Introduction
Liver fibrosis is a compensatory response in the
process of tissue repair following liver damage and inflammation
caused by various chronic pathogenic factors (including viral
infection, immune attack and drug toxicity) (1,2). If
liver fibrosis is diagnosed and treated at an early stage, normal
liver tissue structure and function may be restored. However, if
left untreated it progresses to irreversible end-stage cirrhosis
and liver cancer and has an extremely high mortality (3). The pathogenesis of liver fibrosis is
complex. Although different etiological factors involve diverse
pathophysiological processes, the activation of hepatic stellated
cells (HSCs) is a common process in the development of liver
fibrosis (4). A variety of immune
factors activate HSCs and increase the expression levels of
α-smooth muscle actin (α-SMA) and collagen type 1 α 1 (Col1α1),
thereby forming collagen fibers and extracellular matrices that
lead to liver fibrosis (5). At
present, various drugs have been used for the treatment of liver
fibrosis, including vitamins, nucleoside analogs, liver-protecting
enzymes and interferon treatment (6,7).
However, their efficacy remains unsatisfactory, and the prevention
and treatment of liver fibrosis are challenging (7,8).
Therefore, it is of great practical significance to elucidate the
mechanisms underlying the occurrence and development of liver
fibrosis and to identify effective strategies for its
treatment.
Plantamajoside (PMS), a natural compound extracted
from Psyllium asiatica, has long been used in folk medicine
(9). PMS is a phenylpropanoid
glycoside and previous studies have revealed that it has a wide
range of biological activities, including antioxidant,
anti-inflammatory and antitumor effects (10–13). A
recent study demonstrated that PMS suppresses the progression of
respiratory inflammatory diseases by inhibiting the inflammatory
response (14). Furthermore, PMS
regulates human umbilical vein endothelial cell adhesion function
via the mitogen-activated protein kinase/nuclear factor-κb
signaling pathway (15). However,
the potential effects of PMS on liver fibrosis have not been
investigated.
The activation and proliferation of HSCs as well as
their synthesis and secretion of extracellular matrix serve
important roles in the development of liver fibrosis (16). Activated HSCs may transform into
myofibroblasts, secreting a large amount of collagen fibers to
promote the formation of liver fibrosis (5,17).
Inhibiting the activity of HSCs may therefore effectively delay the
progression of liver fibrosis (5,18).
Activated HSCs have been widely used as a cell model of liver
fibrosis (19–21). Platelet-derived growth factor BB
(PDGF-BB)-induced HSC-T6 cell activation has been previously used
to study liver fibrosis in vitro (20,22).
The present study aimed to investigate whether PMS
may prevent liver fibrosis by affecting the activation and survival
of HSCs, and to explore the underlying molecular mechanism. The
results obtained in the current study may provide a theoretical
basis for the development of novel treatment strategies for the
clinical treatment of liver fibrosis.
Materials and methods
Cell culture and treatment
HSC-T6 cells (cat no. R-HSC-T6; Shanghai Kalang
Biological Technology Co., Ltd., Shanghai, China) were cultured in
Dulbecco's modified Eagle medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine
serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were incubated at 37°C with 5%
CO2.
To activate HSC-T6 cells, cells were serum-starved
in FBS-free DMEM for 24 h and then exposed to 10 ng/ml PDGF-BB
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as previously
described (19,20).
HSC-T6 cells were subsequently treated with
increasing concentrations (20, 40, 80 and 160 µg/ml) of PMS (purity
>99%; Best-Reagent Company, Chengdu, China), or equal amount of
solvent control, at 37°C for the time indicated in the different
experiments. PMS was dissolved in a solution of equal ratio ethanol
and ultrapure water.
Cell proliferation assay
The Cell Counting Kit-8 (CCK-8) assay was performed
to determine the effect of PMS on the proliferation of HSC-T6
cells. First, 1×104 HSC-T6 cells without PDGF-BB
treatment were plated per well in a 96-well plate and treated with
increasing concentrations (0, 20, 40, 80 and 160 µg/ml) of PMS for
48 h. In addition, PDGF-BB treated HSC-T6 cells (1×104
cells per well) were plated into a 96-well plate and treated with
increasing concentrations (0, 20, 40, 80, and 160 µg/ml) of PMS for
48 h, or treated with 160 µg/ml PMS for 12, 24 and 48 h. A total of
10 µl CCK-8 solution was added to each well and incubated at 37°C
for an additional 2 h. Cell proliferation was assessed by measuring
the absorbance at a wavelength of 450 nm using a
FLUOstar® Omega microplate reader (BMG Labtech GmbH,
Ortenberg, Germany).
Cell invasion and migration assay
The effect of PMS on the migration and invasion of
PDGF-activated HSC-T6 cells was determined using Transwell assay.
In brief, chamber inserts (24-well transwell plate; 8 mm pore size)
were coated with 200 mg/ml Matrigel® at 37°C and dried
overnight under sterile conditions for the invasion assay. A total
of 1×104 cells HSC-T6 cells were suspended in serum-free
DMEM containing various concentrations of PMS (0, 20, 40, 80 and
160 µg/ml) and seeded into the upper chamber. A total of 600 µl
DMEM containing 20% FBS was added to the lower chamber. Cells were
incubated for 48 h at 37°C. Migratory or invasive cells on the
basolateral side of the insert were fixed with 100% methanol at
room temperature for 10 min and stained with 0.1% crystal violet at
room temperature for 15 min. The migratory or invasive cells in
five random fields were counted using a light microscope
(magnification, ×200).
Cell apoptosis assay
Cell apoptosis in the present study was analyzed
using the Annexin V-Fluorescein Isothiocyanate (FITC)/Propidium
Iodide (PI) Apoptosis Detection kit (cat no. 70-AP101-100;
MultiSciences, Hangzhou, China) following the manufacturer's
protocol. After treating with various concentrations (0, 20, 40,
80, and 160 µg/ml) of PMS for 48 h, PDGF-activated HSC-T6 cells
were stained with 5 µl Annexin V-FITC and 5 µl PI for 30 min at
room temperature in the dark. A flow cytometer was used to analyze
cell apoptosis. The early and late apoptosis rates were calculated
in the current study using WinMDI software (version 2.5; http://www.cyto.purdue.edu/flowcyt/software/Winmdi.htm).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HSC-T6 cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scienfitic,
Inc.), according to the manufacturer's protocol, and
reverse-transcribed into cDNA using the miScript Reverse
Transcription kit (Qiagen GmbH) according to the manufacturer's
protocol. qPCR was subsequently performed using the
SYBR® Green PCR Master mix (Thermo Fisher Scientific,
Inc.). The sequences of the primer pairs used are presented in
Table I. Amplification conditions
for qPCR were as follows: 5 min at 95°C, followed by 35 cycles at
95°C for 15 sec, 40 sec at 55°C, and 72°C for 1 min. mRNA levels
were quantified using the 2−ΔΔCq method and normalized
to the internal reference gene GAPDH (23).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
|
| Sequence
(5′-3′) |
|---|
|
|
|
|---|
| Primer | Forward | Reverse |
|---|
| BCL2 |
TTGGATCAGGGAGTTGGAAG |
TGTCCCTACCAACCAGAAGG |
| BAX |
CGTCCACCAAGAAGCTGAGCG |
CGTCCACCAAGAAGCTGAGCG |
| α-SMA |
TCCAGAGTCCAGCACAATACCAG |
AATGACCCAGATTATGTTTGAGACC |
| Col1α1 |
GTGAGACAGGCGAACAGG |
GACCAGCAGGACCAGAGG |
| GAPDH |
CTTTGGTATCGTGGAAGGACTC |
GTAGAGGCAGGGATGATGTTCT |
Western blot assay
Total protein was extracted from HSC-T6 cells
treated with or without PDGF-BB, or from PDGF-activated HSC-T6
cells treated with various concentrations (0, 20, 40, 80 and 160
µg/ml) of PMS for 48 h, using radioimmunoprecipitation buffer (cat
no. P0013E; Beyotime Institute of Biotechnology, Shanghai, China).
Total protein was quantified using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Protein samples (30
µg/lane) were separated via SDS-PAGE on a 12% gel. The separated
proteins were subsequently transferred onto a polyvinylidene
difluoride membrane and blocked in 5% skimmed milk at room
temperature for 1.5 h. The membranes were incubated with the
following primary antibodies: Anti-p-AKT (cat no. 4060), anti-AKT
(cat no. 4685), anti-BCL-2 (cat no. 4223), anti-Bax (cat no. 5023)
anti-α-SMA (cat no. 19245), anti-Col1α1 (cat no. 84336) and β-actin
(cat no. 4970; all 1:1,000; Cell Signaling Technology Inc.,
Danvers, MA, USA) overnight at 4°C. Membranes were subsequently
incubated with a horseradish peroxidase-conjugated secondary
antibody (cat no. 7074; 1:2,000; Cell Signaling Technology Inc.) at
room temperature for 2 h. Protein bands were visualized using the
Enhanced Chemiluminescence Detection system (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
band densities of p-AKT were analyzed using Gel-Pro-Analyzer
software (version 6.3; Media Cybernetics, Inc., Rockville, MD,
USA), and the relative protein level of p-AKT was shown as fold of
the control group. Each experiment was performed three times.
Statistical analysis
Results were expressed as the mean values ± standard
deviation. All data analyses were performed using SPSS software
(version 17; IBM Corp., Armonk, NY, USA). Comparisons between two
groups were made using Student's t-test and comparisons between
multiple groups were analyzed by one-way analysis of variance with
a Tukey's post-hoc test. All experiments were repeated three times.
P<0.05 indicated a statistically significant difference.
Results
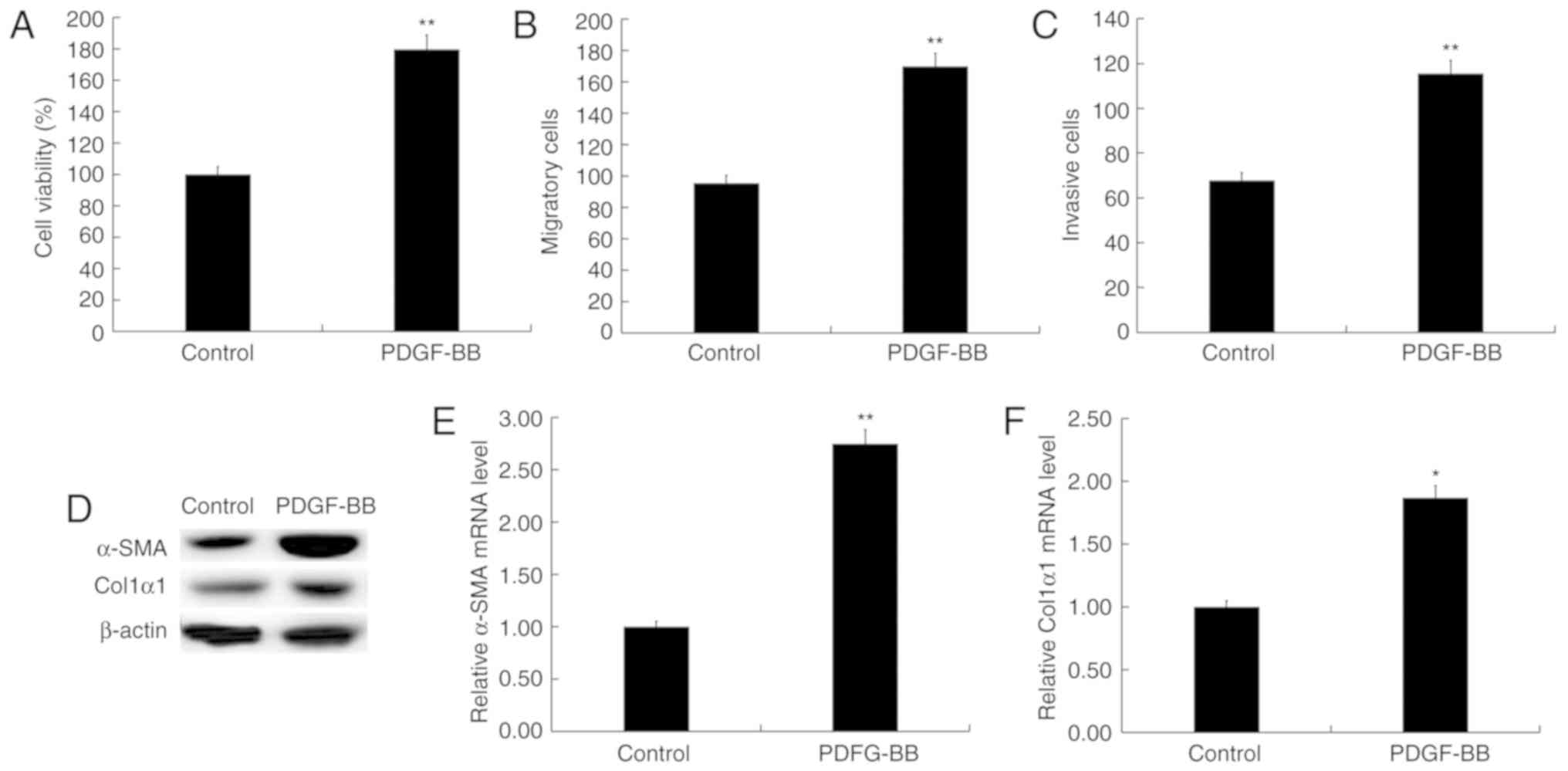
PDGF-BB activates HSC-T6 cells
HSC-T6 cells were activated by treatment with 10
ng/ml PDGF-BB as previously described (19,20).
PDGF-BB significantly increased HSC-T6 cell proliferation (Fig. 1A), migration (Fig. 1B) and invasion (Fig. 1C). Furthermore, compared with HSC-T6
cells which were not treated with PDGF-BB, α-SMA and Col1α1,
markers of activated HSCs, were significantly increased in
PDGF-BB-treated HSC-T6 cells (Fig.
1D-F). Taken together, the data indicated that HSC-T6 cells
were activated by treatment with PDGF-BB.
PMS inhibits activated HSC-T6 cell
proliferation
HSC-T6 cells were treated with various
concentrations (0, 20, 40, 80 and 160 µg/ml) of PMS for 48 h. Cell
viability was subsequently measured. Results indicated that none of
the concentrations of PMS used had significant effects on the cell
viability of HSC-T6 cells (Fig. 2A).
Activated HSC-T6 cells were treated with various concentrations (0,
20, 40, 80, and 160 µg/ml) of PMS for 48 h, or treated with 160
µg/ml PMS for 12, 24 and 48 h. CCK-8 assay results revealed that
PMS inhibited HSC-T6 cell proliferation ability in what appeared to
be a dose- and time-dependent manner (Fig. 2B and C).
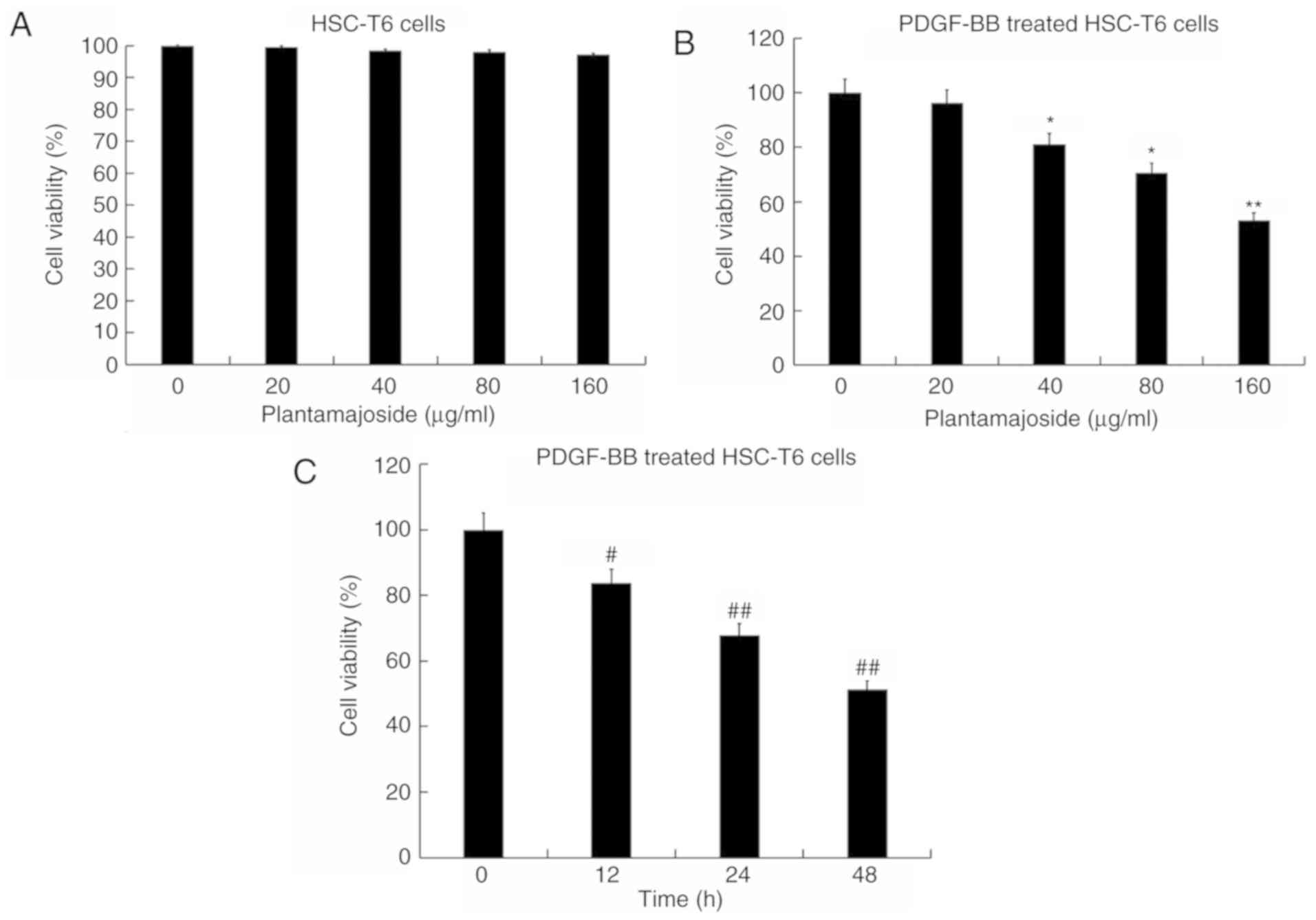 | Figure 2.Effect of PMS on the cell viability
of PDGF-BB treated HSC-T6 cells as determined by the Cell Counting
Kit-8 assay. (A) HSC-T6 cells were treated with various
concentrations (0, 20, 40, 80 and 160 µg/ml) of PMS for 48 h.
Activated HSC-T6 cells were treated with (B) 0, 20, 40, 80 and 160
µg/ml of PMS for 48 h or (C) 160 µg/ml PMS for 12, 24 and 48 h.
Data are presented as the mean ± standard deviation. *P<0.05,
**P<0.01 vs. the 0 µg/ml PMS treated group;
#P<0.05, ##P<0.01 vs. 0 h treatment
group. PMS, plantamajoside; PDGF-BB, platelet-derived growth factor
BB; α-SMA, α-smooth muscle actin; Col1α1, collagen type 1 α 1. |
PMS induces apoptosis in activated
HSC-T6 cells
PDGF-activated HSC-T6 cells were treated with
various concentrations (0, 20, 40, 80, and 160 µg/ml) of PMS for 48
h, then cell apoptosis was evaluated using the Annexin V-FITC/PI
apoptosis detection kit. PMS induced HSC-T6 cell apoptosis in what
appeared to be a dose-dependent manner (Fig. 3A and B). The protein and mRNA levels
of the apoptosis-associated genes BCL-2 and BAX were measured. The
results obtained suggested that the protein and mRNA levels of
BCL-2 and BAX were significantly decreased and increased,
respectively, in activated HSC-T6 cells, in what appeared to be a
dose-dependent manner, following PMS treatment (Fig. 3C-E).
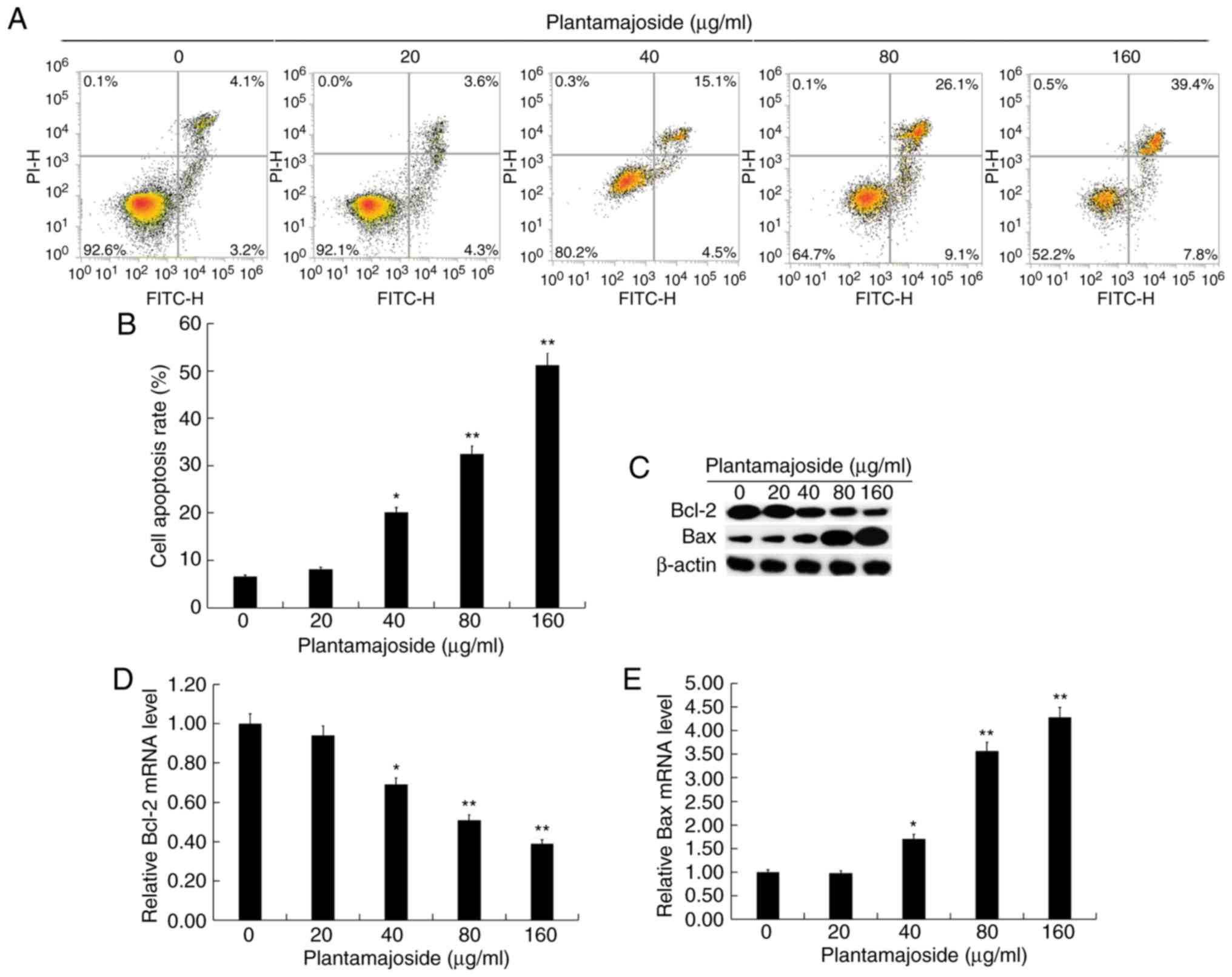 | Figure 3.PMS induces apoptosis in
platelet-derived growth factor BB treated HSC-T6 cells. Activated
HSC-T6 cells were treated with 0, 20, 40, 80 and 160 µg/ml of PMS
for 48 h. (A) Representative plots and (B) quantification from flow
cytometry analysis of apoptosis. The early and late apoptotic cells
were calculated and expressed as % of total cells. (C) The protein
levels of BCL2 and BAX were detected using western blotting. The
mRNA levels of (D) BCL2 and (E) BAX were determined using the
reverse transcription-quantitative polymerase chain reaction. Data
are expressed as the mean ± standard deviation. *P<0.05,
**P<0.01 vs. control group. PMS, plantamajoside; BCL2, BCL2
apoptosis regulator; BAX, BCL2 associated X, apoptosis regulator;
PI, propidium iodide; FITC, fluorescein isothiocyanate. |
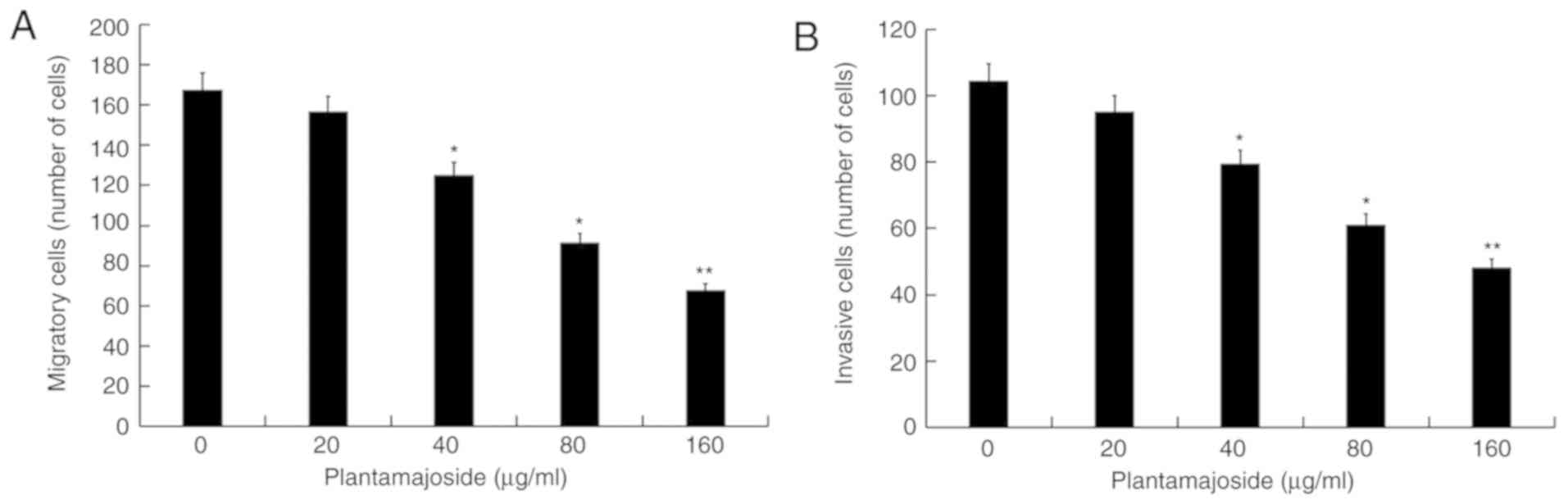
PMS inhibits activated HSC-T6 cell
migration and invasion
The effect of PMS on HSC-T6 cell migration and
invasion was investigated using a Transwell assay. As presented in
Fig. 4A and B, compared with the
control group, PMS treatment appeared to dose-dependently reduce
the migratory and invasive abilities of activated HSC-T6 cells.
This suggested that PMS had an inhibitory effect on the migration
and invasion of activated HSC-T6 cells.
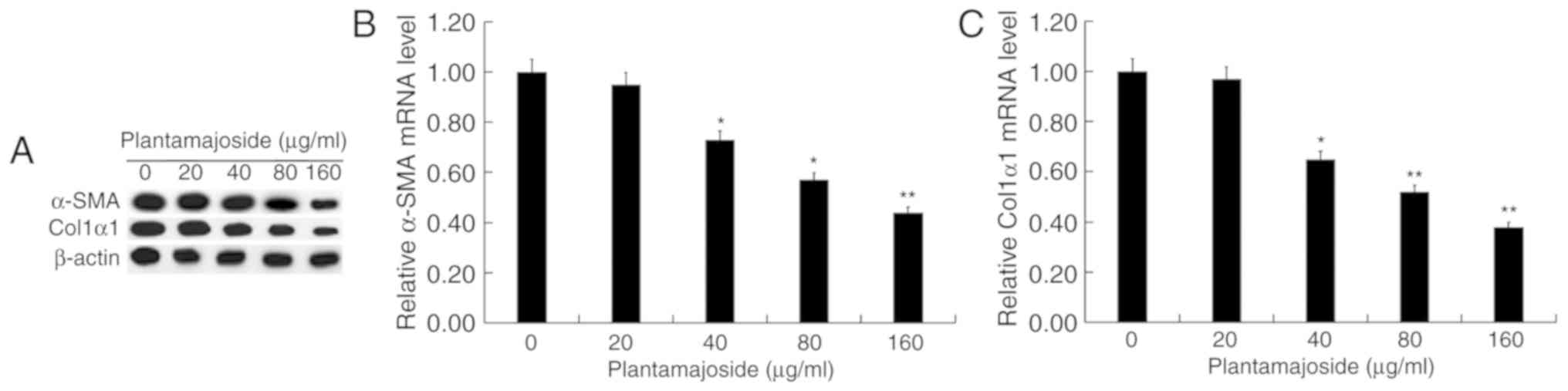
PMS inhibits α-SMA and Col1α1
expression in activated HSCT6 cells
As presented in Fig.
5, the protein (Fig. 5A) and
mRNA (Fig. 5B and C) levels of α-SMA
and Col1α1 were increased in PDGF-BB-treated HSC-T6 cells compared
with the cells from the control group. PMS treatment appeared to
dose-dependently reduce the protein and mRNA levels of α-SMA and
Col1α1 in PDGF-BB-treated HS-T6 cells.
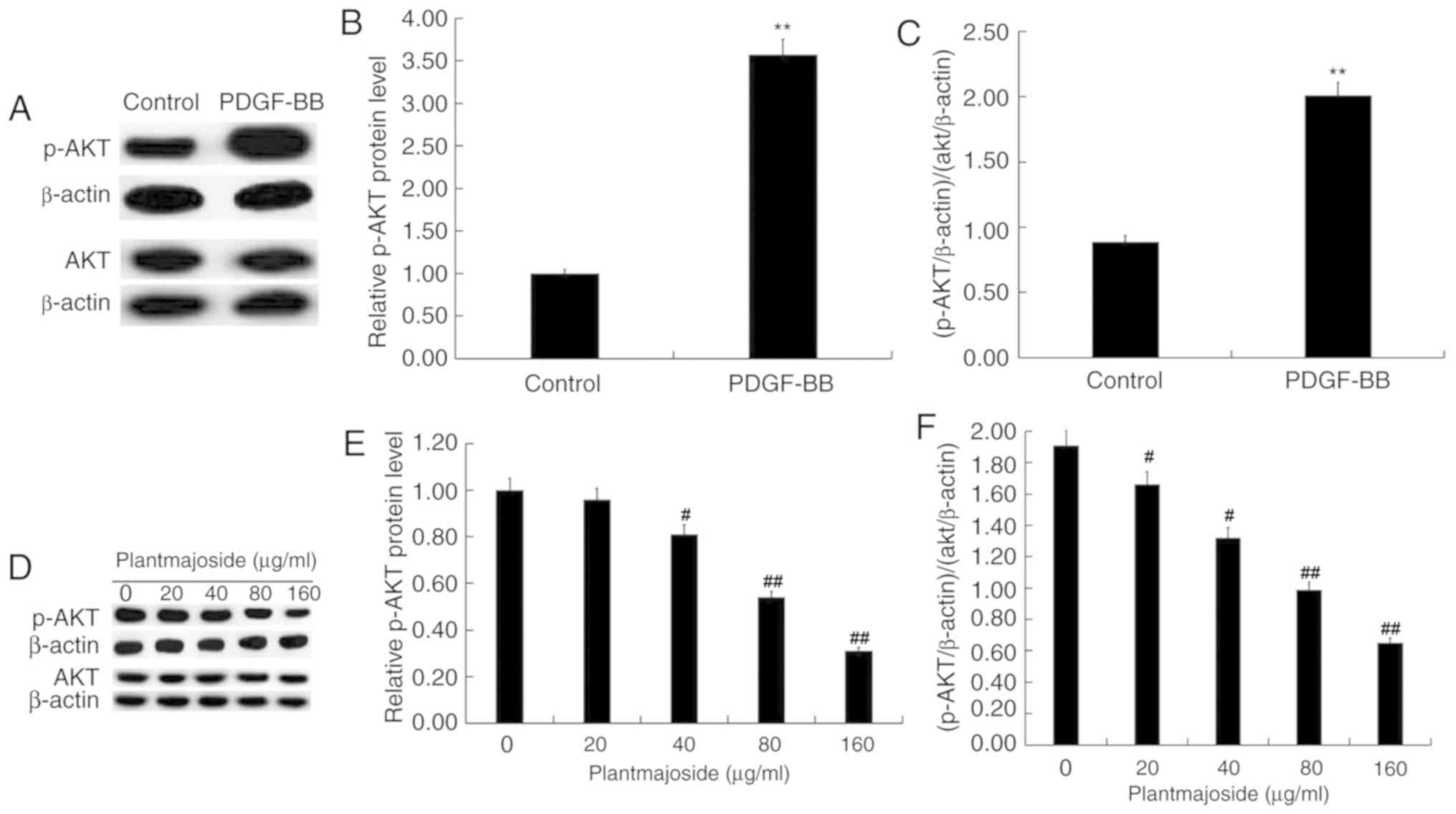
PMS inhibits the activation of the
PI3K/AKT signaling pathway in activated HSC-T6 cells
As presented in Fig.
6, compared with HSC-T6 cells which were not exposed to
PDGF-BB, PDGF-BB treatment significantly enhanced the protein level
of p-AKT in HSC-T6 cells (Fig.
6A-C). Compared with the control group, the protein level of
p-AKT was reduced in what appeared to be a dose-dependent manner in
PDGF-BB-treated HSC-T6 cells following exposure to PMS (Fig. 6D-F).
Discussion
The present study demonstrated that PMS might
dose-dependently inhibit the activation and proliferation in
PDGF-BB-treated HSC-T6 cells. Additionally, it increased apoptosis
and reduced cell migration and invasion. Furthermore, PMS inhibited
the protein expression of p-AKT in what appeared to be a
dose-dependent manner in PDGF-BB-treated HSC-T6 cells. The results
obtained demonstrated that PMS inhibited HSC activation and
survival, suggesting that PMS may exhibit an antifibrotic effect in
the liver.
Liver fibrosis is a healing response to various
insults (24,25). Advanced liver fibrosis results in a
number of pathological and biochemical changes, including
distortions in the normal structure of the liver, which lead to
metabolic abnormalities or hepatocellular carcinoma (26,27).
Liver fibrosis is associated with significant morbidity (28). However, there are currently no
effective therapeutic interventions for liver fibrosis (8,28).
Therefore, it is of great significance to identify new and
effective strategies for the treatment of liver fibrosis. There is
increasing evidence indicates that several cell types are involved
in the development of liver fibrosis (29,30).
However, activation of HSCs is a central process in the
pathogenesis of liver fibrosis (4),
and the activation of HSCs is mediated by a variety of inflammatory
factors and growth factors (5). PMS,
a major natural compound extracted from Psyllium asiatica,
was demonstrated to regulate the inflammatory response and
influence cell survival in vitro and in vivo
(10–15). However, the potential effects of PMS
on liver fibrosis had not been investigated. Therefore, the present
study explored whether PMS exerts an antifibrotic effect by
affecting the activation and survival of HSCs.
PDGF-BB is the most potent mitogenic cytokine for
HSCs (29) and it inhibits HSC
apoptosis, senescence or quiescence (31). PDGF-BB has been widely used for the
activation of HSCs to study liver fibrosis in vitro
(19,20). In the present study, PDGF-BB was used
to activate HSC-T6 cells. Consistent with previous studies
(19,20,32),
PDGF-BB significantly activated HSC-T6 cells in the current study,
demonstrated by increased cell proliferation, enhanced cell
migration and invasion ability, and increased α-SMA and Col1α1,
markers of activated HSCs (33,34). The
activated HSC-T6 cells were treated with various concentrations (0,
20, 80 and 160 µg/ml) of PMS, and cell proliferation, apoptosis,
migration and invasion were determined by the CCK-8 assay, flow
cytometry and the Transwell assay, respectively. The results of the
present study revealed that PMS might dose-dependently inhibit
proliferation, induce cell apoptosis and decrease migration and
invasion in PDGF-BB-treated HSC-T6 cells. Furthermore, PMS may
dose-dependently reduce the protein and mRNA levels of α-SMA and
Col1α1 in HSC-T6 cells treated with PDGF-BB.
Multiple studies have revealed that the PI3K/AKT
signaling pathway is activated during liver fibrosis and that it
serves important roles in the activation of HSCs (35–38).
Therefore, to explore the molecular mechanism of the antifibrotic
effect of PMS, the PI3K/AKT signaling pathway was investigated in
the present study. The PI3K/AKT signaling pathway activated by
PDGF-BB treatment was inhibited by PMS treatment.
In summary, the results obtained in the current
study suggested that PMS exerted an antifibrotic effect in the
liver by inhibiting the activation of HSC and decreasing their
survival via the PI3K/AKT signaling pathway. Therefore, PMS may be
a promising therapeutic agent for the treatment of liver fibrosis.
However, the present study had a number of limitations. The cell
morphology prior to and following cell with PDGF-BB and PMS was not
investigated. Only one HSC cell line was investigated in the
current study. Furthermore, in vitro cell experiments differ
from liver fibrosis in humans. The aim of the current study was to
provide preliminary results of the effect of PMS on liver fibrosis.
Further studies are required to substantiate the results obtained
in the current study. Future studies may demonstrate the effects of
PMS on the clonogenic capacity of HSCs and on other HSC cell lines
as well as the effect of higher doses of PMS. Additionally, in
vivo and clinical studies of the effect of PMS on liver
fibrosis are required to demonstrate the effect of PMS on liver
fibrosis. Furthermore, the mechanisms underlying the effect of PMS
on PDGF-BB-treated HSC-T6 cells require further investigation.
Acknowledgements
Not applicable.
Funding
This study was supported by the Social Development
Foundation of Nantong, Jiangsu, P.R. China (grant no.
MS3201517).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW contributed to study design, data collection and
data analysis. DY contributed to data collection and data analysis.
All authors contributed to interpreting the results and writing the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee UE and Friedman SL: Mechanisms of
hepatic fibrogenesis. Best Pract Res Clinical Gastroenterol.
25:195–206. 2011. View Article : Google Scholar
|
|
2
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Popov Y and Schuppan D: Targeting liver
fbrosis: Strategies for development and validation of antifibrotic
therapies. Hepatology. 50:1294–1306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown-Clay JD, Shenoy DN, Timofeeva O,
Kallakury BV, Nandi AK and Banerjee PP: PBK/TOPK enhances
aggressive phenotype in prostate cancer via
β-catenin-TCF/LEF-mediated matrix metalloproteinases production and
invasion. Oncotarget. 6:15594–15609. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang CY, Yuan WG, He P, Lei JH and Wang
CX: Liver fibrosis and hepatic stellate cells: Etiology,
pathological hallmarks and therapeutic targets. World J
Gastroenterol. 22:10512–10522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuppan D: Liver fibrosis: Common
mechanisms and antifibrotic therapies. Clin Res Hepatol
Gastroenterol. 39 (Suppl):S51–S59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koyama Y, Xu J, Liu X and Brenner DA: New
developments on the treatment of liver fibrosis. Dig Dis.
34:589–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Samuelsen AB: The traditional uses,
chemical constituents and biological activities of plantago major
L. A review. J Ethnopharmacol. 71:1–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang DF, Tang YF, Nie SP, Wan Y, Xie MY
and Xie XM: Effect of phenylethanoid glycosides and polysaccharides
from the seed of Plantago asiatica L. on the maturation of murine
bone marrow-derived dendritic cells. Eur J Pharmacol. 620:105–111.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu H, Zhao G, Jiang K, Chen X, Zhu Z, Qiu
C, Li C and Deng G: Plantamajoside ameliorates
lipopolysaccharide-induced acute lung injury via suppressing NF-κB
and MAPK activation. Int Immunopharmacol. 35:315–322. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pei S, Yang X, Wang H, Zhang H, Zhou B,
Zhang D and Lin D: Plantamajoside, a potential anti-tumor herbal
medicine inhibits breast cancer growth and pulmonary metastasis by
decreasing the activity of matrix metalloproteinase-9 and −2. BMC
Cancer. 15:9652015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Chen D, Li M, Gao X, Shi G and Zhao
H: Plantamajoside inhibits lipopolysaccharide-induced
epithelial-mesenchymal transition through suppressing the
NF-κB/IL-6 signaling in esophageal squamous cell carcinoma cells.
Biomed Pharmacother. 102:1045–1051. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma C and Ma W: Plantamajoside inhibits
lipopolysaccharide-induced MUC5AC expression and inflammation
through suppressing the PI3K/Akt and NF-κB signaling pathways in
human airway epithelial cells. Inflammation. 41:795–802. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Son WR, Nam MH, Hong CO, Kim Y and Lee KW:
Plantamajoside from Plantago asiatica modulates human umbilical
vein endothelial cell dysfunction by glyceraldehyde-induced AGEs
via MAPK/NF-κB. BMC Complement Altern Med. 17:662017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Puche JE, Saiman Y and Friedman SL:
Hepatic stellate cells and liver fibrosis. Compr Physiol.
3:1473–1492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Higashi T, Friedman SL and Hoshida Y:
Hepatic stellate cells as key target in liver fibrosis. Adv Drug
Deliv Rev. 121:27–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Panebianco C, Oben JA, Vinciguerra M and
Pazienza V: Senescence in hepatic stellate cells as a mechanism of
liver fibrosis reversal: A putative synergy between retinoic acid
and PPAR-gamma signalings. Clin Exp Med. 17:269–280. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin X, Kong LN, Huang C, Ma TT, Meng XM,
He Y, Wang QQ and Li J: Hesperetin derivative-7 inhibits
PDGF-BB-induced hepatic stellate cell activation and proliferation
by targeting Wnt/β-catenin pathway. Int Immunopharmacol.
25:311–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu X, Zhi F, Lun W, Deng Q and Zhang W:
Baicalin inhibits PDGF-BB-induced hepatic stellate cell
proliferation, apoptosis, invasion, migration and activation via
the miR-3595/ACSL4 axis. Int J Mol Med. 41:1992–2002.
2018.PubMed/NCBI
|
|
21
|
Yang Y, Chen XX, Li WX, Wu XQ, Huang C,
Xie J, Zhao YX, Meng XM and Li J: EZH2-mediated repression of Dkk1
promotes hepatic stellate cell activation and hepaticfibrosis. J
Cell Mol Med. 21:2317–2328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L, Zhang CZ and Zhu QJ: Kangxian
ruangan keli inhibits hepatic stellate cell proliferation mediated
by PDGF. World J Gastroenterol. 9:2050–2053. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schuppan D: Structure of the extracellular
matrix in normal and fibrotic liver: Collagens and glycoproteins.
Semin Liver Dis. 10:1–10. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anthony PP, Ishak KG, Nayak NC, Poulsen
HE, Scheuer PJ and Sobin LH: The morphology of cirrhosis.
Recommendations on defnition, nomenclature, and classifcation by a
working group sponsored by the world health organization. J Clin
Pathol. 31:395–414. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mormone E, George J and Nieto N: Molecular
pathogenesis of hepatic fbrosis and current therapeutic approaches.
Chem Biol Interact. 193:225–231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han YP, Zhou L, Wang J, Xiong S, Garner
WL, French SW and Tsukamoto H: Essential role of matrix
metalloproteinases in interleukin-1-induced myofibroblastic
activation of hepatic stellate cell in collagen. J Biol Chem.
279:4820–4828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Altamirano-Barrera A, Barranco-Fragoso B
and Méndez-Sánchez N: Management strategies for liver fibrosis. Ann
Hepatol. 16:48–56. 2017. View Article : Google Scholar
|
|
29
|
Gressner AM: Transdifferentiation of
hepatic stellate cells (Ito cells) to myofibroblasts: A key event
in hepatic fibrogenesis. Kidney Int. 54 (Suppl):S39–S45. 1996.
|
|
30
|
Drescher HK, Schumacher F, Schenker T,
Baues M, Lammers T, Hieronymus T, Trautwein C, Streetz KL and Kroy
DC: c-Met signaling protects from nonalcoholic
steatohepatitis-(NASH-) induced fibrosis in different liver cell
types. Oxid Med Cell Longev 2018. 69574972018.
|
|
31
|
Wong L, Yamasaki G, Johnson RJ and
Friedman SL: Induction of beta-platelet-derived growth factor
receptor in rat hepatic lipocytes during cellular activation in
vivo and in culture. J Clin Invest. 94:1563–1569. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu CI, Hoffman JA, Shy BR, Ford EM, Fuchs
E, Nguyen H and Merrill BJ: Function of Wnt/β-catenin in
counteracting Tcf3 repression through the Tcf3-β-catenin
interaction. Development. 139:2118–2129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang L, Zhan S, Huang C, Cheng X, Lv X, Si
H and Li J: TRPM7 channel regulates PDGF-BB-induced proliferation
of hepatic stellate cells via PI3K and ERK pathways. Toxicol Appl
Pharmacol. 272:713–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tao H, Huang C, Yang JJ, Ma TT, Bian EB,
Zhang L, Lv XW, Jin Y and Li J: MeCP2 controls the expression of
RASAL1 in the hepatic fibrosis in rats. Toxicology. 290:327–333.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Woodhoo A, Iruarrizaga-Lejarreta M, Beraza
N, García-Rodríguez JL, Embade N, Fernández-Ramos D, Martínez-López
N, Gutiérrez-De Juan V, Arteta B, Caballeria J, et al: Human
antigen R contributes to hepatic stellate cell activation and liver
fibrosis. Hepatology. 56:1870–1882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
El-Mihi KA, Kenawy HI, El-Karef A,
Elsherbiny NM and Eissa LA: Naringin attenuates
thioacetamide-induced liver fibrosis in rats through modulation of
the PI3K/Akt pathway. Life Sci. 187:50–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Q, Chen L, Wu X, Zhang F, Jin H, Lu
C, Shao J, Kong D, Wu L and Zheng S: Dihydroartemisinin prevents
liver fibrosis in bile duct ligated rats by inducing hepatic
stellate cell apoptosis through modulating the PI3K/Akt pathway.
IUBMB Life. 68:220–231. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Chu ES, Chen HY, Man K, Go MY,
Huang XR, Lan HY, Sung JJ and Yu J: microRNA-29b prevents liver
fibrosis by attenuating hepatic stellate cell activation and
inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget.
6:7325–7338. 2015.PubMed/NCBI
|