Introduction
Colorectal cancer (CRC) has been reported as the
third most common cancer type and the fourth most common cause of
cancer-associated death worldwide, affecting ~1.2 individuals and
leading to 600,000 deaths per year (1,2). The
mortality of patients with CRC has decreased in a number of
countries over the last decade, which is thought to be attributed
to increased CRC screening and decreased prevalence of risk
factors, as well as the development and implementation of novel
treatments (3). However, 50% of
patients still develop metastasis and eventually succumb to their
disease, and most of them develop psychological conditions at
varying degrees, including anxiety and depression, and present with
poor quality of life (QoL) due to the psychological burden
associated with cancer diagnosis, the pain and the side effects of
therapy (4,5). For patients with CRC, chemotherapy is
able to decrease the recurrence risk and prolong survival to a
certain extent, but most patients experience complications
including pain and diarrhea; furthermore, CRC may be associated
with a significant economic burden. Hence, the emotional state and
social life of the patients (and their family) is frequently
negatively affected, thereby leading to the occurrence of anxiety
and depression (6,7). Previous studies have estimated the
occurrence of post-surgical anxiety and depression in patients with
CRC to be 8–23 and 16–39%, respectively (5,8–10). Therefore, it is necessary to explore
strategies to decrease anxiety/depression and improve or maintain
good QoL in patients with CRC.
Care intervention has been considered a common and
efficacious method to ameliorate the adverse effects of carcinoma
and its treatment, and is usually designed to promote improvements
in physical and mental health (11).
A large number of studies have confirmed the favorable effect of
care intervention, including patients' health education, dietary
patterns and physical activity on the functional outcomes in
patients with CRC; however, most of them focus on the functional
outcomes in these patients, while the effect of care intervention
on the prognosis of patients with CRC remains to be determined,
particularly regarding psychosocial problems and QoL (12,13).
Although parts of Chinese clinical trials have revealed the
efficacy of intervention care in improving mental health issues
including anxiety and depression in CRC patients, the role of the
care intervention in patients with CRC receiving adjuvant
chemotherapy remains elusive (14,15). In
addition, most of these previous studies have been performed on
relatively small cohorts, and additional studies with a larger
sample size for validation are urgently required. Considering the
aforementioned points, an incremental patient care program (IPCP)
was designed, consisting of patient health education, physical
exercise, telephone counselling, regular examination as well as
care activities, and the purpose of the present study was to assess
the effects of IPCP on anxiety, depression and QoL in patients with
CRC receiving adjuvant chemotherapy.
Materials and methods
Participants
A total of 298 consecutive patients with CRC who
underwent surgery at the 2nd Affiliated Hospital of Harbin Medical
University (Harbin, China) between January 2014 and December 2016
were recruited for the present randomized, controlled study. The
inclusion criteria were as follows: a) Diagnosis of primary CRC
confirmed by clinicopathologic examinations; b) age of 18–80 years;
c) tumor-nodes-metastasis (TNM) stage, II or III; d) scheduled for
CRC resection followed by adjuvant chemotherapy; e) prognosis of
survival for at least 12 months; and f) ability to complete the
anxiety, depression and QoL assessments. The exclusion criteria
were as follows: a) Neoadjuvant therapies; b) intake of
anti-depressant or anti-anxiety medication within the past 3
months; c) a comorbidity of severe primary mental disorder, or
severe liver or renal disease; d) history of other malignant tumor
types or hematological malignancy; e) no availability for regular
follow-up; f) pregnancy or lactation; g) refusal to provide written
informed consent. The reasons for the collection of data over a
long period (from January 2014 to December 2016) were as follows:
First, at our hospital, numerous patients were diagnosed at the
early stage and did not require adjuvant chemotherapy, and certain
patients at the advanced stage received neoadjuvant therapy; thus,
a considerable proportion of patients were not eligible for the
present study. Furthermore, 300 patients were required to reach
sufficient statistical power, and it took this long until the
number of eligible consecutive patients collected reached this
number. In addition, at the preliminary stage, a total of 625
patients were invited to participate in the present study, but a
considerable amount were excluded due to not satisfying the
inclusion or exclusion criteria. There were 5 patients with severe
depressive disorder and 11 patients with severe anxiety disorder
who were excluded, and these patients received psychotherapy, but
no anti-depressant or anti-anxiety medicines.
Randomization
In the present randomized, controlled study, a
blocked randomization method was adopted, and a randomization code
was generated using Statistical Analysis System (SAS) 9.3 software
(SAS Institute, Carey, NC, USA). The randomization was performed by
an independent statistical analyst who was otherwise not involved
in the study, and the documents were deposited at the medical and
statistical service company Shanghai Qeejen Biotech Co. (Shanghai,
China). When a patient was eligible for the study, a call was made
to Qeejen Biotech Co. and a unique subject identification number
was provided from the randomizing module.
Adjuvant chemotherapy
At 2–6 weeks following CRC resection, all patients
received adjuvant chemotherapy based on their disease condition.
For patients with TNM stage II, according to risk stratification
and T stage, one of the following adjuvant chemotherapy regimens
was selected: i) Capecitabine 1,250 mg/m2 twice daily on
days 1–14, repeated every 3 weeks for a total of 24 weeks; ii)
leucovorin (LV) 400 mg/m2 over 2 h on day 1, followed by
a 5-fluorouracil (5-FU) bolus 400 mg/m2 and then 1,200
mg/m2/day for 2 days (a total continuous infusion of
2,400 mg/m2 over 46–48 h), repeated every 2 weeks for a
total of 24 weeks; iii) oxaliplatin 130 mg/m2
intravenously (IV) over 2 h on day 1 and capecitabine 1,000
mg/m2 twice daily on days 1–14, repeated every 3 weeks
for a total of 24 weeks; iv) oxaliplatin 85 mg/m2 IV
over 2 h on day 1, LV 400 mg/m2 IV over 2 h on day 1,
5-FU 400 mg/m2 IV bolus on day 1 and then 1,200
mg/m2/day for 2 days (total 2,400 mg/m2 over
46–48 h) continuous infusion, repeated every 2 weeks for a total of
24 weeks. For patients with TNM stage III, one of following
adjuvant chemotherapy regimens was selected: i) Oxaliplatin 130
mg/m2 IV over 2 h, day 1, capecitabine 1,000
mg/m2 twice daily on days 1–14, repeated every 3 weeks
for a total of 24 weeks; ii) oxaliplatin 85 mg/m2 IV
over 2 h on day 1, LV 400 mg/m2 IV over 2 h on day 1,
5-FU 400 mg/m2 IV bolus on day 1, then 1,200
mg/m2/day for 2 days (a total continuous infusion of
2,400 mg/m2 over 46–48 h) repeated every 2 weeks for a
total of 24 weeks.
Interventions
After randomization, patients were randomly assigned
to the IPCP group or the control group in a 1:1 ratio. In the IPCP
group, patients received IPCP and conventional care, whereas in the
control group, patients received only conventional care, which
included instructions of post-operative medicine management,
regular examinations (every 3 months) and usual advice regarding
post-operative rehabilitation. The intervention was performed for
six months. The details of IPCP were as follows (Table I): i) Patient health education: Over
the first 2 weeks, patients were provided with general health
education materials, including information about nutrition,
physical activity and mental health care. Furthermore,
comprehensive health education was provied by a designated research
therapist, and detailed instruction was given once every two months
for six months. ii) Physical exercise: Over the first 3 months,
patients participated in low-intensity physical exercise comprised
of three components: Relaxation (30 min five times a week), body
awareness and restorative exercise (90 min once a week) and massage
(30 min twice a week). Over the subsequent 3 months, patients
participated in high-intensity physical exercise for 90 min
followed by 30 min of relaxation exercise three times a week. The
high-intensity physical exercise sessions comprised three
components: 30 min of warm-up exercises, 45 min of resistance
exercise and 15 min of cardiovascular exercise. All physical
exercise was supervised by trained specialist nurses. Details on
low- and high-intensity physical exercise are provided in Table SI. iii) Telephone counseling: Each
participant was assigned a nurse for the 6-month study period.
Counseling sessions were performed weekly during the first three
weeks, every other week for one month thereafter and then monthly.
Each telephone session had a duration of 15–30 min and served to
provide social support and enhance self-efficacy (encourage
patients to actively perform activities to promote their health).
During each telephone call, the nurse worked with the patient to
monitor progress, explore strategies for overcoming barriers and
asked relevant questions about rehabilitation. iv) Regular
examination: Patients were instructed to regularly undergo the
scheduled examinations, including imaging, blood analysis and
colonoscopy, once every two months for six months to manage any
abnormalities. v) Care activities: Patients and family members as
the primary caregivers were invited to the join the monthly
workshop and the nurse communicated with them to resolve issues
that they encountered in the interim period and provided detailed
advice on points including how to maintain a healthy diet, control
body weight and keep a positive mood. In addition, during the care
activities, caregivers were provided with lessons regarding how to
help patients complete their daily physical exercise.
 | Table I.Contents of the incremental patient
care program. |
Table I.
Contents of the incremental patient
care program.
| Item | Frequency and
duration | Core content |
|---|
| Patient health
education | Once every 2 months
for 6 months | Training for
knowledge of disease and self-care |
| Physical
exercise | Low-intensity
physical exercise for 3 months, followed by high-intensity physical
exercise for 3 months | Recovery of physical
function |
| Telephone
counselling | Weekly for 3 weeks,
every other week for 1 month and monthly for 4 months | Emotional
support |
| Regular
examination | Once every 2 months
for 6 months | Surveillance for
disease |
| Care activities | Monthly for 6
months | Close communication
with patients to help them to solve problems |
Information collection
After enrolment in the present study, baseline
information was collected from all patients, which included the
following: a) Demographic characteristics: Age, gender and highest
education; and b) Clinical and pathological characteristics:
Smoking and drinking status, hypertension, hyperlipidemia,
diabetes, pathological grade, tumor size and TNM stage (according
to the 7th edition of the American Joint Committee on Cancer
Staging Manual) (16). In the
present study, the personal information of the patients, including
the demographic and clinicopathological characteristics, were
collected from the patients' medical records, and the Hospital
Anxiety and Depression Scale-anxiety (HADS-A; Table SII), HADS-depression (HADS-D;
Table SII) as well as QoL score
(Table SIII) were from
questionaires completed at the clinic.
Evaluation of anxiety and
depression
Anxiety refers to an emotion that is featured by an
unpleasant state of inner turmoil and is often accompanied by
nervous behavior, including pacing back and forth, somatic
complaints and rumination, or unpleasant feelings of dread over
anticipated events (17). Depression
refers to a state of low mood and aversion to activity, which may
affect a person's thoughts, behavior (sucha as total loss of
interest in enjoyable activities or socializing), feelings (such as
feelings of loneliness, sadness, guilt or worthlessness) and sense
of well-being (18). HADS-A scores
and HADS-D scores were determined to assess anxiety and depression
among the patients. These were assessed at baseline [month 0 (M0)],
M1, M3 and M6. The HADS-A and HADS-D subscales consisted of seven
questions that are scored individually from 0 to 3 points,
resulting in subscale scores ranging from 0 to 21 points, with the
following grade classification: 0–7, no anxiety/depression; 8–10,
light anxiety/depression; 11–14, moderate anxiety/depression;
15–21, severe anxiety/depression (19).
Evaluation of QoL
QoL refers to the satisfaction of patients,
outlining negative and positive features of life. (20). The European Organization for Research
and Treatment of Cancer Quality of Life Questionnaire (EORTC
QLQ-C30 Scale) was used to assess the QoL of patients. The EORTC
QLQ-C30 Scale consists of 30 items, including five functions,
symptoms and financial implications subscales. The first 28 items
of the questionnaire used a 4-point Likert-type response scale
ranging from 1 (not at all) to 4 (very much). Items 29 and 30,
which assess global health status/QoL, use a response scale ranging
from 1 (very poor) to 7 (excellent). All raw data were transformed
to a 0–100-point scale. Higher mean scores for the functional
scales and the global health status/QoL scales indicate better
functioning and overall QoL, whereas a high score for the symptom
scale/single-item scale represents a high level of symptom distress
(21).
Statistical analysis
Sample size calculation was based on a previous
study (6). Under the requirement of
a 90% statistical power to detect a difference of 10% in the
percentage of patients with depression at M6 between the IPCP group
and control group, assuming 25% patients in the IPCP group and 35%
patients in the control group exhibited depression at M6, with a
two-sided 5% level of significance (α), a sample size of 110
participants was required in each group. Accounting for loss to
follow-up of ~26%, 149 patients were enrolled in each group.
Statistical analyses were performed using SPSS 22.0 software (IBM
Corp., Armonk, NY, USA), Office 2010 software (Microsoft Corp.,
Redmond, WA, USA) and GraphPad prim 6.0 (GraphPad Inc., La Jolla,
CA, USA). Values are expressed as the mean ± standard deviation or
n (%). Comparisons between groups were performed using Student's
t-tests or Chi-square tests. Correlations were determined using
Pearson correlation analysis. In addition, the statistics were
based on an intent-to-treat analysis (22). P<0.05 was considered to indicate
statistical significance.
Results
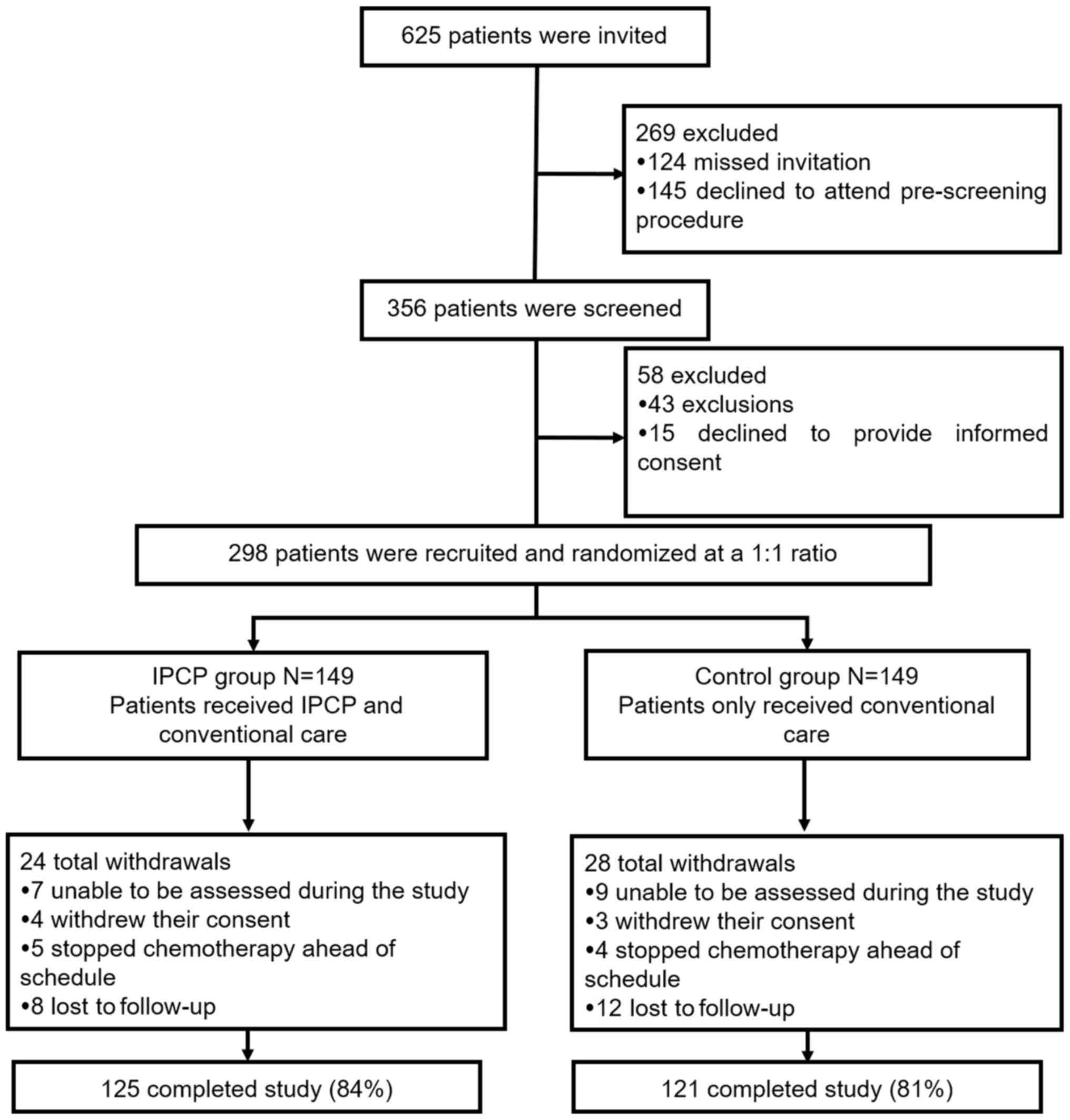
Study flow
A total of 625 patients were invited to participate
in the present study, of which while 269 patients were excluded,
including 124 who missed their invitation and 145 who declined to
attend the pre-screening. A total of 356 patients screened for
eligibility, 58 of which were excluded, including 43 who did not
meet all of the inclusion criteria and 15 who refused to provide
informed consent. The remaining 298 patients were then randomized
at a 1:1 ratio. Among these, 149 received IPCP and conventional
care (IPCP group), whereas the other 149 received only conventional
care (control group). In the IPCP group, 24 patients dropped out
during the study period, including 7 who were unable to be assessed
during the study, 4 who withdrew their consent, 5 who stopped
chemotherapy ahead of schedule and 8 who were lost to follow-up,
resulting in 125 patients (84%) who completed the entire study. In
the control group, there were a total of 28 dropouts, including 9
cases who were unable to be assessed during the study, 3 who
withdrew their consent, 4 who stopped chemotherapy ahead of
schedule and 12 who were lost to follow-up, resulting in 121
patients (81%) who ultimately completed the study (Fig. 1). In the present study, the
statistics were based on an intent-to-treat analysis (22). For patients who did not complete the
entire study, the last available values were used to represent
subsequent missing evaluation indexes.
Baseline characteristics
The baseline characteristics of the patients in the
control and IPCP groups are provided in Table II. No differences were observed in
demographic and clinical characteristics between the IPCP group and
the control group (all P>0.05). The mean age was 60.06±11.00
years in the IPCP group and 58.47±12.52 years in the control group.
The amount of patients with hypertension, hyperlipidemia and
diabetes was 48 (32.2%), 35 (23.5%) and 11 (7.4%), respectively, in
the IPCP group and 58 (38.9%), 36 (24.2%) and 14 (9.4%),
respectively, in the control group. In addition, there were no
differences between groups in HADS-A scores, HADS-A grade, HADS-D
score, HADS-D grade and EORTC QLQ-C30 scale scores (all
P>0.05).
 | Table II.Baseline characteristics of patients
in the IPCP group and the control group. |
Table II.
Baseline characteristics of patients
in the IPCP group and the control group.
| Item | IPCP group
(n=149) | Control group
(n=149) | P-value |
|---|
| Age (years) | 60.06±11.00 | 58.47±12.52 | 0.245 |
| Gender
(male/female) | 98/51 | 91/58 | 0.400 |
| Highest
education |
|
| 0.362 |
| Primary
school or less | 74 (48.3) | 73 (49.0) |
|
| High
school | 42 (28.2) | 50 (33.6) |
|
|
Undergraduate | 35 (23.5) | 26 (17.4) |
|
|
Graduate or above | 0 (0.0) | 0 (0.0) |
|
| Smoking | 58 (38.9) | 68 (45.6) | 0.241 |
| Drinking | 57 (38.3) | 60 (40.3) | 0.722 |
| Hypertension | 48 (32.2) | 58 (38.9) | 0.226 |
| Hyperlipidemia | 35 (23.5) | 36 (24.2) | 0.892 |
| Diabetes | 11 (7.4) | 14 (9.4) | 0.531 |
| Pathological
grade |
|
| 0.466 |
| 1 | 14 (9.4) | 13 (8.7) |
|
| 2 | 88 (59.1) | 98 (65.8) |
|
| 3 | 47 (31.5) | 38 (25.5) |
|
| Tumor size
(cm) | 4.68±1.28 | 4.67±1.34 | 0.940 |
| TNM stage |
|
| 0.104 |
| II | 72 (48.3) | 86 (57.7) |
|
|
III | 77 (51.7) | 63 (42.3) |
|
| HADS-anxiety
score |
|
|
|
| No
anxiety (score, 0–7) | 110 (73.8) | 118 (79.2) | 0.274 |
| Anxiety
(score, 8–21) | 39 (26.2) | 31 (20.8) |
|
| HADS-anxiety
grade |
|
| 0.308 |
| Light
grade (8–10 score) | 26 (17.4) | 15 (10.1) |
|
|
Moderate grade (11–14
score) | 11 (7.5) | 13 (8.7) |
|
| Severe
grade (15–21 score) | 2 (1.3) | 3 (2.0) |
|
| HADS-depression
score |
|
|
|
| No
depression (score, 0–7) | 98 (65.8) | 103 (69.1) | 0.536 |
|
Depression (score, 8–21) | 51 (34.2) | 46 (30.9) |
|
| HADS-depression
grade |
|
|
|
| Light
(score, 8–10) | 33 (22.1) | 24 (16.1) | 0.278 |
|
Moderate (score, 11–14) | 15 (10.1) | 14 (9.4) |
|
| Severe
(score, 15–21) | 3 (2.0) | 8 (5.4) |
|
| EORTC QLQ-C30
scale |
|
|
|
| Global
Health Status score | 63.7±14.5 | 62.9±15.8 | 0.630 |
|
Functions score | 69.9±16.5 | 69.9±18.9 | 0.995 |
|
Symptoms score | 33.3±16.9 | 30.8±14.9 | 0.189 |
Comparison of anxiety scores between
the IPCP group and control group
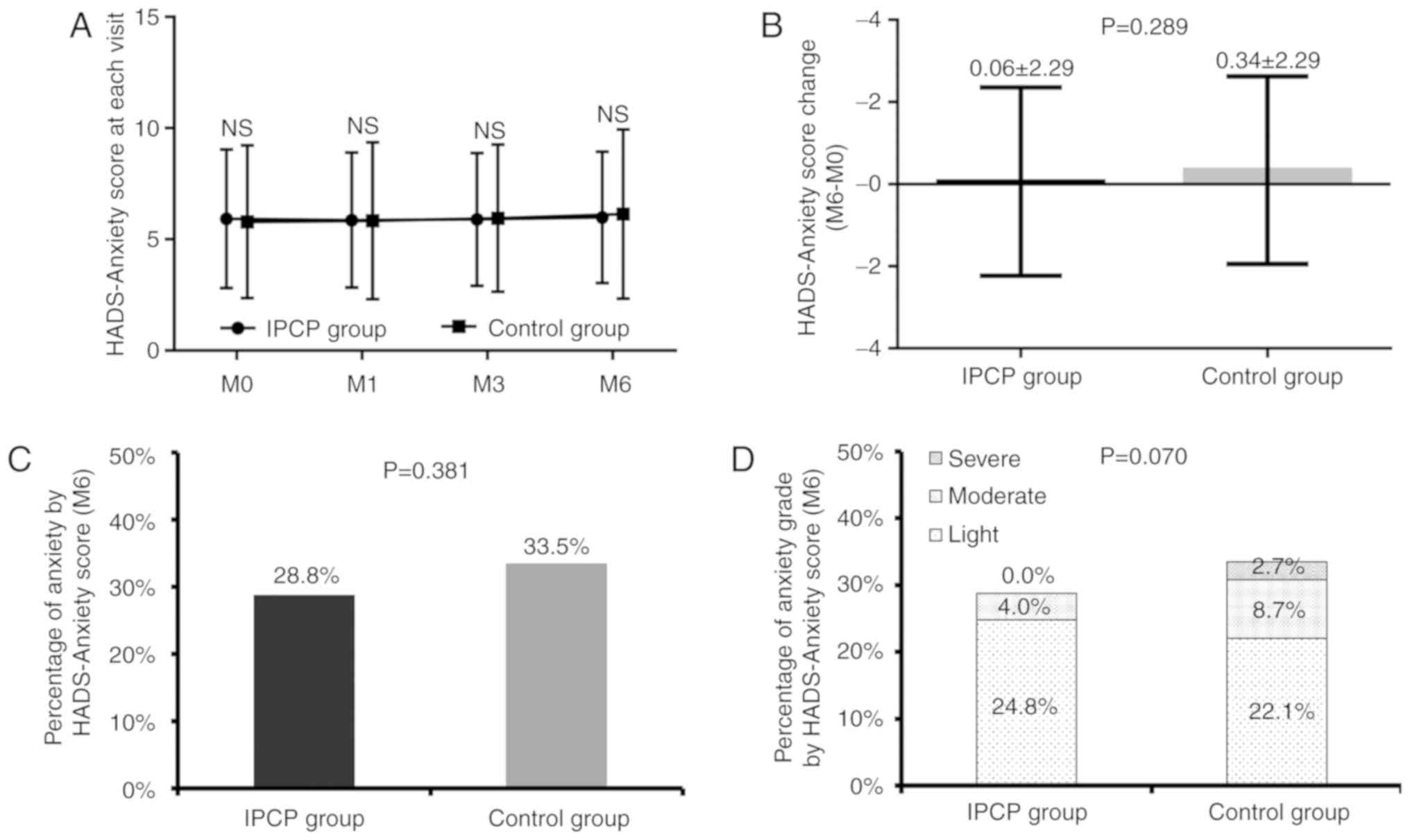
There were no differences between groups in HADS-A
scores at each visit (all P>0.05; Fig. 2A), HADS-A change score from baseline
(M0) to M6 (P=0.289; Fig. 2B) or in
the percentage of patients with anxiety according to HADS-A score
at M6 (P=0.381; Fig. 2C). Compared
with that in the control group, the anxiety score at M6 in IPCP
participants appeared to have decreased but the difference was not
statistically significant (P=0.070; Fig.
2D).
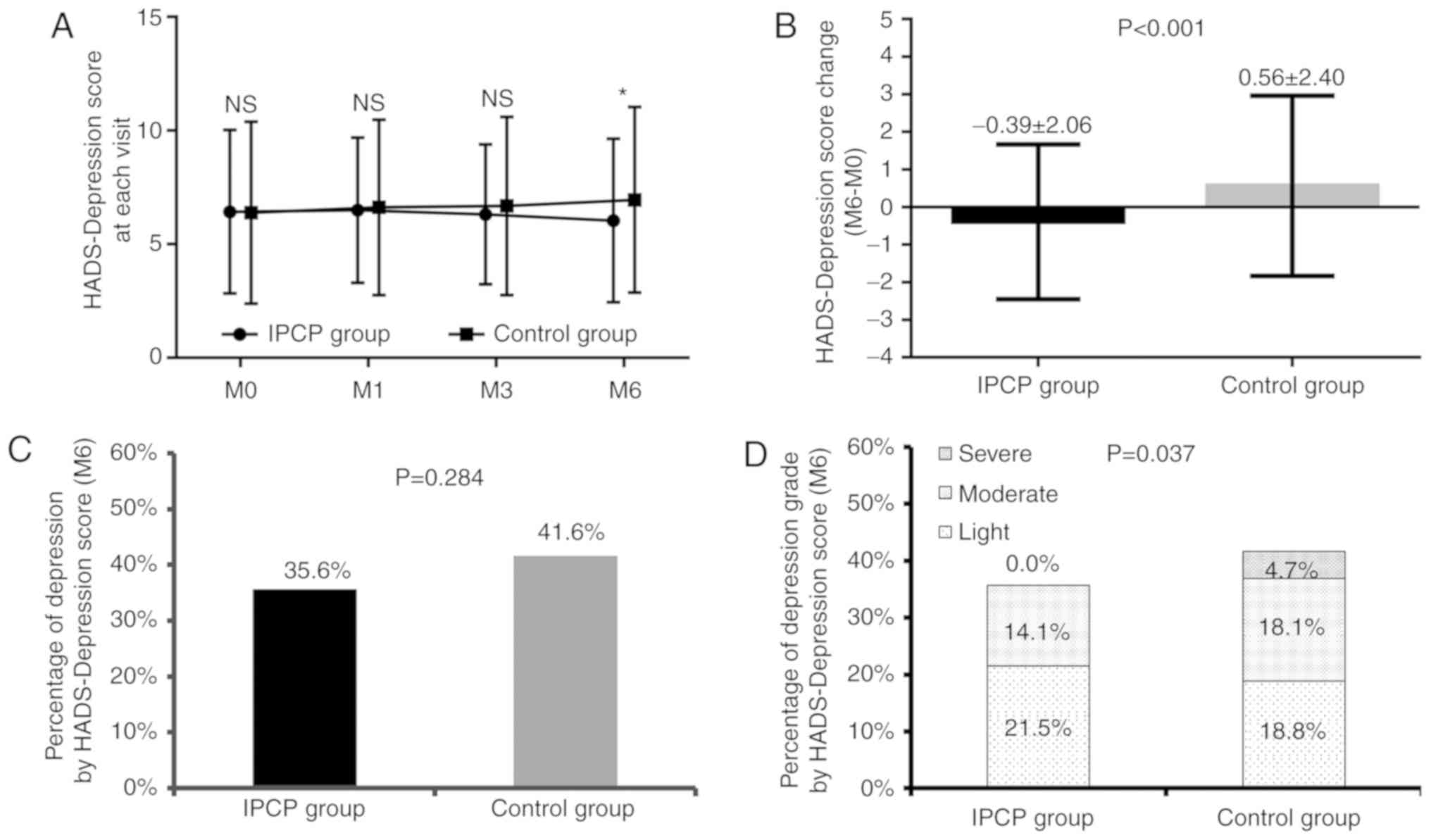
Comparison of depression scores
between the IPCP group and control group
No differences were identified in HADS-D scores at
M0, M1 or M3 between the IPCP and control groups (all P>0.05;
Fig. 3A), although they were lower
in the IPCP group than those in the control group at M6 (P<0.05;
Fig. 3A). As for HADS-D change
scores (M6-M0) between the two groups, they were decreased in the
IPCP group compared with those in the control group (P<0.001;
Fig. 3B). There was no difference
between the groups in the percentage of participants with
depression at M6 according to their HADS-D scores (P=0.284;
Fig. 3C). In addition, the
depression grade was reduced in the IPCP group compared with the
control group (P=0.037; Fig.
3D).
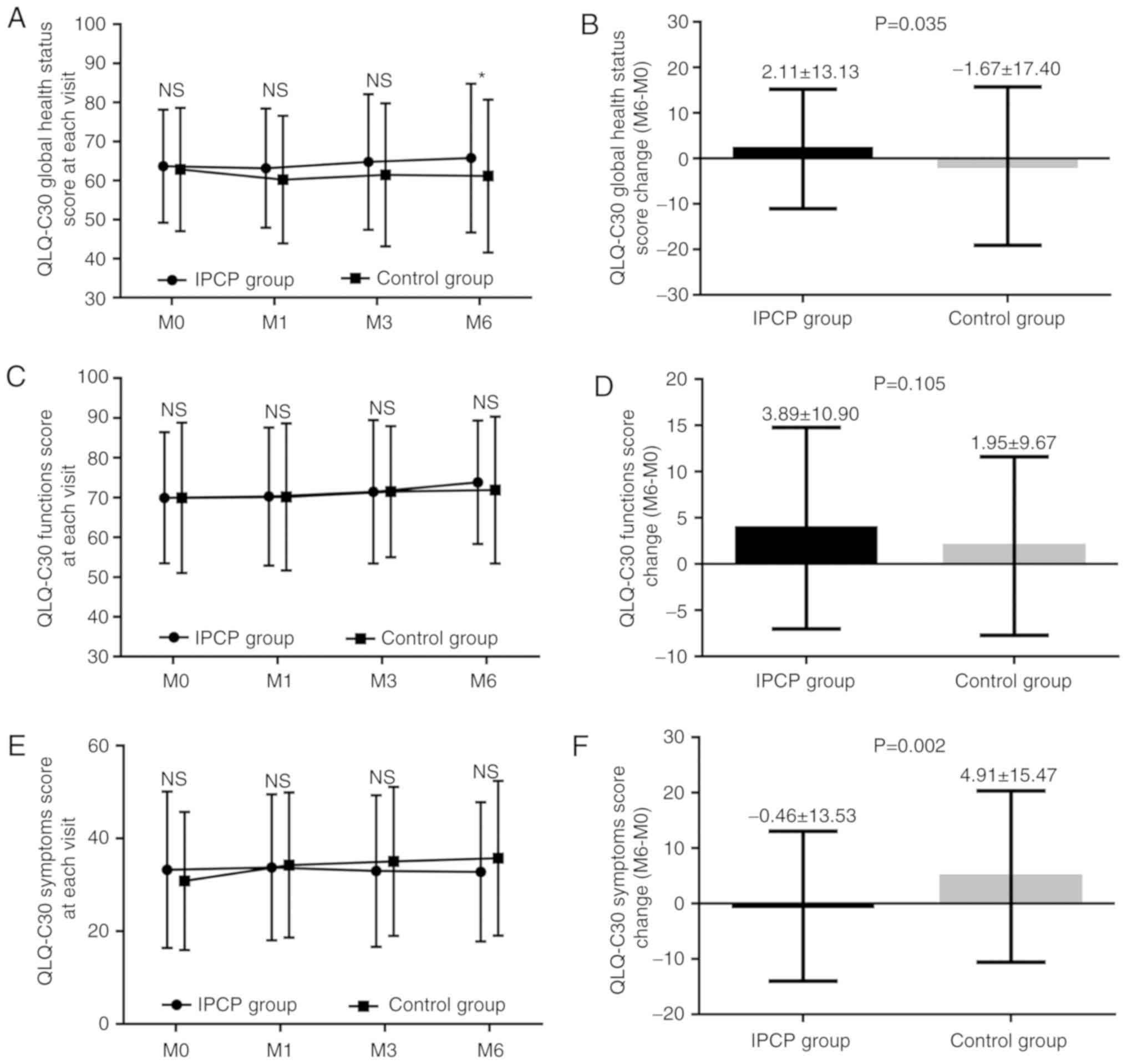
Comparison of QoL between the IPCP
group and control group
No difference in the QLQ-C30 global health status
scores was identified between the IPCP and control groups at M0, M1
or M3 (all P>0.05; Fig. 4A),
although they were higher in the IPCP group at M6 compared with
those in the control group (P<0.05; Fig. 4A). The QLQ-C30 global health status
change scores (M6-M0) exhibited a greater increase in the IPCP
group compared with that in the control group (P=0.035; Fig. 4B). Regarding the QLQ-C30 function
scores, there were no differences between the groups at each visit
(all P>0.05; Fig. 4C), and there
was also no significant difference in the change score (M0-M6)
between the two groups (P=0.105; Fig.
4D). As for the QLQ-C30 symptom scores, no differences between
the groups were present at each visit (all P>0.05), but the
QLQ-C30 symptom change scores (M6-M0) were decreased in the IPCP
group compared with those in the control group (P=0.002; Fig. 4F).
Subgroup analysis between TNM stage II
and III patients
To assess the impact of the TNM stage on the above
analysis, the patients were stratified into TNM stage II and III
groups (Fig. 5). For TNM stage II
patients, the HADS-D change scores (M6-M0) (P=0.002; Fig. 5B) and QLQ-C30 symptom change scores
(M6-M0) (P=0.050; Fig. 5E) were
decreased in the IPCP group compared with those in the control
group. However, no difference was observed in the change in HADS-A
scores (P=0.464; Fig. 5A), QLQ-C30
global health status scores (P=0.264; Fig. 5C) or QLQ-C30 function scores
(P=0.544; Fig. 5D) from baseline to
M6 between the two groups.
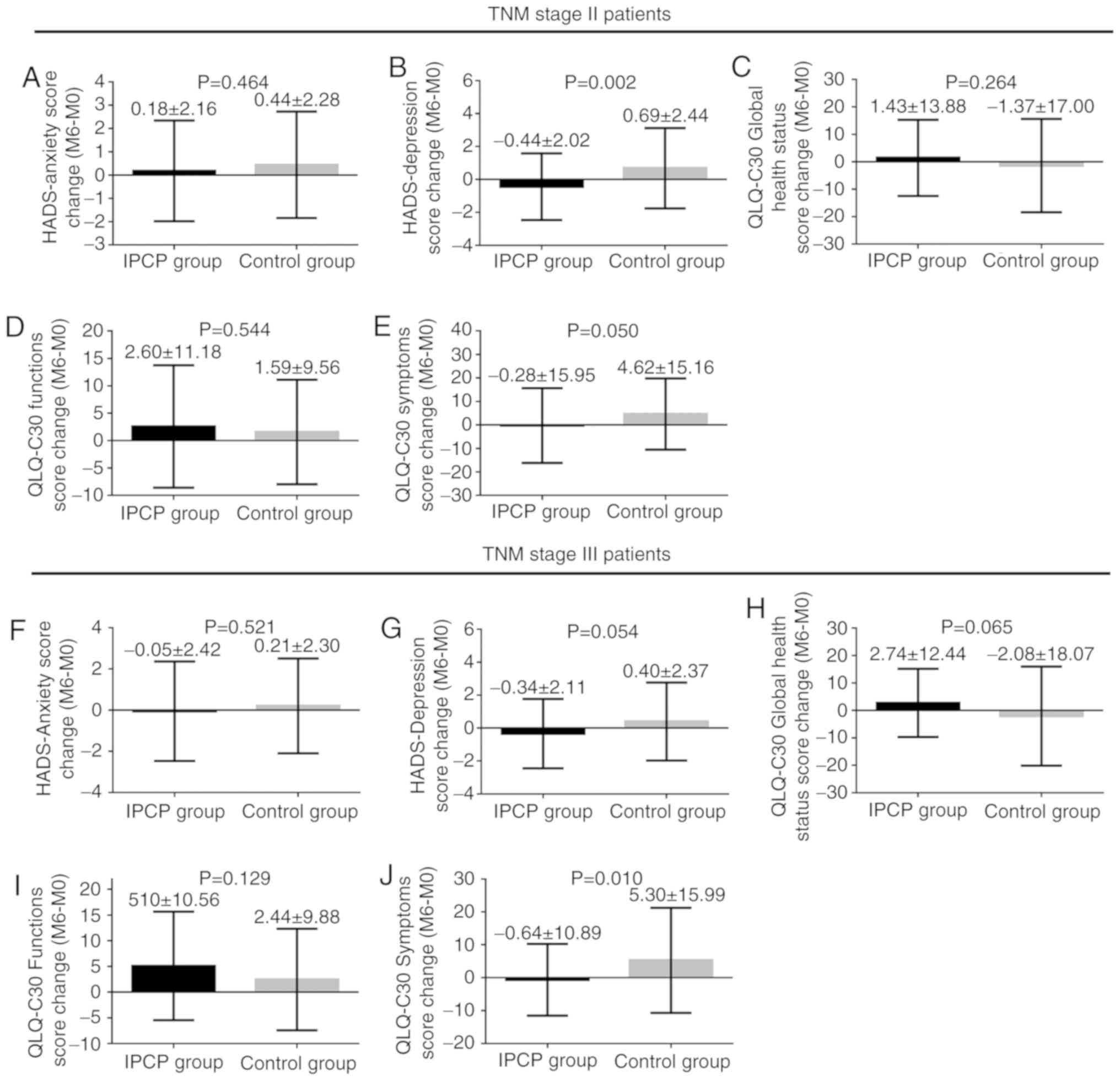 | Figure 5.Subgroup analysis between TNM stage II
and stage III patients. (A-E) For TNM stage II patients, no
difference was observed in the change of (A) HADS-anxiety score,
(C) QLQ-C30 global health status score and (D) QLQ-C30 function
score from baseline (M0) to M6 between groups. (B) The
HADS-depression score change (M6-M0) and (E) the QLQ-C30 symptoms
score (M6-M0) was decreased in the IPCP group compared with those
in the control group. (F-J) As for TNM stage III patients, (J) the
QLQ-C30 symptoms score (M6-M0) was decreased in the IPCP group
compared with that in the control group and (G) the HADS-depression
score change (M6-M0) was numerically decreased, while (H) the
QLQ-C30 global health status score change (M6-M0) was numerically
increased in the IPCP group compared with that in the control
group. (F and I) There was no difference in the change of
HADS-anxiety score and QLQ-C30 functions score from baseline (M0)
to M6 between groups. Values are expressed as the mean ± standard
deviation. NS, no significance; IPCP, incremental patient care
program; M, month; HADS, Hospital Anxiety and Depression Scale;
TNM, tumor-nodes-metastasis; QLQ-C30, European Organization for
Research and Treatment of Cancer Quality of Life Questionnaire. |
For TNM stage III patients, the QLQ-C30 symptom
scores (M6-M0) exhibited a greater decrease in the IPCP group
compared with those in the control group (P=0.010; Fig. 5J), as did the HADS-depression change
scores (M6-M0) (P=0.054; Fig. 5G),
whereas the QLQ-C30 global health status scores (M6-M0) (P=0.065;
Fig. 5H) exhibited a greater
increase in the IPCP group compared with those in the control
group. There was no difference in the change of HADS-A scores or
QLQ-C30 function scores from baseline to M6 between the groups
(P=0.129; Fig. 5F and I). The effect
of IPCP intervention on anxiety, depression and QoL scores and
their rate is also provided in Tables
SIV and SV. In addition,
changes in anxiety, depression and QoL score in the subgroups are
also presented in Table SVI.
Furthermore, correlations between HADS-A score, HADS-D score and
QLQ-C30 scores at M0 and M6 are displayed in Table SVII; however, no significant
differences were identified in control group at baseline and at 6
months. There was a significant negative correlation between HADS-A
and QLQ-C30 function scores at baseline in the IPCP group
(P=0.024), but not at 6 months.
Discussion
The major results of the present study were as
follows: i) The IPCP group experienced an insignificant decrease in
anxiety, as assessed by the HADS-A scores, and significantly
reduced depression, as assessed by the HADS-D scores; ii)
participants receiving IPCP experienced improved QoL, as assessed
by the QLQ-C30.
Recently, the survival of CRC patients has been
reported to be increased, while affected patients are at high risk
of experiencing psychosocial problems (such as depression and
anxiety), which has a negative impact on their health-associated
QoL (23,24). A previous clinical study illustrates
that CRC patients exhibited an obviously higher prevalence of
depression (19.0% vs. 12.8%) and anxiety (20.9% vs. 11.8%) compared
with cancer-free age- and sex-matched individuals (23). Another study revealed a negative
association between the patients' emotional functioning score and
HADS-A score, as well as HADS-D score, and the correlation between
HADS-D and QoL dimensions was significantly higher compared with
the correlation between QoL and HADS-A (24). These previous clinical studies
indicate that psychosocial disorders (including anxiety and
depression) are conditions that negatively influence the QoL of CRC
patients.
Based on several previous studies, HADS-A score,
HADS-D score and the EORTC QLQ-C30 scale are reliable measurement
tools for evaluating anxiety, depression and QoL of cancer patients
(25,26).
In clinical practice, care interventions have been
confirmed to be beneficial for maintaining psychological health in
cancer patients. For instance, one study reported that the health
education level of patients prior to treatment initiation was
associated with decreased anxiety and depression, as assessed by
HADS-A/D scores (6). Another study
revealed that, compared with the baseline, the HADS-D score was
decreased in patients with CRC after a nurse-assisted screening and
referral for participation in a care program (27). Although certain previous studies have
demonstrated the positive impact of care interventions on mental
health among patients with CRC, only few have investigated the
effects of an IPCP on anxiety and depression in patients with CRC
receiving adjuvant chemotherapy. In the present study, a novel IPCP
was designed in three steps as follows: i) After a literature
search, the common care methods for carcinoma were reviewed. ii)
The detailed program was designed and three nurses with nursing
experience of >10 years at the department of digestion were
invited to rate and make decisions regarding the intervention
program; the final result was selected as the final program. iii) A
preliminary study was performed to confirm the effectiveness of the
IPCP in 10 patients with CRC receiving adjuvant chemotherapy, and
its efficacy was indicated to be good in these patients. The
difference in the contents between IPCP and traditional care were
that i) IPCP was performed more intensively, while, for traditional
care, the post-operative medicine management and usual advice on
post-operative rehabilitation were provided according to patients'
requirements (28); ii) IPCP
consisted of patient health education, physical exercise, telephone
counseling, regular examination as well as care activities. iii)
During the care activities, all caregivers were provided with
lessons with regard to how to help patients complete their daily
exercise. The present study demonstrated the possible efficacy of
IPCP in slightly decreasing anxiety and reducing depression. The
possible reasons were as follows: First, IPCP included regular
patient health education, which may provide correct information and
knowledge on risk factors, treatment and prognosis of CRC to
patients, thereby decreasing their fear and confusion that may
arise from inaccurate information and rumors from unreliable
websites, thus reducing their risk of anxiety and depression.
Furthermore, IPCP is a care intervention with low- and
high-intensity physical exercise, and the physical exercise may
promote the motor and sensory recovery thereby increasing patient
confidence and reducing the risk of anxiety and depression
(29). Finally, IPCP is a program
for patients that relies on assistance from caregivers and nurses,
thus providing more opportunities for patients to increase
communication. Increased communication between patients and nurses
may contribute to increased psychological health, including
decreased anxiety and depression, among patients with CRC receiving
adjuvant chemotherapy.
QoL is a critical outcome measure in the assessment
of health status and treatment efficacy in different patients, in
including patients with cancer and rheumatoid arthritis. According
to accumulating evidence, favorable outcomes of care interventions
regarding QoL have been identified in cancer patients. For
instance, one previous study suggested that QoL, as assessed by the
EORTC QLQ-C30 and EuroQol-5 dimensions scales, was improved among
patients receiving chemotherapy for CRC after they received health
education prior to treatment initiation (6). According to a review of 11 studies,
various forms of psychosocial intervention, including health
educational interventions, cognitive-behavioral therapy, relaxation
exercise and supportive group therapy, contribute to a decreased
length of hospital stay, decreased days to stoma proficiency and
decreased hospital-associated anxiety and depression, as well as
improved QoL outcomes in patients with CRC (30). Therefore, for patients with CRC, care
interventions have a positive effect regarding the improvement of
QoL. In the present study, the influence of IPCP on QoL was also
explored, and assessment via the QLQ-C30 global health status
scores, QLQ-C30 function scores and QLQ-C30 symptom scores
suggested that IPCP contributed to an improvement in QoL among
patients with CRC receiving adjuvant chemotherapy. The first
possible reason for this effect is that IPCP is a safe and
effective way to provide low- and high-intensity physical exercise
that contributes to functional recovery and to achieve long-term
improvements in activities of daily living, thereby increasing
physical health and improving QoL of patients with CRC.
Furthermore, IPCP has an important role in maintaining a good
environment for increasing communication among patients, family
members and nurses, thereby promoting mutual understanding and
cooperation and decreasing anxiety and depression, thus improving
mental health and increasing QoL.
Of note, the present study had certain limitations.
First, the sample size was relatively small, leading to low
statistical power. Furthermore, all patients enrolled in the
present study were from a single centre; thus, further studies
should be performed recruiting patients from multiple centres.
Finally, the duration of follow-up was relatively short; thus, the
long-term effects on IPCP on anxiety, depression and QoL in
patients with CRC remain elusive.
In conclusion, IPCP led to a slight decrease in
anxiety to a certain extent and contributed to a significant
reduction in depression and an improvement in QoL in patients with
CRC receiving adjuvant chemotherapy. The present study provided a
novel IPCP that may serve as an efficient care program for outcome
improvements in CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL designed the experiment, JL and XL performed the
experiments and wrote the manuscript, and XL analyzed the data and
revised the manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
tenets established in the Declaration of Helsinki and was approved
by the Ethics Committee of the 2nd Affiliated Hospital of Harbin
Medical University (Harbin, China). Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonzalez-Saenz de Tejada M, Bilbao A, Baré
M, Briones E, Sarasqueta C, Quintana JM and Escobar A; CARESS-CCR
Group, : Association between social support, functional status, and
change in health-related quality of life and changes in anxiety and
depression in colorectal cancer patients. Psychooncology.
26:1263–1269. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Polat U, Arpaci A, Demir S, Erdal S and
Yalcin S: Evaluation of quality of life and anxiety and depression
levels in patients receiving chemotherapy for colorectal cancer:
Impact of patient health education before treatment initiation. J
Gastrointest Oncol. 5:270–275. 2014.PubMed/NCBI
|
|
7
|
Wan Puteh SE, Saad NM, Aljunid SM, Abdul
Manaf MR, Sulong S, Sagap I, Ismail F and Muhammad Annuar MA:
Quality of life in Malaysian colorectal cancer patients. Asia Pac
Psychiatry. 5 (Suppl 1):S110–S117. 2013. View Article : Google Scholar
|
|
8
|
Tsunoda A, Nakao K, Hiratsuka K, Yasuda N,
Shibusawa M and Kusano M: Anxiety, depression and quality of life
in colorectal cancer patients. Int J Clin Oncol. 10:411–417. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alacacioglu A, Binicier O, Gungor O, Oztop
I, Dirioz M and Yilmaz U: Quality of life, anxiety, and depression
in Turkish colorectal cancer patients. Support Care Cancer.
18:417–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medeiros M, Oshima CT and Forones NM:
Depression and anxiety in colorectal cancer patients. J
Gastrointest Cancer. 41:179–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demark-Wahnefried W and Jones LW:
Promoting a healthy lifestyle among cancer survivors. Hematol Oncol
Clin North Am. 22:319–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morey MC, Snyder DC, Sloane R, Cohen HJ,
Peterson B, Hartman TJ, Miller P, Mitchell DC and Demark-Wahnefried
W: Effects of home-based diet and exercise on functional outcomes
among older, overweight long-term cancer survivors: RENEW: A
randomized controlled trial. JAMA. 301:1883–1891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allgayer H, Owen RW, Nair J, Spiegelhalder
B, Streit J, Reichel C and Bartsch H: Short-term moderate exercise
programs reduce oxidative DNA damage as determined by
high-performance liquid chromatography-electrospray ionization-mass
spectrometry in patients with colorectal carcinoma following
primary treatment. Scand J Gastroenterol. 43:971–978. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao F, Song X, Chen Q, Dai Y, Xu R, Qiu C
and Guo Q: Effectiveness of psychological interventions on
depression in patients after breast cancer surgery: A meta-analysis
of randomized controlled trials. Clin Breast Cancer. 17:171–179.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Geng W, Yin J and Zhang J: Effect of
one comprehensive education course to lower anxiety and depression
among Chinese breast cancer patients during the postoperative
radiotherapy period-one randomized clinical trial. Radiat Oncol.
13:1112018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: AJCC Cancer Staeging Manual. 7th. New
York: Springer; 2010
|
|
17
|
Seligman MEP, Walker EF and Rosenhan DL:
Abnormal Psychology. 4th. W.W. Norton & Company; New York, NY:
2001
|
|
18
|
Diagnostic and Statistical Manual of
Mental Disorders, Fifth Edition (DSM-5). American Psychiatric
Association. 2013.
|
|
19
|
Zigmond AS and Snaith RP: The hospital
anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barcaccia B: ‘Quality Of Life: Everyone
Wants It, But What Is It?’. 2016.PubMed/NCBI
|
|
21
|
Tung HY, Chao TB, Lin YH, Wu SF, Lee HY,
Ching CY, Hung KW and Lin TJ: Depression, fatigue, and QoL in
colorectal cancer patients during and after treatment. West J Nurs
Res. 38:893–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van Vulpen JK, Velthuis MJ, Steins
Bisschop CN, Travier N, Van Den Buijs BJ, Backx FJ, Los M, Erdkamp
FL, Bloemendal HJ, Koopman M, et al: Effects of an exercise program
in colon cancer patients undergoing chemotherapy. Med Sci Sports
Exerc. 48:767–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mols F, Schoormans D, de Hingh I,
Oerlemans S and Husson O: Symptoms of anxiety and depression among
colorectal cancer survivors from the population-based, longitudinal
PROFILES Registry: Prevalence, predictors, and impact on quality of
life. Cancer. 124:2621–2628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aminisani N, Nikbakht H, Asghari
Jafarabadi M and Shamshirgaran SM: Depression, anxiety, and health
related quality of life among colorectal cancer survivors. J
Gastrointest Oncol. 8:81–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas BC, Devi N, Sarita GP, Rita K,
Ramdas K, Hussain BM, Rejnish R and Pandey M: Reliability and
validity of the Malayalam hospital anxiety and depression scale
(HADS) in cancer patients. Indian J Med Res. 122:395–399.
2005.PubMed/NCBI
|
|
26
|
Matsumoto T, Ohashi Y, Morita S, Kobayashi
K, Shibuya M, Yamaji Y, Eguchi K, Fukuoka M, Nagao K, Nishiwaki Y,
et al: The quality of life questionnaire for cancer patients
treated with anticancer drugs (QOL-ACD): Validity and reliability
in Japanese patients with advanced non-small-cell lung cancer. Qual
Life Res. 11:483–493. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livingston PM, Craike MJ, White VM,
Hordern AJ, Jefford M, Botti MA, Lethborg C and Oldroyd JC: A
nurse-assisted screening and referral program for depression among
survivors of colorectal cancer: Feasibility study. Med J Aust 193
(5 Suppl). S83–S87. 2010.
|
|
28
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheiner LB: Is intent-to-treat analysis
always (ever) enough? Br J Clin Pharmacol. 54:203–211. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoon LS, Chi Sally CW and Hong-Gu H:
Effect of psychosocial interventions on outcomes of patients with
colorectal cancer: A review of the literature. Eur J Oncol Nurs.
17:883–891. 2013. View Article : Google Scholar : PubMed/NCBI
|