Introduction
Ewing sarcoma (ES) is a highly aggressive, small,
round cell, malignant neoplasm of bone and soft tissue that
typically manifests in children and young adults (1). It demonstrates an aggressive clinical
behavior with a high-rate of local recurrence and distant
metastasis, with ~25% patients presenting metastases of the bone
marrow and lungs at the time of diagnosis, which contributes to the
high mortality rate (2). Primary
treatment options for ES are traditional methods such as surgical
resection, radiotherapy and chemotherapy, curing ~60% of patients
with localized disease (3). By
contrast, the overall survival rate for patients with distant
metastases treated with traditional methods is <30% (4,5).
Therefore, the pathogenesis of ES, especially the molecular
mechanism involved in metastasis, needs to be elucidated to produce
novel treatments.
NF-κB activating protein (NKAP) was originally
identified as a nuclear localized protein that promotes tumor
necrosis factor- and interleukin-1-induced NF-κB activation
(6). Its protein structure contains
three domains; Serine and arginine repeats at the N-terminus (RS
domain), followed by a basic domain and a C-terminal DUF926 (domain
with unknown function) (7). More
specifically, NKAP has been identified to interact with RNA-binding
proteins through its RS domain in order to regulate RNA splicing
and processing, while its basic domain is essential for nuclear
localization (7). Functional
investigations have found that NKAP is involved in the development,
maturation and acquisition of functional competencies of multiple
immune cells including T cells, Invariant Natural Killer T (iNKT)
cells and regulatory T cells (8–12).
Furthermore, two other studies have demonstrated that NKAP is
required for the maintenance and survival of adult hematopoietic
stem cells and may serve an important role in mouse neurogenesis
(13,14). However, whether NKAP plays a role in
tumor progression remains not clearly understood. Li et al
(15) reported that SUMOylated NKAP
is required for chromosome alignment in mitosis, and that its
dysregulation causes chromosomal instability, potentially
contributing to tumorigenesis. Liu et al (16) reported that NKAP functions as an
oncogene and its expression is induced by CoCl2
treatment in breast cancer via the AKT/mTOR signaling pathway.
In the present study, the role of NKAP in the
proliferation, migration and invasion of ES cells was investigated
using RNA interference technology and pcDNA transfection. In
addition, the potential action mechanisms of NKAP were determined
using signaling pathway investigation. The present study identified
that NKAP has the potential to serve as a promising therapeutic
target for ES.
Materials and methods
Cell culture and transfection
Human mesenchymal stem cells (MSCs; cat. no.
PCS-500-012), and human ES cell lines A673 (cat. no. CRL-1598;
authenticated by short tandem repeat profiling), SK-ES-1 (cat. no.
HTB-86) and RD-ES (cat. no. HTB-166) were purchased from the
American Type Culture Collection. A4573 cells were obtained from
the EuroBoNet cell line panel. TC-71 cells (cat. no. ACC-516) were
obtained from German Collection of Microorganisms and Cell
Cultures. The cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; HyClone; GE Healthcare Life Sciences) and
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.)
under standard culture conditions (37°C; 95% humidified air; 5%
CO2).
In order to knock down or overexpress the expression
of NKAP, ES cells were transfected with either 5 nmol of
NKAP-targeted siRNA (siNKAP) or pcDNA-NKAP (Shanghai GeneChem Co.,
Ltd.) when cells reached 70% confluence using Lipofectamine™ 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) for 5 h in DMEM. Cells
were transferred into DMEM supplemented with 10% FBS and normally
cultured for 24 h before subsequent experimentation. The sequences
of siRNAs were as follows: siNKAP, 5′-GAGAAGAGAGCCCTTGCAT-3′;
non-targeting negative control siRNA (siNC; Shanghai GeneChem
Co.,Ltd.), 5′-UUCUCCGAACGUGUCACGUTT-3′. siNC was transfected into
ES cells as the control of siNKAP.
Reverse transcription-quantitative PCR
(RT-qPCR)
Following transfection for 24 h, total RNA was
isolated from the A673 and RD-ES cells using Ultrapure RNA kit
(Beijing CWBio). A total of 1 mg of RNA was transformed into cDNA
through a RT reaction with oligo (dT) primers using HiFiScript cDNA
Synthesis kit (Beijing CWBio). The liquid mixture was incubated at
42°C for 30–50 min, and then at 85°C for 5 min. Then 1 ng of
first-strand cDNA was used as a template for qPCR using SYBR Premix
Ex Taq II kit (Takara Bio, Inc.; 95°C for 10 min, 40 cycles at 95°C
for 15 sec and at 60°C for 1 min). The relative expression of
target gene was analyzed using the 2−ΔΔCt method
(17). β-actin was used as an
internal control. Sequences of the primers were as follows: NKAP
forward, 5′-CGGCAGAAGAGATTAAGTGAG-3′ and reverse,
5′-CGTTCATACCCCCAGAGGTTTAG-3′, and β-actin forward,
5′-CCCGAGCCGTGTTTCCT-3′ and reverse,
5′-GTCCCAGTTGGTGACGATGC-3′.
Western blot analysis
Following transfection for 48 h, MSC, SK-ES-1,
TC-71, A4573, A673 and RD-ES cells were collected and lysed in
radioimmunoprecipitation assay lysis buffer and protease inhibitors
(CWbio). The lysates were centrifuged at 4°C and 12,396 × g for 30
min, and then protein was quantified using BCA protein assay kit
(Sangon Biotech Co., Ltd.). Equal amounts of proteins (20 mg) from
each group were loaded into the lanes of a 10% gel for separation
by SDS-PAGE then were electrotransferred onto polyvinylidene
fluoride membranes. Non-specific binding was blocked by incubating
the membranes with 5% non-fat milk for 1 h at room temperature. The
membranes were then incubated with primary antibodies overnight at
4°C, washed with TBST (TBS buffer with 0.1% Tween 20) three times,
and incubated with HRP-conjugated secondary antibodies (1:5,000;
cat. no. SA00001-15/SA00001-1; ProteinTech Group, Inc.) for 1 h at
room temperature. After being washed with TBST another four times,
the protein bands were visualized using the Amersham ECL Prime
Western Blotting Detection Reagent (GE Healthcare)., with the gray
values being quantified by Image J software v1.8.0 (National
Institutes of Health). The primary antibodies against Bax (cat. no.
60267-1-lg; 1:1,000), cleaved caspase-3 (cat. no. 19677-1-AP;
1:10,000), Bcl2 (cat. no. 12789-1-AP; 1:1,000), AKT (cat. no.
60203-2-Ig; 1:5,000), phosphorylated (p)-AKT (cat. no. 66444-1-Ig;
1:2,000), cyclin D1 (cat. no. 60186-1-Ig; 1:10,000) and GAPDH (cat.
no. 60004-1-Ig; 1:1,000) were purchased from ProteinTech Group,
Inc. The primary antibodies against NKAP (cat. no. ab229096;
1:1,000) and α-Tubulin (cat. no. ab7291; 1:10,000) were purchased
from Abcam. The primary antibody against Caspase 3 (cat. no. 9662;
1:1,000) was obtained from Cell Signaling Technology. α-Tubulin and
GAPDH were used as internal controls.
Proliferation assays
For the Cell Counting Kit-8 (CCK-8) assay, A673 and
RD-ES cells transfected with siNKAP or pcDNA-NKAP were seeded into
a 96-well plate at a density of 3,000 cells per well, each group
including three wells. Cells were cultured for 24, 48 and 72 h then
were treated with 10 ml CCK-8 solution (Dojindo Molecular
Technologies, Inc.) at 37°C for 2 h. Optical density values with
450 nm were detected using a microplate reader (iMark; Bio-Rad
Laboratories, Inc.).
For the colony formation assay, 500 cells
transfected with siRNA for 24 h were seeded in 6-cm petri dishes
and cultured in DMEM medium supplemented with 10% FBS at 37°C for
two weeks. The colonies were thereafter dried in the air for 1 h
prior to fixing with 4% paraformaldehyde at room temperature for 15
min and staining with 0.1% crystal violet solution at room
temperature for 20 min. The number of colonies was subsequently
counted using a light microscope (magnification, ×4).
Transwell invasion and migration
assays
Transwell chambers (pore size, 8 mm; EMD Millipore)
were used to detect the invasion and migration of the ES cells
transfected with siNKAP or pcDNA-NKAP. For cell invasion, assay the
chambers were pre-coated with Matrigel (BD Biosciences) at 4°C for
30 min. Following transfection, A673 or RD-ES cells were seeded in
the upper chambers at a density of 1×105 in 200 µl of
serum-free DMEM. The lower chambers were then filled with 500 µl of
DMEM with 20% FBS as the chemoattractant. Following 24 h of
incubation at 37°C, the non-invaded cells on the upper Transwell
membrane were removed by scraping, while the invaded cells were
fixed with 4% paraformaldehyde at room temperature for 30 min and
stained with 0.1% crystal violet at room temperature for 20 min.
The stained cells were photographed using a light microscope at
magnification ×100 and counted in three random view fields.
The cell migration experiment followed the
aforementioned protocol however no Matrigel was used in the
Transwell chambers.
Gelatin zymography
The influence of NKAP knockdown on the proteolytic
activities of MMP-9 was detected using gelatin zymography. First,
the A673 and RD-ES cells were transfected with siNC or siNKAP for
24 h. The cells were then harvested and seeded in a 6-cm petri dish
(1×105 cells/dish) with DMEM with 10% FBS. Following
culture for 24 h at 37°C, the supernatants were collected and
centrifuged at 4°C and 2,066 × g for 5 min to remove cell debris.
Protein concentrations were quantified with the BCA protein assay
kit (Sangon Biotech Co., Ltd.). A total of 20 µg of proteins from
each sample were then loaded into a 10% gel with 1 mg/ml gelatin A
(Sigma-Aldrich; Merck KGaA) and separated by SDS/PAGE for 1.5 h.
Finally, the gels were incubated with 0.1% Coomassie Brilliant Blue
at room temperature for 3 h then destained with 45% methanol and
10% (v/v) acetic acid until clear bands suggestive of gelatin
digestion were present. Bands were photographed using a gel imager
(Bio-Rad Laboratories, Inc.) and analyzed with Quantity One
software v4.5 (Bio-Rad Laboratories, Inc.).
Flow cytometry detection for
apoptosis
After being transfected with siNC or siNKAP for 48
h, the apoptosis of ES cells was evaluated using flow cytometry. A
total of 1×106 ES cells were centrifuged at 4°C and
1,033 × g for 5 min and resuspended in 1 ml PBS. A total of 5 µl
Annexin V (1 µg/ml; Aposcreen annexin v-biot; Beckman Coulter,
Inc.) was then added and incubated at room temperature for 15 min.
Subsequently, propidium iodide (PI; 1 µg/ml; Beckman Coulter, Inc.)
was added for 5 min at room temperature. All staining incubation
steps were performed in dark. The apoptosis rate was analyzed by a
flow cytometer and calculated using BD FACSDiva software v4.1 (BD
Biosciences).
Statistical analysis
All experiments were repeated three times with the
data expressed as mean ± standard deviation. Comparisons between
different groups were performed using Student's t-test for two
groups or one-way analysis of variance followed by a Tukey's
post-hoc test for multiple groups. Statistical analysis was
performed using GraphPad Prism 7.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate statistical significance.
Results
Downregulation of NKAP induces growth
inhibition of ES cells
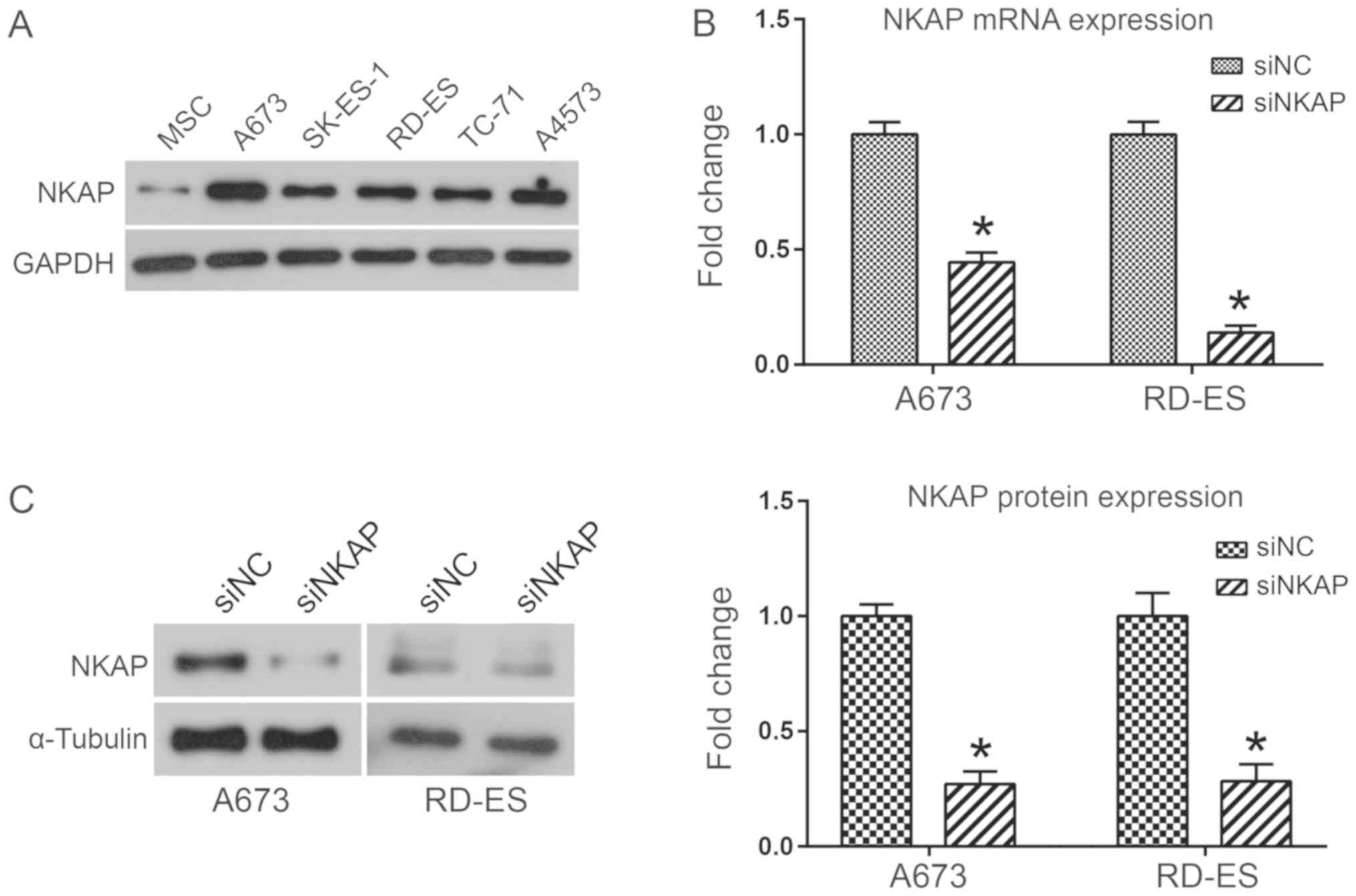
To investigate the biological functions of NKAP in
ES, the expression of NKAP in five different ES cell lines (A673,
SK-ES-1, RD-ES, TC-71 and A4573 cells) and MSCs was analyzed using
western blot analysis. Results indicated that NKAP was markedly
upregulated in ES cell lines when compared with MSCs (Fig. 1A). Due to the high expression of NKAP
in A673 and RD-ES cell lines, NKAP was knocked down in human A673
and RD-ES cell lines via siRNA transfection to investigate the
biological functions of NKAP. The interference effects of siNKAP
were evaluated using RT-qPCR and western blot assays. As
demonstrated in Fig. 1B, NKAP mRNA
expression was significantly decreased in A673 and RD-ES cells
transfected with siNKAP compared with siNC transfection
(P<0.05). Consistent with the mRNA level, NKAP protein
expression was also downregulated when NKAP was silenced
(P<0.05; Fig. 1C). Effects of
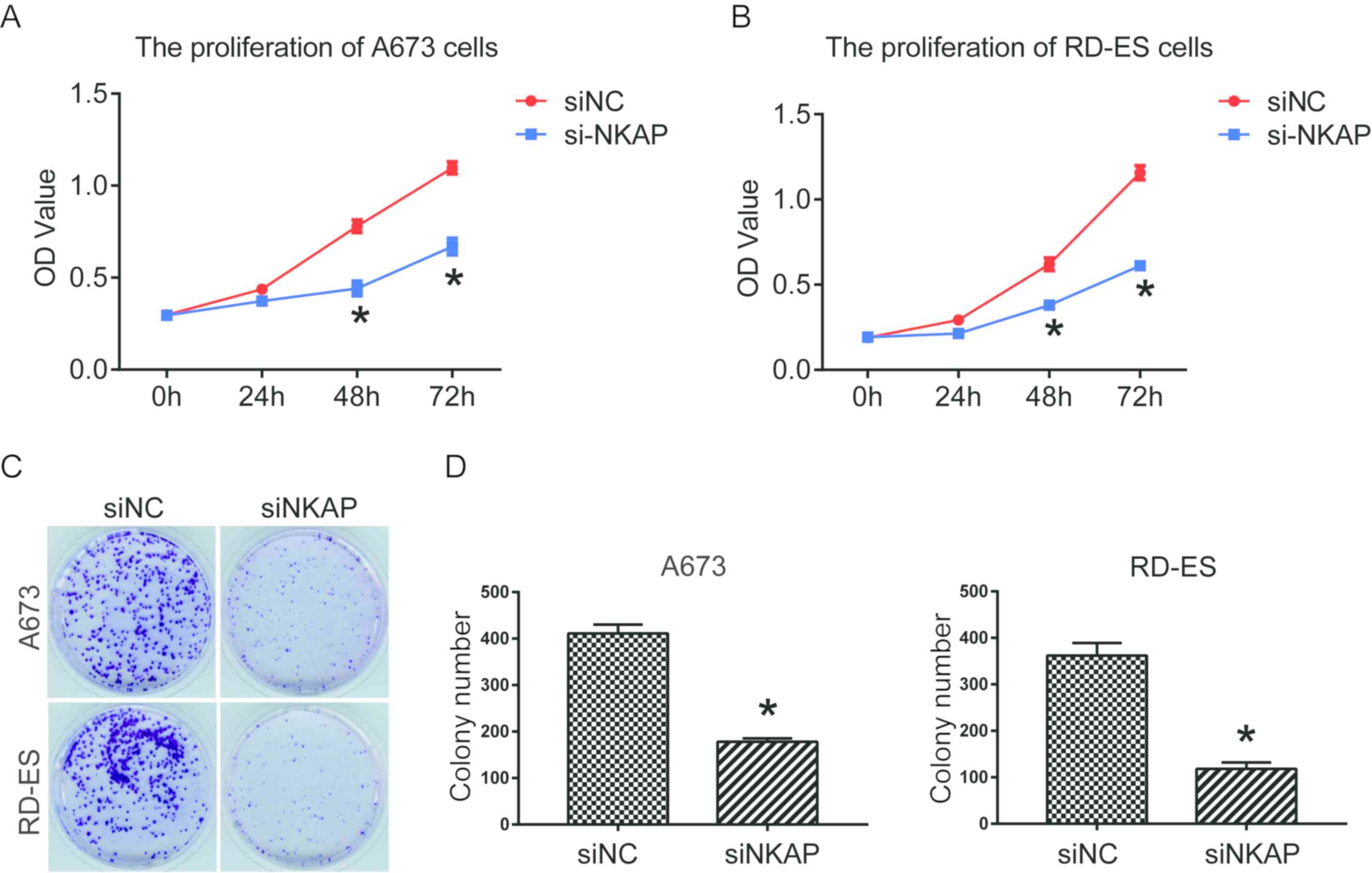
downregulation of NKAP on cell proliferation were investigated
using CCK-8 and colony formation assays. Findings determined that
siNKAP-transfected A673 and RD-ES cells exhibited significantly
decreased cell proliferation and colony formation efficiency when
compared with cells transfected with siNC (P<0.05; Fig. 2).
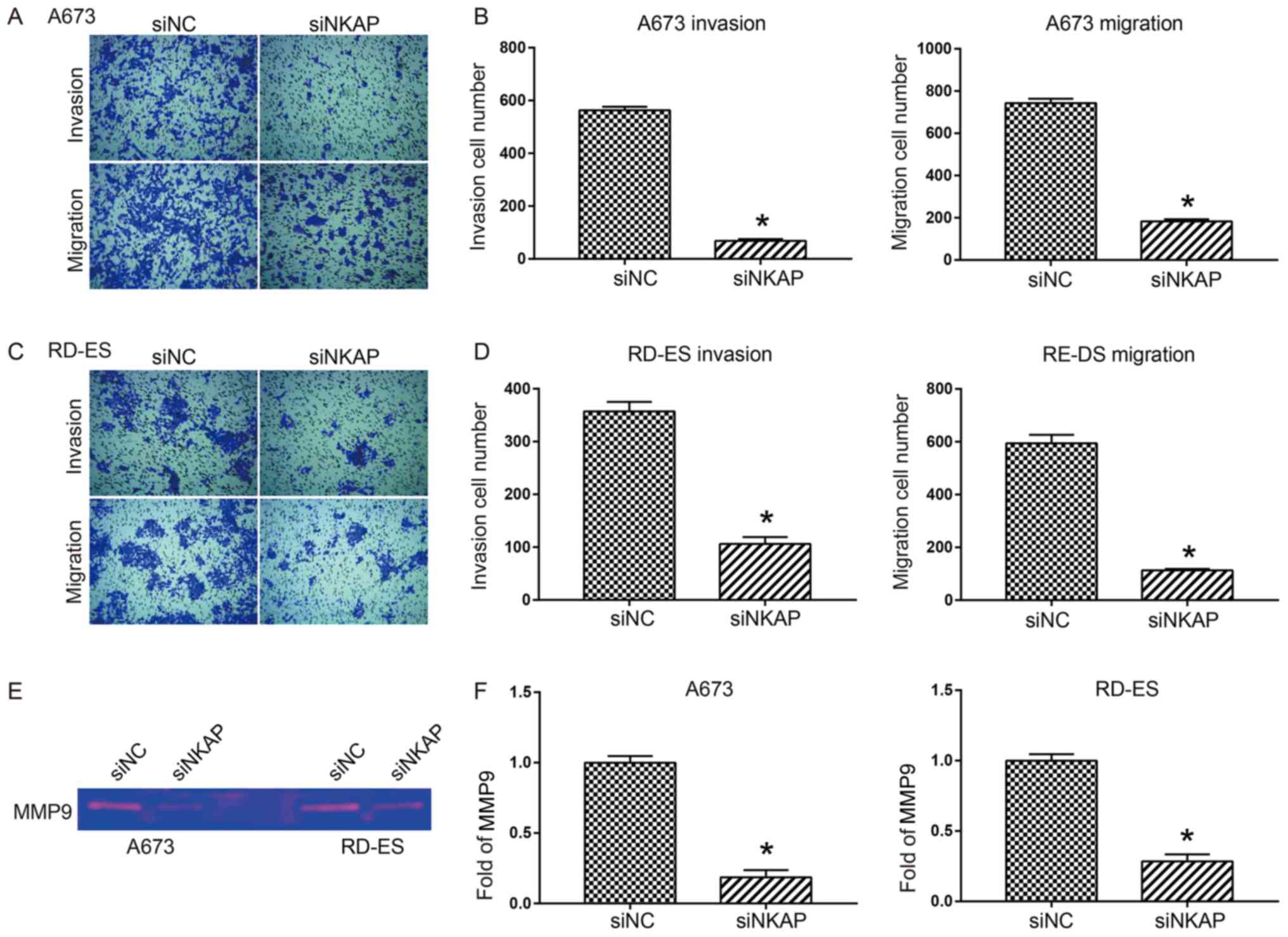
Downregulation of NKAP inhibits the
invasion and migration of ES cells by regulating MMP-9
activity
Invasion and migration are important characteristics
of cancer cells, frequently initiating tumor metastasis in
vivo (18). Therefore, it was
investigated whether NKAP was involved in the invasion and
migration of ES cells. As shown by the Transwell assays, the number
of invasive and migratory A673 cells were significantly decreased
when NKAP was knocked down when compared with the NC group
(P<0.05; Fig. 3A and B). Similar
results were observed in the RD-ES cells (P<0.05; Fig. 3C and D). In order to determine
whether NKAP knockdown induced a decrease in the activity of
secreted MMP-9, which might explain the reduced invasion and
migration, the medium of ES cells was analyzed for MMP-9 activity
by gelatin zymography. As shown in Fig.
3E and F, the degradation of secreted MMP-9 was significantly
decreased in both A673 and RD-ES cells following NKAP silencing
compared with the control (P<0.05).
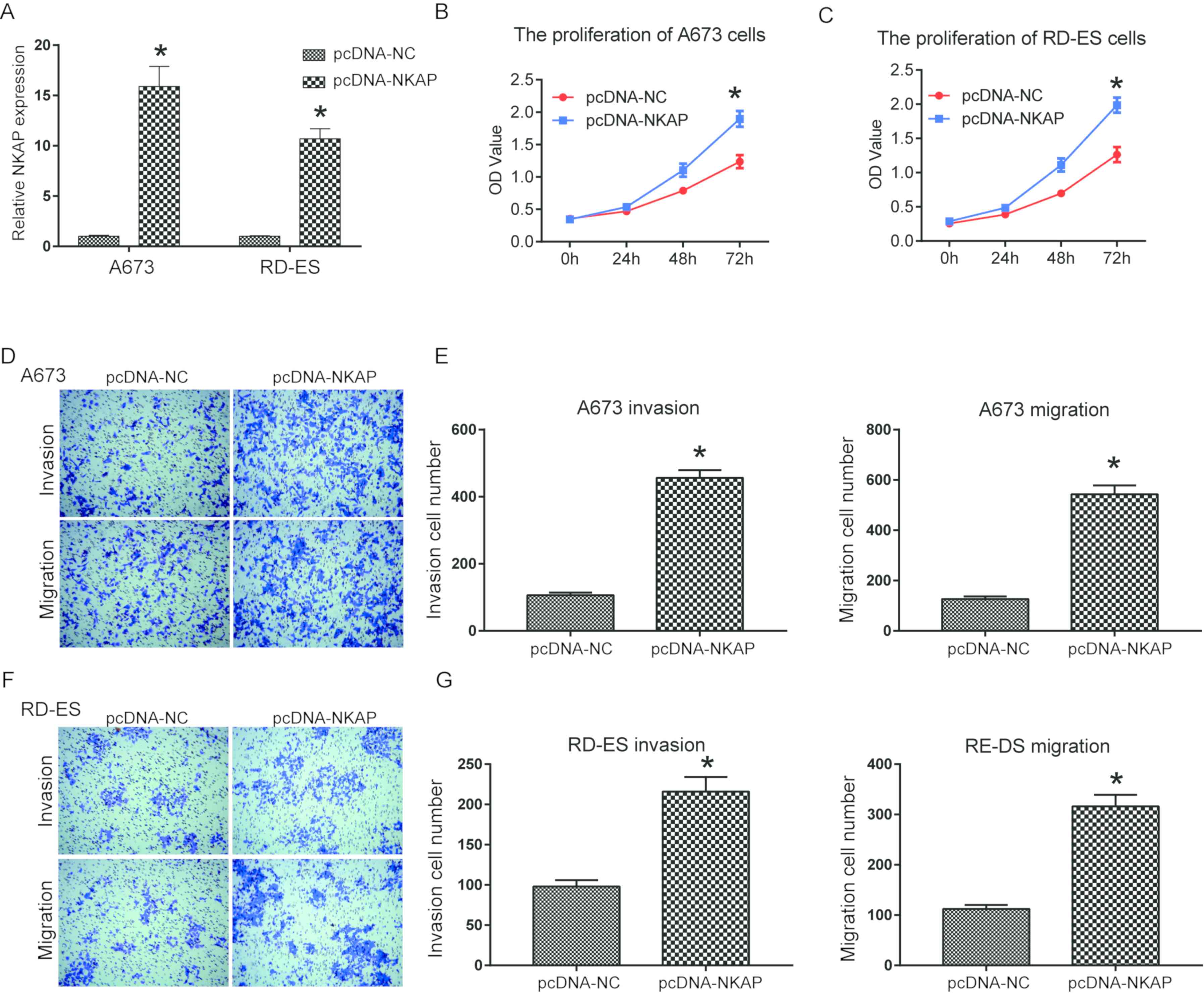
Overexpression of NKAP promotes the
viability, invasion and migration of ES cells
Gain-of-function analysis in ES cells was used to
investigate the function of NKAP more comprehensively.
Overexpression efficiencies of NKAP in A673 and RD-ES cells were
confirmed as NKAP expression in NKAP overexpressing
vector-transfected cells was significantly greater compared with
the control group (P<0.05; Fig.
4A). A673 and RD-ES cell viability significantly increased when
NKAP was overexpressed following pcDNA transfection compared with
the control at 72 h (P<0.05; Fig. 4B
and C). In addition, Transwell assays determined that NKAP
overexpression had a positive role in significantly increasing the
invasion and migration of ES cell lines compared with the control
(P<0.05; Fig. 4D-G). Taken
together, theses results demonstrated that overexpression of NKAP
increased the viability, invasion and migration of ES cells.
Downregulation of NKAP promotes ES
cell apoptosis and activates the mitochondrial apoptosis
pathway
In order to investigate whether cell apoptosis
contributed to the inhibitory effect of NKAP knockdown on ES cells,
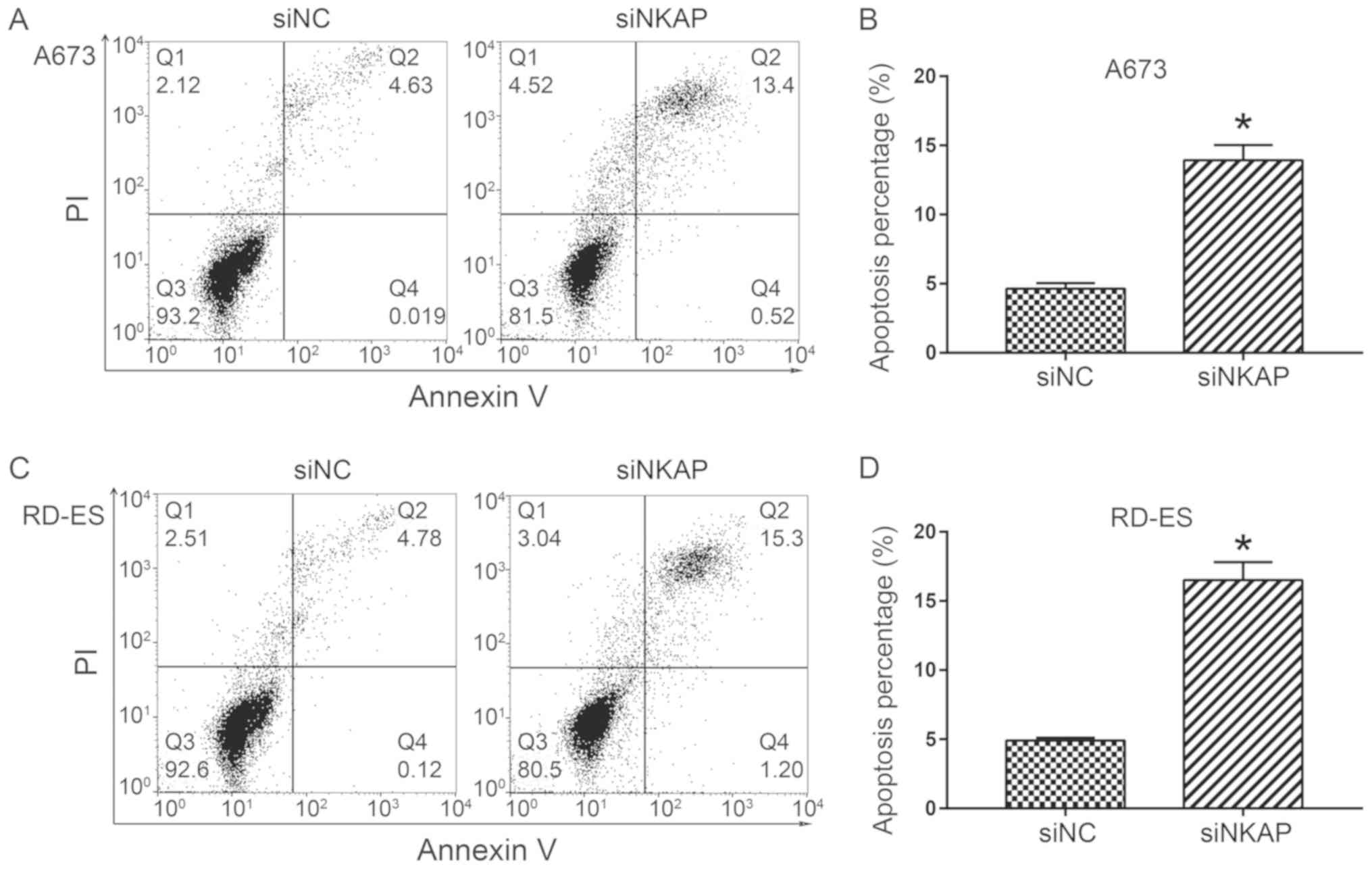
flow cytometry analysis was performed. As demonstrated in Fig. 5, the apoptosis percentage
(Q2+Q4) significantly increased when NKAP was
silenced in A673 and RD-ES cells compared with the control
(P<0.05). Whether the pro-apoptosis effect of NKAP knockdown was
mediated by the mitochondrial apoptosis pathway was investigated
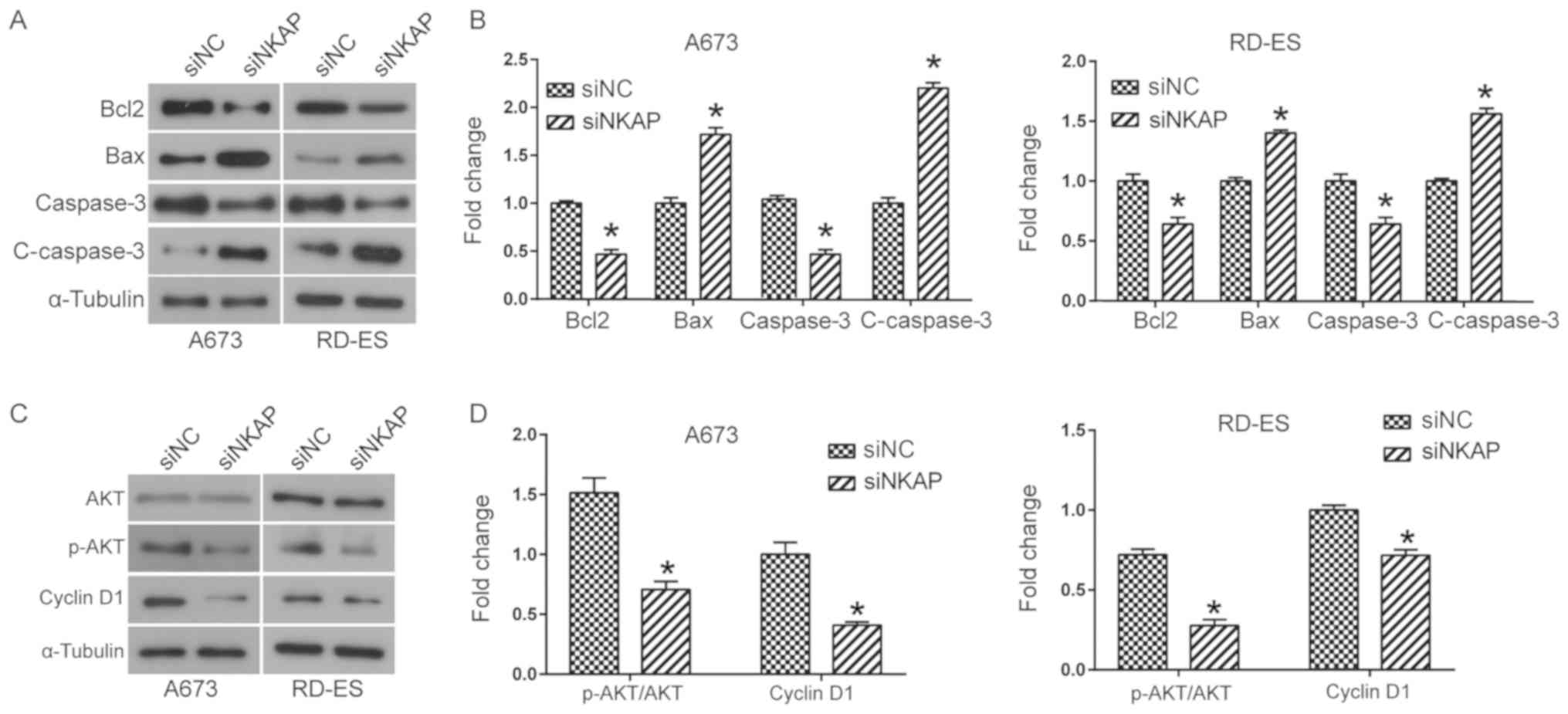
next. Western blot analysis suggested that NKAP knockdown led to a
significant increase in the expression of pro-apoptosis factors,
including Bax and cleaved caspase 3, and a decrease of
anti-apoptotic member Bcl2 compared with the control group
(P<0.05; Fig. 6A and B). Taken
together, NKAP knockdown resulted in the activation of the
mitochondrial apoptosis pathway, increasing the apoptosis of ES
cells.
Downregulation of NKAP inhibits the
activation of the AKT signaling pathway
Finally, the mechanism of action of NKAP in ES cells
was investigated by focusing on the status of the AKT signaling
pathway as it has an important role in tumorigenesis and
progression, being involved in cell proliferation, apoptosis and
metastasis (19,20). Western blot analysis demonstrated
that silencing of NKAP downregulated both the phosphorylation level
of AKT and the expression of its down-stream effector cyclin D1 in
both A673 and RD-ES cells compared with the control (P<0.05;
Fig. 6C and D). These findings
suggested NKAP knockdown led to inactivation of the AKT signaling
pathway.
Discussion
ES is the second-most frequent, primary malignant
bone tumor in children and adolescents (1). Though great improvements have been
achieved in ES therapy, the prognosis for patients with
metastasized ES still remains poor (4). Scientists have thus been committed to
identifying effective prognostic markers or therapeutic targets.
The present study hypothesized that NKAP has the potential to serve
an important role in tumor related functions. Previous studies have
demonstrated that NKAP is a highly conserved protein with various
roles in transcription repression, immune development and
maturation, the maintenance and survival of adult hematopoietic
stem cells, chromosome alignment in mitosis, and RNA splicing and
processing (7,11,14,15).
Furthermore, NKAP deficiency has been identified in soft tissue
sarcomas as well as several other types of human cancer (15). However, the functions and mechanisms
of action of NKAP in tumorigenesis and progression need to be
further elucidated. Therefore, the present study investigated
whether NKAP functions as an important regulator in the
proliferation, migration and invasion of ES cells.
In order to determine this, NKAP was knocked down in
ES cells lines A673 and RD-ES. The effects of NKAP silencing on the
tumor-related phenotypes of ES cells were investigated. Results
determined that NKAP knockdown induced the inhibition of both cell
proliferation and clonogenic abilities in ES cells, whereas NKAP
overexpression promoted cell viability. Flow cytometry indicated
that cell apoptosis was promoted in ES cells following NKAP
silencing. Previous studies demonstrated that NKAP depletion
inhibits the proliferation of iNKT in mice, leading to severe
reductions in thymic and peripheral iNKT cell numbers (8), and decreased proliferation and
increased apoptosis of hematopoietic stem cells (14). This suggests that NKAP functions as
an important regulator in cell proliferation. The present study
identified that NKAP knockdown inhibits migration and invasion in
ES cell lines, most likely mediated by downregulation of MMP-9
secretion. The MMP family has an essential role in regulating cell
mobility by degrading the extracellular matrix to promote tumor
metastasis (21,22). Taken together, the present findings
identified NKAP as a potential novel therapeutic target for tumor
metastasis.
Emerging evidence has identified that NKAP is
involved in the transcription repression of Notch (9). In general, NKAP binds CBF1-interacting
corepressor and recruits histone deacetlylase in T-cell
development, also inducing the activation of the NF-κB signaling
pathway (13). The present study
identified that NKAP knockdown led to the activation of the
mitochondrial apoptosis pathway as evidenced by the increased
levels of Bax and cleaved caspase-3 as well as decreased levels of
Bcl2 detected by western blot analysis. Bax is an established key
apoptosis initiation protein that promotes the permeability of the
mitochondrial outer membrane and triggers the release of cytochrome
C to the cytoplasm (23,24). In turn, cytochrome C induces cell
apoptosis via activation of the caspase cascade (23,24). In
addition, Bcl2 functions as an anti-apoptotic protein by
antagonizing Bax (25,26). The present study determined that the
AKT signaling pathway was inactivated in NKAP-silenced ES cells as
evidenced by the decreased levels of p-AKT and cyclin D1. The AKT
signaling pathway is a key pathway in the regulation of multiple
biological processes such as promoting the proliferation, survival
and migration of tumors (27). When
the AKT pathway is activated, the phosphorylation level of AKT is
elevated, which promotes the expression of a number of its
downstream effectors important in cellular growth such as cyclin D1
(28). The activation of the
Akt/mTOR signaling pathway results in enhanced cell proliferation,
finally leading to tumorigenesis (27).
The present study has some limitations. The
involvement of the mitochondrial apoptosis pathway should be
further confirmed by assessing additional specific markers
(including JC-1), following knockdown of NKAP. In addition, no
rescue experiments were performed to verify that the oncogenic
effects of NKAP on ES cells were indeed mediated by AKT. These
areas of focus should be investigated further in the future
studies.
In conclusion, the present study revealed that NKAP
functions as an oncogenic gene in the progression of ES partly via
the mitochondrial apoptosis and AKT pathways. The findings
suggested that NKAP could be a novel therapeutic target for ES.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL and LQ designed the study and wrote the
manuscript. FL, JW and PW performed the experiments and statistical
analysis. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Esiashvili N, Goodman M and Marcus RB Jr:
Changes in incidence and survival of Ewing sarcoma patients over
the past 3 decades-surveillance epidemiology and end results data.
J Pediatr Hematol Oncol. 30:425–430. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galyfos G, Karantzikos GA, Kavouras N,
Sianou A, Palogos K and Filis K: Extraosseous ewing sarcoma:
Diagnosis, prognosis and optimal management. Indian J Surg.
78:49–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Max D, Kuehnoel CD, Burdach S, Niu L,
Staege MS and Foell JL: Indoleamine-2,3-dioxygenase in an
immunotherapy model for ewing sarcoma. Anticancer Res.
34:6431–6441. 2014.PubMed/NCBI
|
|
4
|
Ladenstein R, Poetschger U, Le Deley MC,
Whelan J, Paulussen M, Oberlin O, van den Berg H, Dirksen U, Hjorth
L, Michon J, et al: Primary Disseminated Multifocal Ewing Sarcoma:
Results of the Euro-EWING 99 Trial. J Clin Oncol. 28:3284–3291.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sand LG, Szuhai K and Hogendoorn PC:
Sequencing Overview of ewing sarcoma: A journey across genomic,
epigenomic and transcriptomic landscapes. Int J Mol Sci.
16:16176–16215. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen D, Li Z, Yang Q, Zhang J, Zhai Z and
Shu HB: Identification of a nuclear protein that promotes NF-kappaB
activation. Biochem Biophys Res Commun. 310:720–724. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burgute BD, Peche VS, Steckelberg AL,
Glöckner G, Gaßen B, Gehring NH and Noegel AA: NKAP is a novel
RS-related protein that interacts with RNA and RNA binding
proteins. Nucleic Acids Res. 42:3177–3193. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thapa P, Chen MW, McWilliams DC, Belmonte
P, Constans M, Sant'Angelo DB and Shapiro VS: NKAP Regulates
Invariant NKT Cell Proliferation and Differentiation into
ROR-gammat-Expressing NKT17 Cells. J Immunol. 196:4987–4998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pajerowski AG, Nguyen C, Aghajanian H,
Shapiro MJ and Shapiro VS: NKAP is a transcriptional repressor of
notch signaling and is required for T cell development. Immunity.
30:696–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thapa P, Das J, McWilliams D, Shapiro M,
Sundsbak R, Nelson-Holte M, Tangen S, Anderson J, Desiderio S,
Hiebert S, et al: The transcriptional repressor NKAP is required
for the development of iNKT cells. Nat Commun. 4:15822013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu FC, Pajerowski AG, Nelson-Holte M,
Sundsbak R and Shapiro VS: NKAP is required for T cell maturation
and acquisition of functional competency. J Exp Med. 208:1291–1304.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dash B, Shapiro MJ, Chung JY, Romero
Arocha S and Shapiro VS: Treg-specific deletion of NKAP results in
severe, systemic autoimmunity due to peripheral loss of Tregs. J
Autoimmun. 89:139–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Worlitzer MM and Schwamborn JC: The Notch
co-repressor protein NKAP is highly expressed in adult mouse
subventricular zone neural progenitor cells. Neuroscience.
266:138–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pajerowski AG, Shapiro MJ, Gwin K,
Sundsbak R, Nelson-Holte M, Medina K and Shapiro VS: Adult
hematopoietic stem cells require NKAP for maintenance and survival.
Blood. 116:2684–2693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li T, Chen L, Cheng J, Dai J, Huang Y,
Zhang J, Liu Z, Li A, Li N, Wang H, et al: SUMOylated NKAP is
essential for chromosome alignment by anchoring CENP-E to
kinetochores. Nat Commun. 7:129692016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Wang H, Yin Y, Li Q and Zhang M:
NKAP functions as an oncogene and its expression is induced by
CoCl2 treatment in breast cancer via AKT/mTOR signaling pathway.
Cancer Manag Res. 10:5091–5100. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Franke TF, Hornik CP, Segev L, Shostak GA
and Sugimoto C: PI3K/Akt and apoptosis: Size matters. Oncogene.
22:8983–8998. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lassen A, Atefi M, Robert L, Wong DJ,
Cerniglia M, Comin-Anduix B and Ribas A: Effects of AKT inhibitor
therapy in response and resistance to BRAF inhibition in melanoma.
Mol Cancer. 13:832014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi BD, Jeong SJ, Wang G, Park JJ, Lim
DS, Kim BH, Cho YI, Kim CS and Jeong MJ: Secretory leukocyte
protease inhibitor is associated with MMP-2 and MMP-9 to promote
migration and invasion in SNU638 gastric cancer cells. Int J Mol
Med. 28:527–534. 2011.PubMed/NCBI
|
|
22
|
Zhang X, Min J, Wang Y, Li Y and Li H, Liu
Q, Liang X, Mu P and Li H: RABEX-5 plays an oncogenic role in
breast cancer by activating MMP-9 pathway. J Exp Clin Cancer Res.
32:522013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gui D, Guo Y, Wang F, Liu W, Chen J, Chen
Y, Huang J and Wang N: Astragaloside IV, a novel antioxidant,
prevents glucose-induced podocyte apoptosis in vitro and in vivo.
PLoS One. 7:e398242012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:922011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-kappaB1/MMP-9 signaling pathway. Cancer Sci.
105:265–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Radha G and Raghavan SC: BCL2: A promising
cancer therapeutic target. Biochim Biophys Acta Rev Cancer.
1868:309–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Polivka J Jr and Janku F: Molecular
targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol
Ther. 142:164–175. 2014. View Article : Google Scholar : PubMed/NCBI
|