Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver cancer. With an age-adjusted incidence of
10.1 cases per 100,000 persons per year, HCC is ranked as the sixth
most common neoplasm and the third leading cause of
cancer-associated death worldwide (1). In China, HCC is ranked as the fourth
most common malignant tumor and the third most deadly disease
(2,3). HCC frequently occurs in patients with
chronic liver diseases, such as cirrhosis, or following infection
of hepatitis B or C virus. Additionally, non-alcoholic fatty liver
disease (NAFLD) has been considered another important risk factor
for HCC. The association between metabolic syndrome, diabetes,
obesity and HCC in NAFLD patients has been supported by
retrospective studies (4–7).
Currently, the main therapeutic strategies for HCC
include surgery (tumor resection or liver transplantation),
radiation therapy and targeted drug therapy. Sorafenib, an oral
multi-kinase inhibitor, was the first drug that was approved for
first-line treatment of advanced HCC. Notably, HCC-associated
mortality can be prevented by avoiding certain risk factors, e.g.
hepatitis B virus vaccination of infants (8) and the prevention of metabolic syndrome
through the use of metformin in patients with diabetes or
propranolol in patients with hepatitis C virus-related cirrhosis
(1,9). These methods could prevent first
primary and second primary recurrence, which is critical to improve
the prognosis (10).
In addition to chemically synthesized agents,
certain natural products, including coffee, vitamin E and fish oil,
as well as phytochemicals, might also reduce the risk of HCC
(10). Matrine is an active alkaloid
extracted from the dry roots of Sophora flavescens, also
known as ‘Ku-Shen’ in traditional Chinese medicine, which has been
used for treatment of various diseases, including HCC and liver
fibrosis (11,12). Matrine has proven effective in
inhibiting HCC proliferation and migration in vitro,
specifically in SMMC-7721 cells (13), Huh-7 cells (14) and HepG2 cells (15) and by inhibiting HCC tumor growth
in vivo (15,16). The anti-tumor effect of matrine has
also been verified in squamous cell carcinomas. Antitumor B, a
Chinese herbal mixture of six plants containing matrine, has been
demonstrated to inhibit the development of
4-nitroquinoline-1-oxide-induced oral squamous cell carcinomas in
mice (17) and lung squamous cell
carcinomas in a mouse model (18).
However, the majority of these studies emphasize the effect of
matrine on an established tumor. The preventive effect of matrine
on the formation of HCC tumors remains to be elucidated.
The Notch signaling pathway is a conserved and
complex signaling pathway. Its key components include Notch
receptor (notch1-4), Notch ligand (delta-like 1, 3, 4, Jagged1 and
Jagged2), and target genes Hes and Hey. The Notch signaling pathway
has a key role in regulating the development of embryos and organs
and controlling cell-fate, including cell proliferation,
differentiation, apoptosis, regeneration and other cellular
activities (19). Notch deregulation
has been indicated to be involved in a number of pathological
processes in the liver, including HCC and HCC-accompanied liver
fibrosis (20). Notably, matrine
inhibits the infiltration of inflammatory monocytes and fibrosis in
CCl4-injured livers (21). The
inhibitory effect on liver fibrosis was also reported for matrine
derivative MD-1 (11). In addition,
previous findings revealed that matrine induces hepatic
differentiation of hepatic progenitor cells, likely by inhibiting
the Notch signaling pathway (22).
Therefore, it is important to determine if matrine could prevent
the development of primary HCC by suppressing the Notch signaling
pathway.
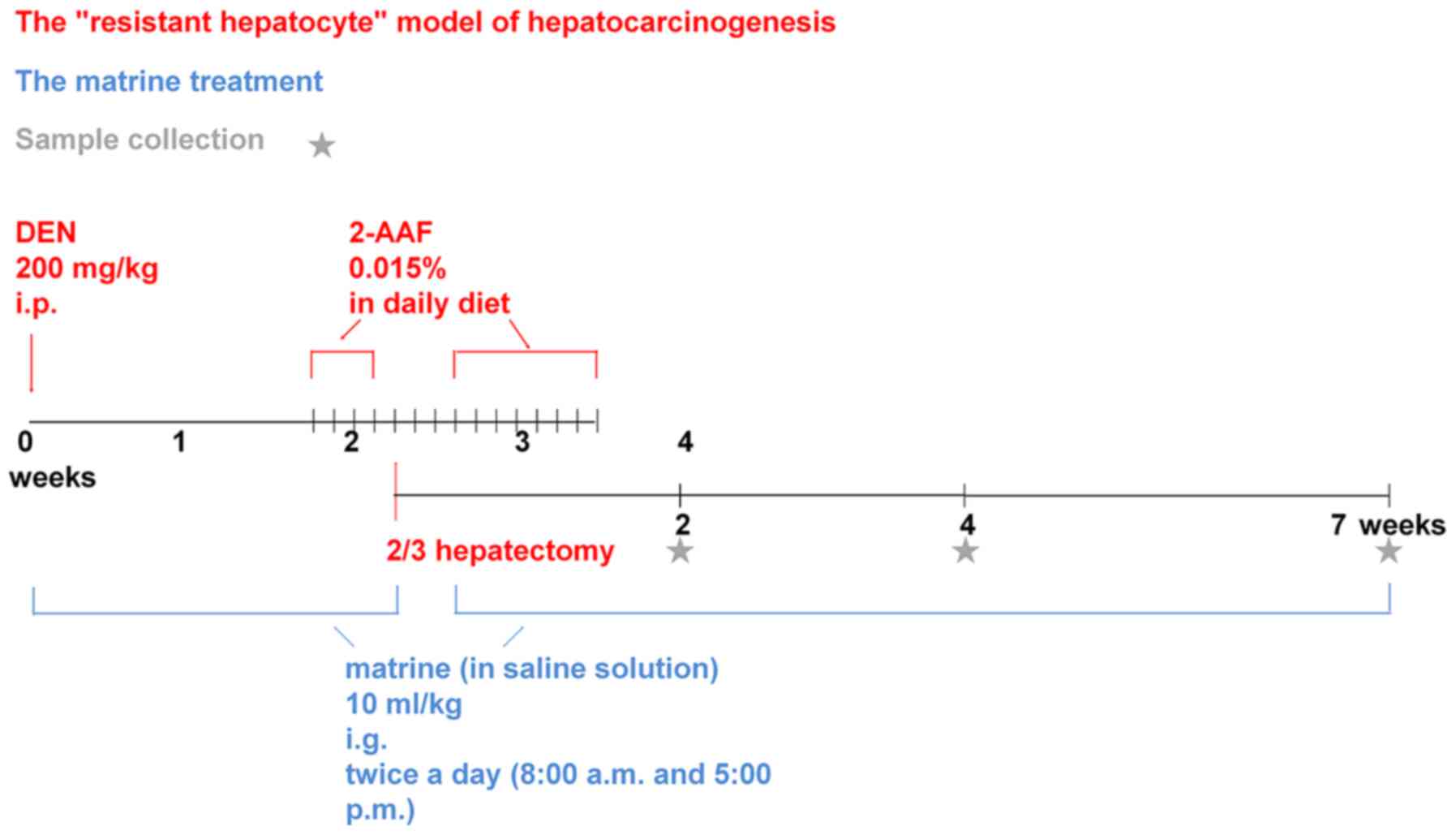
The resistant hepatocyte animal model reflects the
early signs of HCC and is commonly used to determine the preventive
role of chemical agents in HCC initiation (23). In the present study, a resistant
hepatocyte rat model was established by partial hepatectomy
combined with treatment of diethylnitrosamine (DEN) and
2-acetylaminofluorene (2-AAF). The model was further used to
analyze the effect of matrine on the early changes of liver
carcinogenesis.
Materials and methods
Animals models
A total of 48 male Sprague-Dawley rats (clean grade;
6 weeks old; average weight, 120±20 g) were purchased from the
Laboratory Animal Center of the Hebei Medical University. The
animals were maintained in the National Grade II Experimental
Animal Center in a sterile environment (temperature, 22±1°C;
relative humidity, 50±1% and a 12-h light/dark cycle). All animals
had access to drinking water and forage. Additionally animals'
health and behavior were monitored twice a day at 7:30 a.m. and 6
p.m. by a veterinary officer. In addition, all animal studies were
approved and supported by Laboratory Animal Ethics Committee of
Fourth Hospital Hebei Medical University (certificate no.
2017026).
The rats were randomly divided into four groups
(n=12/group): Control, model, low-dose matrine and high-dose
matrine groups. The model, low-dose matrine and high-dose matrine
groups were used to generate preneoplastic lesions for the liver
model. Briefly, rats were intraperitoneally injected with 200 mg/kg
DEN (batch no. 1002279831; Sigma-Aldrich; Merck KGaA) just on the
first day, then low-dose and high-dose matrine groups received
intragastric administration of matrine (batch no. 0301010007160301;
Ningxia bauhinia pharmaceutical Co., Ltd.) at a dosage of 1.25 and
12.5 mg/kg respectively twice per day at 8 a.m. and 5 p.m., which
lasted from intraperitoneal injection of DEN to the killing of
rats. The interval between two processes was no more than 10 min,
so that this experiment can study matrine preventive treatment.
While the rats in the model group were given an equal volume of
saline solution via gavage. Two weeks after DEN injection, rats
received daily intragastric administration of 2-AAF (batch no.
1002176257; Sigma-Aldrich; Merck KGaA) at a dosage of 15 mg/kg for
3 days. Following this, a partial (2/3) hepatectomy was performed.
Preoperatively, rats were anesthetized by intraperitoneal injection
of 10% chloral hydrate (batch no. 20161212; Tianjin Damao Chemical
Reagent Factory) at a dosage of 300 mg/kg, which was much lower
than the LD50 of 480 mg/kg. Three days after the surgery, rats
continued to be administered 2-AAF for 1 week. Each rat was weighed
before each intragastric administration and given the drug
according to its weight to ensure a uniform standard for each
administration. In the control group, equivalent abdominal
opening/closing surgery and intragastric administration of an equal
volume of saline were conducted. However, no DEN injection or 2-AAF
supplementation was provided and no hepatectomy was performed in
the control group. Partial hepatectomy and intraperitoneal
injection of drugs always followed the principle of sterility, and
3 rats died due to postoperative intraperitoneal hemorrhage, which
was caused by the surgical ligation suture falling off, but no
obvious peritonitis was observed. In this experiment, there were 48
rats in total, 4 rats in each group at each time point. The 3 dead
rats were distributed among different experimental groups. In each
group, at least 3 rats were still survived at each time point,
which met the needs of the experimental statistics (24,25).
Each group of rats were sacrificed in 3 batches at 2, 4 and 7 weeks
after hepatectomy. At the end of the administration period, animals
ate and drank freely for 4 h. The rats were anesthetized by
intraperitoneal injection of 10% chloral hydrate (300 mg/kg), then
they were killed by extirpation of eyeballs and exsanguination
(26–28). They were confirmed dead by
observation of respiratory and cardiac arrest and the absence of
any pain response to needle puncture of the extremities. The livers
were then carefully dissected and immediately fixed in 10% formalin
for 24 h at 4°C for subsequent experiments (Fig. 1).
Hematoxylin and eosin (H&E)
staining
Organs were collected, fixed with 10% formalin and
dehydrated using a gradient alcohol series (60, 70, 80, 90, 95 and
100% ethanol) followed by two xylene treatments. The samples were
embedded in paraffin, then tissues were cut into 5-µm-thick
sections. Tissue sections were subsequently deparaffinized in
xylene and rehydrated with a gradient alcohol series (100, 70 and
50% ethanol) and stained using H&E. The sections were stained
with hematoxylin staining for 5 min at 37°C and 1% eosin for 3 min
at 37°C. All liver sections were observed under a light microscope
(magnification, ×400).
Immunohistochemistry
Immunohistochemistry was performed as described
previously (29). Briefly, hepatic
tissue was harvested, fixed in 10% formalin and permeated with
paraffin wax. Subsequently, tissues were cut into 6–7-µm-thick
sections, which was in line with international standards. The
sections were then attached to the slides with polylysine membranes
and incubated for 4.5 h at 65°C. Following this, sections were
dewaxed, rehydrated and treated with hydrogen peroxide to block the
endogenous peroxidase activity. Slides were incubated with rabbit
anti-rat α-1-fetoprotein (AFP) polyclonal antibody (cat. no.
ab46799; 1:80; Abcam), rabbit anti-rat albumin (ALB) monoclonal
antibody (cat. no. ab207327; 1:75; Abcam), rabbit anti-rat Notch1
monoclonal antibody (cat. no. 3608; 1:400; Cell Signaling
Technology, Inc.) and rabbit anti-rat Hes1 monoclonal antibody
(cat. no. BM4488; 1:100; Wuhan Boster Biological Technology Co.,
Ltd.). Following this, slides were washed, incubated with a
ready-to-use horseradish peroxidase-labeled goat anti-rabbit IgG
polymer (cat. no. PV-6001; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) at 37°C for 20 min and then subjected to
incubation with diaminobenzidine chromagen at 37°C for 20 min.
Images were captured using an Olympus BX51T-PHD-J11 light
microscope (Olympus Corporation). Immunohistochemical staining was
quantified with Image-Pro Plus 5.1 for Windows software (Media
Cybernetics, Inc.) using the measurement function. Randomly
selected areas were used for analysis by electing 3 slides for each
rat and 5 fields for each slide, and combined the number of
positive cells with the strong expression of protein and showed the
expression intensity of protein as accurately as possible in the
form of quantitative data. The number of positive cells and the
color intensity of positive cells were obtained, and the
immunohistochemical score (IHS) was calculated as follows: A,
positive cell number grading: 0–1%=0, 1–10%=1, 10–50%=2, 50–80%=3
and 80–100%=4; B, color intensity of positive cells: Grade 0
(negative), 1 (weak positive), 2 (positive), 3 (strong positive);
IHS=A × B (30).
Statistical analysis
Statistical analyses were performed using SPSS 19.00
(IBM Corp). All data were expressed as mean ± standard deviation
and experiments were repeated at least three times. Data were
analyzed using one-way analysis of variance followed by
Student-Newman-Keuls post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Matrine reduces the development of
histopathological HCC-like lesions
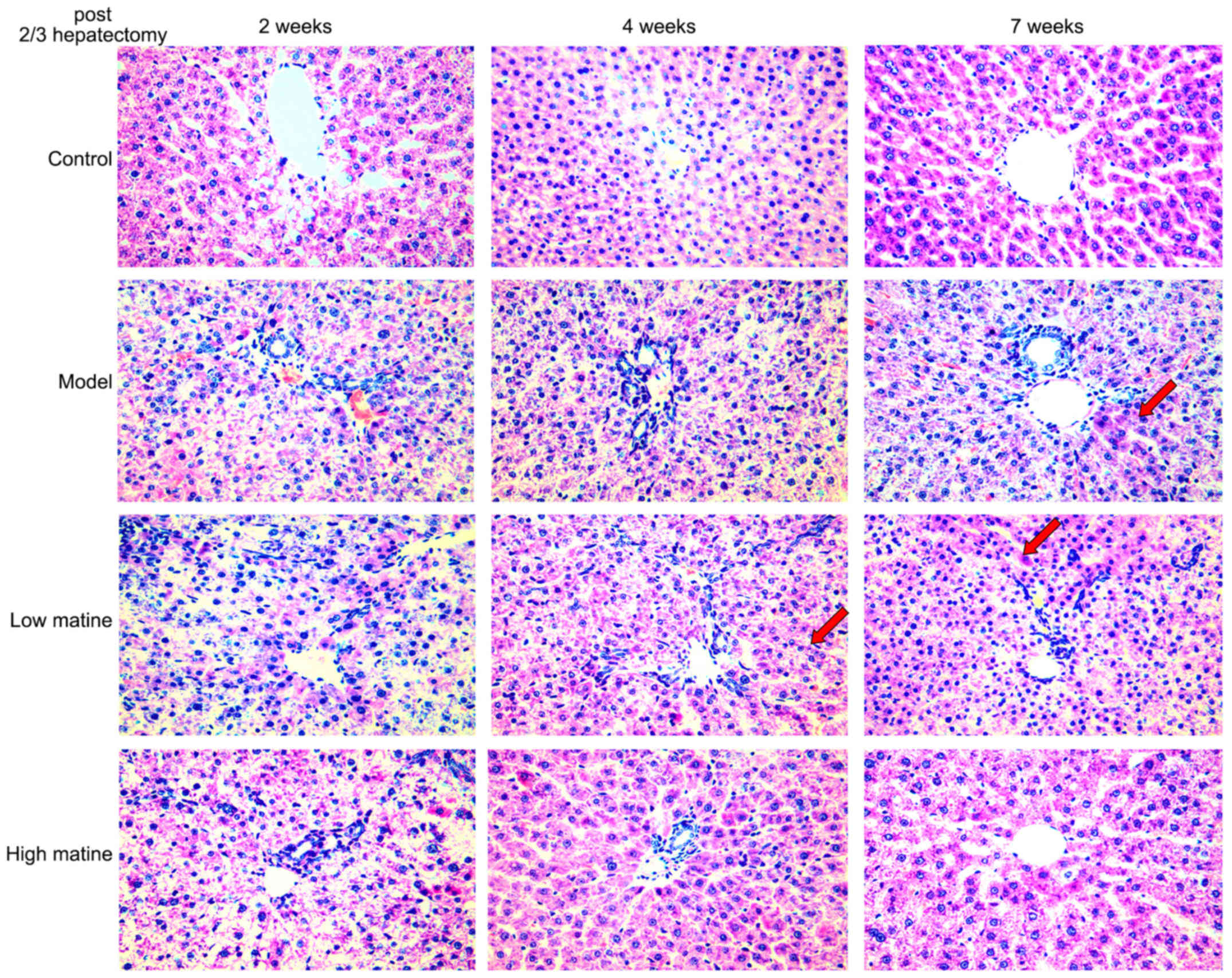
Normal gross morphology and hepatic lobule
structures were indicated in the control group. Furthermore, no
significant infiltration of inflammatory cells in the manifold area
was observed in the control group (Fig.
2). By contrast, the hepatic lobule structures and the
structure of liver cell cords were severely damaged, and the liver
cell membrane was damaged 2 weeks after partial hepatectomy in
model group. Moreover, edema degeneration was observed in a number
of hepatocytes and a large number of inflammatory cells infiltrated
the hepatic portal area in model group. A total of 4 weeks after
surgery in model group, no significant alterations in liver tissue
necrosis or inflammation were observed and injury of hepatic lobule
structure was still visible, together with hepatocyte edema and
necrosis. However, 7 weeks after surgery, a small proportion of
cellular cord structures were detected (Fig. 2).
In the low-dose matrine group, a small proportion of
the hepatocyte cord structures were restored 4 weeks after
operation. 7 weeks after partial hepatectomy, the hepatocyte edema
degeneration was reduced and hepatocyte nodule structures were
recovered (Fig. 2). In the high-dose
matrine group, a higher protective effect was observed in the week
4 and 7 compared with the model group and low-dose matrine group.
Furthermore, discernible lobular structures and a large number of
normal liver cell structures were indicated in the high-dose
matrine group. In addition, edema degeneration of liver cells and
inflammatory cell infiltration were reduced in the high-dose
matrine group (Fig. 2).
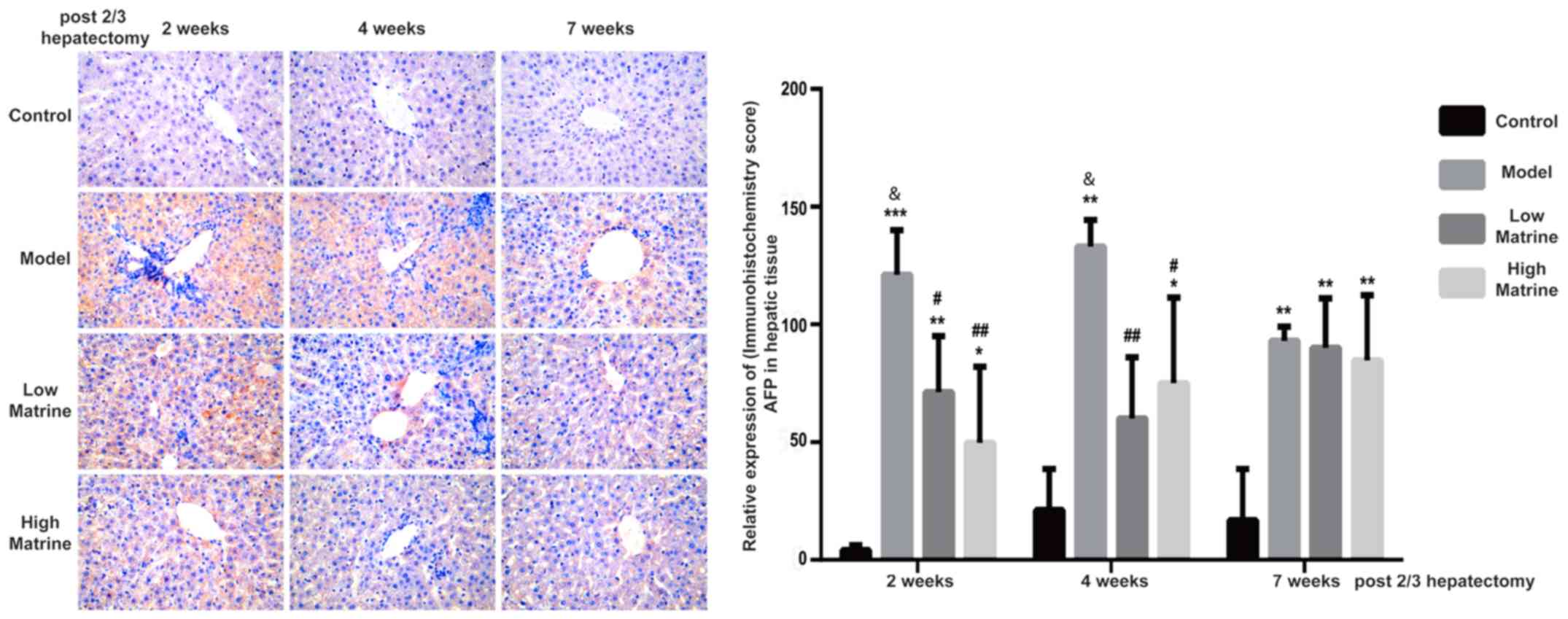
Matrine reduces the expression of AFP
in the liver of resistant hepatocyte rats
AFP is a glycoprotein expressed in hepatoblast stem
cells, hepatic cancer stem cells and hepatoma cells. When the liver
is damaged, an increase of AFP often indicates the severity of
damage. The continuous increase of AFP may indicate that part of
liver tissue has become cancerous (31,32).
According to immunohistochemistry results, AFP was distributed in
the cytoplasm and nuclei of liver cells (Fig. 3). No AFP expression was detected in
the control group. Notably, AFP expression was significantly
upregulated at 2, 4 and 7 weeks after surgery in the model group
(P<0.01; Fig. 3). In addition,
the AFP expression levels in the model groups at 2 and 4 weeks were
significantly increased compared with week 7 in the model group
(P<0.05 at 2 and 4 weeks; Fig.
3). This indicated that HCC-like lesions occurred at earlier
time points.
In the low-dose matrine and high-dose matrine
groups, the expression of AFP was significantly decreased at week 2
and 4 compared with the model group; however, it was still
increased compared with the control group (low-dose matrine group
vs. model group: P<0.05 at 2 weeks and P<0.01 at 4 weeks;
high-dose matrine group vs. model group: P<0.01 at 2 weeks and
P<0.05 at 4 weeks; Fig. 3). In
addition, there was no significant difference in AFP signal between
the model group and low-dose or high-dose matrine groups at week 7
(Fig. 3).
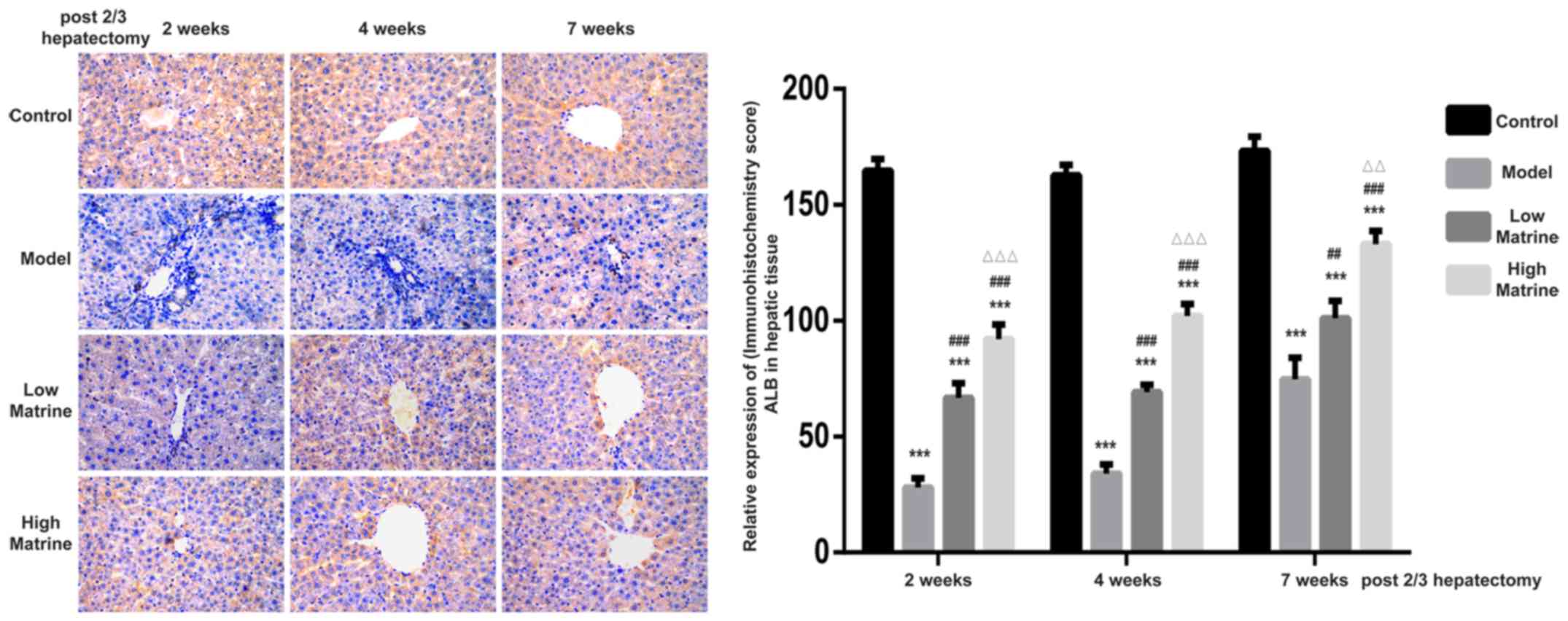
Matrine improves the expression of ALB
in the liver of resistant hepatocyte rats
ALB is secreted by normal hepatocytes and is often
used to distinguish between hepatocytes and hepatoblasts. According
to the immunohistochemistry results, ALB was mainly distributed in
the cytoplasm of liver cells (Fig.
4). Strong ALB expression was detected in the control group.
However, the expression of ALB in the model group was significantly
suppressed at week 2, 4 and 7 compared with the control group
(P<0.05). In the low-dose matrine and high-dose matrine groups,
significantly increased expression was observed at week 2, 4 and 7
compared with the model group (P<0.05). In addition, significant
differences were found between the low-dose matrine and high-dose
matrine groups. Notably, the expression of ALB in the high-dose
matrine group was increased compared with in the low-dose matrine
group at 4 and 7 weeks (Fig. 4).
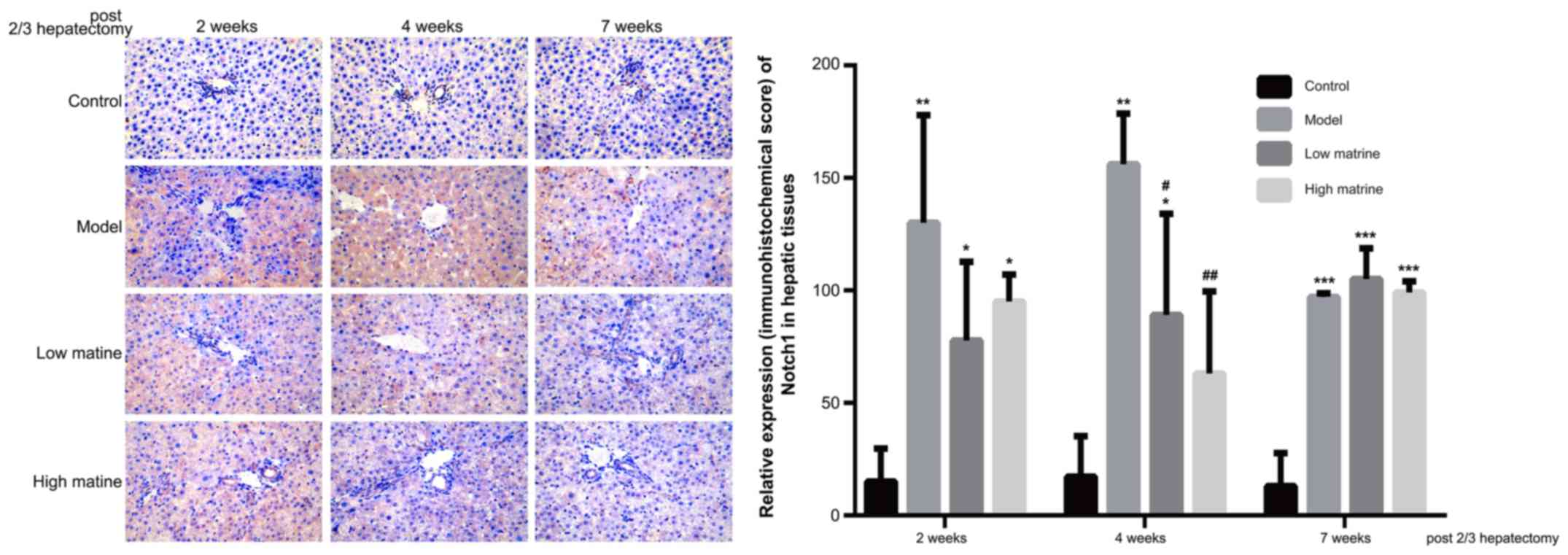
Upregulation of Notch1 expression in
the liver of resistant hepatocyte rats is reduced by matrine
treatment
Notch1 is the primary receptor of the Notch
signaling pathway and was used as the indicator for the activity of
Notch pathway in the present study. According to
immunohistochemistry results, Notch1 was primarily distributed in
the cytoplasm of liver cells (Fig.
5). No Notch1 expression was detected in the control group.
Notably, compared with the control group, Notch1 expression was
significantly upregulated at week 2, 4 and 7 in the model group
(P<0.01 at 2 weeks, P<0.01 at 4 weeks and P<0.001 at 7
weeks; Fig. 5). This suggested that
the HCC-like lesions were accompanied with Notch pathway
activation.
In low-dose matrine and high-dose matrine groups,
significantly reduced Notch1 expression was observed at week 4
compared with the model group (low-dose matrine group vs. model
group at 4 weeks: P<0.05; high-dose matrine vs. model group
weeks: P<0.01; Fig. 5); however,
no significant differences were found at week 4 between low-dose
matrine and high-dose matrine groups. There was no significant
difference in Notch1 staining between the model group and either of
the matrine groups at 2 and 7 weeks (Fig. 5).
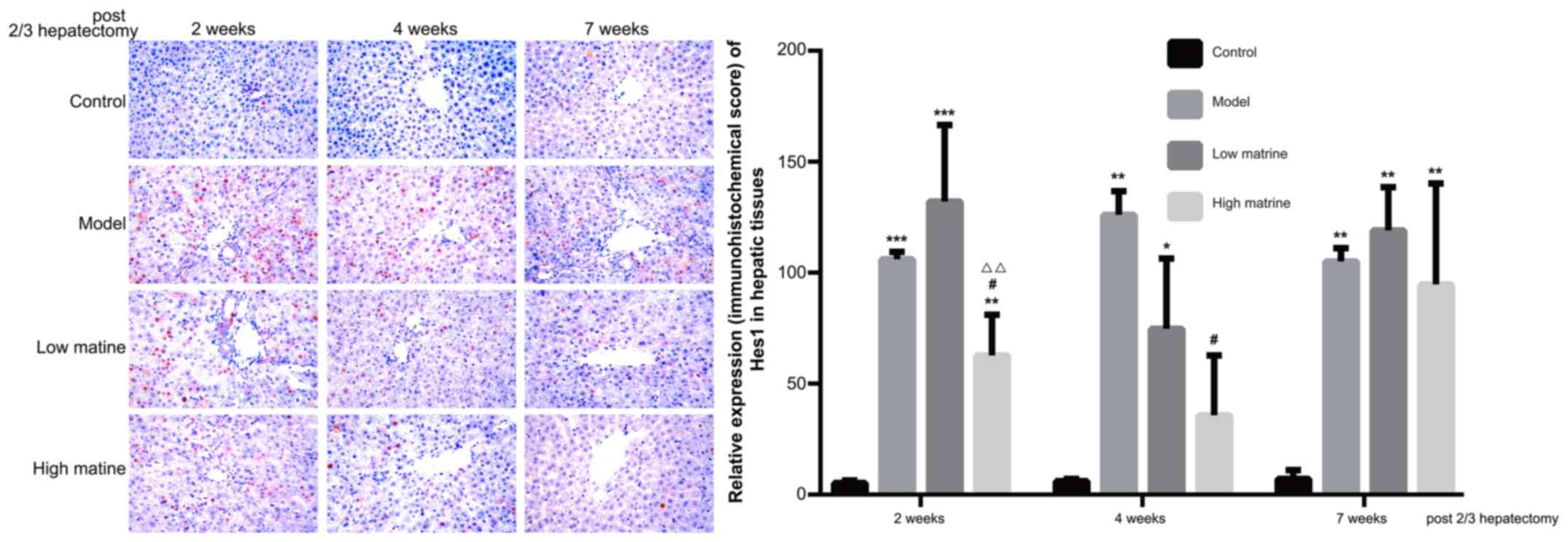
Upregulation of Hes1 expression in
liver of the resistant hepatocyte model is reduced by matrine
treatment
Hes1 is the predominant downstream target gene of
the Notch signaling pathway and its expression level was used as
the indicator for Notch signaling activity. The present data
indicated that Hes1 was primarily distributed in the nuclei of
liver cells (Fig. 6). No Hes1
expression was detected in the control group. Notably, compared
with the control group, Hes1 expression was significantly
upregulated at 2, 4 and 7 weeks after a partial hepatectomy in the
model group (P<0.001 at 2 weeks, P<0.01 at 4 weeks and
P<0.01 at 7 weeks; Fig. 6).
In the low-dose matrine group, Hes1 expression was
not significantly altered compared with the model group. However,
in the high-dose matrine group, significantly reduced Hes1
expression was observed at week 2 and 4 compared with the model
group (P<0.05 at 2 weeks and P<0.05 at 4 weeks; Fig. 6). There was no significant difference
in Hes1 staining between the model group and either of the matrine
groups at 7 weeks (Fig. 6).
Discussion
Traditional Chinese medicine has been shown to be
effective for treating patients with HCC. A number of preclinical
studies have indicated that matrine possesses an anti-tumor effect
against HCC (14,33,34).
However, it remains to be determined if matrine has a
chemopreventive effect against HCC. In this study, a resistant
hepatocyte model of hepatocarcinogenesis (initiation with DEN,
selection/promotion with 2-AAF and partial hepatectomy) was
constructed. This model includes the stimulatory factor associated
with tissue regeneration after hepatectomy (33) and reflects the early signs of HCC.
Thus, this model is commonly used to determine the preventive role
of chemical agents in HCC initiation. For example, this model has
been used to demonstrate the chemopreventive effect of folic acid
on preneoplastic lesions (35).
Similar effects have been observed when applying caffeic acid
phenethyl ester (36) and ethanol
ethanol extract of Phellinus merrillii (37).
In this study, a resistant hepatocyte rat model was
used to explore the chemopreventive effect of matrine on the
development of HCC. Compared with the control group, rats in the
model group exhibited loss of hepatic lobule structure, signs of
damage to structure of liver cell cords and a reduced number of
liver cells. Moreover, infiltration of inflammatory cells in the
hepatic portal area was observed, suggesting the presence of
multiple pre-cancerous characteristics. The present results
indicated that the liver damage of the low-dose matrine and
high-dose matrine groups was mildly alleviated 4 weeks after
partial hepatectomy, and was restored at 7 weeks after operating
(including the restoration of hepatic cord and lobule structures
and reduction of liver cell necrosis) when compared with the model
group. Higher restoration was observed in the high-dose matrine
group compared with the low-dose matrine group. This was consistent
with a previous study, which examined the matrine derivative MD-1
effect on a hepatic fibrosis rat model; their data indicated that
fibrosis, necrosis and inflammatory cell infiltration were all
restored 4 weeks after modeling (11).
In this experiment, higher AFP expression was
detected in the model group when compared with the control group.
Furthermore, ALB expression in the model group was reduced when
compared with the control group. The high AFP expression suggested
that hepatic tissue of the model group exhibited severe damage.
Following matrine treatment, the elevated AFP expression after
modeling was reduced. Regarding the ALB expression, matrine
treatment upregulated the expression when compared with the model
group. In addition, the expression of ALB was increased in the
high-dose matrine group compared with the low-dose matrine group.
The effects of matrine on liver morphology and protein indicated
that matrine reduces liver damage and inhibits the initiation of
HCC-like alterations. This was consistent with a previous study,
which found that matrine derivative MASM may inhibit the
proliferation of HCC in a dose-dependent manner and increase the
expression of ALB, indicating that matrine-like drugs could induce
the transformation of HCC cells into hepatocytes (12). Similarly, matrine was able to
increase the expression of ALB in hepatic oval cells and could
induce their differentiation into hepatocytes (22).
Deregulation of the Notch signaling pathway has been
reported to be involved in a number pathological processes in the
liver, including the development of HCC and HCC-associated liver
fibrosis (20). To explore the
Notch-related mechanism involved in the preventive effect of
matrine, the expression levels of Notch pathway components were
examined. Notch1 is the main receptor of the Notch signaling
pathway, while Hes1 is the main downstream target gene of the
pathway. In the model group, the expression of these two proteins
was upregulated, suggesting that the Notch pathway was activated in
the HCC-mimicking model. Application of high-dose matrine reduced
Notch1 and Hes1 expression, demonstrating that matrine could
inhibit the Notch signaling pathway in the HCC-mimicking model.
Matrine-induced inhibition of the Notch signaling pathway may
alleviate hepatic damage or even reverse hepatic cell
carcinogenesis. Liu et al (38) indicated in a model of liver
transplantation that upregulation of the Notch signaling pathway
could promote differentiation of liver stem cells into
hepatofibrotic cells, thereby increasing the secretion of collagen
Iα1 and fibrillar junction protein, and aggravating liver damage.
However, inhibition of the Notch signaling pathway could reverse
liver damage. Similar to the results of the present study, matrine
was reported to inhibit the expression of Notch signaling pathway
genes in a dose-dependent manner in hepatic oval cells, promoting
hepatic oval cell differentiation into hepatocytes (22,39).
Notably, Liu et al (40) also
found matrine could alleviate myocardial injury and myocardial
fibrosis by inhibiting the Notch signaling pathway.
In this experiment, the classic rat Solt-Farber
model was used, which is often used to assess acute liver damage
and liver cell carcinogenesis. Unfortunately, the typical
early-cancer nodules were not induced, indicating that
preneoplastic lesions were not completely generated. This might be
caused by the inefficient dosage and/or time of the carcinogens
used. Dezsö et al (41) also
found only regenerative, not neoplastic, growth in rat Solt-Farber
experimental hepatocarcinogenesis model. They administered a dosage
of 5 mg/kg 2-AFF to the rats, while a larger dosage of 15 mg/kg
2-AAF was used in the present study, but still did not induce HCC
and the present study believes that the Solt-Farber model need to
be further improved in the future. However, a model of severe liver
damage was made and the application of matrine could alleviate
liver damage to a certain extent, which also demonstrated the
effect of matrine in alleviating liver inflammation and preventing
cancer. In addition, not only has matrine been shown to protect the
liver and prevent cancer, but other extracts from the dry roots of
Sophora flavescnes, such as oxymatrine, have also been shown
to be effective in the treatment of liver disease (42,43).
However, there was a lack of multi-center research. As a result of
its low cost and low side effects, matrine underwent a lot of
research and has potential application in developing countries. In
the future, the authors will further study the detailed mechanism
of matrine and its derivatives in protecting the liver and
preventing cancer.
Taken together, the resistant hepatocyte model in
the present study was used to assess the early changes in HCC.
Furthermore, the present study aimed to determine the effect of
matrine in vivo. The findings indicated that matrine
treatment inhibited the development of pre-cancerous
characteristics, including changes of hepatic lobule structure and
expression changes of AFP and ALB. HCC-associated activation of the
Notch signaling pathway was implicated by the expression of Notch1
and Hes1, which was detected in the model and suppressed by matrine
treatment. Therefore, it was concluded that matrine may be an
effective chemopreventive agent for HCC and can suppress Notch
activation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81072966/H2902),
Hebei Provincial Natural Science Foundation of China (grant no.
C2011206134) and Youth science and technology programme of Hebei
Provincial Health and Family Planning Commission of China (grant
no. 20160645).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS, JX and FY carried out the studies, participated
in collecting data and drafted the manuscript. XH, JW, GH, MZ, XD,
LM, NL, XY, HS, CL and JG performed the experiments and analyzed
the data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal studies were approved and supported by
Laboratory Animal Ethics Committee of Fourth Hospital Hebei Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K, Ma J, Jia X, Ai W, Ma Z and Pan Q:
Advancing the understanding of NAFLD to hepatocellular carcinoma
development: From experimental models to humans. Biochim Biophys
Acta Rev Cancer 1871. 117–125. 2019. View Article : Google Scholar
|
|
5
|
Tessitore A, Cicciarelli G, Del Vecchio F,
Gaggiano A, Verzella D, Fischietti M, Mastroiaco V, Vetuschi A,
Sferra R, Barnabei R, et al: MicroRNA expression analysis in high
fat diet-induced NAFLD-NASH-HCC progression: Study on C57BL/6J
mice. BMC Cancer. 16:32016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian Y, Lyu H, He Y, Xia Y, Li J and Shen
F: Comparison of hepatectomy for patients with metabolic
syndrome-related HCC and HBV-related HCC. J Gastrointest Surg.
22:615–623. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dhar D, Seki E and Karin M: NCOA5, IL-6,
type 2 diabetes, and HCC: The deadly quartet. Cell Metab. 19:6–7.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pazgan-Simon M, Simon KA, Jarowicz E,
Rotter K, Szymanek-Pasternak A and Zuwała-Jagiełło J: Hepatitis B
virus treatment in hepatocellular carcinoma patients prolongs
survival and reduces the risk of cancer recurrence. Clin Exp
Hepatol. 4:210–216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li WQ, Park Y, McGlynn KA, Hollenbeck AR,
Taylor PR, Goldstein AM and Freedman ND: Index-based dietary
patterns and risk of incident hepatocellular carcinoma and
mortality from chronic liver disease in a prospective study.
Hepatology. 60:588–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh S, Singh PP, Roberts LR and Sanchez
W: Chemopreventive strategies in hepatocellular carcinoma. Nat Rev
Gastroenterol Hepatol. 11:45–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng Y, Ying HY, Qu Y, Cai XB, Xu MY and
Lu LG: Novel matrine derivative MD-1 attenuates hepatic fibrosis by
inhibiting EGFR activation of hepatic stellate cells. Protein Cell.
7:662–672. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Qi Y, Bai ZH, Ni CX, Ren QH, Xu WH,
Xu J, Hu HG, Qiu L, Li JZ, et al: A novel matrine derivate inhibits
differentiated human hepatoma cells and hepatic cancer stem-like
cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol
Sin. 38:120–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao L, Wang KX, Zhou YZ, Fang JS, Qin XM
and Du GH: Uncovering the anticancer mechanism of Compound Kushen
Injection against HCC by integrating quantitative analysis, network
analysis and experimental validation. Sci Rep. 8:6242018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Zhang S, Liu J, Fang B, Yao J and
Cheng B: Matrine inhibits the invasive and migratory properties of
human hepatocellular carcinoma by regulating epithelial-mesenchymal
transition. Mol Med Rep. 18:911–919. 2018.PubMed/NCBI
|
|
15
|
Zhou H, Xu M, Gao Y, Deng Z, Cao H, Zhang
W, Wang Q, Zhang B, Song G, Zhan Y and Hu T: Matrine induces
caspase-independent program cell death in hepatocellular carcinoma
through bid-mediated nuclear translocation of apoptosis inducing
factor. Mol Cancer. 13:592014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X and Yu H: Matrine inhibits
diethylnitrosamine-induced HCC proliferation in rats through
inducing apoptosis via p53, Bax-dependent caspase-3 activation
pathway and down-regulating MLCK overexpression. Iran J Pharm Res.
15:491–499. 2016.PubMed/NCBI
|
|
17
|
Wang Y, Yao R, Gao S, Wen W, Du Y, Szabo
E, Hu M, Lubet RA and You M: Chemopreventive effect of a mixture of
Chinese Herbs (antitumor B) on chemically induced oral
carcinogenesis. Mol Carcinog. 52:49–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Zhang Z, Garbow JR, Rowland DJ,
Lubet RA, Sit D, Law F and You M: Chemoprevention of lung squamous
cell carcinoma in mice by a mixture of Chinese herbs. Cancer Prev
Res (Phila). 2:634–640. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bigas A and Espinosa L: The multiple
usages of Notch signaling in development, cell differentiation and
cancer. Curr Opin Cell Biol. 55:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ni MM, Wang YR, Wu WW, Xia CC, Zhang YH,
Xu J, Xu T and Li J: Novel Insights on Notch signaling pathways in
liver fibrosis. Eur J Pharmacol. 826:66–74. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi D and Zhang J, Qiu L, Li J, Hu Z and
Zhang J: Matrine inhibits infiltration of the inflammatory Gr1(hi)
monocyte subset in injured mouse liver through inhibition of
monocyte chemoattractant Protein-1. Evid Based Complement Alternat
Med 2013. 5806732013.
|
|
22
|
Yang Z, Wang L and Wang X: Matrine induces
the hepatic differentiation of WB-F344 rat hepatic progenitor cells
and inhibits Jagged 1/HES1 signaling. Mol Med Rep. 14:3841–3847.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Anilkumar TV, Golding M, Edwards RJ,
Lalani EN, Sarraf CE and Alison MR: The resistant hepatocyte model
of carcinogenesis in the rat: The apparent independent development
of oval cell proliferation and early nodules. Carcinogenesis.
16:845–853. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Williams JM, Oh SH, Jorgensen M, Steiger
N, Darwiche H, Shupe T and Petersen BE: The role of the wnt family
of secreted proteins in rat oval ‘Stem’ cell-based liver
regeneration: Wnt1 drives differentiation. Am J Pathol.
176:2732–2742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yovchev MI, Grozdanov PN, Joseph B, Gupta
S and Dabeva MD: Novel hepatic progenitor cell surface markers in
the adult rat liver. Hepatology. 45:139–149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang K, Zhang J, Yin C, Zhou X and Zhou
S: Protective effects and mechanism of TPX2 on neurocyte apoptosis
of rats in Alzheimer's disease model. Exp Ther Med. 13:576–580.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu JH, Feng D, Zhang YF, Shang Y, Wu Y,
Li XF and Pei L: Chloral hydrate preconditioning protects against
ischemic stroke via upregulating Annexin A1. CNS Neurosci Ther.
21:718–726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng X, Zhang L, Sun L, Zhang D, Zhao H,
Jia J and Wang W: Recovery from rat sciatic nerve injury in
vivo through the use of differentiated MDSCs in vitro.
Exp Ther Med. 5:193–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perks WV, Singh RK, Jones GW, Twohig JP,
Williams AS, Humphreys IR, Taylor PR, Jones SA and Wang ECY: Death
receptor 3 promotes chemokine-directed leukocyte recruitment in
acute resolving inflammation and is essential for pathological
development of mesothelial fibrosis in chronic disease. Am J
Pathol. 186:2813–2823. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li CH, Wang YJ, Dong W, Xiang S, Liang HF,
Wang HY, Dong HH, Chen L and Chen XP: Hepatic oval cell lines
generate hepatocellular carcinoma following transfection with HBx
gene and treatment with aflatoxin B1 in vivo. Cancer Lett.
311:1–10. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yovchev MI, Xue Y, Shafritz DA, Locker J
and Oertel M: Repopulation of the fibrotic/cirrhotic rat liver by
transplanted hepatic stem/progenitor cells and mature hepatocytes.
Hepatology. 59:284–295. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Farber E: Cellular biochemistry of the
stepwise development of cancer with chemicals: G. H. A. Clowes
memorial lecture. Cancer Res. 44:5463–5474. 1984.PubMed/NCBI
|
|
34
|
Zhang J, Gao Y, Han H, Zou C, Feng Y and
Zhang H: Matrine suppresses lung metastasis of human hepatocellular
carcinoma by directly targeting matrix metalloproteinase-9. Biochem
Biophys Res Commun. 515:57–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chagas CE, Bassoli BK, de Souza CA,
Deminice R, Jordão Júnior AA, Paiva SA, Dagli ML, Ong TP and Moreno
FS: Folic acid supplementation during early hepatocarcinogenesis:
Cellular and molecular effects. Int J Cancer. 129:2073–2082. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beltran-Ramírez O, Pérez RM,
Sierra-Santoyo A and Villa-Trevino S: Cancer prevention mediated by
caffeic acid phenethyl ester involves cyp2b1/2 modulation in
hepatocarcinogenesis. Toxicol Pathol. 40:466–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang CH, Chang HY, Chen YC, Lu CC, Huang
SS, Huang GJ and Lai HC: Ethanol extract of Phellinus
merrillii protects against diethylnitrosamine- and
2-acetylaminofluorene-induced hepatocarcinogenesis in rats. Chin J
Integr Med. 23:117–124. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu XB, Lo CM, Cheng Q, NG KT, Shao Y, Li
CX, Chung SK, Ng IOL, Yu J and Man K: Oval cells contribute to
fibrogenesis of marginal liver grafts under stepwise regulation of
aldose reductase and notch signaling. Theranostics. 7:4879–4893.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang ZY, Wang L, Hou YX and Wang XB:
Effects of matrine on oval cellmediated liver regeneration and
expression of RBP-Jκ and HES1. Mol Med Rep. 7:1533–1538. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Z, Wang Y, Zhu H, Qiu C, Guan G, Wang
J and Guo Y: Matrine blocks GEs-induced HCSMCs phenotypic
conversion via suppressing Dll4-Notch pathway. Eur J Pharmacol.
835:126–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dezsö K, Papp V, Bugyik E, Hegyesi H,
Sáfrány G, Bödör C, Nagy P and Paku S: Structural analysis of
oval-cell-mediated liver regeneration in rats. Hepatology.
56:1457–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gu YM, Lu JY, Sun W, Jin RM, Ohira T,
Zhang Z and Tian XS: Oxymatrine and its metabolite matrine
contribute to the hepatotoxicity induced by radix Sophorae
tonkinensis in mice. Exp Ther Med. 17:2519–2528. 2019.PubMed/NCBI
|
|
43
|
Jia Y, Long S, Jiang N, Shan Z, Lu Y, Han
F, Yu J and Feng L: Oxymatrine ameliorates agomelatine-induced
hepatocyte injury through promoting proteasome-mediated CHOP
degradation. Biomed Pharmacother. 114:1087842019. View Article : Google Scholar : PubMed/NCBI
|