Introduction
Acute myeloid leukemia (AML), the most common
phenotype of adult acute leukemia, is a malignancy caused by the
aberrant cloning and hyperplasia of hematopoietic stem cells in the
hemopoietic system (1). Cytogenetic
analysis is considered an important method for prognostic
evaluation (2). Patients with AML
with a normal karyotype contain the largest cytogenetic population
(3). However, in-depth molecular
genetics studies revealed that mutations may be critical
independent risk factors associated with the prognosis of patients
with AML (4); additionally, the
prognosis of patients with AML with a normal karyotype has been
demonstrated to differ from those harboring mutations in different
genes (5).
The DNA methyltransferase 3 α (DNMT3A) gene is
located on chromosome 2p23 and is highly expressed in adults
(6). A recent analysis of genetic
mutations in patients with AML revealed that the DNMT family of
proteins is aberrantly upregulated, with the resulting
hypermethylation of tumor suppressor genes a potential driver in
leukemia development (7). In
addition, a previous study demonstrated that a certain proportion
of DNMT3A mutations exist in the normal karyotypes of patients with
AML, which may be an independent risk factor for the poor prognosis
(8). Although previous studies
demonstrated the key role of DNMT3A-mediated methylation in
patients with AML, the effect of DNMT3A mutation on AML prognosis
remains unclear (9,10). FMS-like tyrosine kinase-3 (FLT3) is a
member of the growth factor receptor family with endonuclease
activity towards tyrosine kinase. In particular, FLT3-internal
tandem duplication (ITD) is the most common mutation found in the
FLT3 gene (11). It has been
reported that patients with AML carrying the FLT3-ITD mutation
display a higher peripheral white blood cell (WBC) count, which
severely affects the rates of complete remission (CR), relapse (RR)
and overall survival (OS) (12).
FLT3-ITD mutation eliminates the autoinhibition of FLT3, resulting
in the activation of downstream proliferative signaling pathways
affecting the proliferation of cells carrying FLT3 mutations n
patients with AML (13). Although
FLT3-ITD is reported at high rates amongst patients with AML
(14), large-sample studies
examining this mutation are lacking.
Therefore, in the present study associations between
DNMT3A and/or FLT3-ITD mutations and prognostic indicators were
analyzed in patients with AML to provide a molecular method for the
prognosis evaluation, and a potential foundation for personalized
therapy.
Materials and methods
General data
A retrospective analysis was performed on data of
the clinical features and prognosis obtained from 133 patients
diagnosed with AML between January 2010 and June 2015. Amongst
these patients, 74 were male and 59 were female with the age range
between 18–61 years old. According to French-American-British (FAB)
classification of AML (15), there
were 3, 13, 50, 27, 14, 22 and 4 patients classified with the M0,
M1, M2, M3, M4, M5 and M6 subtypes, respectively, with 0 mixed
subtypes. Patients were grouped according to genetic mutations
detected by next-generation sequencing: 34 patients harboring
mutations in DNMT3A and FLT3-ITD were enrolled into the DNMT3A +
FLT3-ITD mutation group, 37 patients harboring mutations in
FLT3-ITD only were enrolled into the FLT3-ITD mutation group, 32
patients harboring mutations in DNMT3A only were enrolled into the
DNMT3A mutation only group and 30 patients harboring no DNMT3A and
FLT3-ITD mutations were designated as the control group. Patients
in all groups were administered daunorubicin and cytarabine
chemotherapy regimens. The following inclusion criteria were used:
i) Patients who were diagnosed with AML by bone marrow cell
morphology analysis; and ii) genetic mutation was confirmed with
examination of chromosome and fusion genes. The following exclusion
criteria were used: i) Patients afflicted with severe diseases of
other organs; ii) patients with other tumors; iii) patients with
cognitive dysfunction resulting in difficulties in negotiation
confirmed via face-to-face communication; and iv) patients who can
not participate in the study. All patients and respective families
agreed to participate in the study and signed written informed
consent. This study was approved by the Ethics Committee of The
Affiliated Zhangjiagang Hospital of Soochow University.
Experiment methods
Experimental materials and
reagents
The equipment and reagents used in the current study
included: XE-2100D Automatic Analyzer (Sysmex Asia Pacific Pte.
Ltd.), fetal bovine serum (Hyclone; GE Healthcare), daunorubicin
(State Food and Drug Administration of China [SFDA] no. H33020925;
Zhejiang Hisun Chemical Co., Ltd.), cytarabine (registration no.
H20100594; Actavis; Teva Pharmaceutical Industries Ltd.),
daunorubicin (SFDA no. H20040600; Guangdong Leawell Pharmaceutical
Co., Ltd.), mitoxantrone (SFDA no. H10960119; Zhejiang Ruixin
Pharmaceutical Co., Ltd.) and etoposide (SFDA no. H53021627; KPC
Pharmaceutical, Inc.). Bovine serum albumin (BSA; SFDA no.
A104912-5g; Shanghai Aladdin Biochemical Technology Co., Ltd.),
fluorescein isothiocyanate (CAS Number: 27072-45-3; Sigma-Aldrich;
Merck KGaA), phycoerythrin (552603; BD PharMingen) and
allophycocyanin (553051; BD PharMingen).
Comparison of indicators before and
after treatment
The following were collected from the patients in
the four groups: A total of 4 ml venous blood was collected to
perform peripheral blood cell counts using the XE-2100D automatic
analyzer according to the manufacturer's protocol. Parameters
included WBC, platelet and hemoglobin counts, and proportions of
myeloid progenitor cells, including WBC, platelet and hemoglobin
counts, and proportions of myeloid progenitor cells. A total of 0.1
ml marrow was collected as previously described in the literature
(16) to perform bone marrow smears
for the counting of karyocytes (17)
stained with Wright's stain under a microscope, to calculate the
number of myeloid progenitor cells; and 1 ml marrow was collected
to prepare specimens for immunogenotyping.
Flow cytometry to determine
immunogenotypes
For immunogenotyping, fluorescence-conjugated
antibodies were added to 100 µl marrow specimens in each reaction,
which were then diluted with 100–200 µl phosphate-buffered saline
supplemented with 2% BSA. The fluorescein antibodies labeled with
fluorescein isothiocyanate/phycoerythrin/allophycocyanin were added
at 5 µl respectively.
The samples were subsequently subjected to shaking
incubation in a dark room at room temperature for 25–30 min,
followed by incubation with 1.5 ml red blood cell lysis buffer (1X)
in a dark room at room temperature for 15–20 min. Following
centrifugation at 780 × g under room temperature, the supernatant
was discarded, and the pellet was subsequently mixed with
phosphate-buffered saline supplemented with 2% BSA to determine the
immunogenotypes using flow cytometry.
Chemotherapy regimens
The first treatment course were performed as
follows: Daunorubicin (40–60 mg/m2; 1 time/day; days
1–3) + cytarabine (100–200 mg/m2; 1 time/day; days 1–7)
or darubicin (8–10 mg/m2; 1 day/day; days 1–3) +
cytarabine (100–200 mg/m2; 1 time/day; days 1–7), which
was selected on basis of the patient's economic situation (higher
dose treatment for patients in better economic situation). Doses
were adjusted upon infection or according to results of blood tests
obtained routinely from the patients during treatment. Patients
exhibiting no remission following the first treatment course were
subjected to the mitoxantrone (MEA) regimen in the second treatment
course: Mitoxantrone (4 mg/m2; days 1–3) + etoposide
(100 mg; days 4–6) + cytarabine (150 mg/m2; days 1–7);
whereas patients displaying significant remission were administered
with the same doses for the second treatment course.
Observation indices
WBC, platelet and hemoglobin counts in addition to
the proportion of myeloid progenitor cells at onset time in the
four patient groups were observed and compared (Table I). The immunogenotypes of patients in
the four groups at the time of onset were also observed. RECIST 1.1
criteria (18) were adopted to
evaluate chemotherapy efficacy in patients from the four groups
using the following four categories: Complete remission (CR),
partial remission (PR), stable disease (SD) and progressive disease
(PD). Effectiveness rate=(CR + PR)/total no of patients
(CR+PR+SD+PD) ×100%. The following CR criteria were used: Patients
exhibiting no symptoms or signatures of leukemia with no compromise
to the quality of life or Hb ≥90 g/l, absolute value of neutrophils
≥1.5×109/l, platelet ≥100×109/l, ≤5%
myeloblast (type I+II) and normal erythrocyte and megakaryocytic
series. The following PR criteria were used: Patients demonstrating
5–20% myeloblast (type I+II) or one or more indicators failing to
meet the CR criterion. The following SD criteria were used:
Chemotherapy exerted no overall effects on patients. The following
PD criteria were used: Patients exhibiting no remission but showing
disease progression following chemotherapy. After the CR rates of
the patients in the four groups were calculated, the 1-year RR and
3-year OS rate of patients were subsequently compared through
3-year follow-up. The following were used as criteria for PR:
Patients who achieved complete remission following treatment
exhibiting 5–20% myeloblasts (type I+II) and did not satisfy the CR
criterion specific for myelogram following one course of effective
treatment. Relapse rate=number of relapsed people within 1
year/total number of people.
 | Table I.Comparison of leukocyte count,
platelet count, hemoglobin count and myeloid progenitor cells in
the four groups of patients at onset. |
Table I.
Comparison of leukocyte count,
platelet count, hemoglobin count and myeloid progenitor cells in
the four groups of patients at onset.
| Index | DNMT3A + FLT3-ITH
mutation group n=34 | FLT3-ITH mutation
only group n=37 | DNMT3A mutation only
group n=32 | Control group
n=30 | F statistic | P-value |
|---|
| White blood cells
(×109/l) | 79.31±21.24 |
44.05±12.09a,b |
39.78±11.69a,b |
33.14±9.10a | 68.01 | <0.001 |
| Platelet
(×109/l) | 48.60±35.23 | 44.61±7.97 | 41.40±7.59 | 36.29±6.38 | 2.414 | 0.070 |
| Hemoglobin (g/l) | 71.33±6.87 | 72.24±2.73 | 69.60±4.84 | 62.11±4.23 | 28.11 | <0.001 |
| Myeloid progenitor
cells (%) | 0.91±0.05 |
0.62±0.04a,b |
0.64±0.03a,b |
0.57±0.03a | 518.0.3 | <0.001 |
Following complete remission, the patient's bone
marrow myeloblast I + type II (primary mononuclear + naive
monocytes or primordial lymph + naive lymphocytes) >5 and ≤20%,
and they cannot achieve complete remission following a course of
effective anti-leukemia treatment, or patients displaying
myeloblast (type I+II) >20%. OS time was defined as the time
period the between the day of diagnosis to all-cause mortality or
the end of follow-up.
Statistical analysis
All data were analyzed using SPSS 18.0 software
(SPSS, Inc.). Measurement data are presented as the mean ± standard
deviation, and ≥3 means were compared using one-way analysis of
variance followed by Bonferroni post-hoc test. Nominal data were
compared using the χ2 test. The Kaplan-Meier survival
analysis was prepared using GraphPad Prism 6 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
Baseline data comparison
Comparisons of gender, age and FAB subtypes among
patients of the four groups revealed no statistically significant
differences (P>0.05; Table
II).
 | Table II.General patient data. |
Table II.
General patient data.
| Factor | DNMT3A + FLT3-ITH
mutation group n=34 (%) | FLT3-ITH mutation
only group n=37 (%) | DNMT3A mutation
only group n=32 (%) | Control group n=30
(%) | χ2 | P-value |
|---|
| Gender |
|
|
|
| 0.089 | 0.993 |
|
Male | 19 (55.88) | 21 (56.76) | 18 (56.25) | 16 (53.33) |
|
|
|
Female | 15 (44.12) | 16 (43.24) | 14 (43.75) | 14 (46.67) |
|
|
| Age (years) |
|
|
|
| 0.696 | 0.874 |
|
<50 | 14 (41.18) | 15 (40.54) | 11 (34.38) | 10 (33.33) |
|
|
|
>50 | 20 (58.82) | 22 (59.46) | 21 (65.63) | 20 (66.66) |
|
|
| Body mass index
(kg/m2) |
|
|
|
| 0.905 | 0.824 |
|
<22 | 15 (44.12) | 17 (45.95) | 17 (53.13) | 16 (53.33) |
|
|
|
>22 | 19 (55.88) | 20 (54.05) | 15 (46.88) | 14 (46.67) |
|
|
| Smoking |
|
|
|
| 0.035 | 0.998 |
|
Yes | 16 (47.06) | 18 (48.65) | 15 (46.88) | 14 (46.67) |
|
|
| No | 18 (52.94) | 19 (51.35) | 17 (53.13) | 16 (53.33) |
|
|
| Drinking |
|
|
|
| 0.018 | 0.999 |
|
Yes | 21 (61.76) | 23 (62.16) | 20 (62.50) | 19 (63.33) |
|
|
| No | 13 (38.24) | 14 (37.84) | 12 (37.50) | 11 (17.46) |
|
|
| FAB
classification |
|
|
|
| 1.710 | 1.000 |
| M0 | 1 (2.94) | 1 (2.70) | 0 | 1 (3.33) |
|
|
| M1 | 3 (8.82) | 4 (10.81) | 3 (9.38) | 3 (10.00) |
|
|
| M2 | 13 (38.24) | 14 (37.84) | 12 (37.50) | 11 (36.67) |
|
|
| M3 | 6 (17.65) | 8 (22.86) | 7 (21.88) | 6 (20.00) |
|
|
| M4 | 4 (11.76) | 3 (8.11) | 4 (12.50) | 3 (9.99) |
|
|
| M5 | 6 (17.65) | 6 (16.22) | 5 (15.63) | 5 (16.67) |
|
|
| M6 | 1 (2.94) | 1 (2.70) | 1 (3.13) | 1 (3.33) |
|
|
| Mixed
type | 0 | 0 | 0 | 0 |
|
|
WBC, platelet and hemoglobin counts,
and proportions of myeloid progenitor cells in patients at the time
of onset
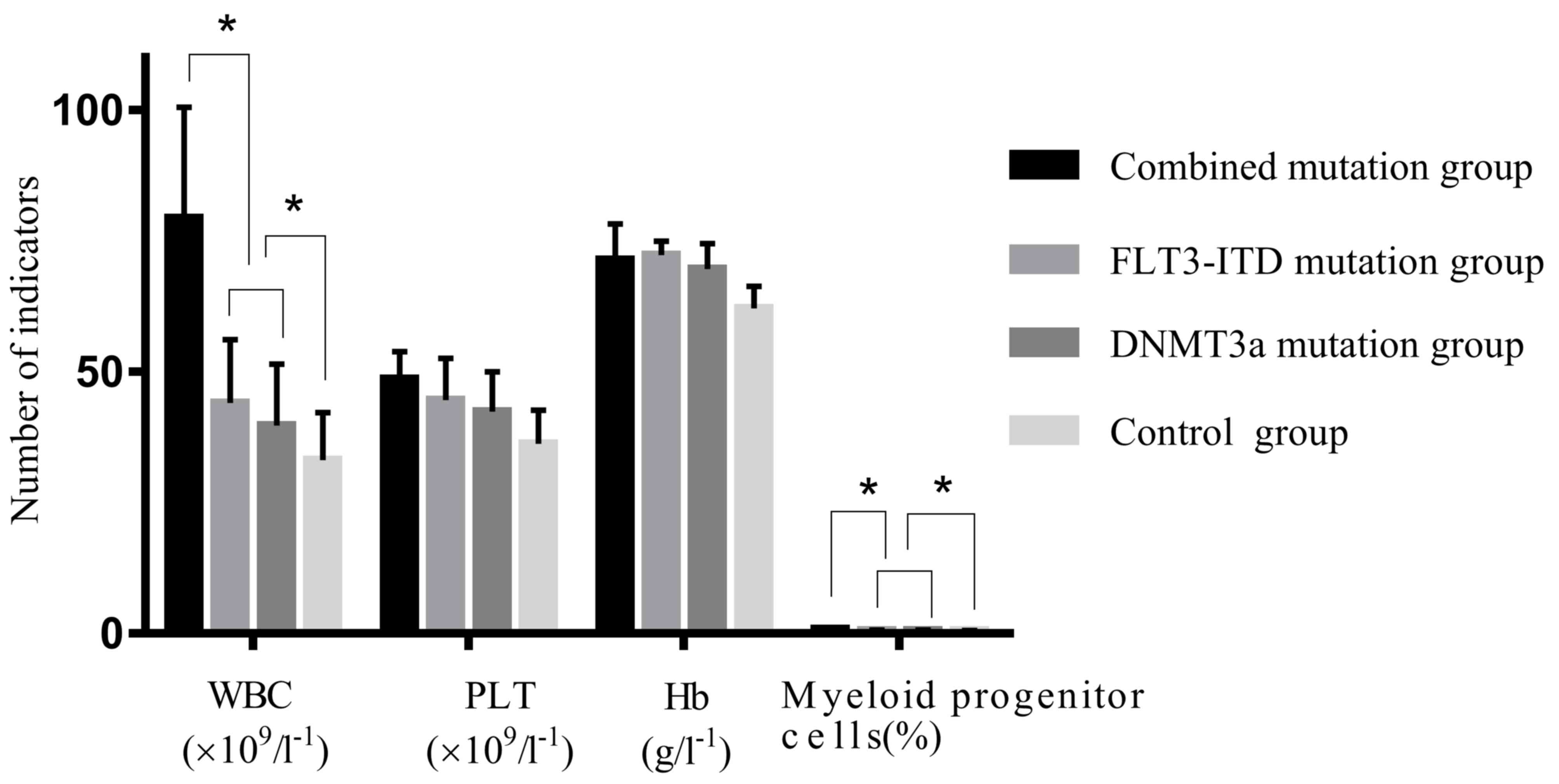
In the DNMT3A + FLT3-ITD group, the peripheral WBC
count and proportion of myeloid progenitor cells were significantly
higher than those in the FLT3-ITD mutation only, DNMT3A mutation
only and control patient groups (all P<0.05), but no significant
differences were observed in the platelet count and hemoglobin
levels in the peripheral blood of patients across the four groups
(P>0.05; Fig. 1; Table I). The WBC count and proportion of
myeloid progenitor cells in the FLT3-ITD mutation only and DNMT3A
mutation only patient groups were significantly higher compared
with the control group (P<0.05); but exhibited no significant
differences when compared with each other (P>0.05; Fig. 1; Table
I).
Comparison of immunogenotypes of
patients across the four groups
The CD15+ and HLA-DR+ rates
were significantly higher in the DNMT3A + FLT3-ITD mutation and
DNMT3A mutation only patient groups compared with those in the
FLT3-ITD mutation only and control groups (P<0.05). In addition,
the rates of CD38+ in the DNMT3A + FLT3-ITD mutation and
FLT3-ITD mutation only patient groups were significantly higher
compared with that in the DNMT3A mutation only and control groups
(P<0.05). Comparison between the four patient groups
demonstrated no significant differences in the rates of
CD34+, CD33+, CD13+,
CD14+ and CD117+ (P>0.05; Table III).
 | Table III.Comparison of immunological positive
rates in the four patient groups. |
Table III.
Comparison of immunological positive
rates in the four patient groups.
| Immunogenotype | DNMT3A + FLT3-ITH
mutation group n=34 (%) | FLT3-ITH mutation
group n=37 (%) | DNMT3A mutation
only group n=32 (%) | Control group n=30
(%) | χ2 | P-value |
|---|
|
CD34+ | 32 (94.12) | 34 (91.89) | 29 (90.63) | 28 (93.33) | 0.339 | 0.953 |
|
CD117+ | 31 (91.18) | 35 (94.59) | 30 (93.75) | 29 (96.67) | 0.884 | 0.829 |
|
CD15+ | 29 (85.29) | 21
(56.76)a,b | 28 (87.50) | 26
(86.67)a,b | 13.87 | <0.050 |
|
HLA-DR+ | 14 (41.18) | 3
(8.11)a,b | 10 (31.25) | 5
(16.67)a,b | 12.41 | <0.050 |
|
CD13+ | 30 (88.24) | 28 (75.68) | 28 (87.50) | 27 (90.00) | 3.540 | 0.316 |
|
CD33+ | 32 (94.12) | 34 (91.89) | 30 (93.75) | 29 (96.67) | 0.673 | 0.880 |
|
CD14+ | 3 (8.82) | 3 (8.11) | 2 (6.25) | 2 (6.67) | 0.207 | 0.976 |
|
CD38+ | 28 (82.35) | 31 (83.78) | 9
(28.13)a,c | 13
(43.33)a,c | 33.03 | <0.001 |
Evaluation of chemotherapy efficacy in
patients across the four groups
The overall rate of chemotherapy effectiveness was
38.24% in the DNMT3A + FLT3-ITD mutation patient group, which was
significantly lower compared with 70.27, 71.88 and 83.33% in the
FLT3-ITD mutation only, DNMT3A mutation only and control patient
groups, respectively (P<0.05). Comparison of the overall
effectiveness rates of chemotherapy between the DNMT3A only and
FLT3-ITD only groups revealed no statistically significant
differences (P>0.05), but were significantly lower when compared
with the control group (P<0.05; Table IV).
 | Table IV.Chemotherapy efficacy in the four
patient groups. |
Table IV.
Chemotherapy efficacy in the four
patient groups.
| Curative
effect | DNMT3A + FLT3-ITH
mutation group n=34 (%) | FLT3-ITH mutation
only group n=37 (%) | DNMT3A mutation
only group n=32 (%) | Control group n=30
(%) | χ2 | P-value |
|---|
| Complete
remission | 4 (11.76) | 14 (37.84) | 13 (40.63) | 15 (50.00) | 13.69 | <0.001 |
| Partial
remission | 9 (26.47) | 12 (32.43) | 10 (31.25) | 10 (33.33) | 0.730 | 0.866 |
| Stability of the
disease | 14 (41.18) | 6 (16.22) | 5 (15.63) | 3 (10.00) | 11.58 | <0.001 |
| Progress of the
disease | 7 (20.59) | 5 (13.51) | 4 (12.50) | 2 (6.67) | 2.684 | 0.443 |
| Total effective
rate | 13 (38.24) | 26
(70.27)a,b | 23
(71.88)a,b | 25
(83.33)a | 16.34 | <0.050 |
Comparison of CR rates, 1-year RR and
3-year OS rates of patients between the four groups
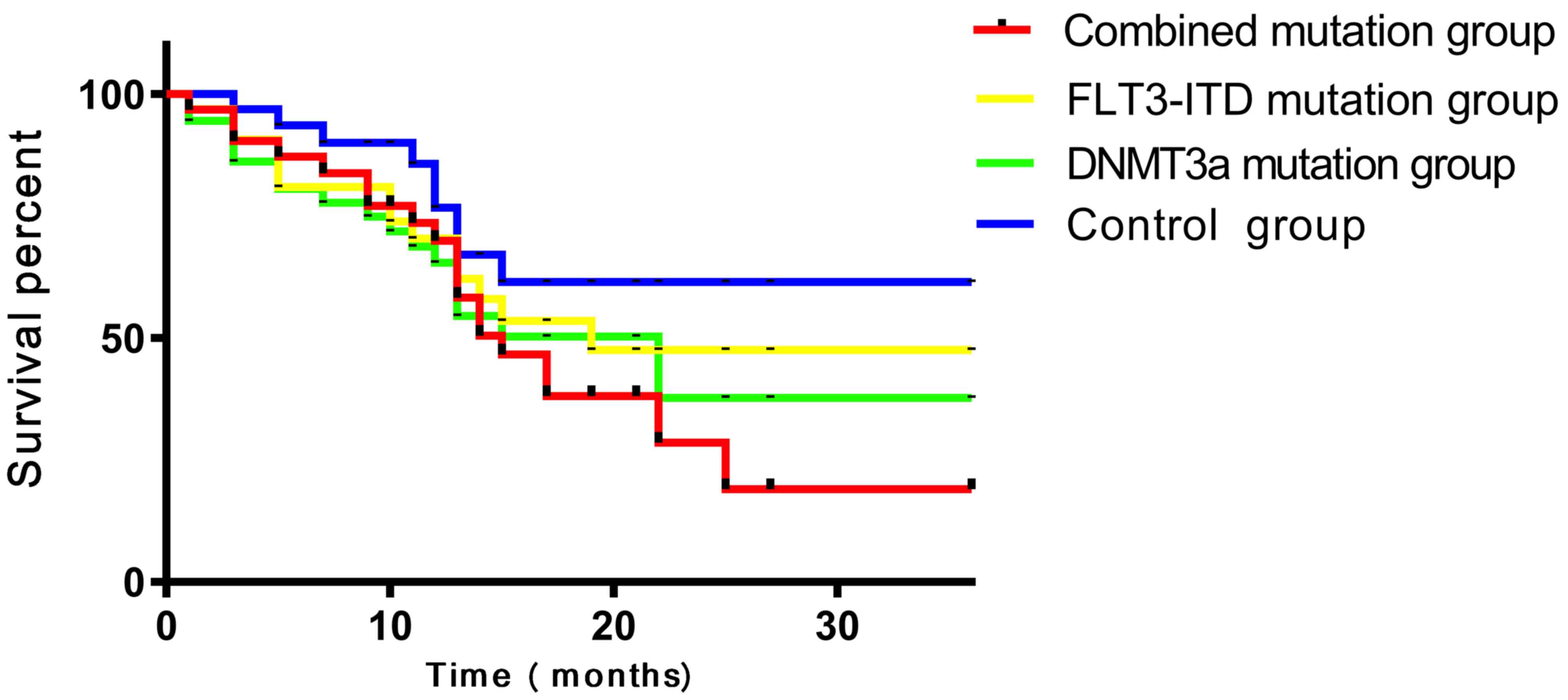
In the DNMT3A + FLT3-ITD group, the CR rate of
patients was 11.76%, which was significantly lower compared with
37.84, 40.63 and 50.00% in the FLT3-ITD mutation only, DNMT3A
mutation only and control patient groups respectively (P<0.05).
Although DNMT3A mutation only and FLT3-ITD mutation only patient
groups displayed no significant differences in the CR rates when
compared with each other (P>0.05), significantly lower CR rates
were exhibited when they were compared with the control patient
group (P<0.05). The 1-year RR of patients in the DNMT3A +
FLT3-ITD mutation patient group was 75.00%, compared with 14.29,
15.38 and 6.67% in the FLT3-ITD mutation only, DNMT3A mutation only
and control patient groups, respectively (P<0.05). The
difference between FLT3-ITD mutation only and DNMT3A mutation only
patient groups was not statistically significant (P>0.05), but
these two groups exhibited significantly higher 1-year RR compared
with the control patient group (P<0.05). In the DNMT3A +
FLT3-ITD mutation group, patients displayed a 3-year OS rate of
11.76%, which was significantly lower compared with the rates of
43.24, 43.75 and 53.33% in the FLT3-ITD mutation only, DNMT3A
mutation only and control patient groups, respectively (P<0.05).
The difference between the FLT3-ITD mutation only and DNMT3A
mutation only groups was not statistically significant (P>0.05),
but these two groups exhibited significantly lower 3-year OS rates
compared with the control patient group (P<0.05; Fig. 2; Table
V).
 | Table V.Comparison of complete remission,
1-year relapse rate, and 3-year overall survival in the four groups
of patients. |
Table V.
Comparison of complete remission,
1-year relapse rate, and 3-year overall survival in the four groups
of patients.
| Item | DNMT3A + FLT3-ITH
mutation group n=34 (%) | FLT3-ITH mutation
only group n=37 (%) | DNMT3A mutation
only group n=32 (%) | Control group n=30
(%) | χ2 | P-value |
|---|
| Complete
remission | 4 (11.76) | 14
(37.84)a,b | 13
(40.63)a,b | 15
(50.00)a | 11.67 | <0.050 |
| 1-year relapse
rate | 3 (75.00) | 2
(20.00)a,b | 2
(22.22)a,b | 1
(6.67)a | 1057 | <0.050 |
| 3-year overall
survival | 4 (11.76) | 16
(43.24)a,b | 14
(43.75)a,b | 16
(53.33)a | 13.86 | <0.050 |
Discussion
AML is a malignant disease of hematopoietic stem
cells, and cytogenetic examination is currently the most common
method applied for the prognostic evaluation of AML (19). Accumulating evidence has reported
that mutations in genes encoding DNMT3A, FLT3-ITD and nucleophosmin
1 occur in AML patients (20). FLT3
encodes a protein that is pivotal for the proliferation and
differentiation of human lymphoid progenitor cells (21). The FLT3-ITD mutation is proposed to
be associated with the poor prognosis of patients with AML
(22). DNMT3A is also a commonly
mutated gene in patients with AML, with the third-highest mutation
rate of all known genes associated with leukemia (23). DNMT3A mutation is an independent
prognostic factor in patients with AML and is useful for risk
stratification in determining prognosis. Studies have demonstrated
that DNMT3A combined with FLT3-ITD mutation is the most frequent
combination of mutations in patients with AML (24,25). A
previous study revealed a close association between DNMT3A and
FLT3-ITD mutations (26), but
studies evaluating the clinical features and prognosis of patients
carrying mutations in DNMT3A and FLT3-ITD in detail are lacking.
Therefore, in the present study patients with AML were separated
into groups according to mutations in these two genes, and the
efficacy of chemotherapy and prognosis for these patients was
subsequently explored.
In the present study, the WBC, platelet and
hemoglobin counts of as well as the proportion of myeloid
progenitor cells among the four patient groups were first compared.
There were no significant differences in the platelet counts and
hemoglobin levels in the peripheral blood of patients in the four
groups (P>0.05), suggesting that simultaneous mutation of DNMT3A
and FLT3-ITD contributes to increased WBC and myeloid progenitor
cells. It was previously demonstrated that FLT3-ITD mutation can
activate a downstream kinase through the continuous activation of
protein tyrosine kinase, thereby triggering signal transduction and
further inducing the spontaneous and non-receptor-dependent
proliferation of leukocytes (27).
However, the role of DNMT3A in cell proliferation remains
unclear.
The current study evaluated the immunogenotypes of
patients in the four groups. A number of studies have indicated
that the immunophenotype may be one of the indicators that can
affect the remission rate of patients with AML (28), so it can be hypothesized that CD15
and HLA-DR expression may be associated with the combined mutation
of DNMT3A and FLT3-ITD genes, which warrants further investigation.
It has been previously reported that, compared with patients
harboring no DNMT3A mutations, patients carrying DNMT3A mutation
were found to have higher CD34+, CD15+ and
HLA-D+ rates (29).
Furthermore, another previous study revealed that patients carrying
FLT3-ITD mutations exhibited higher rates of CD34+ and
CD38+ compared with patients without this mutation
(30). Therefore, chemotherapy
efficacy was compared between the four groups in the current study.
The results suggested that patients with simultaneous mutations are
associated with poorer chemotherapy efficacy compared with those
carrying single mutations. A previous study reported that patients
with AML carrying combined FLT3-ITD and DNMT3A mutations have a
significantly lower rate of complete response to induction
chemotherapy (31), the underlying
mechanism of which has not been adequately explained. Finally, CR,
1-year RR and OS rates of patients between the four groups were
compared. A previous study investigating the effect of FLT3-ITD and
DNMT3A simultaneous mutations on patients with AML suggested that
these mutations affect the survival time and can serve as a
molecular criterion for prognostic evaluation (32). In addition, Ahn et al
(33) evaluated the effect of gene
mutations introduced following hematopoietic stem cell
transplantation in patients with AML with normal karyotypes. This
study revealed that patients harboring simultaneous mutations in
DNMT3A and FLT3-ITD demonstrated significantly higher relapse rates
and lower event-free survival and OS rates compared with patients
with wild-type or DNMT3A mutation. Therefore, the results presented
in the present study are in agreement with the abovementioned
previous observations.
In conclusion, patients with AML carrying
simultaneous mutations of FLT3-ITD and DNMT3A demonstrated poor
prognosis with lower CR, 1-year RR and OS rates compared with those
carrying single mutations in either gene. However, the reason for
the clinical remission rate of patients with FLM3-ITD + DNMT3A
combined mutations being lower compared with that of patients with
FLT3-ITD and DNMT3A single mutations was not explained in the
present study. Further studies are required to develop novel
therapeutic strategies specific to these gene mutations in patients
with AML.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
QRZ and KH were responsible for study conception and
design. Data analysis and interpretation were performed by XW, JC
and FG. QRZ was responsible for drafting the article. Critical
revision of article was performed by KH. XW, JC and FG performed
data collection. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The Affiliated Zhangjiagang Hospital of Soochow University. All
patients and their families agreed to participate in the study and
signed written informed consent.
Patient consent for publication
The patient, or parent, guardian or next of kin (in
case of deceased patients) provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Döhner H, Weisdorf DJ and Bloomfield CD:
Acute myeloid leukemia. N Engl J Med. 373:1136–1152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mrózek K, Heinonen K and Bloomfield CD:
Clinical importance of cytogenetics in acute myeloid leukaemia.
Best Pract Res Clin Haematol. 14:19–47. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papaemmanuil E, Gerstung M, Bullinger L,
Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F,
Bolli N, et al: Genomic classification and prognosis in acute
myeloid leukemia. N Engl J Med. 374:2209–2221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolach O and Stone RM: Acute myeloid
leukemia. Target Ther Transl Cancer Res. 5:892015. View Article : Google Scholar
|
|
5
|
Stone RM, Mandrekar SJ, Sanford BL,
Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K,
Marcucci G, et al: Midostaurin plus chemotherapy for acute myeloid
leukemia with a FLT3 mutation. N Engl J Med. 377:454–464. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kao HW, Liang DC, Kuo MC, Wu JH, Dunn P,
Wang PN, Lin TL, Shih YS, Liang ST, Lin TH, et al: High frequency
of additional gene mutations in acute myeloid leukemia with MLL
partial tandem duplication: DNMT3A mutation only is associated with
poor prognosis. Oncotarget. 6:33217–33225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferreira HJ, Heyn H, Vizoso M, Moutinho C,
Vidal E, Gomez A, Martínez-Cardús A, Simó-Riudalbas L, Moran S,
Jost E and Esteller M: DNMT3A mutations mediate the epigenetic
reactivation of the leukemogenic factor MEIS1 in acute myeloid
leukemia. Oncogene. 35:3079–3082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Debarri H, Lebon D, Roumier C, Cheok M,
Marceau-Renaut A, Nibourel O, Geffroy S, Helevaut N, Rousselot P,
Gruson B, et al: IDH1/2 but not DNMT3A mutations are suitable
targets for minimal residual disease monitoring in acute myeloid
leukemia patients: A study by the acute leukemia french
association. Oncotarget. 6:42345–42353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaidzik VI, Weber D, Paschka P, Kaumanns
A, Krieger S, Corbacioglu A, Krönke J, Kapp-Schwoerer S, Krämer D,
Horst H, et al: DNMT3A mutant transcript levels persist in
remission and do not predict outcome in patients with acute myeloid
leukemia. Leukemia. 32:30–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Im AP, Sehgal AR, Carroll MP, Smith BD,
Tefferi A, Johnson DE and Boyiadzis M: DNMT3A and IDH mutations in
acute myeloid leukemia and other myeloid malignancies: Associations
with prognosis and potential treatment strategies. Leukemia.
28:1774–1783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marhäll A, Heidel F, Fischer T and
Rönnstrand L: Internal tandem duplication mutations in the tyrosine
kinase domain of FLT3 display a higher oncogenic potential than the
activation loop D835Y mutation. Ann Hematol. 97:773–780. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fleischmann M, Schnetzke U, Schrenk KG,
Schmidt V, Sayer HG, Hilgendorf I, Hochhaus A and Scholl S: Outcome
of FLT3-ITD-positive acute myeloid leukemia: Impact of allogeneic
stem cell transplantation and tyrosine kinase inhibitor treatment.
J Cancer Res Clin Oncol. 143:337–345. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ortlepp C, Steudel C, Heiderich C, Koch S,
Jacobi A, Ryser M, Brenner S, Bornhäuser M, Brors B and Hofmann WK:
Autotaxin is expressed in FLT3-ITD positive acute myeloid leukemia
and hematopoietic stem cells and promotes cell migration and
proliferation. Exp Hematol. 41:444–461.e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levis MJ, Perl AE, Altman JK, Gocke CD,
Bahceci E, Hill J, Liu C, Xie Z, Carson AR, McClain V, et al: A
next-generation sequencing-based assay for minimal residual disease
assessment in AML patients with FLT3-ITD mutations. Blood Adv.
2:825–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bashasha SH, Kordofani AA, Osman IM, Musa
OH, Altayb HN and Elmagbool BK: Prevalence of the different FAB sub
type of acute myeloid leukemia related to hematological parameters
in Sudanese. J Hematol Blood Disord. 3:1022017.
|
|
16
|
Meyerson H, Sundaram S and Lazarus HM:
Bone marrow structure and marrow aspiration, biopsy, and collection
for therapeutic intent proceduresLazarus H and Schmaier A: Concise
guide to hematology. Springer; Cham: pp. 233–252. 2019, View Article : Google Scholar
|
|
17
|
Wen H, Yuan L, Wei C, Zhao Y, Qian Y, Ma
P, Ding S, Yang X and Wang X: Effects of combined exposure to
formaldehyde and benzene on immune cells in the blood and spleen in
Balb/c mice. Environ Toxicol Pharmacol. 45:265–273. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz LH, Litière S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ossenkoppele G: Acute myeloid leukemia.
HemaSphere. 2:112018.
|
|
20
|
Tsai CH, Hou HA, Tang JL, Kuo YY, Chiu YC,
Lin CC, Liu CY, Tseng MH, Lin TY, Liu MC, et al: Prognostic impacts
and dynamic changes of cohesin complex gene mutations in de novo
acute myeloid leukemia. Blood Cancer J. 7:6632017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lyman SD, James L, Zappone J, Sleath PR,
Beckmann MP and Bird T: Characterization of the protein encoded by
the flt3 (flk2) receptor-like tyrosine kinase gene. Oncogene.
8:815–822. 1993.PubMed/NCBI
|
|
22
|
Altman JK, Perl AE, Cortes JE, Smith CC,
Litzow MR, Hill JE, Larson RA, Liu C, Ritchie EK, Strickland SA, et
al: Deep molecular response to gilteritinib to improve survival in
FLT3 mutation-positive relapsed/refractory acute myeloid leukemia.
J Clin Oncol. 35:70032017. View Article : Google Scholar
|
|
23
|
Spencer DH, Russler-Germain DA, Ketkar S,
Helton NM, Lamprecht TL, Fulton RS, Fronick CC, O'Laughlin M, Heath
SE, Shinawi M, et al: CpG island hypermethylation mediated by
DNMT3A is a consequence of AML progression. Cell. 168:801–816. e13.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Renneville A, Boissel N, Nibourel O,
Berthon C, Helevaut N, Gardin C, Cayuela JM, Hayette S, Reman O,
Contentin N, et al: Prognostic significance of DNA
methyltransferase 3A mutations in cytogenetically normal acute
myeloid leukemia: A study by the Acute Leukemia French Association.
Leukemia. 26:1247–1254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marcucci G, Metzeler KH, Schwind S, Becker
H, Maharry K, Mrózek K, Radmacher MD, Kohlschmidt J, Nicolet D,
Whitman SP, et al: Age-related prognostic impact of different types
of DNMT3A mutations in adults with primary cytogenetically normal
acute myeloid leukemia. J Clin Oncol. 30:742–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jost E, Lin Q, Weidner CI, Wilop S,
Hoffmann M, Walenda T, Schemionek M, Herrmann O, Zenke M,
Brümmendorf T, et al: Epimutations mimic genomic mutations of
DNMT3A in acute myeloid leukemia. Leukemia. 28:12272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song G, Valdez BC, Li Y, Liu Y, Champlin
RE and Andersson BS: Synergistic cytotoxicity of sorafenib with
busulfan and nucleoside analogs in human FMS-like tyrosine Kinase 3
internal tandem duplications-positive acute myeloid leukemia cells.
Biol Blood Marrow Transplant. 20:1687–1695. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang LJ, Jiang NG, Zhou J, Yuan-Xin YE,
Xiao-Jun LU, Ying BW and Jiang H: Immunophenotypic analysis of
DNMT3A gene mutations in the patients with acute myeloid leukemia.
Chin J Clin Lab Sci. 2014.(In Chinese).
|
|
29
|
Kato N, Kitaura J, Doki N, Komeno Y,
Watanabe-Okochi N, Togami K, Nakahara F, Oki T, Enomoto Y, Fukuchi
Y, et al: Two types of C/EBPα mutations play distinct but
collaborative roles in leukemogenesis: Lessons from clinical data
and BMT models. Blood. 117:221–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schranz K, Hubmann M, Harin E, Vosberg S,
Herold T, Metzeler KH, Rothenberg-Thurley M, Janke H, Bräundl K,
Ksienzyk B, et al: Clonal heterogeneity of FLT3-ITD detected by
high-throughput amplicon sequencing correlates with adverse
prognosis in acute myeloid leukemia. Oncotarget. 9:30128–30145.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang BY: Research progress of combined
mutation of FLT3-ITD and DNMT3A R882 gene in acute myeloid
leukemia. Chin Mod Med. 2018.(In Chinese).
|
|
32
|
Gale RE, Lamb K, Allen C, El-Sharkawi D,
Stowe C, Jenkinson S, Tinsley S, Dickson G, Burnett AK, Hills RK
and Linch DC: Simpson's paradox and the impact of different DNMT3A
mutations on outcome in younger adults with acute myeloid leukemia.
J Clin Oncol. 33:2072–2083. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn JS, Kim HJ, Kim YK, Lee SS, Jung SH,
Yang DH, Lee JJ, Kim NY, Choi SH, Jung CW, et al: DNMT3A R882
mutation with FLT3-ITD positivity is an extremely poor prognostic
factor in patients with normal-karyotype acute myeloid leukemia
after allogeneic hematopoietic cell transplantation. Biol Blood
Marrow Transplant. 22:61–70. 2016. View Article : Google Scholar : PubMed/NCBI
|