Introduction
Bladder cancer is the fifth most common cancer
worldwide. Most bladder cancers are not muscle-invasive and can
generally be managed with only transurethral resection of bladder
tumors (TURBT) and intravesical therapy (1). Patients with high-grade
non-muscle-invasive bladder cancer may eventually develop muscle
invasion, necessitating surgical management with radical cystectomy
or additional therapies (2) relevant
to bladder cancer detection and prognosis.
Although imaging is widely used clinically, its
ability to predict clinical outcomes for patients with bladder
cancer remains limited. Therefore, tumor marker expression is an
important component of the prognosis; however, specific markers to
detect bladder cancer have not yet been identified. In addition,
the prognostic power of a single marker is limited; however, the
identification of a combination of markers to predict bladder
cancer outcomes may have greater prognostic significance. Previous
data have implicated peroxisome proliferator-activated receptor-γ
(PPAR-γ) and phosphatase and tensin homologue deleted on chromosome
ten (PTEN) inactivation in the development of keratinizing squamous
metaplasia, an important process driving malignancy in the
intestinal tract (3). This process
is also significant during urogenital differentiation, and previous
studies using either embryonic urogenital epithelium or adult
bladder urothelium have demonstrated that urothelium can be
programed to form prostate-like acini (4,5). A
previous study identified specific molecular pathways activated
during normal bladder development and urothelial response to injury
(6). The molecular findings from
these studies are relevant to malignant tumor development and
describe potential novel markers that may have clinical
utility.
The PPAR-γ and PTEN pathways are important for
maintaining cellular energy balance and during urothelial
differentiation. PPAR-γ is a type II nuclear receptor encoded by
the PPAR-γ gene in humans that regulates fatty acid and glucose
metabolism, and PPAR-γ activation appears to have important
functions in various human cancer cell lines (7,8). PTEN is
a protein encoded by the PTEN gene, which has been identified to
contain several cancer-causing mutations. It functions as a tumor
suppressor gene through the activation of its phosphatase protein
product. It is also commonly mutated in bladder disease (9), where it has an important role in
regulating cell differentiation and tumorigenesis. It has
previously been suggested that the PPAR-γ agonist pioglitazone is
associated with an increased risk for bladder cancer (10). PTEN inactivation has also been
implicated in bladder cancer development, in which it synergizes
with other genetic changes to drive tumorigenesis. Expression of
these two markers exhibits tissue heterogeneity, and there are no
reports at present examining the association between their
expression and progression of bladder cancer.
In the present study, the expression levels of
PPAR-γ and PTEN protein in bladder cancer and the association
between clinical parameters and PPAR-γ and PTEN protein expression
were examined.
Materials and methods
Patients
Patients were recruited from the North District of
Suzhou Municipal Hospital (Suzhou, China) from January 2010 to
January 2015. Tissues included in the analysis were taken from 94
patients, including 77 male patients and 17 female patients aged
from 33 to 92 years old (mean, 71±11.22), 60 of whom had received
TURBT, whereas 34 were treated by partial cystectomy.
Applying the World Health Organization
classification (2004) (11), tissues
were classified as low-grade urothelial carcinoma (59 cases) and
high-grade urothelial carcinoma (35 cases). Clinical classification
was performed according to the Classification of Malignant Tumors
(TNM) criteria; with superficial tumors (Ta-T1; n=61) and invasive
tumors (T2-T4; n=33). Lymphatic metastasis was present in 31 cases.
A total of 20 normal bladder tissue samples were obtained as
control samples from 13 male patients during radical prostatectomy
and from 7 female patients during biopsy. All patients provided
written informed consent and the present study was approved by
Suzhou Municipal Hospital Ethics Committee.
Bioinformatics analysis
A public database, The Cancer Genome Atlas (TCGA)
(https://cancergenome.nih.gov/) was
employed for this study. In total, information of 994 patients were
collected and the patients with incomplete information were
excluded from this study. The gene expression was analyzed by SPSS
software version 13.0 (SPSS, Inc.,) and GraphPad Software Version 5
(GraphPad Software, Inc.).
Immunohistochemistry (IHC)
Tissues were fixed in 10% formalin at room
temperature for 24–48 h and embedded in paraffin blocks. Sections
(3–5 µm) were prepared from paraffin blocks for staining. During
immunohistochemical analysis, the sections were hydrated in graded
alcohol dilutions. Following heat-mediated antigen retrieval, the
sections were incubated with 3% H2O2 for 15
min at room temperature and blocked with 5% bovine serum albumin
(cat. no. ST023; Beyotime Institute of Biotechnology, Haimen,
China) for 1 h at room temperature. Sections were subsequently
incubated with primary antibodies overnight at 4°C followed by
secondary antibody incubation for 1 h at room temperature. The
primary antibodies used were rabbit anti-PPAR-γ antibody (1:200;
cat. no. ab209350; Abcam, Cambridge, UK) and rabbit anti-PTEN
antibody (1:200; cat. no. ab32199; Abcam). The
horseradish-peroxidase conjugated secondary antibody was goat
anti-rabbit IgG (1:2,000; cat. no. sc-2040; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), which was used at 1:200.
Hematoxylin was used to counterstain nuclei at room temperature for
3 min. Subsequently, images captured from 3–5 fields of view with
the magnification of ×400 from each sample were analyzed under the
light microscope. Samples with >10% positive cells were
considered positive. The negative control for this experiment was
sections incubated without primary antibody and the indicated
antibodies were tested in the positive control samples according to
the protocols provided by the manufacturer (anti-PPAR-γ antibody,
cat. no. ab209350; anti-PTEN antibody, cat. no. ab32199; Abcam).
The microscopes and imaging system, Leica Microsystems GmbH
(Wetzlar, Germany), was used for analysis, and positive rates were
analyzed. Grey levels were analyzed automatically for IHC paraffin
blocks. In the grey level analysis, the level is negatively
correlated with the expression level of proteins. The detailed
methods used are as reported previously (12). In this method, low expression is
reported as a high grey level, and high protein expression is
reported as a low grey level. The grey level data are presented in
the results.
Statistical analysis
All data were analyzed using SPSS software version
13.0 (SPSS, Inc., Chicago, IL, USA). Patient parameters are
presented as the mean ± standard error of the mean for each
characteristic. Pearson correlation analysis was used to determine
the coefficient of association between clinical characteristics and
PPAR-γ or PTEN. The Kruskal-Wallis test was used to analyze the
enumeration data. Measurements between different groups were
statistically analyzed using a two-tailed Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
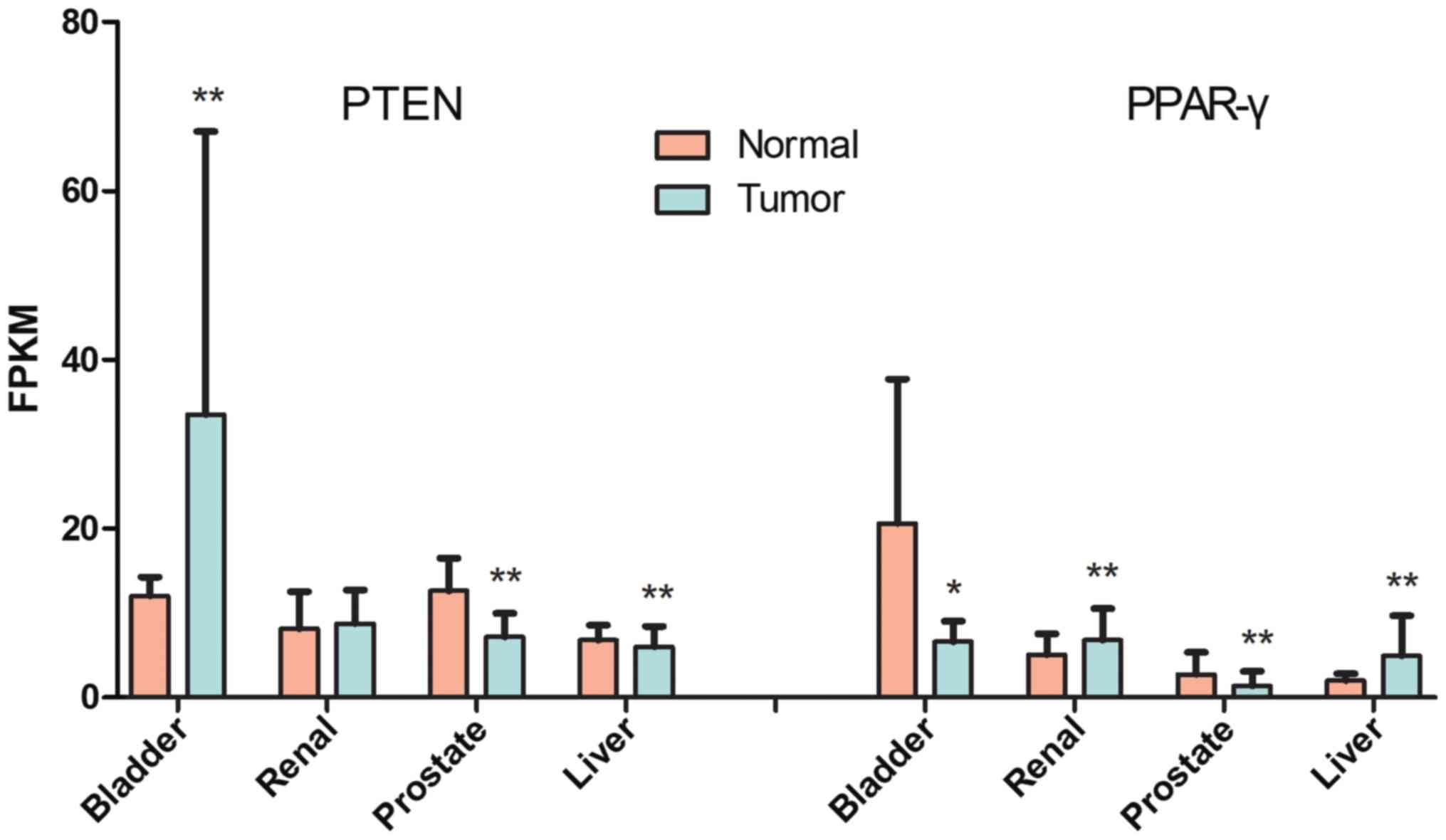
Bioinformatics analysis reveals
distinct expression patterns of PPAR-γ and PTEN in bladder
cancer
As presented in Fig.
1, PTEN expression in bladder cancer is highest among the
cancers examined. PTEN expression was significantly reduced in
prostate and liver cancers compared with normal tissues
(P<0.001), although in bladder cancer, it was significantly
increased compared with normal tissue (P<0.001). PPAR-γ
expression was downregulated in bladder (P<0.05) and prostate
(P<0.001) cancers. In renal and liver tumors, PPAR-γ expression
increased (P<0.001). Therefore, heterogeneity among different
cancers was observed for PPAR-γ and PTEN expression.
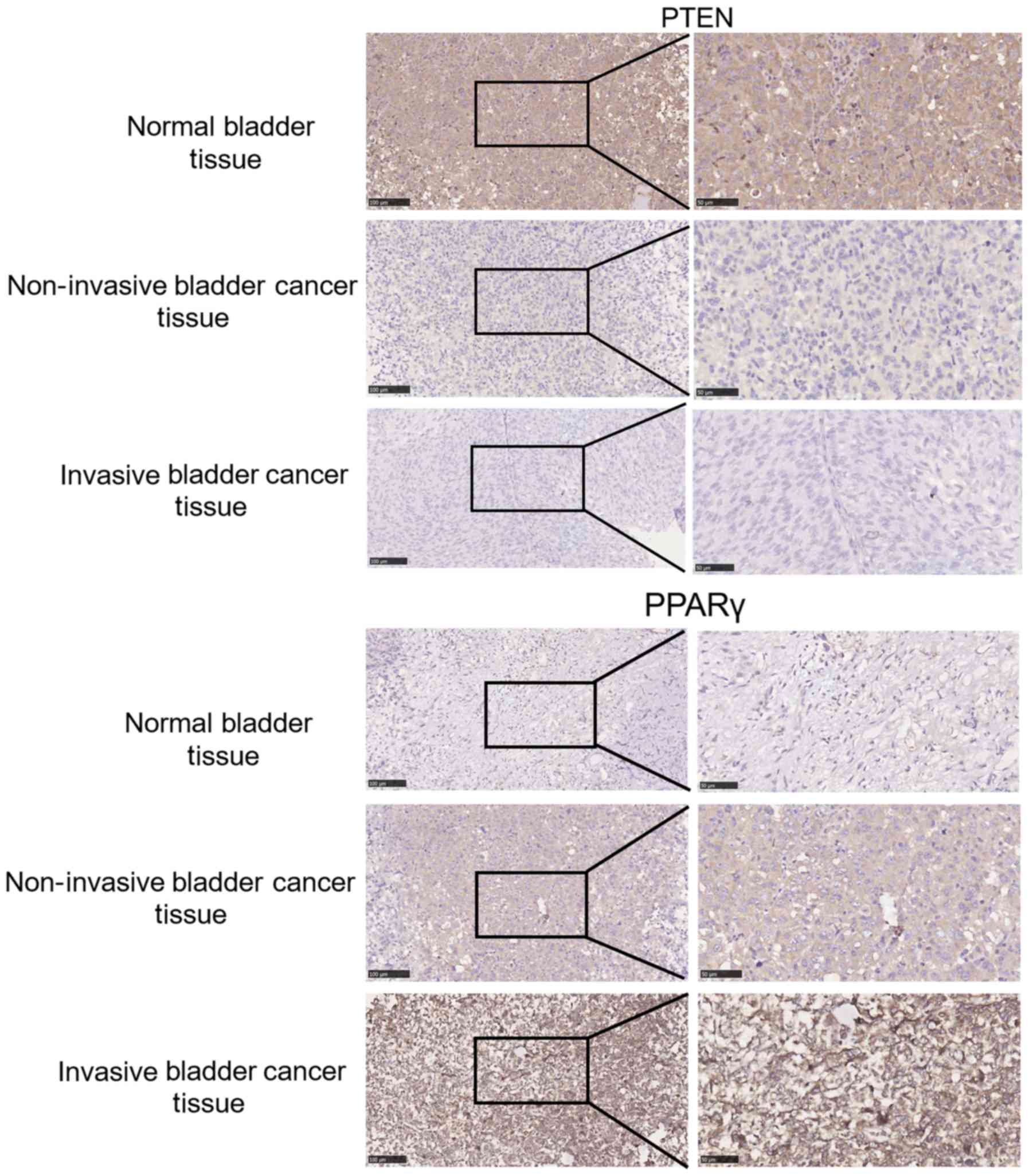
Validation of PTEN and PPAR-γ
expression in clinical bladder cancer samples
IHC was used to evaluate PTEN and PPAR-γ expression
in paraffin slides from bladder cancer samples. As presented in
Fig. 2, PTEN expression was reduced
in bladder cancer tissue compared with normal tissues and was
markedly decreased for the duration of cancer progression. PPAR-γ
levels were markedly increased in bladder cancers and increased as
invasion and bladder carcinoma became more severe. Furthermore, it
was concluded that PTEN and PPAR-γ expression were markedly
negatively associated in clinical samples.
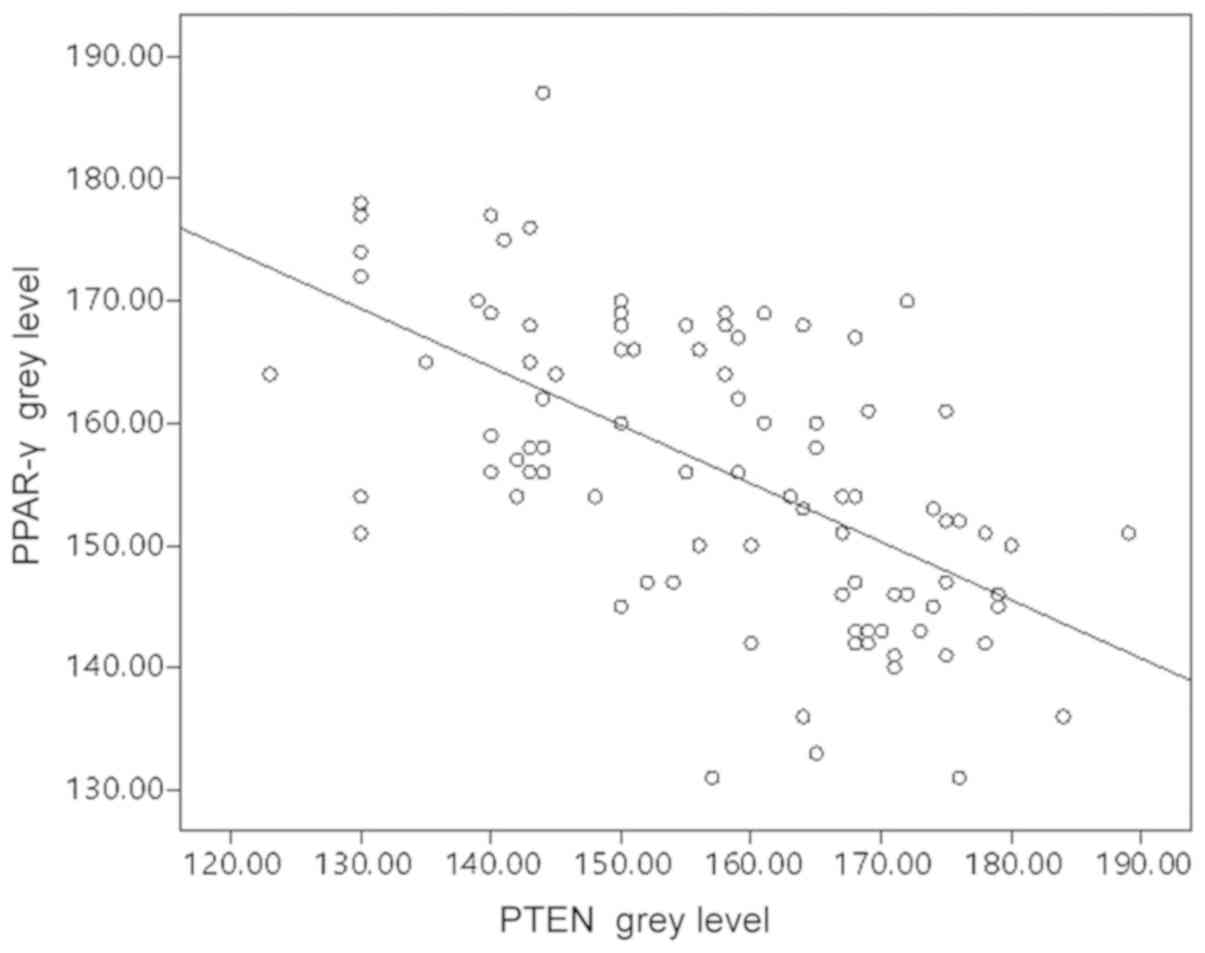
PTEN and PPAR-γ levels are negatively
correlated in bladder cancer
PTEN and PPAR-γ expression were further analyzed in
tissue samples from a cohort of patients with bladder cancer. PTEN
expression was significantly lower in bladder cancer samples than
in normal tissues (P<0.0001; Table
I). However, PPAR-γ levels were significantly elevated in
bladder cancer compared with normal tissues (P<0.0001; Table II). To demonstrate the association
between PTEN and PPAR-γ, scatter diagrams were used to indicate the
distribution of biomarker expression, and Pearson correlation
analysis was used to evaluate statistical significance. As
presented in Fig. 3, a significant
negative correlation was confirmed between PPAR-γ and PTEN
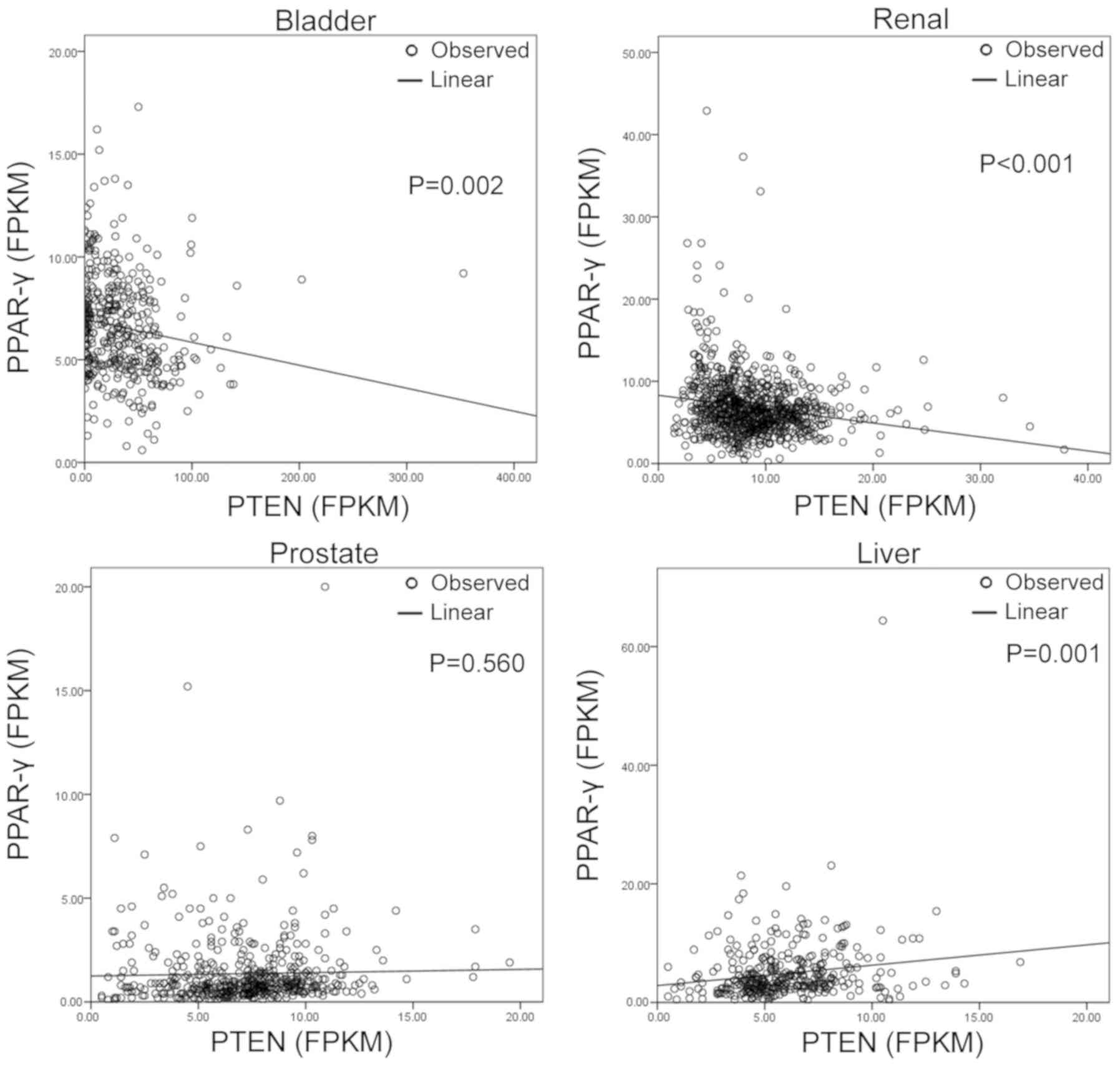
expression (r=−0.604; P<0.05). However, in other cancers, this
association changed based on cancer type. Using data from the TCGA
database (Fig. 4), PPAR-γ and PTEN
were positively correlated in liver (r2=0.033;
P<0.001) and renal cancers (r2=0.03; P<0.001). In
prostate cancer, there was no significant correlation between
PPAR-γ and PTEN expression. The bladder cancer data collected from
the TCGA database, however, generated different results to those
observed in the present clinical samples (P=0.002; Figs. 1 and 4).
 | Table I.PTEN expression in bladder
tissues. |
Table I.
PTEN expression in bladder
tissues.
|
| PTEN expression
(n) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | + | − | PTEN positive rate
(%) | χ2 | P-value |
|---|
| Normal bladder | 20 | 0 | 100 | 15.84 | <0.0001 |
| Bladder cancer | 49 | 45 | 50 |
|
|
 | Table II.PPAR-γ expression in bladder
tissues. |
Table II.
PPAR-γ expression in bladder
tissues.
|
| PPAR-γ expression
(n) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | + | − | PPAR-γ positive rate
(%) | χ2 | P-value |
|---|
| Normal bladder | 4 | 16 | 20 | 15.82 | <0.0001 |
| Bladder cancer | 64 | 30 | 68 |
|
|
PPAR-γ and PTEN are associated with
bladder cancer progression
The mean age of the individuals that provided
clinical samples was 71±11.22 years with a range of 33–92 years.
The PPAR-γ grey level was significantly upregulated in tumor
tissues (189.55±6.85 vs. 156.31±11.83; P=0.002; Table III) compared with normal bladder
tissue. Notably, unlike renal cancer and colorectal cancer, bladder
cancer tissue exhibited PTEN downregulation (133.00±7.69 vs.
157.3±14.98; P=0.001; Table III)
(13,14). Furthermore, although tumor size was
not associated with expression, PPAR-γ levels were higher in
superficial bladder cancers than in invasive tumors (grey level,
162.26±9.40 vs. 150.23±12.56; P< 0.01), and PTEN was expressed
at significantly lower levels in superficial bladder cancers than
in invasive tumors (grey level 145.30±6.98 vs. 170.61±8.88;
P<0.01). PPAR-γ levels were also lower in low-grade bladder
cancer samples than in high-grade cancer tissues (grey level
159.22±10.85 vs. 151.40±11.94; P=0.002), whereas the opposite
association was observed in the PTEN analysis (grey level
153.51±16.25 vs. 163.91±9.67; P=0.001). The association between
lymphatic metastasis and marker expression was also assessed and
revealed that PPAR-γ levels were elevated (grey level, 159.54±10.31
vs. 149.74±12.15; P<0.01) in patients without lymphatic
metastasis, whereas PTEN levels were reduced (grey level,
153.90±15.63 vs. 164.45±10.66; P<0.01) in the same individuals.
No significance was observed between marker expression and number
of local tumors.
 | Table III.PPAR-γ and PTEN grey level in bladder
tissues according to different individual characteristics. |
Table III.
PPAR-γ and PTEN grey level in bladder
tissues according to different individual characteristics.
| Group | n | PPAR-γ (grey
level) | T-value | P-value | PTEN (grey
level) | T-value | P-value |
|---|
| Normal bladder | 20 | 189.55±6.85 | 12.11 | 0.002 | 133.00±7.69 | 7.06 | 0.001 |
| Bladder cancer | 94 | 156.31±11.83 |
|
| 157.30±14.98 |
|
|
| Tumor size
(cm) |
|
|
|
|
|
|
|
| ≤3 | 46 | 158.07±11.97 | 1.42 | 0.160 | 155.48±16.06 | 1.21 | 0.230 |
|
>3 | 48 | 154.63±11.57 |
|
| 159.21±13.79 |
|
|
| Clinic grade |
|
|
|
|
|
|
|
|
Superficial | 61 | 162.26±9.40 | 9.92 | <0.01 | 150.23±12.56 | 9.14 | <0.01 |
|
Invasive | 33 | 145.30±6.98 |
|
| 170.61±8.88 |
|
|
| Pathological
grade |
|
|
|
|
|
|
|
| Low
grade | 59 | 159.22±10.85 | 3.25 | 0.002 | 153.51±16.25 | 3.89 | 0.001 |
| High
grade | 35 | 151.40±11.94 |
|
| 163.91±9.67 |
|
|
| Lymphatic
metastasis |
|
|
|
|
|
|
|
| No | 61 | 159.54±10.31 | 4.08 | <0.01 | 153.90±15.63 | 3.84 | <0.01 |
|
Yes | 33 | 149.74±12.15 |
|
| 164.45±10.66 |
|
|
| Number of local
tumors |
|
|
|
|
|
|
|
|
Single | 47 | 157.85±11.27 | 1.27 | 0.208 | 154.57±16.05 | 1.84 | 0.069 |
|
Multiple | 47 | 154.77±12.30 |
|
| 160.19±13.42 |
|
|
Discussion
In the present study, PTEN and PPAR-γ expression was
analyzed in bladder cancers and a negative correlation was
demonstrated between them. This is the first time, to our
knowledge, that this association has been documented in bladder
cancer, which differs from other cancer types (13,14).
Furthermore, the association between PTEN and PPAR-γ expression and
bladder cancer progression was examined, revealing different
expression correlations during bladder cancer development.
As one of the most common carcinomas of the urinary
tract, bladder cancer is primarily (>90%) a transitional cell
carcinoma (15). Cystoscopy is a
commonly used method that generates a diagnosis by the biopsy of
suspicious lesions. The gold standard for diagnosis is a biopsy
obtained during cystoscopy, during which cancer may be an
incidental finding (16). In many
cases, cystoscopy can overlook a tumor, particularly when it is
small, and is also highly dependent on clinician skill (17). Therefore, biomarker identification is
important for the diagnosis and prognosis of bladder cancer. Many
limits and biases exist for single markers, highlighting the
potential utility of a combination of biomarkers as an effective
predictor of bladder cancer progression.
PPAR-γ is widely known for its influence on
metabolism and inflammation as well as its role in cell
differentiation and apoptosis (18,19). In
addition, it has been studied in oncology, and a number of studies
suggest that PPAR-γ promotes cancer development and is highly
expressed in urinary tumors (20,21). In
contrast, other studies have suggested that the activation of
PPAR-γ indirectly blocks carcinogenesis and aids in anticancer
therapy (22,23). Therefore, PPAR-γ expression and
function exhibits apparent tissue heterogeneity. Based on these
findings, it was hypothesized that it may also be a biomarker for
detecting early stage bladder cancer.
PTEN is important for cell growth and regulates
metabolic processes, cell motility and polarity via the
phosphoinositide 3-kinase (PI3K) pathway (24). Furthermore, PTEN also inhibits tumor
angiogenesis through the PI3K/protein kinase B (AKT) pathway, which
regulates vascular endothelial growth factor and its receptor as
well as matrix metalloproteinases. PTEN mutations remove a
contribution to the negative regulation of cell growth, leading to
tumor development (25). PTEN has
also been widely studied in the progression of prostatic, gastric,
renal and breast cancer, among others, and is an important
regulator and marker for carcinoma occurrence and development
(20).
In the present study, PTEN and PPAR-γ expression was
analyzed in different tissues using TCGA. In the present analysis,
PTEN expression was significntly elevated in bladder cancers, and
PPAR-γ was downregulated; the association of these two proteins
varied across tumor type. Notably, PTEN and PPAR-γ expression were
positively correlated in prostate and liver cancer. Although a
different view for PPAR-γ and PTEN expression in renal clear cell
cancer has been suggested, this discrepancy may be caused by
differences in metabolic subtypes of cancer. Bladder cancer tissue
from 94 clinical cases was included and different results to the
TCGA data were obtained. This may be because the normal tissue and
tumor data are collected from two different databases. Furthermore,
there may be discrepancies between RNA level and protein expression
as indicated in previous reports (26,27).
Furthermore, PTEN and PPAR-γ expression was
significantly correlated with clinical stage and pathological
progression, indicating that these molecules have potential as
markers of bladder cancer tumorigenesis and development.
Furthermore, Pearson analysis indicated that PTEN and PPAR-γ
expression was negatively correlated, which is distinct from the
association observed in liver cancer. This association may be
associated with tumorigenesis and progression. During colorectal
carcinogenesis, PPAR-γ and PTEN expression are positively
correlated, which may indicate an interaction between these two
proteins, with PTEN being a potential downstream regulatory factor
of PPAR-γ (13). However, the
present results were not in accordance with those of a previous
report (13). It is generally
accepted that PPAR-γ activates the expression of genes such as
PTEN, c-myc and P27, which inhibit cancer proliferation, metastasis
and invasion (28). It has been
reported previously that after PPAR-γ activation, PTEN expression
is elevated in colorectal cancers (13), which contributes to
phosphatidylinositol (3,4,5)-trisphosphate (PIP3) dephosphorylation,
inhibiting the activation of PIP3 and PI3K/AKT signaling.
Therefore, the cell cycle is generally blocked in the G1 stage,
inhibiting proliferation and invasion (28). In the present study, however, PTEN
expression was decreased, and PPAR-γ was increased in bladder
cancer tissue. First, although PPAR-γ is highly expressed, its
activation may not be elevated. Second, elevated expression may be
a bladder cancer adaptation that ensures the ligand level is
insufficient to generate an anti-tumor effect. Third, this may
result from a PPAR-γ mutation in bladder cancer, which requires
validation. Finally, as PTEN and PPAR-γ are both important
metabolism markers, the variation observed may be caused by
differences between the metabolic profiles of different
cancers.
However, there are also limitations in the present
study. Firstly, the patients are all from one hospital and further
studies are required to confirm the effect of these markers.
Secondly, more detailed molecular mechanisms should be further
studied for PTEN and PPAR-γ in bladder cancer. Thirdly, in order to
detect bladder cancer more concisely, more markers should be
supplied to this multifactorial analysis.
In conclusion, the present study suggests that
PPAR-γ and PTEN expression are closely associated with bladder
cancer prognosis and are negatively correlated with each other.
Therefore, PPAR-γ and PTEN signaling may be a potential therapeutic
target in bladder cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Project
of Rejuvenating Public Health through Science and Education of
Suzhou (grant no. KJXW2014023) and the Precision Medicine Program
of Second Military Medical University (grant no. 2017JZ35).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ and FW designed the study. HX and JJ wrote the
manuscript. XS performed data collection. JL, YZ and HY performed
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by Suzhou Municipal
Hospital Ethics Committee. All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TCGA
|
The Cancer Genome Atlas
|
|
PPAR-γ
|
peroxisome proliferator-activated
receptor-γ
|
|
PTEN
|
phosphatase and tensin homologue
deleted on chromosome ten
|
|
TURBT
|
transurethral resection of bladder
tumors
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Comperat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strand DW, DeGraff DJ, Jiang M, Sameni M,
Franco OE, Love HD, Hayward WJ, Lin-Tsai O, Wang AY, Cates JM, et
al: Deficiency in metabolic regulators PPARgamma and PTEN
cooperates to drive keratinizing squamous metaplasia in novel
models of human tissue regeneration. Am J Pathol. 182:449–459.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aboseif S, El-Sakka A, Young P and Cunha
G: Mesenchymal reprogramming of adult human epithelial
differentiation. Differentiation. 65:113–118. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Liu W, Hayward SW, Cunha GR and
Baskin LS: Plasticity of the urothelial phenotype: Effects of
gastro-intestinal mesenchyme/stroma and implications for urinary
tract reconstruction. Differentiation. 66:126–135. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castillo-Martin M, Domingo-Domenech J,
Karni-Schmidt O, Matos T and Cordon-Cardo C: Molecular pathways of
urothelial development and bladder tumorigenesis. Urol Oncol.
28:401–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Michalik L, Auwerx J, Berger JP,
Chatterjee VK, Glass CK, Gonzalez FJ, Grimaldi PA, Kadowaki T,
Lazar MA, O'Rahilly S, et al: International union of pharmacology.
LXI. peroxisome proliferator-activated receptors. Pharmacol Rev.
58:726–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krishnan A, Nair SA and Pillai MR: Biology
of PPAR gamma in cancer: A critical review on existing lacunae.
Curr Mol Med. 7:532–540. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Platt FM, Hurst CD, Taylor CF, Gregory WM,
Harnden P and Knowles MA: Spectrum of phosphatidylinositol 3-kinase
pathway gene alterations in bladder cancer. Clin Cancer Res.
15:6008–617. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dormandy JA, Charbonnel B, Eckland DJ,
Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre
PJ, Murray GD, et al: Secondary prevention of macrovascular events
in patients with type 2 diabetes in the PROactive Study
(PROspective pioglitAzone Clinical Trial In macroVascular Events):
A randomised controlled trial. Lancet. 366:1279–1289. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Montironi R and Lopez-Beltran A: The 2004
WHO classification of bladder tumors: A summary and commentary. Int
J Surg Pathol. 13:143–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Francisco JS, Moraes HP and Dias EP:
Evaluation of the Image-Pro Plus 4.5 software for automatic
counting of labeled nuclei by PCNA immunohistochemistry. Braz Oral
Res. 18:100–1004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin MS, Huang JX, Chen WC, Zhang BF, Fang
J, Zhou Q, Hu Y and Gao HJ: Expression of PPARgamma and PTEN in
human colorectal cancer: An immunohistochemical study using tissue
microarray methodology. Oncol Lett. 2:1219–1224. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Wei J, Tian X, Li Y and Li X:
Prognostic role of PPAR-gamma and PTEN in the renal cell carcinoma.
Int J Clin Exp Pathol. 8:12668–12677. 2015.PubMed/NCBI
|
|
15
|
Chalasani V, Chin JL and Izawa JI:
Histologic variants of urothelial bladder cancer and nonurothelial
histology in bladder cancer. Can Urol Assoc J. 3:S193–S198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walid MS and Heaton RL: Can
posthysterectomy cystoscopy be utilized as a screening test for
bladder cancer? Ger Med Sci. 6:132008.
|
|
17
|
Lotan Y and Roehrborn CG: Sensitivity and
specificity of commonly available bladder tumor markers versus
cytology: Results of a comprehensive literature review and
meta-analyses. Urology. 61:109–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forootan FS, Forootan SS, Malki MI, Chen
D, Li G, Lin K, Rudland PS, Foster CS and Ke Y: The expression of
C-FABP and PPARgamma and their prognostic significance in prostate
cancer. Int J Oncol. 44:265–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuentes E, Guzman-Jofre L, Moore-Carrasco
R and Palomo I: Role of PPARs in inflammatory processes associated
with metabolic syndrome (Review). Mol Med Rep. 8:1611–1616. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee C, Ramirez JA, Guitart J and Diaz LK:
Expression of cyclooxygenase-2 and peroxisome
proliferator-activated receptor gamma during malignant melanoma
progression. J Cutan Pathol. 35:989–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshimura R, Matsuyama M, Segawa Y, Hase
T, Mitsuhashi M, Tsuchida K, Wada S, Kawahito Y, Sano H and
Nakatani T: Expression of peroxisome proliferator-activated
receptors (PPARs) in human urinary bladder carcinoma and growth
inhibition by its agonists. Int J Cancer. 104:597–602. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li MY, Yuan H, Ma LT, Kong AW, Hsin MK,
Yip JH, Underwood MJ and Chen GG: Roles of peroxisome
proliferator-activated receptor-alpha and -gamma in the development
of non-small cell lung cancer. Am J Respir Cell Mol Biol.
43:674–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sullivan PF, Pedersen NL, Jacks A and
Evengard B: Chronic fatigue in a population sample: Definitions and
heterogeneity. Psychol Med. 35:1337–1348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edfors F, Danielsson F, Hallstrom BM, Kall
L, Lundberg E, Ponten F, Forsström B and Uhlén M: Gene-specific
correlation of RNA and protein levels in human cells and tissues.
Mol Syst Biol. 12:8832016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greenbaum D, Colangelo C, Williams K and
Gerstein M: Comparing protein abundance and mRNA expression levels
on a genomic scale. Genome Biol. 4:1172003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Langle Y, Lodillinsky C, Belgorosky D,
Sandes EO and Eijan AM: Role of peroxisome proliferator activated
receptor-gamma in bacillus Calmette-Guerin bladder cancer therapy.
J Urol. 188:2384–2390. 2012. View Article : Google Scholar : PubMed/NCBI
|