Introduction
Thyroid cancer incidence is rapidly increasing in
the USA. In 2017, an estimated annual diagnosis rate of 56 870
people and an annual mortality rate of 2 010 thyroid cancer cases
were reported (1). Thyroid cancers
are typically classified as papillary, follicular and anaplastic
carcinomas. Anaplastic thyroid cancer (ATC) accounts for 1 to 2% of
all thyroid tumours (2). ATC is
characterized by aggressive, local invasion and common distant
metastases. Available therapies for ATCs include chemotherapy,
radiotherapy and surgery. However, no effective target treatment is
available. ATC is still one of most fatal types of cancer, with a
mean survival time of 6 months after diagnosis (3). Therefore, improved understanding of the
molecular mechanisms underlying ATC carcinogenesis and progression
will contribute to find novel diagnosis markers and therapeutic
targets.
MicroRNA (miRNA) is a group of small noncoding RNAs
that regulate gene expression by translation repression or
messenger RNA (mRNA) degradation by binding to the 3′-untranslated
region (3′-UTR) of the target Mrna (4). Increasing evidence indicate that miRNAs
are involved in the regulation of cell survival, proliferation and
migration through mediating the expression of their target genes.
Recent studies on microRNA in thyroid tumours have provided new
insights for the development of novel biomarkers that can be used
to diagnose thyroid cancer and optimize its management (5–8). To
date, however, our understanding of how miRNAs affect ATC
development and progression remains unclear.
In the present study, we identified T-cell
intracellular antigen (TIA1) as a direct target gene of miR-599 in
ATC. Moreover, we provided evidence that miR-599 can promote ATC
cell proliferation and metastasis in vitro and accelerate
tumour growth in vivo by targeting TIA1.
Materials and methods
Cell culture and tissue
collection
The ATC cell lines (SW1736 and KAT-18) and human
immortalized follicular cell line (Nthy-ori3-1) were obtained from
the ATCC (Manassas, VA, USA). The cell lines were authenticated via
short-tandem repeat profiling performing by BMR Genomics. The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM, HyClone,
Beijing, China) supplemented with 10% foetal bovine serum (FBS)
(HyClone, Gaithersburg, Maryland, USA). All the cells were
incubated at 37°C in a humidified atmosphere with 5%
CO2. Human ATC specimens and adjacent normal thyroid
tissues (10 pairs) were obtained from patients who underwent
surgery according to an approved human protocol at the WeiHaiWei
people' hospital. All of the patient materials were obtained with
written informed consent.
Cell transfection
A lentiviral Phblv-u6-puro vector was purchased from
GenePharma (Shanghai, China). Lentiviruses carrying miR-599 or
miR-NC were packaged following the manufacturer's instructions. The
sequences were as follows: miR-599 mimic,
5′-CUGUCCACAGUGUGUUUGAUAAG-3′; miR-NC 5′-ACUACUGAGUGACAGUAGA-3′.
KAT-18 cells were grown in 6-well plates until they reached 50%
confluency. The medium was replaced with 1 ml of fresh culture
medium supplemented with 100 µl viral supernatant (1×108
UT/ml) and 8 µg/ml Polybrene for 24 h. The KAT-18 cells were
further cultured in medium containing puromycin at 3 µg/ml.
Individual puromycin-resistant colonies were isolated during drug
screening. Mammalian TIA1 expression plasmids were purchased from
Genescript (Nanjing, China). An empty plasmid served as a negative
control (control plasmid). SiRNAs designed to specifically silence
TIA1 were purchased from GenePharma (Shanghai, China). A scrambled
siRNA served as a control. The siRNA sequences were as follows:
si-TIA1: TGCACAACAAATTGGCCAGTA. Transient transfection was carried
out by using Lipofectamine 2000 Transfection Reagent (Invitrogen,
CA, USA) according to the manufacturer's instructions.
Cell viability assay
Cell viability was detected via CCK-8 assay
(Beyotime Institute of Biotechnology). The cells were trypsinized
and seeded at 3,000 cells/well in a 96-well plate. After culturing
for indicated time (0, 24, 48 and 72 h), 10 µl of the CCK-8 was
added into each well. The resulting mixtures were incubated at 37°C
for 3 h. Then, the absorbance of each well was examined by using a
Multi-skan MK3 spectrophotometer set at a wavelength of 450 nm.
Colony formation assay
KAT-18 cells transfected with miR-599 or miR-NC or
siTIA1 were seeded at 400 cells in six-well plates and cultured for
approximately 10 d until colony formation was observed. Colonies
were fixed with methanol and stained with 0.5% crystal violet
(Sigma, USA). The colonies were photographed, and scored.
Analysis of apoptosis
The fraction of apoptotic cells was detected via
Annexin V and propidium iodide (PI) staining method according to
the manufacturer's protocol (KeyGEN BioTHCH, Nanjing, Jiangsu,
China). Apoptotic cells were analyzed by using a Flow cytometer
(Beckman, CA, USA). The results are presented as the percentage of
apoptotic cells relative to the total number of cells.
Cell migration and cell invasion
assays
Cell migration and invasion ability of KAT-18 were
analyzed by polycarbonate membrane transwell inserts (BD
Biosciences, Bedford, MA, USA). Briefly, the upper sides of the
filters were coated with 50 µl Matrigel solution (matrigel:
DMEM=1:8) for invasion. Cells were harvested at 48 h post
transfection, and 1×104 cells with 200 µl of serum-free
medium were seeded in the upper chamber. The lower chamber was
filled with medium supplemented with 5% FBS (invasion) or not
(migration). Following incubation for 8 h (migration) or 12 h
(invasion) at 37°C with 5% CO2, cells on the lower
filter were fixed with methanol, stained with crystal violet, and
then counted under a light microscope (CKX41; Olympus
Corporation).
Real-time quantitative PCR (qPCR)
Trizol (Invitrogen Life Technologies, Carlsbad, CA,
USA) were implemented for extracting the total RNA from clinical
tissues and ATC cells. Transcriptional First Strand cDNA Synthesis
Kit was employed for reverse transcription reactions, and SYBR
Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) was
utilized to operate qRT-PCR experiment. The primers used for
amplification were: TIA1 forward 5′-TCCCGCTCCAAAGAGTACATATGAG-3′,
and reverse 5′-AAACAATTGCATGTGCTGCACTTTC-3′; miR-599 forward
5′-GUUGUGUCAGUUUAUCAAAC-3′, and reverse 5′-GUUGUGUCAGUUUAUCAAAC-3′;
U6 forward 5′-TGCGGGTGCTCGCTTCGCAGC-3′, and reverse
5′-CCAGTGCAGGGTCCGAGGT-3′; β-actin forward
5′-GATCATTGCTCCTCCTGAGC-3′, and reverse
5′-ACTCCTGCTTGCTGATCCAC-3′.
Western blot assay
The method used for Western blot analysis has been
described in our previous study (9).
The primary antibodies were TIA1 antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA), TGFβ2 (Abcam, Cambridge, MA,
USA) and β-actin antibody (Beyotime, Nantong, China).
In vivo tumorgenesis assay
All experiments involving mice were performed in The
Model Animal Research Center of Weifang medical University. Animal
experiments were approved by the Animal Management Rule of the
Chinese Ministry of Health and were performed in accordance with
the approved guidelines and experimental protocols of Weifang
medical University (Weifang, China). Female nude mice (4-week-old)
were obtained from Jilin Laboratory Animal Center (Changchun,
China), and bred in special pathogen-free (SPF) condition.
1×106 KAT-18 cells transfected with lentiviral miR-599
mimic or lentiviral control were suspended in 100 µl of serum-free
medium and subcutaneously injected into the back of nude mice.
Xenograft volume (V) was monitored by measuring the length (L) and
width (W) with calipers and was calculated as V=0.5 × L (length) ×
W2 (width). The tumor tissues were dissected, weighted,
and stored at −80°C until use.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
software. All experiments were performed in triplicate. Unless
otherwise indicated, the data were evaluated as mean ± SD (standard
deviation). Differences between two groups were assessed using
Student's t-test (two-tailed). Data of more than two groups were
analyzed using one way ANOVA with post hoc test by Tukey's test.
Correlations between TIA1 and miR-599 were analyzed using Spearman
rank correlation. P<0.05 was considered to indicate a
statistically significant difference.
Results
MiR-599 is downregulated in ATC
tissues and cell lines
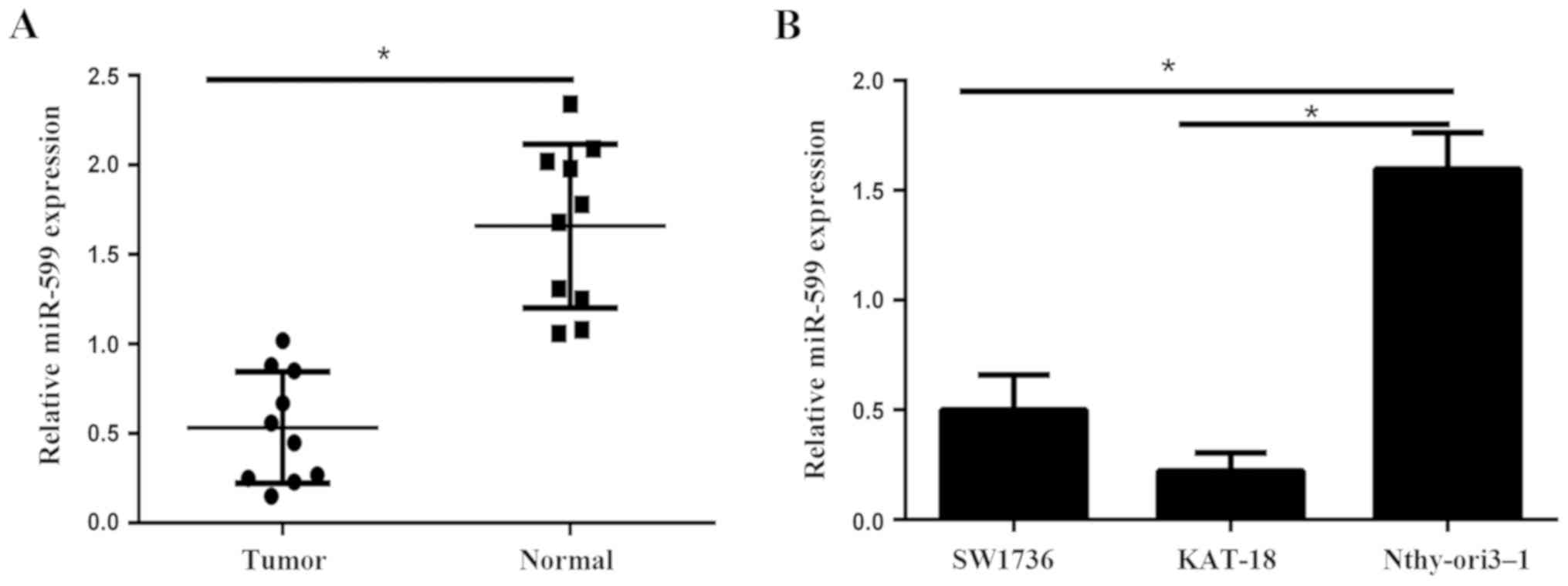
In the present study, we firstly evaluated the
expression level of miR-599 in 10 cases of human ATC tissues and
their matched adjacent non-tumor tissues via qPCR. As indicated in
Fig. 1A, miR-599 was markedly lower
in ATC tissues compared to that in adjacent non-tumor tissues.
Furthermore, qPCR results showed that miR-599 expression were also
downregulated in ATC cell lines (SW1736 and KAT-18) compared to
human immortalized follicular cell line Nthy-ori3-1 (Fig. 1B). The lower expression of miR-599
was detected in the KAT-18 cells. Then KAT-18 cell line was
selected for further studies because of the lower expression levels
of miR-599.
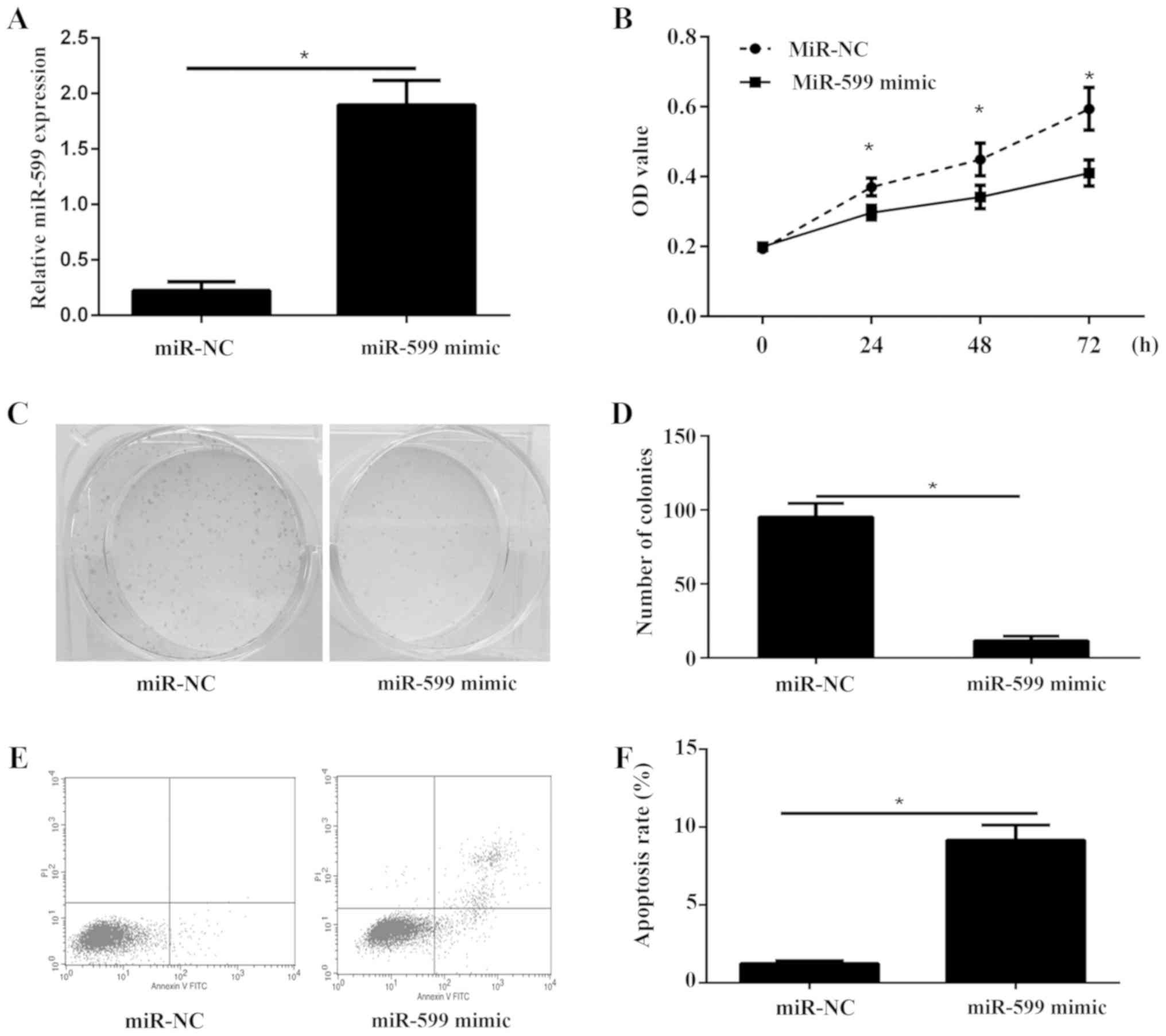
MiR-599 reduces cell viability,
proliferation, induces cell apoptosis in ATC cell line
To examine the biological role of miR-599 in ATC,
KAT-18 cells were transfected with miR-599 mimic or miR-NC. The
data showed that miR-599 was significantly increased in cells
transfected with miR-599 mimic compared to cells transfected with
miR-NC by using qPCR (Fig. 2A). Cell
viability of KAT-18 was detected via CCK-8 assay. The results
showed that overexpression of miR-599 in KAT-18 cells significantly
inhibited cell viability (Fig. 2B).
Furthermore, the colony formation assay illustrated that the
proliferation of miR-599 overexpression cells was severely
inhibited (Fig. 2C and D). In
addition, cell apoptosis was investigated in KAT-18 cells
transfected with miR-599 mimic or miR-NC. As showed in Fig. 2E and F, overexpression of miR-599
could significantly increase cell apoptosis ratio in KAT-18 cell
line.
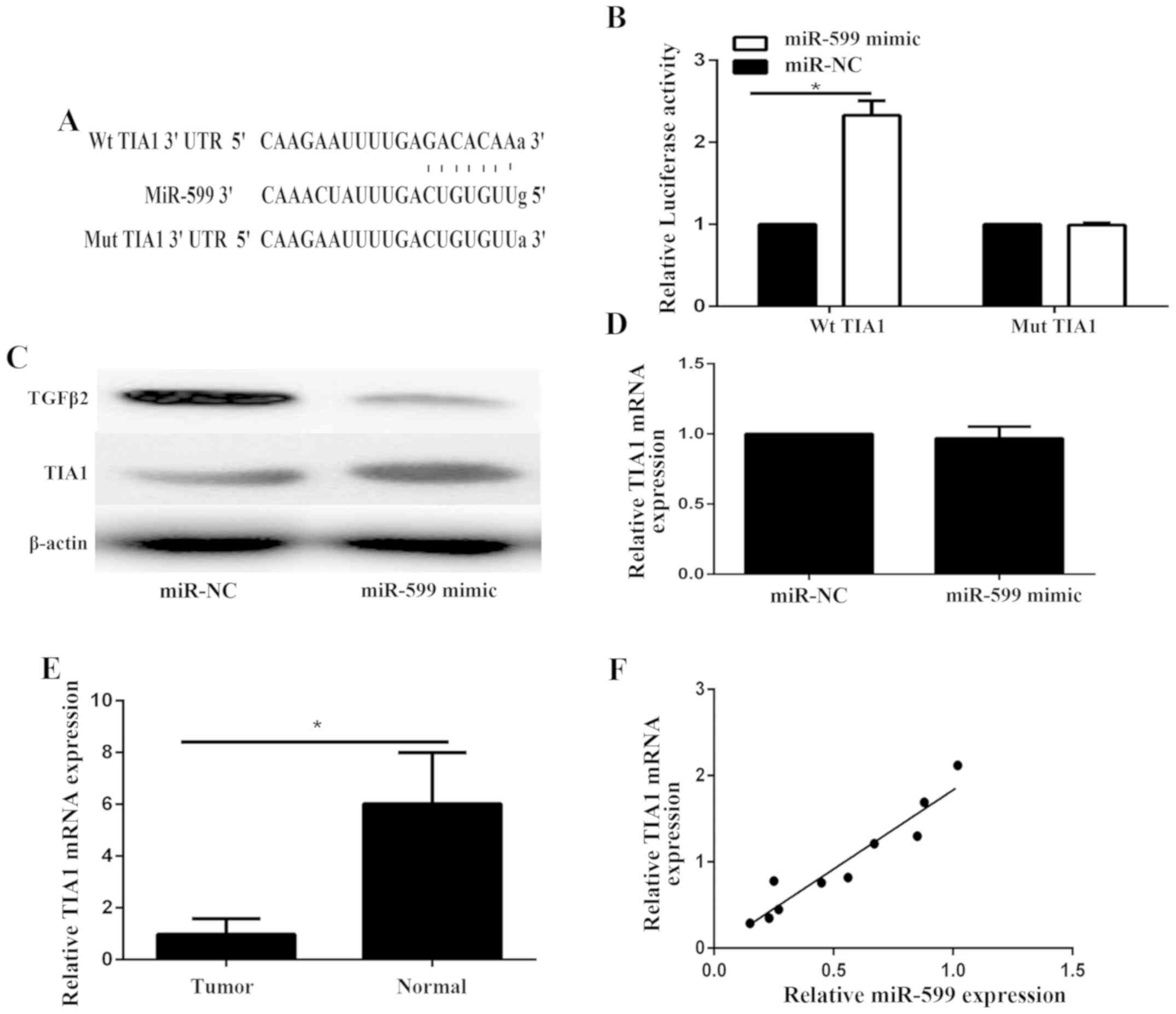
TIA1 is a direct target of
miR-599
TargetScan was used to identify the target of
miR-599. TIA1 was predicted to be a target gene of miR-599
(Fig. 3A). The WT-TIA1-3′-UTR
(Fig. 3A) or MT-TIA1-3′-UTR
(Fig. 3A) luciferase reporter vector
was generated. The luciferase reporter assay was performed to
confirm the relationship of miR-599 and TIA1 in KAT-18 cells. The
cells were co-transfected with miR-599 and either wild type or
mutated TIA1-3′-UTR reporter. As shown in Fig. 3B, the luciferase activity was
markedly enhanced only in KAT-18 cells co-transfected with miR-599
mimics and WT-TIA1-3′-UTR vector, illustrating that miR-599 could
directly bind to the 3′-UTR of TIA1 mRNA in ATC cells. Furthermore,
we examined the expression of TIA1 in KAT-18. Western blot assay
showed that the protein level of TIA1 was markedly enhanced in
miR-599-overexpressing KAT-18 cells compared to the control group
(Fig. 3C). However, the mRNA
expression of KAT-18 was not affected after overexpression of
miR-599 (Fig. 3D). Therefore,
miR-599 inhibited the expression of its target TIA1 at the
post-transcriptional level. Furthermore, we found that the mRNA
expression of TIA1 in ATC was significantly downregulated compared
with adjacent noncancerous tissues (Fig.
3E) and was positively correlated with miR-599 in ATC tissues
(Fig. 3F). To exclude the off-target
effect of miR-599 on TIA1, western blot was used to examine the
expression level of TGFβ2, which has been identified as a direct
target gene of miR-599 (10). The
results showed that the expression of TGFβ2 was significantly
decreased in KAT-18 transfected with miR-599 mimic (Fig. 3C).
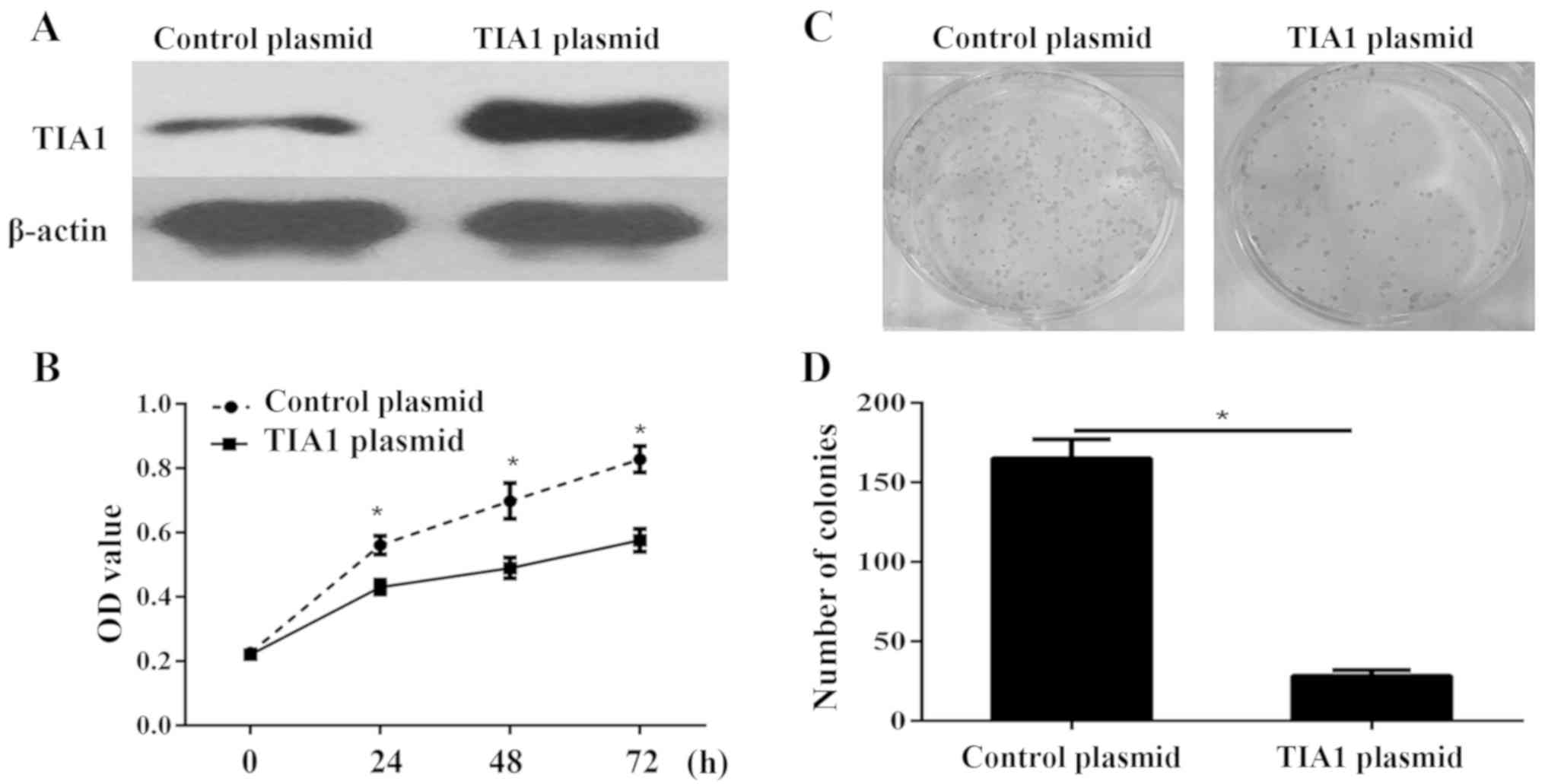
TIA1 overexpression inhibits ATC cell viability,
proliferation, migration and invasion in vitro.
To further determine whether TIA1 plays a critical
role in cell proliferation, we performed in vitro
gain-of-function analyses by overexpressing TIA1 with a plasmid in
KAT-18 cells. The western blot results showed that TIA1 was
significantly overexpressed in KAT-18 cells (Fig. 4A). Cell viability of KAT-18 was
detected via CCK-8 assay. The results showed that overexpression of
TIA1 in KAT-18 cells significantly inhibited cell viability
(Fig. 4B). Furthermore, the colony
formation assay illustrated that the proliferation of TIA1
overexpression cells was severely inhibited (Fig. 4C and D). In addition, the transwell
migration and invasion assay showed that the TIA1 silencing
significantly promoted the migration and invasion of KAT-18 cell
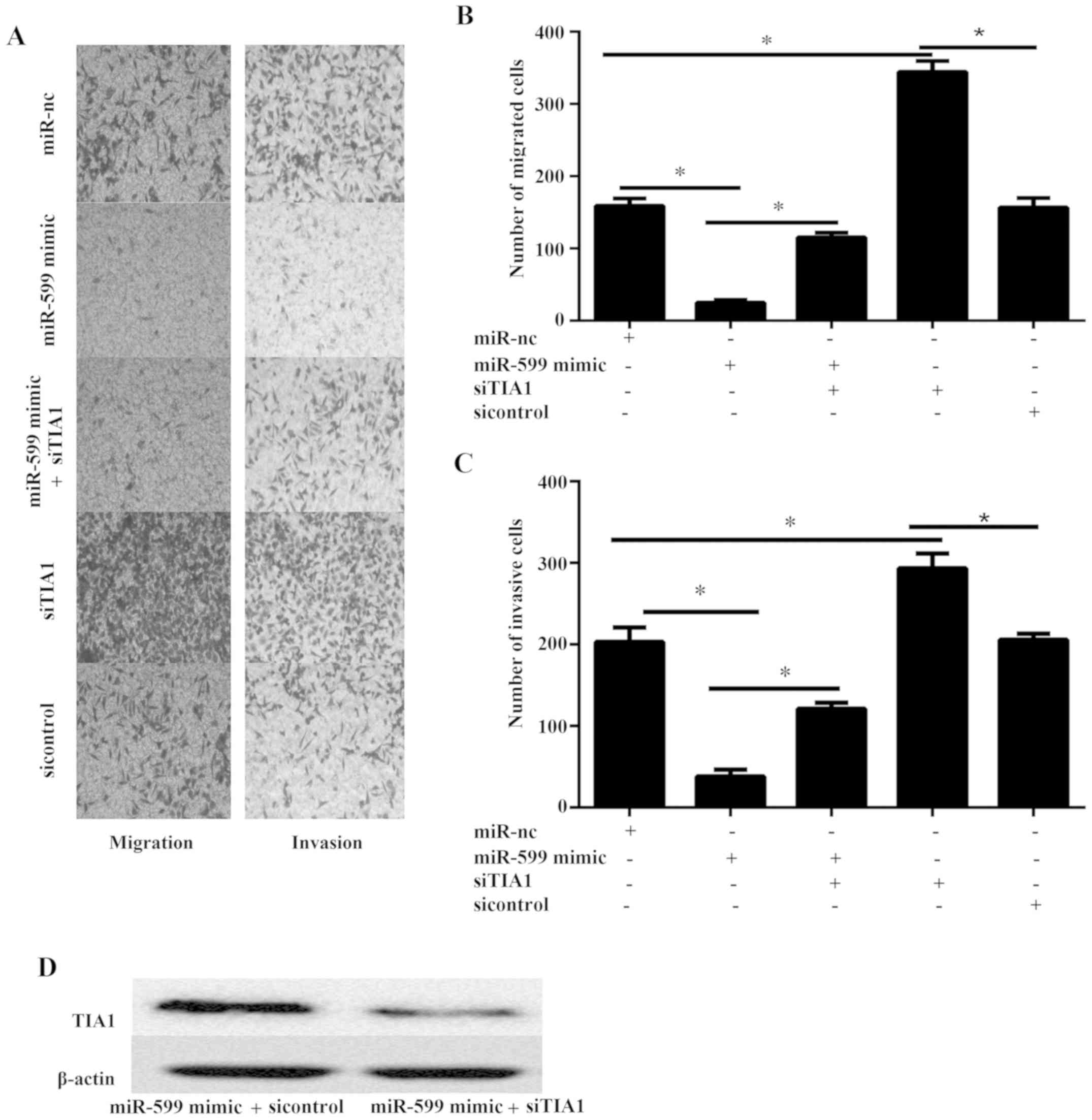
(Fig. 5A-C).
MiR-599 inhibits cell migration and invasion via
TIA1 in KAT-18 cell line.
The migration and invasion of cell plays an
important role in tumor metastasis. Thus, the effect of miR-599
overexpression on the migration and invasion of ATC cells was
examined in vitro. The ability of migration and invasion was
examined via transwell migration and invasion assay. The data
illustrated that miR-599 mimic markedly inhibited the cell
migration and invasion (Fig.
5A-C).
To discover the functional correlation of TIA1
targeting by miR-599 in ATC cell, we assessed whether TIA1
silencing reversed miR-599 biologic function of cell migration and
invasion. KAT-18 cells were co-transfected with miR-599 mimic or
miR-NC and siTIA1 or sicontrol. The western blot results
illustrated that the protein level of TIA1 was markedly
downregulated in miR-599 TIA1 mimic combination with siTIA1
compared to miR-599 combination with sicontrol (Fig. 5D). Furthermore, the results showed
that siTIA1 could partially abrogated effect of miR-599 mimic on
cell migration and invasion (Fig.
5A-C), suggesting that miR-599 suppressed human ATC cell
migration and invasion via the activation of TIA1.
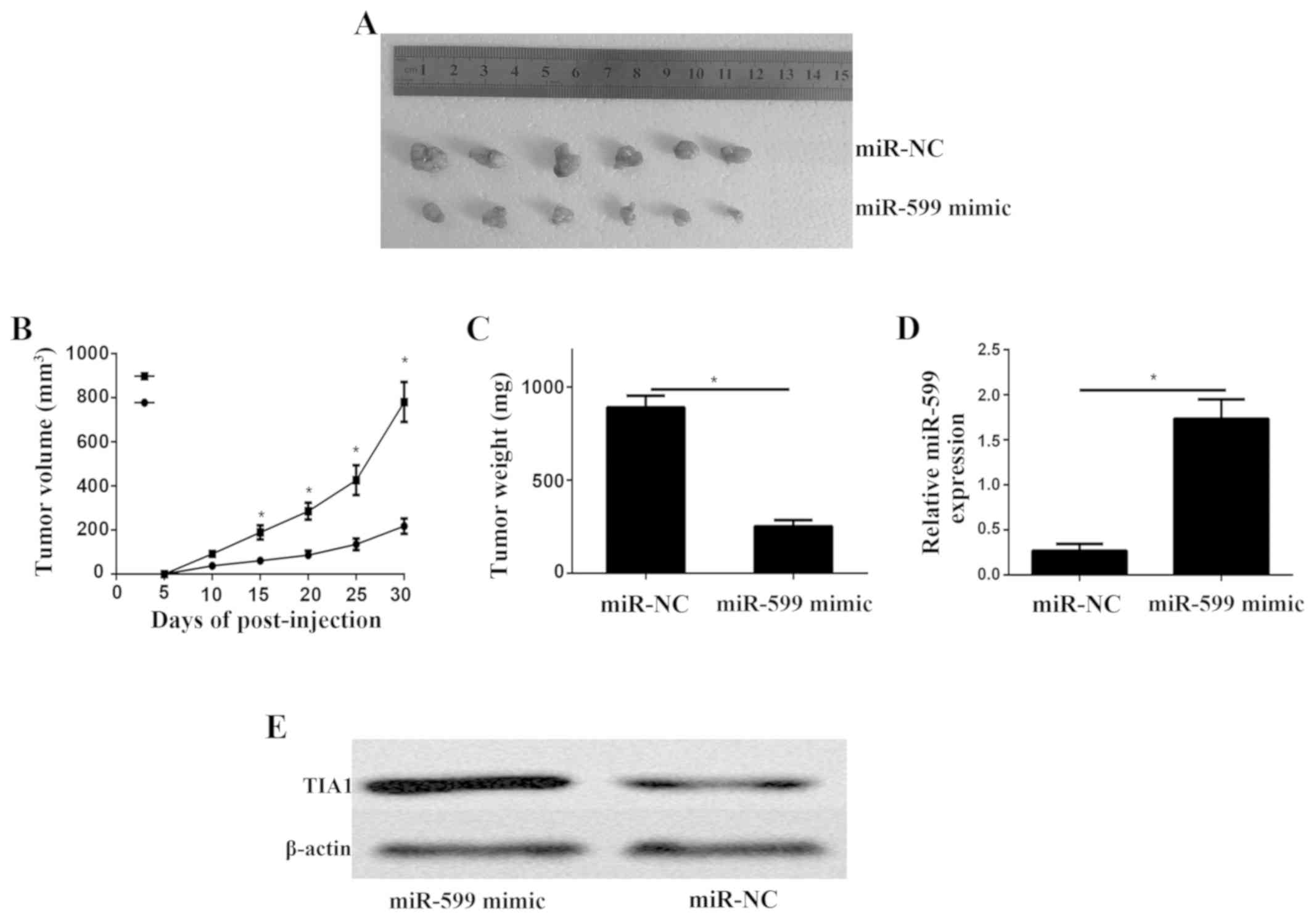
MiR-599 promotes ATC growth in vivo by
targeting TIA1
At last, we showed the function of miR-599 in the
growth of ATC xenograft. Nude mice were subcutaneously implanted
with KAT-18 cells transfected with lentiviral miR-599 and
lentiviral control. On day 30 after implantation, all mice were
sacrificed and obtained the tumor xenograft (Fig. 6A). Moreover, the growth rate
(Fig. 6B) and weight (Fig. 6C) of the tumor xenograft were
significantly decreased in the miR-599-overexpressing group when
compared to the control group. qPCR analysis of miR-599 expression
levels in the miR-599 mimics tumor xenograft mice and control tumor
xenograft mice (Fig. 6D). The
western blot results showed that the protein level of TIA1 was
higher in the tumor transfected miR-599 mimic (Fig. 6E).
Discussion
A comprehensive description of the molecular
mechanisms underlying ATC initiation and progression will
facilitate the novel biomarker identification for early ATC
diagnosis and therapy, thereby improving the outcome of patients
with ATC. Over the past decade, miRNAs have emerged as a new class
of gene regulators involved in a variety of cancers (11). The present study demonstrated for the
first time that miR-599 inhibits the tumour suppressor gene TIA1 to
promote cancer proliferation in ATC. This study also firstly showed
TIA1's function in ATC. Thus, miR-599/TIA1 axis can be used for
early diagnosis and it can also be used as a potential target to
treat ATC.
Previous studies have suggested the role of miR-599
as a potential anti-onco-miRNA in various types of cancers. A
previous study has shown that miR-599 is frequently downregulated
in human gastric cancer, and low miR-599 expression is associated
with lymph node metastasis. In addition, miR-599 overexpression
suppresses gastric cancer cell migration and invasion and the
epithelial–mesenchymal transition process dramatically (11). Furthermore, miR-599 may act as a
tumour suppressor, playing an important role in regulating lung
cancer, glioma, hepatocellular carcinoma and breast cancer
metastasis (12–15). However, the function and regulatory
mechanisms of miR-599 in ATC remains obscure. Here, miR-599
expression was significantly downregulated in human ATC tissues and
cell lines compared with the adjacent normal tissues and cell
lines. Moreover, miR-599 overexpression significantly inhibited the
ATC cell viability, colony formation, migration and invasion in
vitro. MiR-599 overexpression also suppressed ATC tumour growth
in vivo. These data suggested that miR-599 may function as a
tumour suppressor in ATC.
One of our new findings in the current study
indicated that TIA1 is the target gene of miR-599 during the ATC
progression inhibition. TIA1 is a RNA binding protein, which is
linked to multiple biologic processes associated with RNA
metabolism both in the nucleus and in the cytoplasm (16). A recent study has shown that TIA1
serves as a tumour suppressor gene. In addition, TIA1 regulates or
interacts with many types of mRNA involved in cancer cell
proliferation, apoptosis, angiogenesis, invasiveness and metastasis
in many types of cancer (17–20).
However, a subsequent study has shown that TIA1 may be a novel
oncogenic function of TIA1 in oesophageal squamous cell carcinoma
(21). Therefore, whether or not
TIA1 is a tumour promoter or suppressor remains controversial.
These contradictory results reveal that the function of TIA1 in
different tissues depends on the cancer type and tumorigenesis
mechanism. Therefore, future research must demonstrate the
conclusive function of TIA1 in different types of cancer. The
present study demonstrated that TIA1 may be a tumour-suppressor
gene, which inhibits ATC cell viability, proliferation and
metastasis. Furthermore, because of the myriad of TIA1
tumour-suppressor functions, we elucidated the mechanisms
underlying TIA1 regulation during tumorigenesis in ATC. In this
study, we firstly demonstrated that TIA1 was regulated by miR-599
in ATC. Furthermore, we demonstrated that TIA1 is a direct target
gene of miR-599 via luciferase assay in ATC. However, miRNAs have
unique ability to regulate many protein-coding genes. A single
miRNA can target a number of genes, thereby regulating numerous
biological processes. Therefore, future studies need to identify
the new molecular pathways regulated by the tumour-suppressive
miR-599.
In conclusion, miR-599 is significantly
downregulated in the ATC clinical specimens. MiR-599 also functions
as a tumour suppressor in ATC through the regulation of TIA1
expression. Identification of the tumour-suppressive and
miRNA-mediated cancer pathways in human ATC can provide new
information on the potential therapeutic targets to treat ATC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JWB and WBC conceived and designed the experiments.
WBC wrote and revised the manuscript. YLZ and JTQ conducted all
experiments. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Weihaiwei People's Hospital (Weihai, China) and
written informed consent was obtained from all patients prior to
their inclusion within the study.
Patient consent for publication
All patients provided written informed consent for
the publication of their data and any accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagaiah G, Hossain A, Mooney CJ,
Parmentier J and Remick SC: Anaplastic thyroid cancer: A review of
epidemiology, pathogenesis, and treatment. J Oncol 2011.
5423582011.
|
|
3
|
O'Neill JP and Shaha AR: Anaplastic
thyroid cancer. Oral Oncol. 49:702–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fuziwara CS and Kimura ET: MicroRNAs in
thyroid development, function and tumorigenesis. Mol Cell
Endocrinol. 456:44–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosignolo F, Memeo L, Monzani F, Colarossi
C, Pecce V, Verrienti A, Durante C, Grani G, Lamartina L, Forte S,
et al: MicroRNA-based molecular classification of papillary thyroid
carcinoma. Int J Oncol. 50:1767–1777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boufraqech M, Klubo-Gwiezdzinska J and
Kebebew E: MicroRNAs in the thyroid. Best Pract Res Clin Endocrinol
Metab. 30:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Celano M, Rosignolo F, Maggisano V, Pecce
V, Iannone M, Russo D and Bulotta S: MicroRNAs as biomarkers in
thyroid carcinoma. Int J Genomics 2017. 64965702017.
|
|
9
|
Wang X, Jin Y, Zhang H, Huang X, Zhang Y
and Zhu J: MicroRNA-599 inhibits metastasis and
epithelial-mesenchymal transition via targeting EIF5A2 in gastric
cancer. Biomed Pharmacother. 97:473–480. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie B, Zhang C, Kang K and Jiang S:
miR-599 inhibits vascular smooth muscle cells proliferation and
migration by targeting TGFB2. PLoS One. 10:e01415122015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nelson KM and Weiss GJ: MicroRNAs and
cancer: Past, present, and potential future. Mol Cancer Ther.
7:3655–3660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Sui Y, Zhu Q and Sui X:
Hsa-miR-599 suppresses the migration and invasion by targeting BRD4
in breast cancer. Oncol Lett. 14:3455–3462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang T, Ma G, Zhang Y, Huo H and Zhao Y:
miR-599 inhibits proliferation and invasion of glioma by targeting
periostin. Biotechnol Lett. 39:1325–1333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian W, Wang G, Liu Y, Huang Z, Zhang C,
Ning K, Yu C, Shen Y, Wang M, Li Y, et al: The miR-599 promotes
non-small cell lung cancer cell invasion via SATB2. Biochem Biophys
Res Commun. 485:35–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tian J, Hu X, Gao W, Zhang J, Chen M,
Zhang X, Ma J and Yuan H: Identification a novel tumor-suppressive
hsa-miR-599 regulates cells proliferation, migration and invasion
by targeting oncogenic MYC in hepatocellular carcinoma. Am J Transl
Res. 8:2575–2584. 2016.PubMed/NCBI
|
|
16
|
Sánchez-Jiménez C and Izquierdo JM: T-cell
intracellular antigens in health and disease. Cell Cycle.
14:2033–2043. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reyes R, Alcalde J and Izquierdo JM:
Depletion of T-cell intracellular antigen proteins promotes cell
proliferation. Genome Biol. 10:R872009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Izquierdo JM, Alcalde J, Carrascoso I,
Reyes R and Ludeña MD: Knockdown of T-cell intracellular antigens
triggers cell proliferation, invasion and tumour growth. Biochem J.
435:337–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hamdollah Zadeh MA, Amin EM,
Hoareau-Aveilla C, Domingo E, Symonds KE, Ye X, Heesom KJ, Salmon
A, D'Silva O, Betteridge KB, et al: Alternative splicing of TIA-1
in human colon cancer regulates VEGF isoform expression,
angiogenesis, tumour growth and bevacizumab resistance. Mol Oncol.
9:167–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Liu R, Yang F, Cheng R, Chen X, Cui
S, Gu Y, Sun W, You C, Liu Z, et al: miR-19a promotes colorectal
cancer proliferation and migration by targeting TIA1. Mol Cancer.
16:532017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hamada J, Shoda K, Masuda K, Fujita Y,
Naruto T, Kohmoto T, Miyakami Y, Watanabe M, Kudo Y, Fujiwara H, et
al: Tumor-promoting function and prognostic significance of the
RNA-binding protein T-cell intracellular antigen-1 in esophageal
squamous cell carcinoma. Oncotarget. 7:17111–17128. 2016.
View Article : Google Scholar : PubMed/NCBI
|