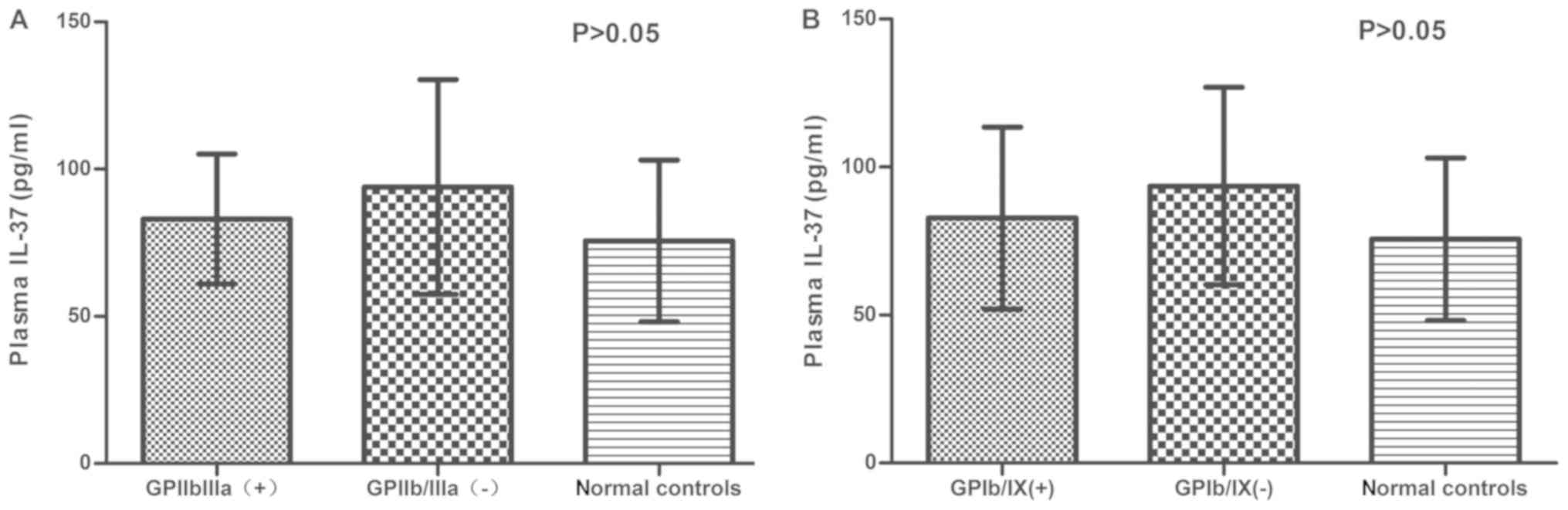
Since anti-platelet autoantibodies have an important
role in the pathogenesis of ITP, the concentration of IL-37 was
further compared in anti-platelet autoantibody-positive and
-negative patients. Fig. 3A presents
the concentration of IL-37 in anti-GPIIb/IIIa-positive and
-negative ITP patients. No significant difference was identified
between anti-GPIIb/IIIa-positive (83.06±22.02 pg/ml) and -negative
ITP patients (93.93±36.47 pg/ml; P>0.05), or in the plasma
levels of IL-37 between anti-GPIIb/IIIa-positive and -negative ITP
patients, as compared with that in normal controls (75.62±27.52
pg/ml; P>0.05). Similar results were obtained for
anti-GPIb/IX-positive/negative ITP patients (P>0.05; Fig. 3B).
Influence of glucocorticoids on plasma
IL-37 concentration in ITP patients
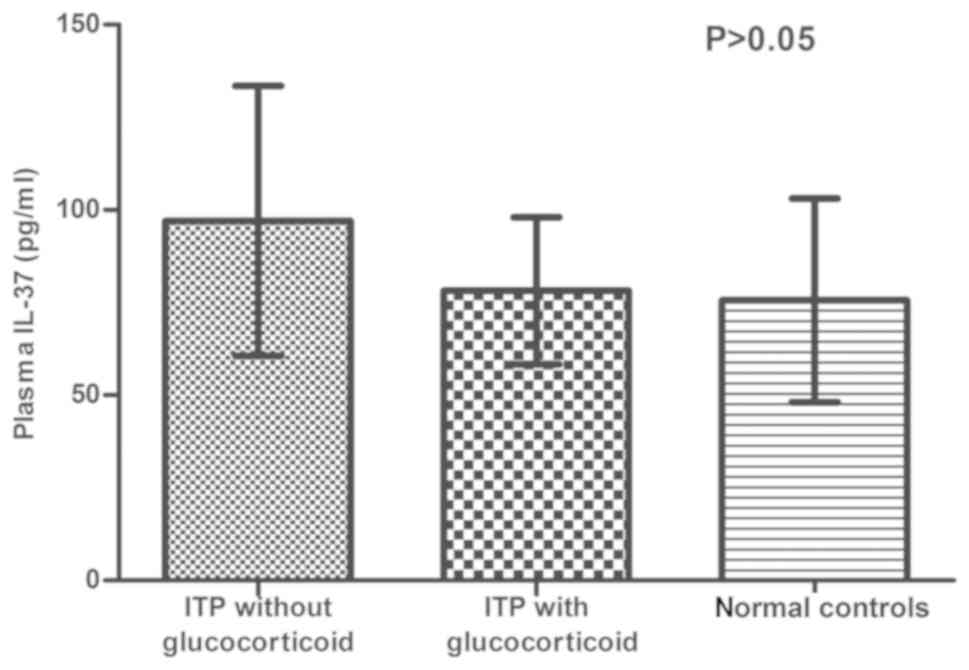
Glucocorticoids are used as a first-line therapy for
ITP. In order to investigate any potential difference between
patients treated with and without glucocorticoids, the
concentration of plasma IL-37 was analyzed in different groups. The
concentration of IL-37 was as follows: ITP patients treated without
glucocorticoids (97.06±36.45 pg/ml), ITP patients treated with
glucocorticoids (78.23±19.84 pg/ml) and normal controls
(75.62±27.52 pg/ml); no significant difference was observed
(P>0.05; Fig. 4).
Correlation between IL-37 and platelet
count in ITP patients
The correlation between plasma levels of IL-37 and
platelet count in patients with active ITP was assessed by
Pearson's correlation analysis and no significant correlation was
identified (P>0.05).
Discussion
Autoimmune diseases, including ITP, are
characterized by impaired function caused by an immune response, in
which abnormal antibodies are produced and attack the body itself.
IL-37, a novel member of the IL-1 family, has been recognized as an
important anti-inflammatory cytokine expressed by immune cells.
Abnormal expression of IL-37 has been reported in various
autoimmune diseases, including SLE, RA, IBD, GD and AS (18–27), as
a potential negative factor influencing the development of these
disorders. According to Ye et al (22), increased IL-37 expression was
associated with SLE disease activity, and IL-37 levels were
significantly higher in patients with renal disease. Xia et
al (20) indicated that the
expression of IL-37 was markedly increased in RA patients, and a
significant correlation was identified between IL-37 levels and
disease activity. In addition, patients with active AS and GD had
higher levels of IL-37 than those with inactive AS and GD and
healthy controls (26,27). All aforementioned data suggest that
the expression of IL-37 is associated with disease activity of the
above autoimmune diseases. Furthermore, inflammatory cytokine
expression was higher in patients with active disease, as compared
with that in patients with inactive disease and healthy controls.
Functional analysis indicated that certain pro-inflammatory
cytokines, including IL-1/6/10 and TNF-α, may be involved in
promoting the expression of IL-37, while high IL-37 expression may
inhibit the overproduction of pro-inflammatory cytokines in
autoimmune diseases through a negative feedback mechanism (31).
While most studies have reported an increased IL-37
expression in autoimmune diseases, decreased IL-37 expression was
also identified in other autoimmune conditions, including Behcet's
disease (32), asthma (33), Vogt-Koyanagi-Harada disease (34) and allergic rhinitis (35). The inconsistent expression of IL-37
among different autoimmune diseases may be due to differences in
their immunological mechanisms. As mentioned above, IL-37 levels
frequently exhibit a positive correlation with disease activity.
This means that infection or inflammation increases IL-37
expression, while a high level of IL-37 may help to reduce the
disease severity through a negative feedback mechanism. Conversely,
low levels of IL-37 may indicate more severe inflammation.
To date, the role of IL-37 in ITP patients has
remained elusive. In the present study, the plasma concentration of
IL-37 and its mRNA expression in PBMCs of ITP patients was
determined for the first time by using ELISA and RT-qPCR,
respectively. The results indicated no significant difference in
IL-37 levels between ITP patients and controls. In addition, no
correlation was identified between IL-37 and anti-platelet
autoantibodies. The correlation between IL-37 and the platelet
count was also analyzed, with no statistical significance observed.
However, the mean value of plasma IL-37 in ITP, particularly active
ITP, was much higher than that in the controls. However, the
P-value for the comparison of plasma IL-37 between patients with
active ITP and controls was 0.107, as determined by Bonferroni's
post-hoc test. Further studies with a bigger sample size are
required to confirm these results, which may obtain a higher
statistical significance.
It has been reported that IL-37 is able to
translocate into the nucleus and downregulate pro-inflammatory
cytokines (36), suggesting that
IL-37 may also have an intracellular, in addition to its
extracellular, function. Further studies examining the expression
of intracellular IL-37 by methods including flow cytometry may be
required.
Glucocorticoids, including conventional prednisone
and HD-DEX, have been recommended as first-line therapy for ITP
patients. Song et al (23)
reported that glucocorticoids are able to downregulate the
increased expression of IL-37 in SLE. The concentration of IL-37
was analyzed in ITP patients treated with and without
glucocorticoids, with no significant difference observed between
the two groups, which indicates that glucocorticoids may not
regulate IL-37 in ITP patients. There are several explanations for
this. First, the patients included in the present study were
treated with glucocorticoids days, months or years ago, and thus,
the glucocorticoid medication or blood sample collection time was
inconsistent. Furthermore, the patients treated with or without
glucocorticoids were not the same patients, and in a future study,
assessment of the concentration of IL-37 in the same patients prior
to and after glucocorticoid treatment may be more meaningful.
Accumulating evidence suggests that IL-37 has a
pivotal role in autoimmune diseases. In the present study, the
expression of IL-37 in ITP patients was evaluated for the first
time, but no significantly abnormal expression of IL-37 was
identified in these patients. It was therefore concluded that IL-37
may not have a pivotal role in the development of ITP. However, the
lack of significant differences may be due to the limited number of
patients in different groups. Larger number of ITP patients should
be enrolled in the future work and achieve more accurate
results.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81100335).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
FZ contributed to experimental design, data analysis
and manuscript writing; X-JuZ contributed to case collection,
literature search and manuscript writing; X-JiZ and TY performed
the experiments and statistical analysis; YL contributed to case
collection, data interpretation and figure creation; QY was
responsible for data interpretation, literature search, paper
revision and submission.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Shandong Provincial Hospital affiliated to Shandong
University (Jinan, China). Written informed consent was obtained
from all patients and/or their guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
|
1
|
Stasi R, Evangelista ML, Stipa E,
Buccisano F, Venditti A and Amadori S: Idiopathic thrombocytopenic
purpura: Current concepts in pathophysiology and management. Thromb
Haemost. 99:4–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Panitsas FP, Theodoropoulou M, Kouraklis
A, Karakantza M, Theodorou GL, Zoumbos NC, Maniatis A and Mouzaki
A: Adult chronic idiopathic thrombocytopenic purpura (ITP) is the
manifestation of a type-1 polarized immune response. Blood.
103:2645–2647. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Ma D, Zhu X, Qu X, Ji C and Hou
M: Elevated profile of Th17, Th1 and Tc1 cells in patients with
immune thrombocytopenic purpura. Haematologica. 94:1326–1329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu B, Zhao H, Poon MC, Han Z, Gu D, Xu M,
Jia H, Yang R and Han ZC: Abnormality of CD4(+)CD25(+) regulatory T
cell in idiopathic thrombocytopenic purpura. Eur J Haematol.
78:139–143. 2007.PubMed/NCBI
|
|
5
|
Yu J, Heck S, Patel V, Levan J, Yu Y,
Bussel JB and Yazdanbakhsh K: Defective circulating CD25 regulatory
T cells in patients with chronic immune thrombocytopenic purpura.
Blood. 112:1325–1328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao C, Li X, Zhang F, Wang L, Peng J and
Hou M: Increased cytotoxic T-lymphocyte-mediated cytotoxicity
predominant in patients with idiopathic thrombocytopenic purpura
without platelet autoantibodies. Haematologica. 93:1428–1430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nold MF, Nold-Petry CA, Zepp JA, Palmer
BE, Bufler P and Dinarello CA: IL-37 is a fundamental inhibitor of
innate immunity. Nat Immunol. 11:1014–1022. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo Y, Cai X, Liu S, Wang S, Nold-Petry
CA, Nold MF, Bufler P, Norris D, Dinarello CA and Fujita M:
Suppression of antigen-specific adaptive immunity by IL-37 via
induction of tolerogenic dendritic cells. Proc Natl Acad Sci USA.
111:15178–15183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dinarallo CA and Bufler P: Interleukin-37.
Semin Immunol. 25:466–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bufler P, Gamboni-Robertson F, Azam T, Kim
SH and Dinarello CA: Interleukin-1 homologues IL-1F7b and IL-18
contain functional mRNA instability elements within the coding
region responsive to lipopolysaccharide. Biochem J. 381:503–510.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao W, Kumar S, Lotze MT, Hanning C,
Robbins PD and Gambotto A: Innate immunity mediated by the cytokine
IL-1 homologue 4 (IL-1H4/IL-1F7) induces IL-12-dependent adaptive
and profound antitumor immunity. J Immunol. 170:107–113. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McNamee EN, Masterson JC, Jedlicka P,
McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P,
Dinarello CA and Rivera-Nieves J: Interleukin 37 expression
protects mice from colitis. Proc Natl Acad Sci USA.
108:16711–16716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakai N, Van Sweringen HL, Belizaire RM,
Quillin RC, Schuster R, Blanchard J, Burns JM, Tevar AD, Edwards MJ
and Lentsch AB: Interleukin-37 reduces liver inflammatory injury
via effects on hepatocytes and non-parenchymal cells. J
Gastroenterol Hepatol. 27:1609–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ballak DB, van Diepen JA, Moschen AR,
Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P,
Boekschoten MV, Müller M, et al: IL-37 protects against
obesity-induced inflammation and insulin resistance. Nat Commun.
5:47112014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coll-Miro M, Francos-Quijorna I,
Santos-Nogueira E, Torres-Espin A, Bufler P, Dinarello CA and
López-Vales R: Beneficial effects of IL-37 after spinal cord injury
in mice. Proc Natl Acad Sci USA. 113:1411–1416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Neff CP, Barber K, Hong J, Luo Y,
Azam T, Palmer BE, Fujita M, Garlanda C, Mantovani A, et al:
Extracellular forms of IL-37 inhibit innate inflammation in vitro
and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc
Natl Acad Sci USA. 112:2497–2502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lunding L, Webering S, Vock C, Schröder A,
Raedler D, Schaub B, Fehrenbach H and Wegmann M: IL-37 requires
IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation
in mice. Allergy. 70:366–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Zhang J, Tao J and Lu T: Elevated
serum levels of Interleukin-37 are associated with inflammatory
cytokines and disease activity in rheumatoid arthritis. APMIS.
123:1025–1031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xia L, Shen H and Lu J: Elevated serum and
synovial fluid levels of interleukin-37 in patients with rheumatoid
arthritis: Attenuated the production of inflammatory cytokines.
Cytokine. 76:553–557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia T, Zheng XF, Qian BH, Fang H, Wang JJ,
Zhang LL, Pang YF, Zhang J, Wei XQ, Xia ZF and Zhao DB: Plasma
interleukin-37 is elevated in patients with rheumatoid arthritis:
Its correlation with disease activity and Th1/Th2/Th17-related
cytokines. Dis Markers. 2015:7950432015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan
YX and Jiang YF: Plasma levels of IL-37 and correlation with TNF-α,
IL-17A, and disease activity during DMARD treatment of rheumatoid
arthritis. PLoS One. 9:e953462014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye L, Ji L, Wen Z, Zhou Y, Hu D, Li Y, Yu
T, Chen B, Zhang J, Ding L, et al: IL-37 inhibits the production of
inflammatory cytokines in peripheral blood mononuclear cells of
patients with systemic lupus erythematosus: Its correlation with
disease activity. J Transl Med. 12:692014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song L, Qiu F, Fan Y, Ding F, Liu H, Shu
Q, Liu W and Li X: Glucocorticoid regulates interleukin-37 in
systemic lupus erythematosus. J Clin Immunol. 33:111–117. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farrokhi M, Rezaei A, Amani-Beni A,
Etemadifar M, Kouchaki E and Zahedi A: Increased serum level of
IL-37 in patients with multiple sclerosis and neuromyelitis optica.
Acta Neurol Belg. 115:609–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imaeda H, Takahashi K, Fujimoto T, Kasumi
E, Ban H, Bamba S, Sonoda H, Shimizu T, Fujiyama Y and Andoh A:
Epithelial expression of interleukin-37b in inflammatory bowel
disease. Clin Exp Immunol. 172:410–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen B, Huang K, Ye L, Li Y, Zhang J,
Zhang J, Fan X, Liu X, Li L, Sun J, et al: Interleukin-37 is
increased in ankylosing spondylitis patients and associated with
disease activity. J Transl Med. 13:362015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Wang Z, Yu T, Chen B, Zhang J, Huang
K and Huang Z: Increased expression of IL-37 in patients with
Graves' disease and its contribution to suppression of
proinflammatory cytokines production in peripheral blood
mononuclear cells. PLoS One. 9:e1071832014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
British Committee for Standards in
Haematology General Haematology Task Force, . Guidelines for the
investigation and management of idiopathic thrombocytopenic purpura
in adults, children and in pregnancy. Br J Haematol. 120:574–596.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou M, Peng J, Shi Y, Zhang C, Qin P, Zhao
C, Ji X, Wang X and Zhang M: Mycophenolatemofetil (MMF) for the
treatment of steroid-resistant idiopathic thrombocytopenic purpura.
Eur J Haematol. 70:353–357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu WD, Zhao Y and Liu Y: Insights into
IL-37, the role in autoimmune diseases. Autoimmun Rev.
14:1170–1175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bouali E, Kaabachi W, Hamzaoui A and
Hamzaoui K: Interleukin-37 expression is decreased in Behçet's
disease and is associated with inflammation. Immunol Lett.
167:87–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Charrad R, Berraïes A, Hamdi B, Ammar J,
Hamzaoui K and Hamzaoui A: Anti-inflammatory activity of IL-37 in
asthmatic children: Correlation with inflammatory cytokines TNF-α,
IL-β, IL-6 and IL-17A. Immunobiology. 221:182–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ye Z, Wang C, Tang J, Zhou Y, Bai L, Liu
Y, Kijlstra A and Yang P: Decreased interleukin-37 expression in
Vogt-Koyanagi-Harada disease and upregulation following
immunosuppressive treatment. J Interferon Cytokine Res. 35:265–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu W, Deng L, Chen Y, Sun C, Wang J, Zhou
L, Li H and Luo R: Anti-inflammatory effect of IL-37b in children
with allergic rhinitis. Mediators Inflamm. 2014:7468462014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sharma S, Kulk N, Nold MF, Gräf R, Kim SH,
Reinhardt D, Dinarello CA and Bufler P: The IL-1 family member 7b
translocates to the nucleus and down-regulates proinflammatory
cytokines. J Immunol. 180:5477–5482. 2008. View Article : Google Scholar : PubMed/NCBI
|