Introduction
In emergency medical treatment, patients with
femoral neck fracture often suffer from severe pain, resulting in
some complications, especially in the elderly. In response to this,
several non-steroidal anti-inflammatory drugs (NSAIDs), including
acetaminophen, have been applied to control the pain (1), although the actual effect is not
satisfying. NSAIDs can cause cardio-renal toxicity (2), gastrointestinal bleeding (3) and platelet inhibition (4,5). In
addition, it has been demonstrated that the application of opioids,
such as fentanyl and morphine, often cause nausea and vomiting, as
well as other common side-effects, including excessive sedation,
respiratory depression and delirious symptoms (6–9). Up to
now, for most of the patients in the emergency room effective and
secure analgesia were unavailable, which was one of the factors of
acute deliria (10).
Fascia iliaca compartment block (FICB) has been
reported to provide a more extensive blocking range and better
analgesic effect compared with the three-in-one femoral nerve block
(11,12). FICB has been widely used in
perioperative analgesia by anesthesiologists with a good effect
(13,14). Randomized controlled trials (RCTs)
and low quality results have suggested that FICB could be well used
in the management of acute pain in patients with femoral neck
fracture (15–17). However, in a previous clinical study
it was reported that the analgesic effect of FICB mainly depends on
the capacity of the local anesthetic solution. Occasionally, in
order to obtain a longer duration of analgesia, a higher
concentration of local anesthetics has been considered to apply
clinically. However, the incidence of local anesthetic systemic
toxicity (LAST) may increase with the dose increase (18).
Ropivacaine, a stereo-specific levorotary local
anesthetic, has been widely utilized in peripheral nerve block
analgesia (19). Compared with
bupivacaine, ropivacaine exerts vasoconstrictor effects and has a
lower cardiac toxicity risk, and therefore has been widely used in
surgical analgesia (20,21). A previous report by Paut et al
(22) showed that a maximum plasma
concentration (Cmax) of >50% in children with FICB is
more than twice the alert concentration of ropivacaine (2.2 µg/ml),
without the occurrence of serious LAST, indicating that FICB may
have a higher risk of LAST in specific populations. Also, a case
report revealed that a severe LAST occurred after the interscalene
brachial plexus block with 75 mg ropivacaine in a 76-year old
female patient (23). In addition to
the local anesthetic dose, the occurrence of LAST is closely
related to the blood flow of the injection region. However, it has
been reported that compared with young adults, elderly patients
have poor blood vessels in tissue surrounding the nerves, thus, a
slower absorption of local anesthetic in the elderly causes a low
Cmax and prolonged time to reach the maximum plasma
concentration (Tmax) (24,25).
Free plasma concentration of local anesthetic is known to be the
key factor of LAST. Torup et al (26) have reported that although the total
plasma concentration of ropivacaine of ~30% in patients exceeded
the alert of neurotoxicity after bilateral transverse fascia block,
no toxicity reaction occurred clinically, as the free plasma
concentration was below the warning level. Therefore, the effective
and safe dose of ropivacaine in patients, and especially the
elderly, is important to be investigated comprehensively.
Patients and methods
Case selection and grouping
After obtaining the approval from the Ethics
Committee of the First Affiliated Hospital of Zhejiang Chinese
Medicine University (Hangzhou, China) (no. 2013-k-058), 40 patients
at American Society of Anesthesiologists (ASA) I–II stage with
femoral neck fracture, 60–85 years of age, were enrolled in the
study. Patients with the following conditions were excluded from
the study: patients under treatment for chronic pain; with local
anesthetic allergy; with injection site infection; body mass index
>30 kg/m2; lower extremity nerve disease;
comprehension barriers on the Visual Analogue Scale (VAS). All the
patients were informed of the study and signed a consent form.
According to the random Digital Envelope method, patients were
randomly assigned to receive FICB with a 0.7 ml/kg of solution
containing 0.375% ropivacaine (group L) or 0.5% ropivacaine (group
H), as previously described (27–29).
FICB operation
In the emergency room, patients were given oxygen
saturation (SPO2), non-invasive arterial blood pressure
and electrocardiograph (ECG) monitoring and recording were carried
out, and vein channels were established. A needle was placed 1 cm
below one-third the distance from the pubic tubercle toward the
anterior superior iliac spine (ASIS). The skin was pierced with a
large diameter needle and then inserted into a blunt needle (16G
Tuohy) at 45 degrees until iliac fascia. Prior to the infusion of
ropivacaine, the position of the needle was confirmed by
ultrasound. Subsequently, group L received 0.7 ml/kg of 0.375%
ropivacaine (Naropine®; AstraZeneca AB), and group H
received 0.7 ml/kg of 0.5% ropivacaine.
VAS scoring
Pain was evaluated based on VAS, with 0
characterizing no pain and 10 used for the most intense pain. VAS
scores were recorded before and at 20 min after the block. To
guarantee the objectivity of the data, VAS and block were assessed
in a blinded manner to patient treatment, and statistical analysis
was carried out after the separate assessments.
Block scope assessment
The block range of lateral, anterior and medial
thigh, corresponding to the lateral femoral cutaneous nerve,
femoral nerve and obturator nerve area, was tested with the cold
sensation disappearance method. The detailed data on nerve blocking
at the three sites were recorded at 20 min after the operation,
including failure, in one, two or three nerve innervation
areas.
Assessment of cardiovascular and
nervous system toxicity
The effect of local anesthetics on auditory and/or
visual impairment, perioral numbness, tingling, paresthesia and/or
paralysis, muscle twitching and/or muscle stiffness, and
articulation disorders, were evaluated before, and at 1 or 2 h
after FICB operation.
Blood sample collection and
testing
Samples (3 ml) of venous blood were obtained
immediately, and at 15, 30, 45, 60, 90 and 120 min after FICB.
Plasma was isolated within an hour and frozen immediately at −80°C.
The liquid chromatography-electrospray ionization-tandem mass
spectrometry (LC-ESI-MS/MS) was applied to determine the total and
free plasma concentration of ropivacaine. The free plasma
concentration was obtained by equilibrium dialysis. The
concentration-vs.-time curve of plasma ropivacaine was simulated
using DAS 3.0 pharmacokinetic software (Anhui Provincial Drug
Clinical Evaluation Center) to calculate the pharmacokinetic
parameters, namely, Cmax, Tmax, elimination
half-time (T1/2z), area under the plasma
concentration-time curve (AUC0-t), area under the plasma
concentration-time curve extrapolated to infinity
(AUC0-∞), and clearance (CLz/F).
Statistical analysis
SPSS 19.0 statistical software (IBM Corp.) was used
for statistical analysis. All quantitative measurement data were
expressed as the mean ± standard deviation (SD) and analyzed by
Student's t-test. Dichotomous data (age, sex, thigh sensory blocks)
were analyzed using Chi-square test. P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics
There was no significant difference in sex ratio,
age, ASA classification, height and weight between the groups, as
shown in Table I.
 | Table I.General characteristics of patients in
both groups. |
Table I.
General characteristics of patients in
both groups.
| Variables | Group L (n=20) | Group H (n=20) | P-value |
|---|
| Age (years) | 76.4±5.6 | 73.8±6.3 | 0.176 |
| Sex (M/F) | 8/12 | 10/10 | 0.751 |
| Weight (kg) | 57.1±10.8 | 62.3±8.6 | 0.100 |
| Height (cm) | 162.5±8.3 | 160.9±12.6 | 0.636 |
| ASA physical status
(I/II) | 7/13 | 5/15 | 0.730 |
Effect of analgesia, block and nervous
system toxicity
FICB achieved good analgesic effect in groups L and
H. The VAS scores were recorded before FICB and at 20 min after
FICB. As shown in Table II, the VAS
score was reduced from 6.30±0.97 to 2.87±0.73 in group L, while at
the same time it was reduced from 6.46±1.02 to 2.27±0.82 in group H
(P<0.05). Also, the VAS score was lower in group H than that in
group L (P<0.05) at 20 min after FICB. The lateral femoral
cutaneous nerve and femoral nerve were completely blocked at 20 min
after the operation in both groups. In group H, the simultaneous
blocking rate of lateral femoral cutaneous nerve, femoral nerve and
obturator nerve was 85%, similar to 75% of group L (P>0.05), as
shown in Table III. After FICB,
none of the patients in either group was reported with
auditory/visual deficits or articulation disorders, or other
neurological problems.
 | Table II.VAS scores before and at 20 min after
FICB. |
Table II.
VAS scores before and at 20 min after
FICB.
| Variables | Group L (n=20) | Group H (n=20) | P-value |
|---|
| Before FICB | 6.30±0.97 | 6.46±1.02 | 0.614 |
| At 20 min after
FICB |
2.87±0.73a |
2.27±0.82a,b | 0.020 |
 | Table III.Thigh sensory blocks at 20 min after
FICB in the two groups [n (%)]. |
Table III.
Thigh sensory blocks at 20 min after
FICB in the two groups [n (%)].
| Sensory block | Group L (n=20) | Group H (n=20) | P-value |
|---|
| LFC+F | 20 (100.0) | 20 (100) | 1.00 |
| LFC+F+O | 15 (75.0) | 17 (85) | 0.695 |
Influence of pharmacokinetic
parameters of ropivacaine
The results of the detection of the pharmacokinetics
parameters of total and free plasma ropivacaine are shown in
Tables IV and V. No detectable differences were observed
in t1/2z, CLz/F and Tmax of
exposed total and free plasma of ropivacaine between groups H and L
(P>0.05). The AUC of exposed total and free plasma of
ropivacaine was higher in group H than that in group L (P<0.05).
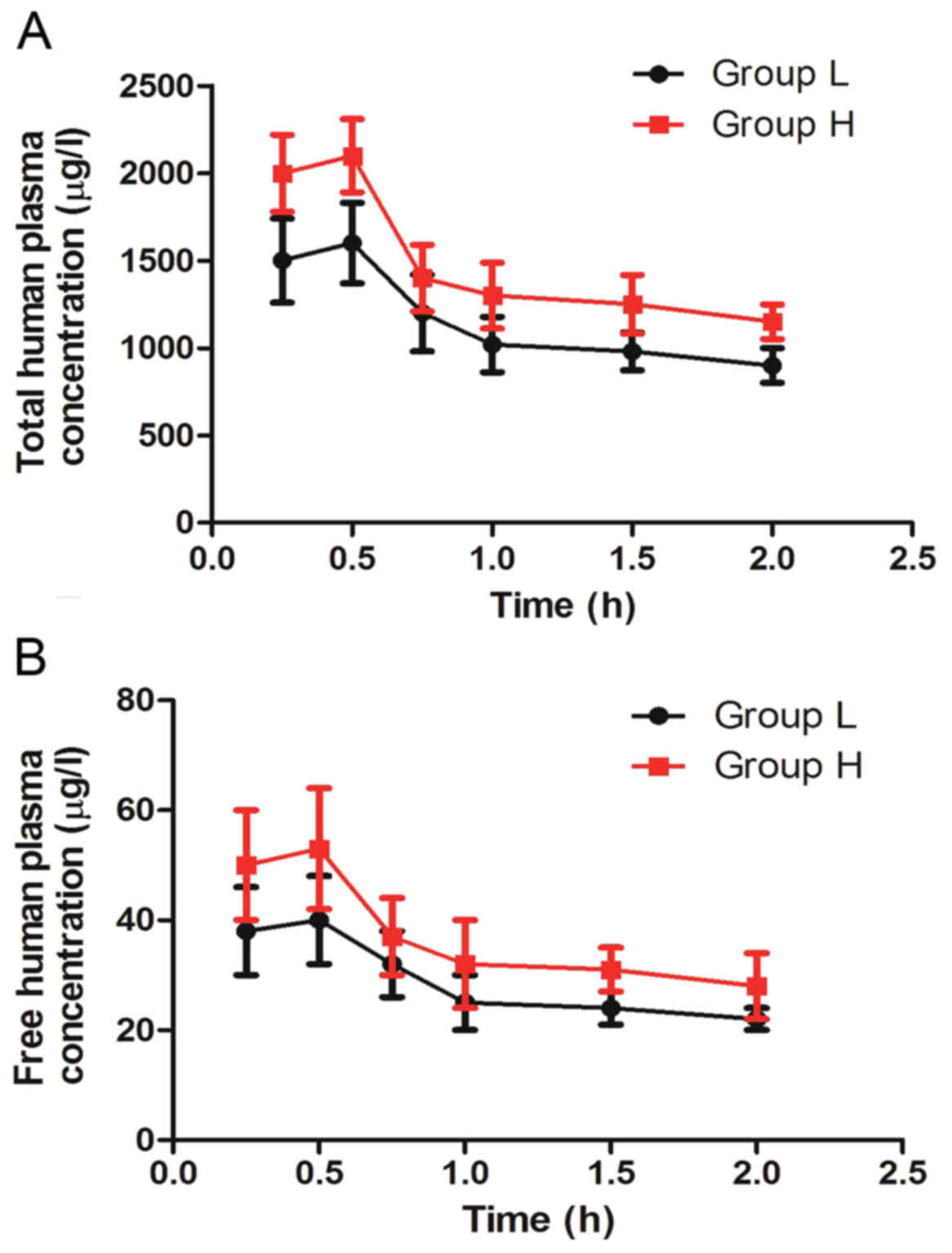
Tmax of ropivacaine was 0.56±0.09 and 0.53±0.12 in
groups H and L, respectively. The total Cmax of
ropivacaine was 2.17±0.56 in group H, higher than 1.56±0.42 in
group L (P<0.05). The free Cmax of ropivacaine was
53.4±13.1 in group H, higher than 43.5±14.6 in group L (P<0.05).
There were 4 patients with total plasma concentration of
ropivacaine >2.2 µg/ml at some points in each group. The highest
value was 3.13 and 3.34 µg/ml in groups L and H, respectively.
Neither group showed signs of central nervous system and
circulatory system poisoning.
 | Table IV.Comparison of the kinetic parameters
of total plasma pharmacokinetics of ropivacaine in the two
groups. |
Table IV.
Comparison of the kinetic parameters
of total plasma pharmacokinetics of ropivacaine in the two
groups.
| Parameters | Group L (n=20) | Group H (n=20) | P-value |
|---|
| AUC0-t
(mg/l × h) | 2.48±0.76 | 3.36±0.63 | <0.01 |
| AUC0-∞
(mg/l × h) | 7.82±1.37 | 8.68±1.04 |
0.034 |
| t1/2z
(h) | 2.78±1.02 | 2.28±0.61 |
0.072 |
| Tmax
(h) | 0.53±0.12 | 0.56±0.09 |
0.428 |
| CLz/F
(m3/h/kg) | 40.8±14.7 | 38.9±12.0 |
0.662 |
| Cmax
(mg/l) | 1.56±0.42 | 2.17±0.56 | <0.01 |
 | Table V.Comparison of the kinetic parameters
of free plasma pharmacokinetics of ropivacaine in the two
groups. |
Table V.
Comparison of the kinetic parameters
of free plasma pharmacokinetics of ropivacaine in the two
groups.
| Parameters | Group L (n=20) | Group H (n=20) | P-value |
|---|
| AUC0-t
(µg/l × h) | 50.82±16.40 | 72.61±21.21 | <0.01 |
| AUC0-∞
(µg/l × h) | 91.63±23.20 | 207.12±44.61 | <0.01 |
| t1/2z
(h) | 1.66±0.52 | 1.87±0.68 | 0.280 |
| Tmax
(h) | 0.52±0.15 | 0.59±0.17 | 0.175 |
| CLz
(m3/h/kg) | 2,134.0±658.2 | 1,900.6±723.1 | 0.291 |
| Cmax
(µg/l) | 43.5±14.6 | 53.4±13.1 | 0.030 |
The changes of total and free plasma ropivacaine
concentration were shown to be time-dependent (Fig. 1). That is, total and free plasma
ropivacaine concentrations declined gradually with the prolongation
of the operation time.
Discussion
The present study was designed to evaluate the
efficacy and safety of ropivacaine at different concentrations on
analgesia in elderly with femoral neck fracture after FICB. The
results revealed that both concentrations, 0.375 and 0.5% of
ropivacaine, demonstrate a favorable effect in analgesia for
FICB.
It is universally understood that the incidence of
femoral neck fractures in older adults, both men and women,
increases exponentially with age (30). It has been demonstrated that the
systemic function of the elderly patients gradually declines, and
the pain caused by the fracture decreases diagnostic accuracy while
increasing therapeutic difficulty for clinicians, even leading to
severe hemodynamic changes or triggering of cardiovascular and
cerebrovascular events. In light of these outcomes, it is
indispensable to develop early analgesia and active treatment. The
FICB technique, improving the patient activities and reducing
complications, is used more widely as preoperative analgesia in the
elderly patients with femoral neck fractures (31). In line with the aforementioned
research, our study suggests that FICB has a positively analgesic
effect in elderly patients.
A previous report demonstrated that ropivacaine
possesses evident postoperative analgesia capacity as reflected by
the postoperative VAS score reduction (32). Consistent with this finding, our
study showed that the VAS scores were obviously declined both in
group L and H after FICB, and VAS score in group H was remarkably
lower than that in group L. Additionally, the lateral femoral
cutaneous nerve and femoral nerve were completely blocked at 20 min
after operation in both groups. Simultaneously blocking rates of
lateral cutaneous nerve, femoral nerve and obturator nerve in group
H and L, were 85 and 75%, respectively, higher than the results of
Yun et al (33). Their study
showed that 40% of lateral femoral cutaneous nerve, femoral nerve
and obturator nerve were simultaneously blocked and 60% of lateral
femoral cutaneous nerve and femoral nerve were blocked. The
possible reason for this inconformity is that the blocking needle
used in the study by Yun et al was 22 G, compared with the
16 G needle of the present study, which may caused the needle to
pierce the muscle group. In the present study we used ultrasound to
confirm the final injection location of ropivacaine. Consistent
with our data, two other studies found that ultrasound-guided FICB
significantly improved the three nerve block success rate, hinting
that ultrasound can improve the success rate of puncture even with
a fine needle (34,35). Overall, the evidence showed that a
higher level of ropivacaine, remaining within the safe-dose range,
influenced indexes of postoperative analgesia more intensely.
It has been suggested that a high-dose longitudinal
supra-inguinal FICB with 0.5% ropivacaine enables the reduction of
morphine consumption after total hip arthroplasty, although, the
safety of this dose is not settled (36). Another report has shown that high
plasma concentration of ropivacaine is also prone to produce a
certain toxic reaction (37). In the
present study, we found that the Cmax of ropivacaine was
2.17±0.56 in group H, higher than 1.56±0.42 in group L (P<0.05).
Over 2.2 µg/ml is thought to cause anesthetics poisoning (29). There were 4 patients with total
plasma concentrations of ropivacaine >2.2 µg/ml at some points
in each group. Neither group showed signs of central nervous system
and circulatory system poisoning. The free Cmax of
ropivacaine was rather low in both groups, specifically 53.4±13.1
and 43.5±14.6 in group H and L, respectively. Besides, the
Tmax was 0.56±0.09 and 0.53±0.12 in group H and L,
respectively. However, it was found that the peak concentration of
11 patients appeared in 15 min, due to the experimental design, and
no point before 15 min was chosen. There may be some bias in the
pharmacokinetic analysis of DAS 3.0, and therefore the peak value
of drugs may occur earlier. The rich vascular muscle between iliac
fascia space may lead to rapid absorption, suggesting that high
dose and high-dose injection between iliac fascia space may bring
the risk of local anesthetics poisoning. Different from the results
of Paut et al (22), reported
in pediatric FICB, the results of the present study revealed that
the maximum concentration occurred at 20–90 min. The heart and
nervous system toxicity from the free section, and the plasma
concentrations of ropivacaine to induce toxic reaction is still not
unified. In spite of this, it is realized that the concentration of
0.5% ropivacaine remains a risk for further extensive analgesia
treatment based on our data. However, our results are based on a
small sample size without young subjects as control, so the
difference between the elderly and young people is unclear.
Sampling time also needs further study. For elder patients, the
most important factor involved in drug pharmacokinetics is the
decreased liver function.
In conclusion, 0.375% and 0.5% of ropivacaine have a
favourable effect in analgesia for FICB. Nonetheless, there is a
theoretical risk of local anesthetics poisoning in both groups,
suggesting that a lower dose might be a good choice for a single
large volume of FICB.
Acknowledgements
Not applicable.
Funding
This study was supported by the Projects of Medical
and Health Technology Development Program in Zhejiang Province (no.
2014kyb).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XWG and FFZ designed the study, wrote, drafted and
revised the manuscript. CL and LYY performed the experiments. FFZ,
SPW and MZ collected the samples, analyzed the data and made the
diagrams. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics committee of
the First Affiliated Hospital of Zhejiang Chinese Medicine
University (Hangzhou, China) (no. 2013-k-058). All patients were
informed of the study and signed a consent form.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wiffen PJ, Derry S, Moore RA, McNicol ED,
Bell RF, Carr DB, McIntyre M and Wee B: Oral paracetamol
(acetaminophen) for cancer pain. Cochrane Database Syst Rev.
7:CD0126372017.PubMed/NCBI
|
|
2
|
Aghazadeh-Habashi A, Asghar W and Jamali
F: Drug-disease interaction: Effect of inflammation and
nonsteroidal anti-inflammatory drugs on cytochrome P450 metabolites
of arachidonic acid. J Pharm Sci. 107:756–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sostres C, Carrera-Lasfuentes P and Lanas
A: Non-steroidal anti-inflammatory drug related upper
gastrointestinal bleeding: Types of drug use and patient profiles
in real clinical practice. Curr Med Res Opin. 33:1815–1820. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrowska H: Inhibition of human platelet
cathepsin A by non-steroidal anti-inflammatory drugs - in vitro
study. Pol J Pharmacol. 48:113–116. 1996.PubMed/NCBI
|
|
5
|
Scharf RE: Drugs that affect platelet
function. Semin Thromb Hemost. 38:865–883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicholson BD: Economic and clinical burden
of opioid-induced nausea and vomiting. Postgrad Med. 129:111–117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fox LM, Hoffman RS, Vlahov D and Manini
AF: Risk factors for severe respiratory depression from
prescription opioid overdose. Addiction. 113:59–66. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomes T, Juurlink DN, Antoniou T, Mamdani
MM, Paterson JM and van den Brink W: Gabapentin, opioids, and the
risk of opioid-related death: A population-based nested
case-control study. PLoS Med. 14:e10023962017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Webster LR: Risk factors for opioid-use
disorder and overdose. Anesth Analg. 125:1741–1748. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kenes MT, Stollings JL, Wang L, Girard TD,
Ely EW and Pandharipande PP: Persistence of delirium after
cessation of sedatives and analgesics and impact on clinical
outcomes in critically Ill patients. Pharmacotherapy. 37:1357–1365.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capdevila X, Biboulet P, Bouregba M,
Barthelet Y, Rubenovitch J and d'Athis F: Comparison of the
three-in-one and fascia iliaca compartment blocks in adults:
Clinical and radiographic analysis. Anesth Analg. 86:1039–1044.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tran DQ, Clemente A and Finlayson RJ: A
review of approaches and techniques for lower extremity nerve
blocks. Can J Anaesth. 54:922–934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eastburn E, Hernandez MA and Boretsky K:
Technical success of the ultrasound-guided supra-inguinal fascia
iliaca compartment block in older children and adolescents for hip
arthroscopy. Paediatr Anaesth. 27:1120–1124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Odor PM, Chis Ster I, Wilkinson I and Sage
F: Effect of admission fascia iliaca compartment blocks on
post-operative abbreviated mental test scores in elderly fractured
neck of femur patients: A retrospective cohort study. BMC
Anesthesiol. 17:22017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pinson S: Fascia Iliaca (FICB) block in
the emergency department for adults with neck of femur fractures: A
review of the literature. Int Emerg Nurs. 23:323–328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chesters A and Atkinson P: Fascia iliaca
block for pain relief from proximal femoral fracture in the
emergency department: a review of the literature. Emerg Med J.
31:e84–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mittal R and Vermani E: Femoral nerve
blocks in fractures of femur: Variation in the current UK practice
and a review of the literature. Emerg Med J. 31:143–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satsumae T, Tanaka M, Saito S and Inomata
S: Convulsions after ropivacaine 300 mg for brachial plexus block.
Br J Anaesth. 101:860–862. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hansen TG: Ropivacaine: A pharmacological
review. Expert Rev Neurother. 4:781–791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das NT and Deshpande C: Effects of
intraperitoneal local anaesthetics bupivacaine and ropivacaine
versus placebo on postoperative pain after laparoscopic
cholecystectomy: A randomised double blind study. J Clin Diagn Res.
11:UC08–UC12. 2017.PubMed/NCBI
|
|
21
|
Rao KG, Misra S and Shukla A: Comparison
between epidural ropivacaine versus ropivacaine with clonidine in
patients undergoing abdominal hysterectomy: A randomized study.
Anesth Essays Res. 11:334–339. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paut O, Schreiber E, Lacroix F, Meyrieux
V, Simon N, Lavrut T, Camboulives J and Bruguerolle B: High plasma
ropivacaine concentrations after fascia iliaca compartment block in
children. Br J Anaesth. 92:416–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dhir S, Ganapathy S, Lindsay P and Athwal
GS: Case report: Ropivacaine neurotoxicity at clinical doses in
interscalene brachial plexus block. Can J Anaesth. 54:912–916.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung HJ, Ok SH, Sohn JY, Son YH, Kim JK,
Lee SH, Han JY, Lim DH, Shin IW, Lee HK, et al: Vasoconstriction
potency induced by aminoamide local anesthetics correlates with
lipid solubility. J Biomed Biotechnol 2012. 1709582012.
|
|
25
|
Xiao J, Cai MH and Wang XR, He P and Wang
XR: Time course of action and pharmacokinetics of ropivacaine in
adult and elderly patients following combined lumbar plexus-sciatic
nerve block. Int J Clin Pharmacol Ther. 48:608–613. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Torup H, Mitchell AU, Breindahl T, Hansen
EG, Rosenberg J and Møller AM: Potentially toxic concentrations in
blood of total ropivacaine after bilateral transversus abdominis
plane blocks; a pharmacokinetic study. Eur J Anaesthesiol.
29:235–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koköfer A, Nawratil J, Felder TK, Stundner
O, Mader N and Gerner P: Ropivacaine 0.375% vs. 0.75% with
prilocaine for intermediate cervical plexus block for carotid
endarterectomy: A randomised trial. Eur J Anaesthesiol. 32:781–789.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peng PW, Coleman MM, McCartney CJ, Krone
S, Chan VW, Kaszas Z and Vucemilo I: Comparison of anesthetic
effect between 0.375% ropivacaine versus 0.5% lidocaine in forearm
intravenous regional anesthesia. Reg Anesth Pain Med. 27:595–599.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun N, Wang S, Ma P, Liu S, Shao A and
Xiong L: Postoperative analgesia by a transversus abdominis plane
block using different concentrations of ropivacaine for abdominal
surgery: A meta-analysis. Clin J Pain. 33:853–863. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Senohradski K, Markovic-Denic L, Lesic A,
Bumbasirevic V and Bumbasirevic M: Trends in the incidence of hip
fractures. Osteoporosis Int. 24:1759–1763. 2013. View Article : Google Scholar
|
|
31
|
Miller GW, Godrey JJ, Sagmeister ML and
Lewis TL: Provision of fascia iliaca compartment block in the acute
management of proximal femoral fractures: A national observational
study of UK hospitals. Injury. 47:2490–2494. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su Y, Zhang Z, Zhang Y, Li H and Shi W:
Efficacy of ropivacaine by the concentration of 0.25%, 0.5%, and
0.75% on surgical performance, postoperative analgesia, and
patient's satisfaction in inguinal hernioplasty: A randomized
controlled trial. Patient Prefer Adherence. 9:1375–1379. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yun MJ, Kim YH, Han MK, Kim JH, Hwang JW
and Do SH: Analgesia before a spinal block for femoral neck
fracture: Fascia iliaca compartment block. Acta Anaesthesiol Scand.
53:1282–1287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kumar D, Hooda S, Kiran S and Devi J:
Analgesic efficacy of ultrasound guided FICB in patients with hip
fracture. J Clin Diagn Res. 10:UC13–UC16. 2016.PubMed/NCBI
|
|
35
|
Dolan J, Williams A, Murney E, Smith M and
Kenny GN: Ultrasound guided fascia iliaca block: A comparison with
the loss of resistance technique. Reg Anesth Pain Med. 33:526–531.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Desmet M, Vermeylen K, Van Herreweghe I,
Carlier L, Soetens F, Lambrecht S, Croes K, Pottel H and Van de
Velde M: A longitudinal supra-inguinal fascia iliaca compartment
block reduces morphine consumption after total hip arthroplasty.
Reg Anesth Pain Med. 42:327–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Graf BM, Abraham I, Eberbach N, Kunst G,
Stowe DF and Martin E: Differences in cardiotoxicity of bupivacaine
and ropivacaine are the result of physicochemical and
stereoselective properties. Anesthesiology. 96:1427–1434. 2002.
View Article : Google Scholar : PubMed/NCBI
|