Introduction
Prostate cancer is one of the most common tumors
observed in male genitourinary system, and is the second leading
cause of cancer-associated mortality in men with the highest
prevalence in older men (1,2). Chemotherapy is a commonly applied
method for cancer treatment. At present, a large number of drugs,
including abiraterone acetate, bicalutamide and cabazitaxel have
been approved by the U.S. Food and Drug Administration for the
treatment of human prostate cancer (3,4).
Androgen deprivation therapy has demonstrated a degree of
effectiveness against androgen-dependent prostate malignancies
(5,6). However, as prostate cancer advances it
frequently transforms into androgen-independent prostate cancer
(AIPC), reducing the efficacy of androgen deprivation therapy
(7). In addition, drug resistance
remains an obstacle to successful therapy, as it may lead to an
aggressive and lethal form of prostate cancer, for which there is a
lack of effective treatment.
Proto-oncogene serine/threonine-protein kinase pim-1
(pim-1) is a proto-oncogene encoded by the pim-1 gene (8). It has been reported that pim-1 serves
important roles in apoptosis, proliferation and differentiation of
cancer cells and the progression of cancer (9). In particular, pim-1 serves an important
role in the induction or suppression of cell cycle progression and
apoptosis. In a previous study, immunohistochemical analysis was
used to characterize the expression patterns of pim-1 in high grade
prostatic cancer tissues and normal tissues (10). The expression of pim-1 in prostate
cancer tissues was demonstrated to be significantly higher compared
with that in normal prostate tissues and benign prostatic
hyperplasia (9). In addition, pim-1
expression has been demonstrated to negatively correlate with
clinical outcome after therapy.
Gene therapy has brought about a breakthrough for
the treatment of prostate cancer, and a number of studies have
identified a substantial number of genes that may be potential
targets for the treatment of this malignancy (11,12). In
the present study, AIPC cell lines PC3 and DU145, which do not
respond to androgens, glucocorticoids or epidermal/fibroblast
growth factors, were selected as models (13,14). In
addition, the cisplatin-resistant subline of PC3, PC3/DDP, was
used. In the present study, an RNA-interference approach was
utilized to investigate the effects of pim-1 knockdown on PC3 and
DU145 cell proliferation. In addition, the effects of pim-1 on
PC3/DDP cell sensitivity to chemotherapeutic drugs were
investigated. The results of the present study may provide new
ideas for the therapy of AIPC.
Materials and methods
Cell lines, reagents and
antibodies
Human prostate cancer cell lines PC3 and DU145 were
purchased from American Type Culture Collection and cultured in
DMEM (HyClone; GE Healthcare Life Sciences) supplemented with 10%
fetal calf serum (cat. no. SH30071.03; HyClone; GE Healthcare Life
Sciences), 100 U/ml penicillin and 100 µg/ml streptomycin (cat. no.
15140122, Gibco ThermoFisher Scientific, Inc.) at 37°C and the
concentration of CO2 was 5%. MTT was obtained from
Sigma-Aldrich (Merck KGaA). Pim-1 short hairpin RNA (shRNA) plasmid
(human; cat. no. sc-36225-SH) and control shRNA plasmid-A (cat. no.
sc-108060) were purchased from Santa Cruz Biotechnology Inc. The
Lipofectamine® 3000 transfection reagent was obtained
from Invitrogen (Thermo Fisher Scientific, Inc.).
Cell transfection
PC3 or DU145 cells were seeded into 24-well plates
at a density of 1×105 cells/well. After 8 h of culture,
the cells were transfected with pim-1-specific or control shRNA for
6 h using Lipofectamine 3000 according to manufacturer's protocols.
Briefly, 1 µg of pim-1 or control shRNA plasmid and 2 µl of
Lipofectamine 3000 were diluted in 100 µl Opti-MEM (Gibco;
ThermoFisher Scientific, Inc.) each and incubated at room
temperature for 5 min. The two solutions were mixed gently at room
temperature to a total volume of 200 µl and incubated for a further
15 min. This transfection mixture was then gently added into each
well. Media containing the transfection mixture was subsequently
replaced with fresh medium supplemented with 10% FBS in each well 6
h after transfection. The cells were then cultured for 24, 48, 72
and 96 h before detection.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from pim-1 shRNA and control
shRNA-transfected cells (1×106 cells) using RNApure kit
(BioTeke Corporation) and reverse transcribed using RevertAid RT
Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific,
Inc.) for 5 min at 25°C, 60 min at 42°C and 5 min at 70°C. Pim-1
mRNA levels were detected by qPCR amplification using SYBR green
(cat. no. 4385610; Thermo Fisher Scientific, Inc.) and an Applied
Biosystems 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Human pim-1 primer pair (cat. no.
OOCA01281) was purchased from BioPike, LLC. β-actin was used as the
internal reference gene. The primers for β-actin were: Forward,
5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse,
5′-CAATAGTGATGACCTGGCCGT-3′. The thermocycling conditions were:
94°C for 2 min, followed by 30 cycles of 95°C for 10 sec and 57°C
for 30 sec. Each sample was run in triplicate. Relative expression
levels were determined using the 2−∆∆Cq method (15).
MTT viability assay
MTT assay was performed as previously described
(16,17). Briefly, PC3 and DU145 cells
(1×105 cells/well) were seeded into 48-well plates.
After 8 h incubation, cells were transfected with pim-1 or control
shRNA for 6 h, before being subsequently cultured for 48, 72 and 96
h. Next, 20 µl MTT agent (stock concentration 5 mg/ml) was added
into each well followed by a further 4 h of incubation. Finally,
200 µl DMSO was added into the medium to dissolve the formazan
crystals and incubated for 15 min at 37°C, and formazan was
measured at a wavelength of OD490. The plates were read on a
microplate reader (iMark™; Bio-Rad Laboratories, Inc.) before the
data were analyzed. Survival rate (%)=100 × (OD 490 value of tested
sample/OD 490 value of untreated cells).
IC50 determination
PC3 and their drug resistant counterpart PC3/DDP
cells (Shanghai Aulu Biological Technology, Co., Ltd.) were first
seeded into 96-well plates (3×104 cells/well). Following
8-h culture at 37°C, PC3 and PC3/DDP cells were treated with drugs
used for chemotherapy, including cisplatin, 5-fluorouracil and
doxorubicin at 37°C for 48 h. The drug concentrations of each drug
applied were as follows: 1000, 500, 250, 125, 62.5, 31.25, 15.62,
7.81, 3.90 and 1.95 µg/ml, as previously described (18,19).
Cells treated with 0.1% DMSO were used as the negative controls.
Cell viability was determined using MTT assay and the
IC50 values were calculated using GraphPad Prism 5
software (GraphPad Software, Inc.).
Western blotting
Cells (1×106 cells/well) were collected
and lysed using RIPA buffer (Beyotime Institute of Biotechnology),
and the concentration of total proteins were determined using
bicinchoninic acid assay. The proteins (25 µg/lane) were separated
by 10% SDS-PAGE as previously described (20,21), and
transferred to PVDF membranes at a constant current of 300 mA for 1
h. The PVDF membranes were blocked using 5% non-fat milk diluted in
TBS supplemented with 0.1% Tween-20 (TBST) for 1 h at room
temperature. The membranes were subsequently incubated overnight at
4°C with primary antibodies against pim-1 (cat. no. ab94603;
1:10,000; Abcam), anti-β-actin (mouse monoclonal; cat. no.
sc-47778; 1:10,000; Santa Cruz Biotechnology, Inc.) and anti-p-gp
(cat. no. ab242104; 1:500; Abcam). The membranes were washed three
times using TBST before they were incubated with horseradish
peroxidase-conjugated goat anti-mouse (cat. no. sc-2031) or goat
anti-rabbit secondary antibodies(cat. no. sc-2004) (1:10,000; Santa
Cruz Biotechnology, Inc.) for 1 h at room temperature. The membrane
was washed a further three times with TBST buffer and were
subsequently visualized using chemiluminescent ECL reagent kit
(cat. no. WBKLS0500; EMD Millipore; Merck KGaA). β-actin used as
the loading control and for normalization. ImageJ software (version
1.8.0; National Institutes of Health) was used for
densitometry.
Annexin V-FITC/propidium iodide (PI)
staining analysis
PC3 and PC3/DDP cells (5×105 cells/per
well) were first seeded into 6-well plates. Following 8 h
incubation, the cells were transfected for 6 h with pim-1 or
control shRNA and cultured for 24 h and treated with 0.1 mg/ml of
cisplatin for 48 h. Cell apoptosis was assessed using annexin
V-FITC/PI staining according to manufacturer's protocol (Annexin V
kit; sc-4252 AK; Santa Cruz Biotechnology, Inc.). Briefly, the
cells were digested using 0.25% trypsin for 1–2 min before being
washed twice with ice-cold PBS. The cells were then collected by
centrifugation at 800 × g for 5 min at 4°C and subsequently
resuspended in binding buffer (10 mM HEPES-NaOH; 25 mM
CaCl2; 144 mM NaCl; pH 7.4). Annexin V-FITC (0.1 µg/µl)
and PI (0.05 µg/µl) were subsequently added to the cells, followed
by incubation in the dark for 15 min at room temperature.
Fluorescence-activated cell sorting (FACS) analysis was performed
by collecting 10,000 cells for each sample using BD LSRFortessa
X-20 flow cytometer and BD FACSDiva™ software version 6.0 (BD
Biosciences).
Statistical analysis
Data were analyzed using SPSS statistical package
(version 13; SPSS, Inc.). Two sets of independent samples were
analyzed using Student's t-test. Comparisons between multiple
groups were analyzed using ANOVA followed by Tukey test. The
experiments were repeated three times, and the results were
expressed as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
Pim-1 knockdown by shRNA inhibits
proliferation of prostate cancer cell lines PC3 and DU145
The role of pim-1 in prostate cancer cell viability
was examined. PC3 and DU145 cells were transfected with
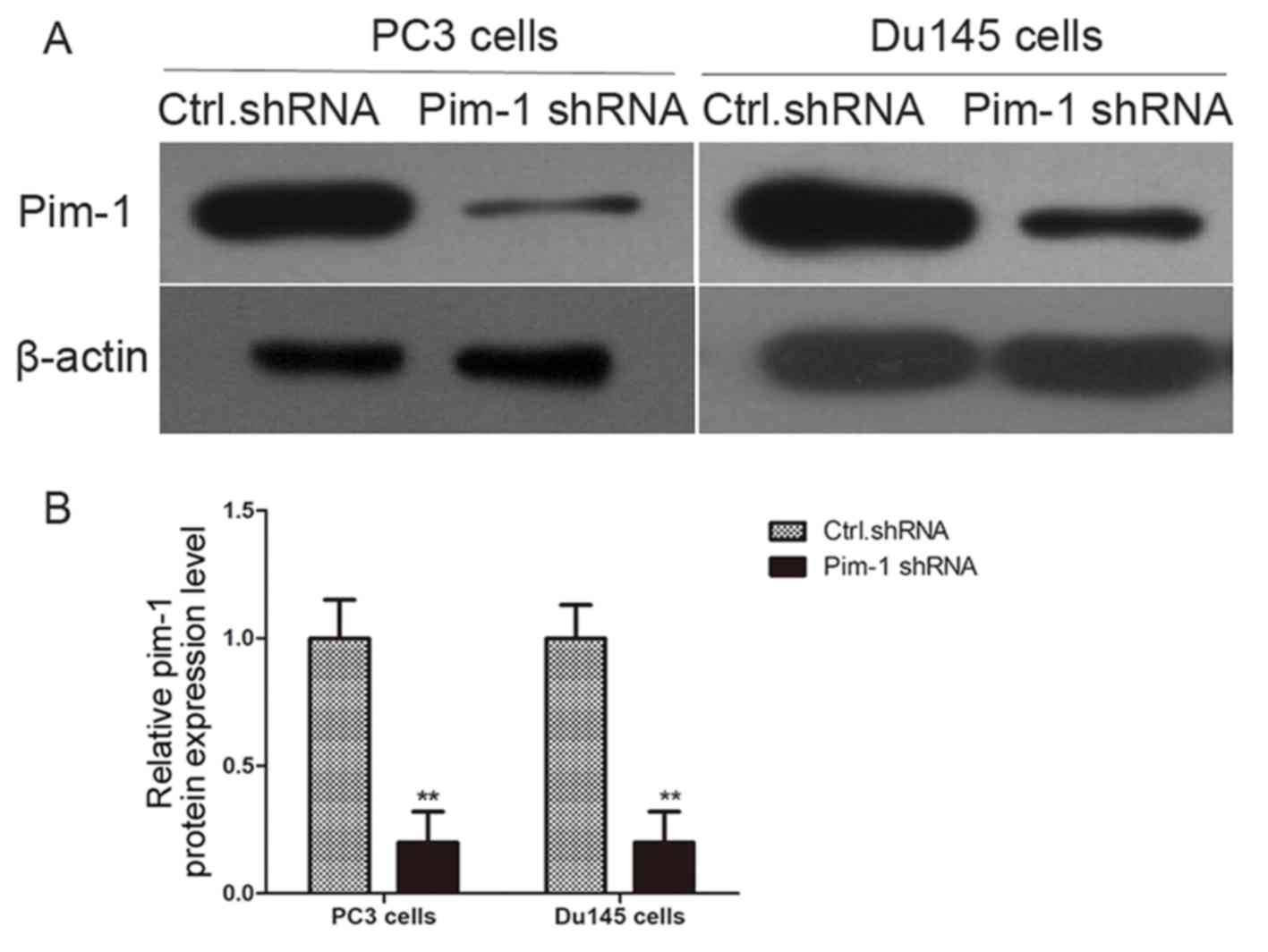
pim-1-specific shRNA or control shRNA. Pim-1 protein expression was
successfully knocked down by pim-1 shRNA in PC3 and Du145 cells 48
h following transfection (Fig. 1).
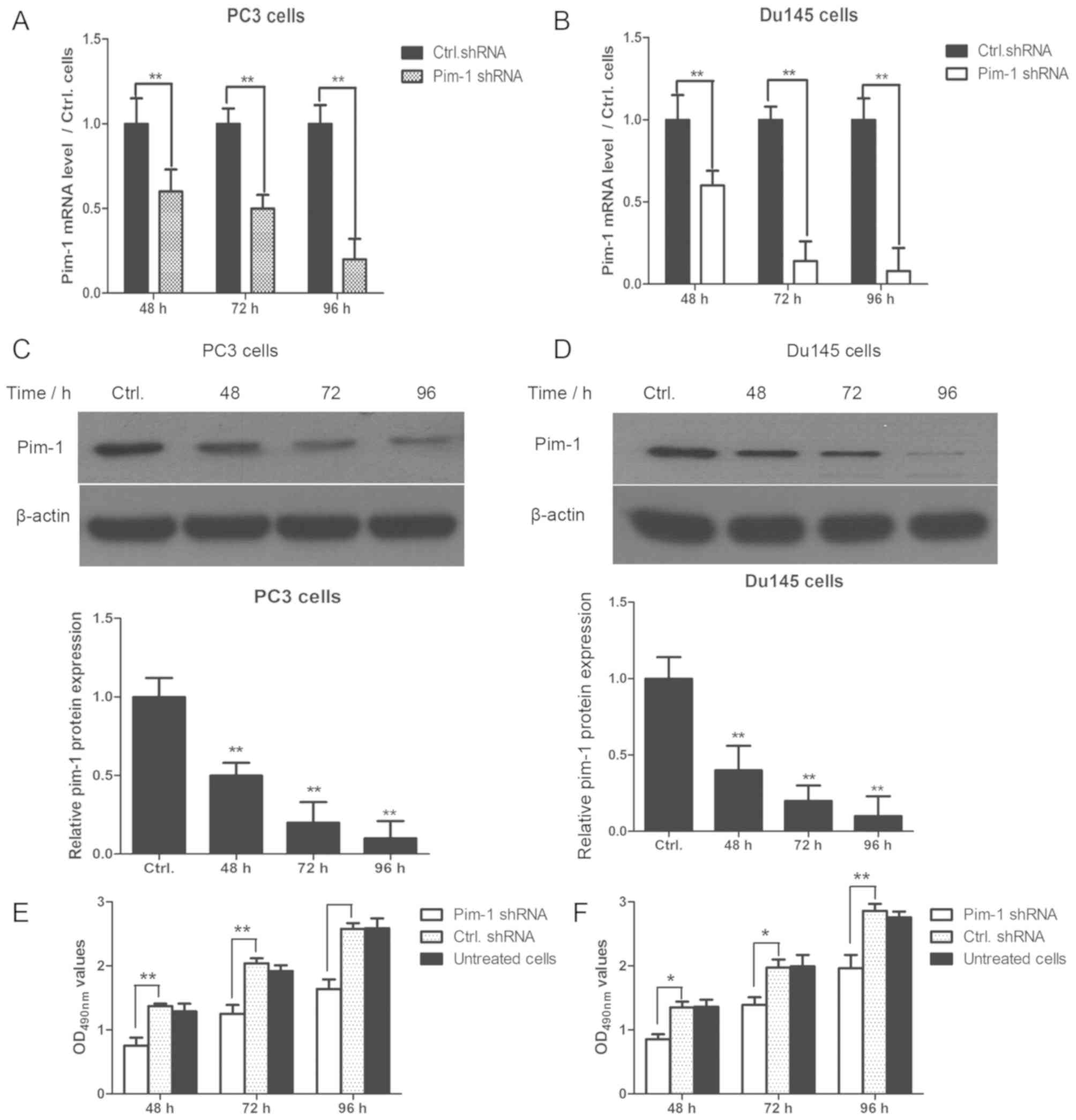
The pim-1 shRNA-transfected PC3 and DU145 cells were subsequently
cultured for 48, 72 and 96 h after transfection. Pim-1 mRNA and
protein expression were significantly reduced by pim-1 shRNA
transfection in PC3 and DU145 cells at each timepoint; compared
with cells transfected with control shRNA (Fig. 2A-D). In addition, cell viability was
significantly reduced in PC3 and DU145 cells transfected with pim-1
shRNA compared with cells transfected with control shRNA after 48,
72 and 96 h (P<0.05; Fig. 2E). No
statistically significant differences were observed between
untreated cells and those transfected with control shRNA in terms
of cell viability (P>0.05).
Expression of pim-1 and permeability
glycoprotein (p-gp) is markedly higher in PC3/DDP cells than that
in PC3 cells
Drug resistance is an obstacle to successful therapy
in a number of human malignancies. In the present study, the PC3
cell line and its cisplatin-resistant subline PC3/DDP were used as
models to investigate the role of pim-1 in prostate cancer drug
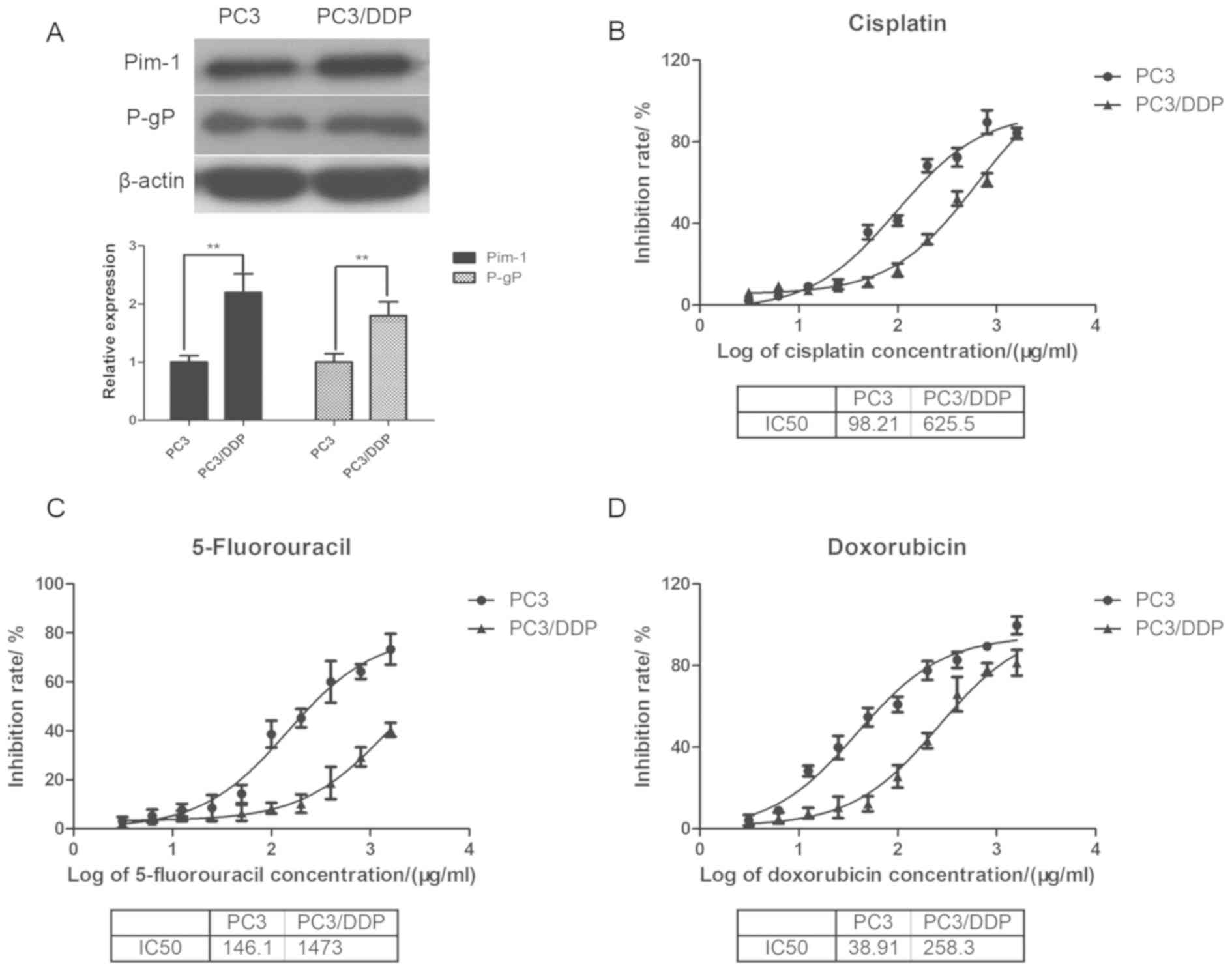
resistance. Pim-1 and p-gp protein levels were determined using
western blotting in cisplatin-sensitive PC3 and resistant PC3/DDP
cells. The expression of p-gp was significantly increased in
PC3/DDP cells compared with the PC3 cells (P<0.01; Fig. 3A). Importantly, pim-1 expression was
also demonstrated to be significantly higher in PC3/DDP cells
compared with that in PC3 cells (P<0.01).
Determination of IC50 values to
cisplatin, 5-flurouracil and doxorubicin in PC3 and PC3/DDP
cells
The half maximal inhibitory concentration
(IC50) was subsequently determined for cisplatin,
5-fluorouracil and doxorubicin in inhibiting prostate cancer cell
viability using MTT assay. The IC50 values for cisplatin
in PC3 cells and PC3/DDP cells were 98.21 and 625.50 µg/ml,
respectively (Fig. 3B); whereas the
IC50 values for 5-fluorouracil in PC3 cells and PC3/DDP
cells were 146.1 and 1473.0 µg/ml, respectively (Fig. 3C). The IC50 values for
doxorubicin in PC3 and PC3/DDP cells were 38.91 and 258.3 µg/ml,
respectively (Fig. 3D). Thus, the
IC50 values of PC3/DDP cells for the three chemotherapeutic drugs
were markedly higher than that of PC3 cells. Altogether, these
observations indicated that the drug-resistant PC3/DDP and its
parental drug-sensitive PC3 cell lines were suitable cell models
for studying chemotherapeutic drug resistance.
Pim-1 knockdown increases
chemotherapeutic drug sensitivity in PC3/DDP cells
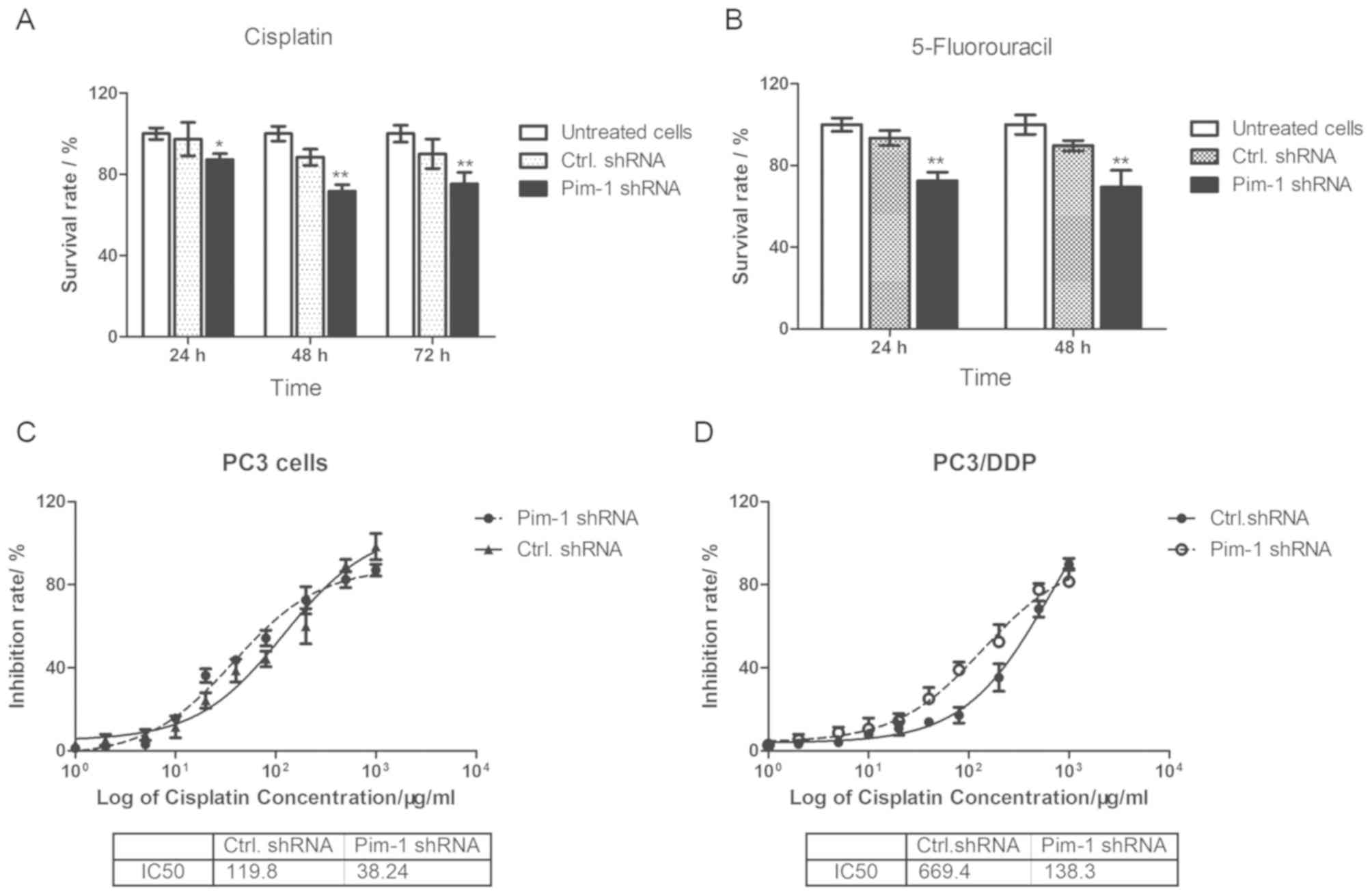
To investigate whether pim-1 serves a role in
chemotherapeutic drug sensitivity in PC3/DDP cells, cells
transfected with pim-1 shRNA were treated with concentrations of
cisplatin or 5-fluorouracil lower than IC50. Pim-1 or control
shRNA-transfected PC3/DDP cells were first treated with 0.1 mg/ml
cisplatin for 24, 48 and 72 h before cell viability was assessed
using MTT assay. The viability of PC3/DDP cells transfected with
pim-1 shRNA was significantly reduced compared with cells
transfected with control shRNA in the presence of cisplatin at all
timepoints tested (Fig. 4A). In the
presence of 0.5 mg/ml 5-fluorouracil, PC3/DDP cells transfected
with pim-1 shRNA exhibited lower survival rates compared with cells
transfected with control shRNA transfected after 24 and 48 h
(Fig. 4B). The IC50
values of cisplatin in PC3 and PC3/DDP cells transfected with pim-1
shRNA and control shRNA were calculated as 38.24 µg/ml and 119.8
µg/ml, respectively (3.13-fold; Fig.
4C). For PC3/DDP cells, the IC50 value for cisplatin
following pim-1 shRNA transfection was calculated to be 138.3
µg/ml, compared with 669.4 µg/ml in cells transfected with control
shRNA (4.84-fold; Fig. 4D). In
conclusion, this observation suggests that pim-1 knockdown
increased the sensitivity of PC3/DDP cells to cisplatin and
5-fluorouricil.
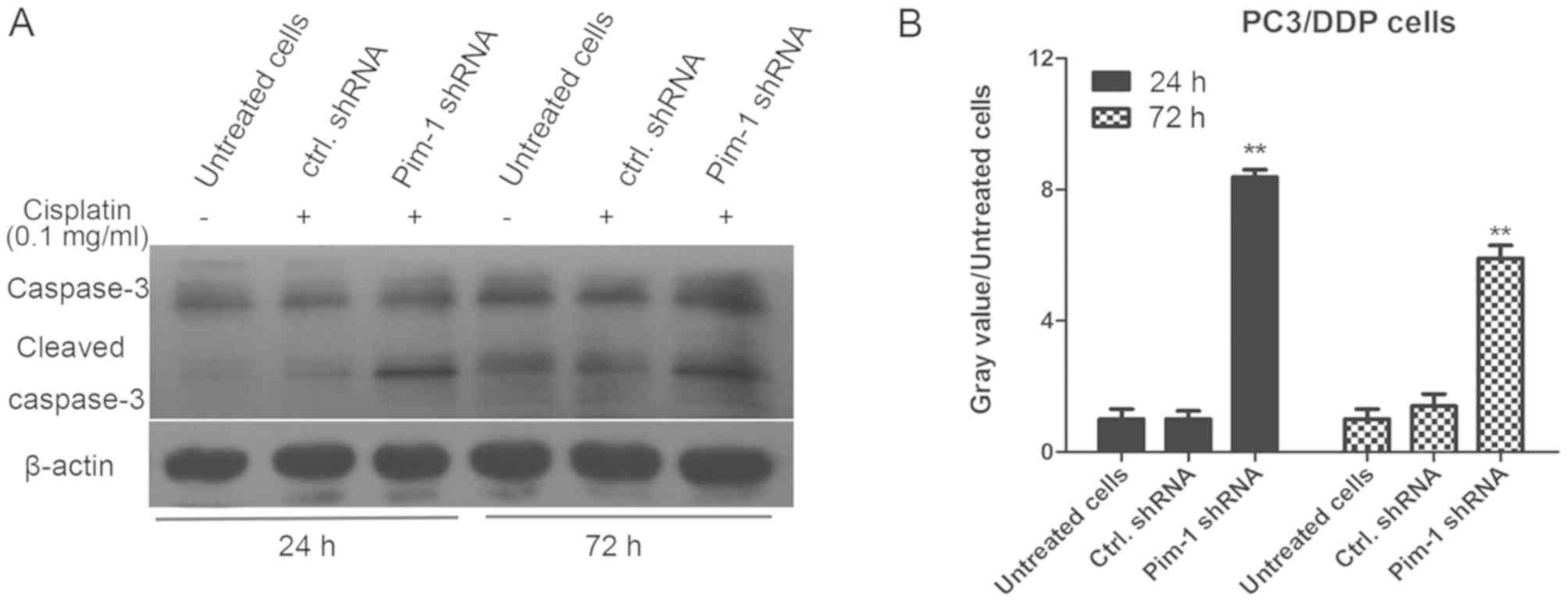
Pim-1 knockdown promotes caspase-3
activation and induces apoptosis in PC3/DDP cells
To clarify the molecular mechanism of pim-1 in
inducing chemotherapeutic drug resistance in PC3/DDP cells, the
effect of pim-1 knockdown on cell apoptosis was examined. PC3/DDP
cells were transfected with pim-1 or control shRNA and subsequently
treated with 0.1 mg/ml cisplatin for 24 and 72 h. The levels of
caspase-3 and cleaved caspase-3 were then determined using western
blotting analysis. Following 0.1 mg/ml cisplatin treatment, the
levels of cleaved caspase-3 in PC3/DDP cells transfected with pim-1
shRNA were significantly higher compared with cells transfected
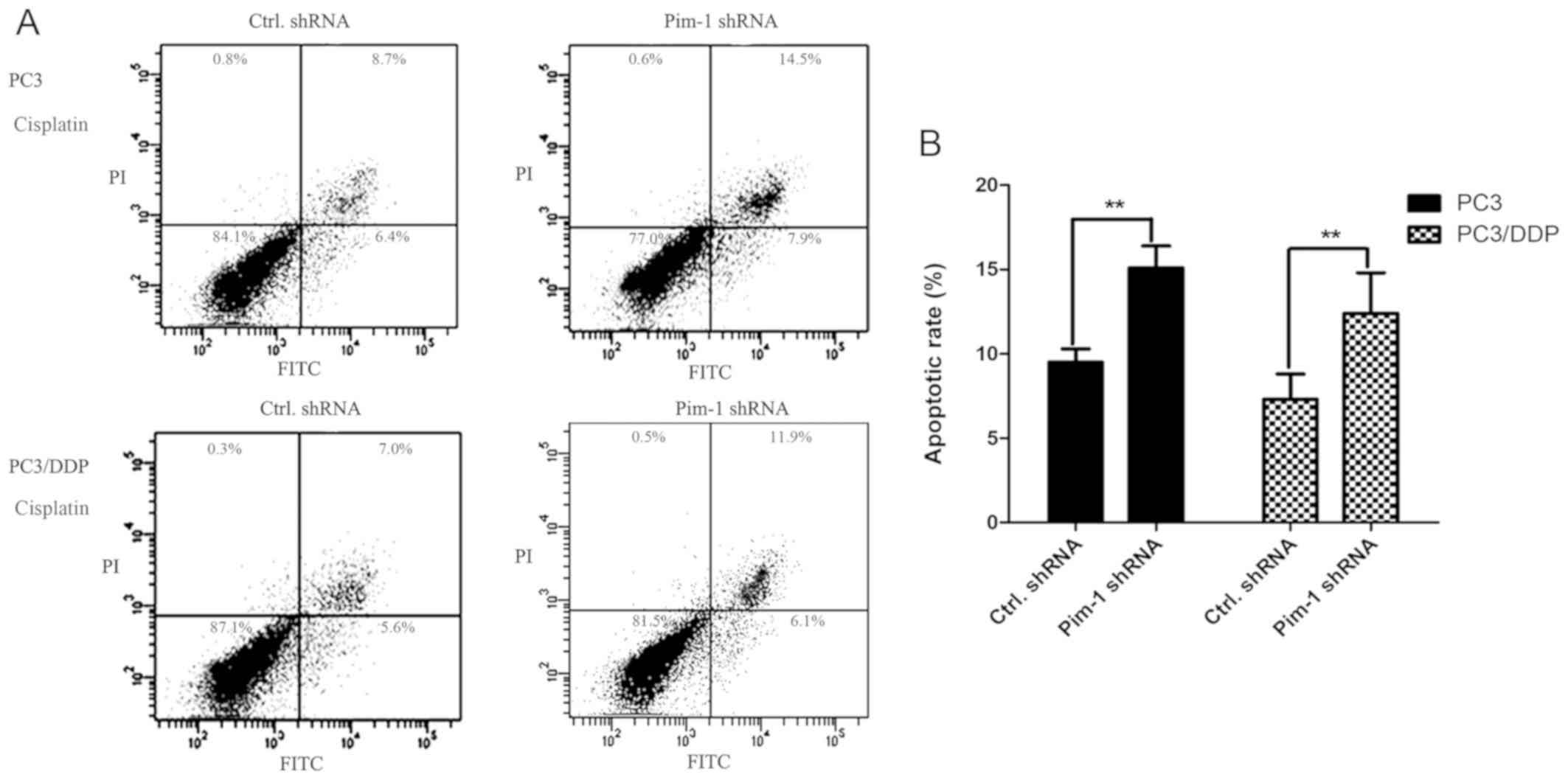
with control shRNA after 24 and 72 h (P<0.01; Fig. 5). To support this finding, FACS
analysis was performed to measure the apoptotic rate of PC3 and
PC3/DDP cells transfected with pim-1 or control shRNA, following 48
h of 0.1 mg/ml cisplatin treatment. In the presence of cisplatin,
the apoptotic rates of PC3 and PC3/DDP cells transfected with pim-1
shRNA were significantly higher compared with those transfected
with control shRNA (Fig. 6). This
observation indicates further that pim-1 knockdown increased
apoptotic rates in cisplatin-resistant PC3/DDP cells.
Discussion
Prostate cancer is a heterogeneous disease in male
reproductive system, which consists of androgen-dependent and
androgen-independent varieties (22). Androgen deprivation therapy has
achieved remarkable results in the treatment of advanced prostate
cancer, and hormone therapy has gradually become an important
method for the treatment of prostate cancer (23). For androgen-dependent prostate
cancer, surgical castration therapy has a certain therapeutic
effect (24). However, in patients
with advanced prostate cancer, the disease usually turns into
non-androgen-dependent prostate cancer after a period of time,
which makes castration treatment less effective. There is currently
no effective treatment for androgen-independent prostate cancer
(24). The purpose of the present
study was to find a new and effective method for the prevention and
treatment of advanced prostate cancer and AIPC. In particular, the
effects of pim-1 on resistance to chemotherapy were explored in
PC3/DDP cells and its parental cell line PC3. However, the current
study had certain limitations. Comparisons between androgen
dependent and androgen-independent prostate cancer cells, primary
cell lines and animal models were not included in this study. In
future research, clinical specimens should be collected and used to
test and compare the levels of pim-1 in androgen dependent and
androgen-independent prostate cancer. Furthermore, an animal model
should be used to test the tumorigenesis of pim-1 shRNA- and
control shRNA-transfected cells.
Three members of proto-oncogene serine/threonine
kinase family have been identified, including pim-1, pim-2 and
pim-3 (25,26). It has been reported that pim-1 is
involved in regulating cell cycle progression and apoptosis
(26); and has been detected to be
heavily expressed in numerous cancers including prostate cancer,
Burkitt's lymphoma, oral cancer and a variety of hematopoietic
lymphomas (27,28). Therefore, to study the role of pim-1
in the prostate cancer physiology, endogenous pim-1 expression was
knocked down in androgen-independent prostate cancer cells,
including PC3, DU145 and PC3/DDP, using the shRNA approach. The
present study revealed that pim-1 knockdown resulted in reduced
viability in AIPC cell lines. Pim-1 knockdown markedly increased
the activation of caspase-3 and promoted cell apoptosis of
chemotherapeutic drug-resistant PC3/DDP cells, which was also
confirmed by FACS assay. It has been reported that pim-1 is the
target gene of the Janus kinase and STAT signaling pathway.
Additionally, pim-1 is involved in the regulation of cell apoptosis
and cell cycle progression by interacting with the PI3K/Akt
signaling pathway (29).
The role of pim-1 in chemotherapeutic drug
sensitivity of prostate cancer cells was also tested. P-gp, encoded
by the multi-drug resistance protein 1 gene, has been reported to
contribute to drug resistance in cancer cells, which serves an
important role in drug disposition and distribution (30). It was found that pim-1 knockdown
significantly increased PC3/DDP cell sensitivity to cisplatin,
5-fluorouracil and doxorubicin, which could potentially reduce the
drug dose required for chemotherapy and increase its antitumor
potency, whilst reducing the risk of drug toxicity.
In conclusion, results from the present study
suggested that pim-1 knockdown suppressed AIPC cell viability and
increased chemotherapeutic drug sensitivity in cisplatin-resistant
PC3/DDP cells. These results may be helpful for the clinical
therapy of AIPC and addressing resistance to chemotherapy drugs in
prostate cancers.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The
Natural Science Foundation of Jiangsu Province (grant no.
BK20160481), The Natural Science Foundation of Yangzhou (grant no.
YZ2015111), Project for Jiangsu Commission of Health (grant nos.
QNRC2016360 and H2018108) and Six Talent Peaks Project in Jiangsu
Province (grant no. WSW-258).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SWL designed the studies and carried out literature
research; XZ performed the experimental studies; YYS and PW
analyzed the data and performed the statistical analysis; CFY
assisted in performing the experiments and prepared the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mahmoud AM, Yang W and Bosland MC: Soy
isoflavones and prostate cancer: A review of molecular mechanisms.
J Steroid Biochem Mol Biol. 140:116–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma X, Xiao Z, Li X, Wang F, Zhang J, Zhou
R, Wang J and Liu L: Prognostic role of circulating tumor cells and
disseminated tumor cells in patients with prostate cancer: A
systematic review and meta-analysis. Tumour Biol. 35:5551–5560.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nevedomskaya E, Baumgart SJ and Haendler
B: Recent advances in prostate cancer treatment and drug discovery.
Int J Mol Sci. 19:E13592018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenberger M: Research in drug
development for advanced prostate cancer. Clin Adv Hematol Oncol.
16:42–44. 2018.PubMed/NCBI
|
|
5
|
González Á, García de Durango C, Alonso V,
Bravo B, Rodríguez de Gortázar A, Wells A, Forteza J and
Vidal-Vanaclocha F: Distinct osteomimetic response of
androgen-dependent and independent human prostate cancer cells to
mechanical action of fluid flow: Prometastatic implications.
Prostate. 77:321–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams RM, Hajiran CJ, Nayeem S and
Sooter LJ: Identification of an antibody fragment specific for
androgen-dependent prostate cancer cells. BMC Biotechnol.
14:812014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang C, Li P, Wen Y, Feng G, Liu Y, Zhang
Y, Xu Y and Zhang Z: The promotion on cell growth of
androgen-dependent prostate cancer by antimony via mimicking
androgen activity. Toxicol Lett. 288:136–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K, Li Y, Zhou D, Fan Y, Guo H, Ma T,
Wen J, Liu D and Zhao L: Synthesis and biological evaluation of
quinoline derivatives as potential anti-prostate cancer agents and
Pim-1 kinase inhibitors. Bioorg Med Chem. 24:1889–1897. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JE, Son JE, Jeong H, Joon Kim D, Seo
SK, Lee E, Lim TG, Kim JR, Chen H, Bode AM, et al: A novel
cinnamon-related natural product with Pim-1 inhibitory activity
inhibits leukemia and skin cancer. Cancer Res. 75:2716–2728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Li G, Li B, Song H, Shang Z, Jiang
N and Niu Y: Androgen deprivation therapy has no effect on Pim-1
expression in a mouse model of prostate cancer. Oncol Lett.
13:4364–4370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamura RE, de Luna IV, Lana MG and Strauss
BE: Improving adenoviral vectors and strategies for prostate cancer
gene therapy. Clinics (Sao Paulo). 73 (Suppl 1):e476s2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grozescu T and Popa F: Immunotherapy and
gene therapy in prostate cancer treatment. J Med Life. 10:54–55.
2017.PubMed/NCBI
|
|
13
|
Souza AG, Bastos VAF, Silva IBB, Marangoni
K and Goulart VA: Different gene therapy strategies: A overview for
prostate cancer. Curr Gene Ther. 16:287–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai Z, Lv H, Cao W, Zhou C, Liu Q, Li H
and Zhou F: Targeting strategies of adenovirusmediated gene therapy
and virotherapy for prostate cancer (Review). Mol Med Rep.
16:6443–6458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abel SDA and Baird SK: Honey is cytotoxic
towards prostate cancer cells but interacts with the MTT reagent:
Considerations for the choice of cell viability assay. Food Chem.
241:70–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the MTT Assay. Cold Spring Harb
Protoc 2018. 2018. View Article : Google Scholar
|
|
18
|
Ma S, Tan W, Du B, Liu W, Li W, Che D and
Zhang G: Oridonin effectively reverses cisplatin drug resistance in
human ovarian cancer cells via induction of cell apoptosis and
inhibition of matrix metalloproteinase expression. Mol Med Rep.
13:3342–3348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tian J, Liu R and Qu Q: Role of
endoplasmic reticulum stress on cisplatin resistance in ovarian
carcinoma. Oncol Lett. 13:1437–1443. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Swellmeen L, Shahin R, Al-Hiari Y, Alamiri
A, Hasan A and Shaheen O: Structure based drug design of Pim-1
kinase followed by pharmacophore guided synthesis of
quinolone-based inhibitors. Bioorg Med Chem. 25:4855–4875. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Zhang Q, Wuu YR, Zou S and Hei TK:
Cytoplasmic irradiation induces metabolic shift in human small
airway epithelial cells via activation of Pim-1 kinase. Radiat Res.
187:441–453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Etheridge T, Liou J, Downs TM, Abel EJ,
Richards KA and Jarrard DF: The impact of celecoxib on outcomes in
advanced prostate cancer patients undergoing androgen deprivation
therapy. Am J Clin Exp Urol. 6:123–132. 2018.PubMed/NCBI
|
|
23
|
Thamilselvan V, Menon M, Stein GS,
Valeriote F and Thamilselvan S: Combination of carmustine and
selenite inhibits EGFR mediated growth signaling in
androgen-independent prostate cancer cells. J Cell Biochem.
118:4331–4340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha S, Shin DH, Seok JR and Myung JK:
Differential proteome expression analysis of androgen-dependent and
-independent pathways in LNCaP prostate cancer cells. Exp Cell Res.
359:215–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu J, Xiong G, Cao Z, Huang H, Wang T, You
L, Zhou L, Zheng L, Hu Y, Zhang T and Zhao Y: PIM-1 contributes to
the malignancy of pancreatic cancer and displays diagnostic and
prognostic value. J Exp Clin Cancer Res. 35:1332016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Z, Liu H, Yuan X, Wang Y, Li L, Wang
G, Song J, Shao Z and Fu R: Downregulation of Pim-2 induces cell
cycle arrest in the G0/G1 phase via the p53-non-dependent p21
signaling pathway. Oncol Lett. 15:4079–4086. 2018.PubMed/NCBI
|
|
27
|
Tursynbay Y, Zhang J, Li Z, Tokay T,
Zhumadilov Z, Wu D and Xie Y: Pim-1 kinase as cancer drug target:
An update. Biomed Rep. 4:140–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ouhtit A, Muzumdar S, Gupta I,
Shanmuganathan S and Tamimi Y: Understanding the functional
discrepancy of Pim-1 in cancer. Front Biosci (Elite Ed). 7:208–214.
2015.PubMed/NCBI
|
|
29
|
Jiang W, Chen Y, Song X, Shao Y, Ning Z
and Gu W: Pim-1 inhibitor SMI-4a suppresses tumor growth in
non-small cell lung cancer via PI3K/AKT/mTOR pathway. OncoTargets
Ther. 12:3043–3050. 2019. View Article : Google Scholar
|
|
30
|
Ma X, Hu M, Wang H and Li J: Discovery of
traditional Chinese medicine monomers and their synthetic
intermediates, analogs or derivatives for battling P-gp-mediated
multi-drug resistance. Eur J Med Chem. 159:381–392. 2018.
View Article : Google Scholar : PubMed/NCBI
|