Introduction
Atrial fibrillation (AF) is among the most common
arrhythmia seen in clinical practice, with a prevalence approaching
2% of the general population. Its prevalence raises with advancing
age, and is expected to enhance 3-fold in the next 3 decades
(1). AF is associated with impaired
functional status, hospitalization, decreased quality of life and
augmented mortality. Furthermore, clinical characteristics of
ischemic stroke from AF are severe and thromboembolism is
considered the most significant cause (2). Risk factors for developing AF include
both intrinsic cardiac disease such as left ventricular
hypertrophy, valvular pathology, myocardial infarction and
congestive heart failure and non-cardiac risk factors such as
diabetes, smoking, hypertension, and obesity. It has been reported
that AF is linked with a 2.3-fold risk of ischemic stroke, 2-fold
risk of cardiovascular mortality, and 5-fold risk of incident
congestive heart failure (3). AF
development can be a multifactorial process, including
susceptibility related to co-morbidities that promote early atrial
enlargement, inflammation, ion channel abnormalities, conduction
heterogeneity due to atrial fibrosis, and autonomic remodeling.
However, the exact molecular mechanisms leading to AF are still
unknown. In addition to conventional risk factors, a genetic
predisposition has been shown to contribute to AF risk (4), and the genes responsible may provide
important clues toward therapy. Additionally, treatment to restore
sinus rhythm among patients with AF has limited long-term success
rates. Therefore, identifying potential new therapeutic approaches
and targets based on molecular mechanisms of AF is very
important.
Rho-kinase (ROCK) is known as an effector of the
small GTP-binding Rho proteins. Rho/ROCK pathway is involved in
diverse cellular functions, including endothelial dysfunction,
smooth muscle contraction, gene expression, actin cytoskeletal
organization, apoptosis, proliferation, migration, inflammation,
and cell polarity (5). Rho/ROCK
system contributes to the pathogenesis of cardiovascular diseases
including heart failure, hypertension, and angina pectoris
(5). The Rho/ROCK pathway has also
been involved in regulating cardiac conduction system (6). It has been suggested that leukocyte
ROCK activity may be a useful surrogate marker for cardiovascular
outcomes and that ROCK inhibition may be a therapeutic target for
inhibition of cardiovascular events (7). It has been shown that administration of
selective ROCK inhibitor produces antiarrhythmic effects in
experimental studies (8,9). Since there is no clinical study showing
the contribution of leukocyte Rho/ROCK pathway on AF development,
the goal of the present study was to determine the role of
leukocyte RHO/ROCK gene expressions in patients with
non-valvular atrial fibrillation (NVAF).
Materials and methods
A total of 37 NVAF patients followed up in Gaziantep
25 Aralik State Hospital were enrolled in this study. NVAF
diagnosis was made by surface electrocardiogram and all of the
patients had atrial fibrillation when the blood samples were taken.
Patients who had heart failure, peripheral artery disease, valvular
heart disease, coronary artery disease, diabetes mellitus, kidney
failure, thyroid disorder, autoimmune disorder, pregnancy,
hypertension, dyslipidemia, and cancer were excluded. Patients who
had had prior cardiac surgery or an ablation procedure for AF
management were also excluded. All the patients had persistent AF.
Age and sex matched 47 healthy controls were included to the study.
The control group was consisted of healthy individuals who had no
history of cardiac arrhythmias or AF. Patients stopped taking
medications for at least 24 h prior to venous blood sample
collection, and blood samples were taken between 9:00 and 10:00
a.m. Medications used by the patients are presented in Table I. Written informed consent was
obtained from subjects according to the Declaration of Helsinki,
and the Ethics Committee of Gaziantep University approved the study
with approval number 2015/193.
 | Table I.Baseline demographic and clinical
characteristics of patients with NVAF and controls. |
Table I.
Baseline demographic and clinical
characteristics of patients with NVAF and controls.
|
Characteristics | Controls
(n=47) | NVAF Patients
(n=37) | P-value |
|---|
| Age (years) | 57.76±6.05 | 55.82±8.19 | 0.2154 |
| Sex |
|
| 0.8669 |
| Male, n
(%) | 25 (53.2) | 19 (51.4) |
|
| Female,
n (%) | 22 (46.8) | 18 (48.6) |
|
| Smoking status |
|
Current, n (%) | 8 (17.0) | 9 (24.3) | 0.4415 |
| Never,
n (%) | 33 (70.2) | 21 (56.8) |
|
| Past, n
(%) | 6 (12.8) | 7 (18.9) |
|
| BMI
(kg/m2) | 25.52±4.28 | 24.47±6.21 | 0.3624 |
| Systolic BP (mm
Hg) | 118.03±9.76 | 120.93±13.05 | 0.2473 |
| Diastolic BP (mm
Hg) | 77.92±8.46 | 79.84±13.61 | 0.4303 |
| Total cholesterol
(mg/dl) | 146.73±16.32 | 157.04±32.58 | 0.0622 |
| Low density
lipoprotein cholesterol (mg/dl) | 99.72±11.62 | 109.02±30.93 | 0.0609 |
| High density
lipoprotein cholesterol (mg/dl) | 43.18±7.06 | 41.32±8.79 | 0.2852 |
| Triglyceride
(mg/dl) | 126.10±28.53 | 135.11±39.62 | 0.2293 |
| Medications |
|
Antiplatelets, n (%) | – | 25 (67.6) |
|
|
Anticoagulants, n (%) | – | 12 (32.4) |
|
Gene expression studies
Blood samples (5 ml) were obtained from subjects,
and RNA was purified from leukocytes using the High Pure RNA
Isolation Kit (Roche Diagnostics) as described by the manufacturer.
RNA quantities were spectrophotometrically measured by using a
microplate spectrophotometer (Epoch; BioTek). Concentrations were
kept constant according to measurements, and equal sample aliquots
were stored at −80°C until further use. RNA was converted to cDNA
using the Qiagen miScript Reverse Transcription Kit (Qiagen)
according to manufacturer's protocol.
PCR was performed using the high-throughput platform
BioMark HD System (Fluidigm) that utilizes a fluorescent based PCR
method. Expression of each gene was determined by performing
primary probe design. Real-time PCR was done in BioMark 96.96
Dynamic Array (Fluidigm) using a set of TaqMan Gene Expression
Assays (Life Technologies; Thermo Fisher Scientific, Inc.). mRNA
expressions were determined by comparison with housekeeping gene
β-actin (ACTB) and GAPDH from the same sample as
internal control. We studied 2 ROCK and 21 Rho GTPases genes for
expression study. Data were analyzed using the 2−ΔΔCt
method, according to the formula:
ΔCt=CtRHO/ROCK-CtACTB/GAPDH, where
Ct is threshold cycle.
Statistical analysis
Data are expressed as the mean ± SD, SEM or
percentage. Statistics were carried out using GraphPad Instat
version 3.05 (GraphPad Software Inc.). Differences in the mean
values of the two groups were determined using the unpaired
Student's t-test. Categorical data were analyzed with Chi-square
test. The gene expression analysis was performed by using online
program, QIAGEN GeneGlobe (http://www.qiagen.com/geneglobe), and unpaired
Student's t test was used to compare gene expression data. The
level of statistical significance was set at P<0.05.
Results
General characteristics of study
population
Table I shows
clinical and demographic characteristics of the study groups. The
average age, sex, percentages of smokers, body mass index, blood
pressure, total cholesterol, low density lipoprotein cholesterol,
high density lipoprotein cholesterol, and triglyceride levels in
the NVAF group were similar when compared to the controls (Table I). The mean ejection fraction of the
left ventricle was 61.28±2.47%, and the mean left atrium diameter
was 33.96±1.74 mm in the patient group.
Comparision of gene expression between atrial
fibrillation patients and healthy controls. Gene expression studies
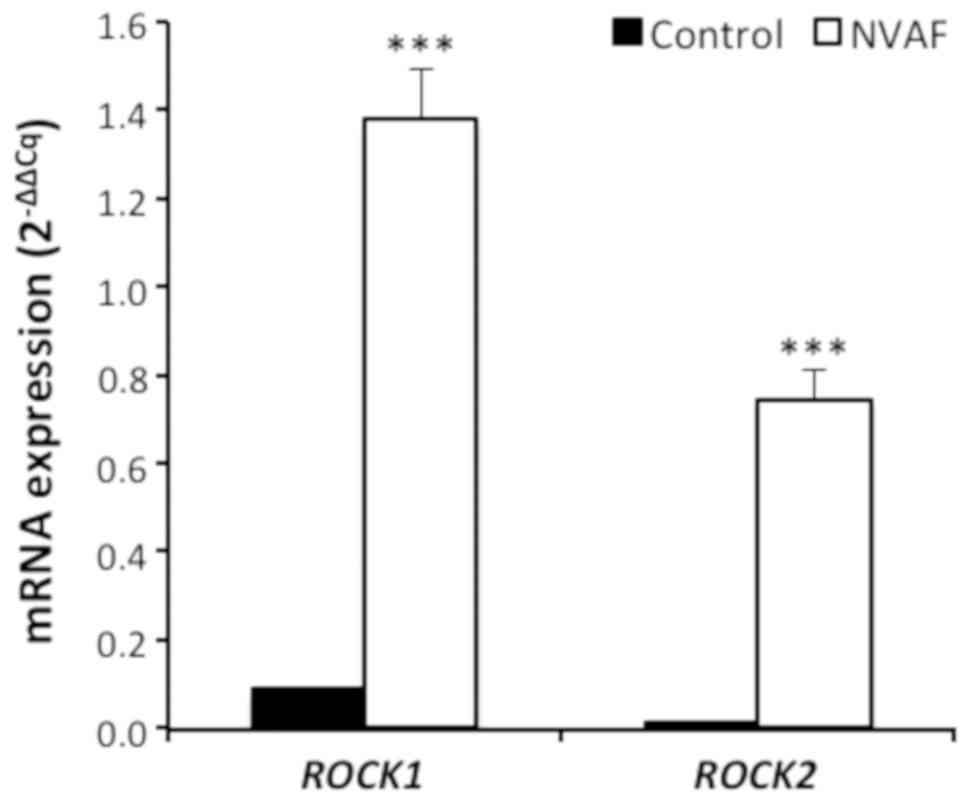
revealed that ROCK1 and ROCK2 mRNA contents in
leukocytes were markedly elevated in NVAF patients when compared to
the control groups (P<0.0001 for ROCK1 and ROCK2
(Fig. 1). There were also
significant elevations in RHOBTB2, RND3 (RHOE), RHOC, RHOG,
RHOH, RAC3, RHOB, RHOD, RHOV, RHOBTB1, RND2, RND1, and
RHOJ gene expressions in NVAF patients (Fig. 2). However, there were marked
depressions in CDC42, RAC2, and RHOQ gene expressions
in NVAF patients (Fig. 3). No
significant changes in gene expressions were detected in other Rho
GTPase proteins (RHOA, RAC1, RHOF, RHOU, and RHOBTB3,
P>0.05 (Figs. 2 and 3).
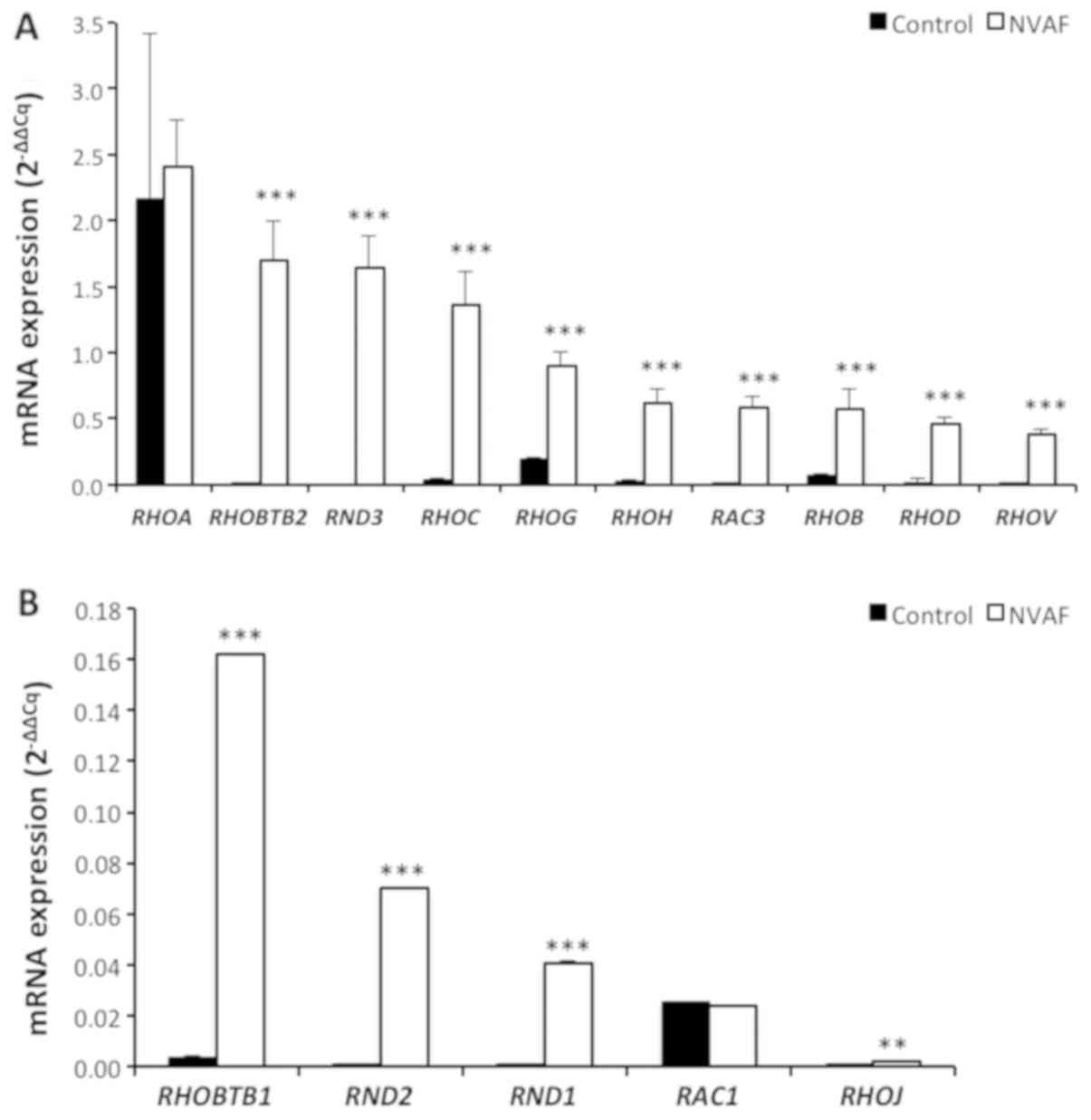 | Figure 2.Comparison of the peripheral blood
mRNA RHOA, RHOBTB2, RND3 (RHOE), RHOC, RHOG, RHOH, RAC3, RHOB,
RHOD, RHOV, (A) and RHOBTB1, RND2, RND1, RAC1, RHOJ (B)
expressions in healthy controls (n=47, solid bars) and in patients
with non-valvular atrial fibrillation (NVAF, n=37, open bars).
Values are given as mean ± SEM. P=0.8478, P<0.0001, P<0.0001,
P<0.0001, P<0.0001, P<0.0001, P<0.0001, P=0.0003,
P<0.0001, P<0.0001, P<0.0001, P<0.0001, P<0.0001,
P=0.9273, and P=0.0084 values were obtained for RHOA, RHOBTB2,
RND3 (RHOE), RHOC, RHOG, RHOH, RAC3, RHOB, RHOD, RHOV, RHOBTB1,
RND2, RND1, RAC1 and RHOJ, respectively. **P<0.01 and
***P<0.001. NVAF, non-valvular atrial fibrillation. |
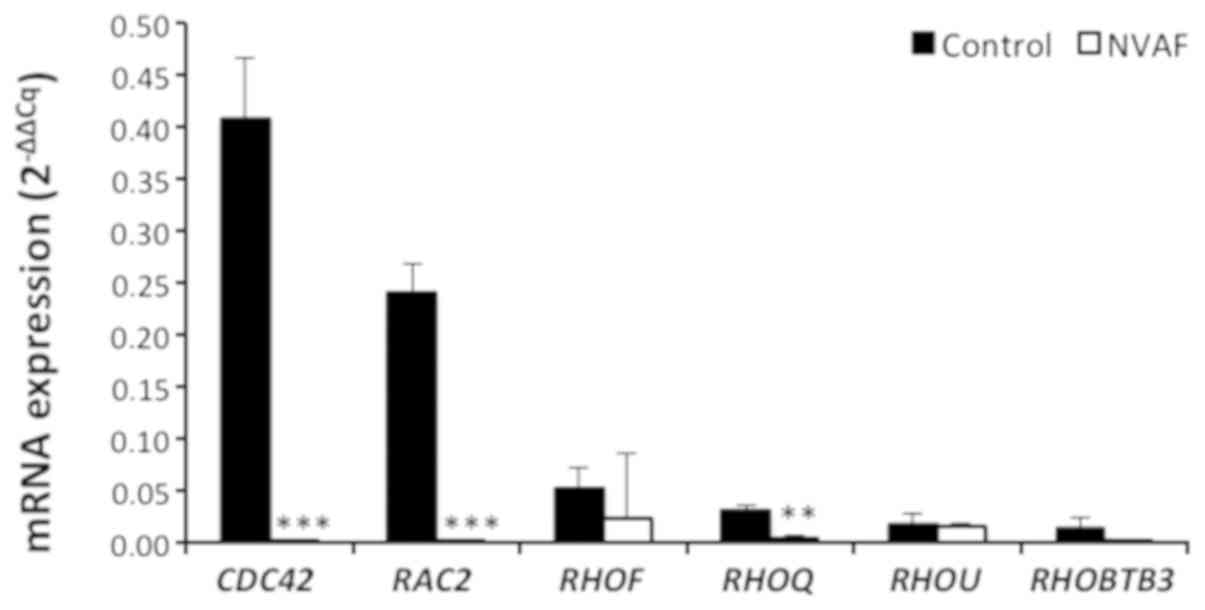 | Figure 3.Comparison of the peripheral blood
mRNA CDC42, RAC2, RHOF, RHOQ, RHOU, and RHOBTB3
expressions in healthy controls (n=47, solid bars) and in patients
with non-valvular atrial fibrillation (NVAF, n=37, open bars).
Values are given as mean ± SEM, P<0.0001, P<0.0001, P=0.1511,
P=0.0060, P=0.7739, and P=0.0511 values were obtained for CDC42,
RAC2, RHOF, RHOQ, RHOU, and RHOBTB3, respectively.
**P<0.01 and ***P<0.001. NVAF, non-valvular atrial
fibrillation. |
Discussion
This study evaluated 2 ROCK and 21 RHO
gene expressions in patients with NVAF and compared to control
subjects in this study. Suppressed (CDC42, RAC2, and
RHOQ,) and elevated (ROCK1, ROCK2, RND3, RHOBTB2, RHOC,
RHOG, RHOH, RAC3, RHOD, RHOB, RHOV, RHOBTB1, RND2, RND1, and
RHOJ) gene expressions were observed in cases with NVAF.
However, we have not observed marked changes in RHOA, RAC1,
RHOF, RHOU, and RHOBTB3 gene expressions. To the best of
our knowledge, there is no published study to evaluate the
leukocyte RHO/ROCK gene expressions in NVAF.
Several studies have suggested that the measurement
of leukocyte ROCK activity is a valuable and alternative method to
determine clinical ROCK activity in patients. ROCK activity in
circulating leukocytes is considered to be a useful biomarker for
the assessment of therapeutic responses and disease severity
(7). Indeed, the elevated leukocyte
ROCK activities in patients with hypertension (10), stable chronic congestive heart
failure (11), metabolic syndrome
(12), coronary artery disease
(13), and angina pectoris (14) have previously been reported. Li et
al (15) showed that ROCK1, but
not ROCK2, activity in circulating leukocytes is increased in
ST-segment elevation myocardial infarction (STEMI) patients with
diabetes mellitus. However, protein expression levels of ROCK1 and
ROCK2 in circulating leukocytes were found to be both greater in
STEMI patients with diabetes than those without diabetes (15). The protein expressions of ROCK1 and
ROCK2 in the myolytic left atrial myocytes of mitral regurgitation
AF patients were found to be markedly higher than that of the
normal subjects (16). We have
observed an increased ROCK1 and ROCK2 gene
expressions in circulating leukocytes of NVAF patients.
Rnd3 (known as RhoE) functions as a repressor of
ROCK1 (17,18). Since members of the Rnd subfamily are
defective in GTPase activity, even in the presence of RhoGAPs, they
bind but do not hydrolyze GTP (19).
It has been demonstrated that Rnd3 (RhoE) is able to bind to and
inhibit the function of ROCK1 but not that of ROCK2 (18). Additionally, Rnd3 (RhoE) binding to
ROCK1 prevents RhoA binding to the Rho-binding domain (18). Rnd3 (RhoE) is the only endogenous
ROCK1 antagonist discovered to date (18). Overexpression of Rnd3 (RhoE)
diminished ROCK1-mediated biological effects including myosin light
chain phosphatase phosphorylation, stress fiber formation, and
apoptosis (18). Rnd3-null mice died
due to fetal arrhythmias at the embryonic stage (20). We have observed augmented both
RND3 (RHOE) and ROCK1 gene expressions in our study.
This data may suggest that Rnd3 (RhoE) inhibition of ROCK1
signaling does not occur at the gene expression level.
Gene expression of RHOA from left atrial
tissue is significantly increased in patients with severe mitral
valve disease and persistent AF when compared to sinus rhythm
(21). However, we have not noted
any significant change in RHOA gene expression in this
study. This may be related to the fact that Rnd proteins function
as RhoA antagonists (17). We have
detected increased expressions of RND1, RND2, RND3 as well
as RHOG genes. Up-regulation RhoG has also been observed to
counteract the effects of RhoA (19).
The atypical Rho GTPases, RhoU and RhoV, which are
constitutively in an active GTP-bound state, may regulate cell-cell
adhesions (22). We detected
elevated RHOV, but not RHOU, gene expression in our
study. Significance of this observation in NVAF remains to be
identified.
The RhoBTB family consists of three members, namely
RhoBTB1, RhoBTB2 and RhoBTB3. They are considered atypical Rho
GTPases, because they are not regulated by the conventional GTPase
cycle (23). Augmentation in
RHOBTB1 and RHOBTB2, but no change in RHOBTB3,
gene expressions was observed in our study. However, their roles in
the NVAF are currently unknown.
AF is frequently associated with cerebral and
cardiac atherothromboembolism (2).
It is known that Rac2 and RhoH are the only Rho GTPases with
expression restricted to the hematopoietic cells (24). We detected depressed RAC2 and
elevated RHOH gene expressions in this study. Significance
of these changes in AF-related thrombogenesis is not known, and
requires further studies.
Rac1 is a membrane-bound signal transducing molecule
involved in the regulation of adhesion and cell motility as well as
mitosis, gene expression, cell cycle progression, and cell death
(25). Rac1 GTPase is one of the
main regulators of cell motility through actin reorganization and
of reactive oxygen species (ROS) formation through regulation of
NADPH oxidase activity (25).
However, we have found no change in leukocyte RAC1 gene
expression in this study.
CDC42 activity is essential for endothelial barrier
repair, adherens junction stability, and restoration of
permeability (26). Thus, depressed
CDC42 gene expression seen in this study may contribute to
the development of AF.
Accumulating evidence indicate that generation of
ROS may play an important role in the induction and maintenance of
AF (27). Levels of the serum
oxidative stress biomarker are elevated in patients with AF
(27). AF in human is linked with a
significant reduction in the expression of antioxidant genes as
well as a significant increase in the expression of 5 genes related
to ROS, supporting a shift toward prooxidation state in AF
(28). There is evidence that
augmented oxidative stress triggers the activation of ROCK
(29). ROCK also up-regulates NADPH
oxidases, and increases Ang II-induced ROS production (30). Taken together, these findings may
imply that ROCK is involved in the pathogenesis of AF.
There are several limitations of this study.
Firstly, the gene expression profiles in this study were measured
in isolated leukocytes, but the ideal tissue for study NVAF is the
left atrium. However, Lin et al (31) studied the association of whole blood
gene expression with AF in a large community-based cohort, and
identified 7 genes markedly upregulated with prevalent AF. Raman
et al (32) also elucidated
peripheral blood gene expression in patients with persistent AF
that underwent electrical cardioversion. In another study,
peripheral monocyte toll-like receptor (TLR) expression levels have
been investigated and elevated TLR-2 and TLR-4 expressions were
detected in patients with AF (33).
Recently, we have also shown that all transient receptor potential
channels gene expressions are upregulated in leukocytes of the NVAF
patients (34). The increased
leukocyte RHO/ROCK gene expressions observed in this study
should be studied at the cardiac level. However, it should be
emphasized that this group patient with NVAF has no indication for
cardiac operation. Additionally, we were not able validate the
Rho/ROCK pathway in AF development using another method such as
western blot analysis. Finally, medications can modulate Rho/ROCK
pathway and can induce gene expressions. However, in order to
eliminate this, subjects stopped taking medications for at least 24
h prior to blood collection.
These data may imply that Rho/ROCK pathway can
contribute to the pathogenesis of NVAF through activated leukocytes
which stimulates the immune or inflammatory cascade. The findings
of this study may provide novel pathophysiological insights for
NVAF, and new potential intervention targets that can be tested in
future studies. Therefore, these findings may also suggest that
Rho/ROCK inhibitors can serve as a potential novel antiarrhythmic
approach for the treatment of AF.
Acknowledgements
The authors wish to thank Ebru Temiz (Gaziantep
University, Department of Medical Biology) for her contribution to
gene expression analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IVD designed and performed experiments. IVD, FY, EV,
ES, FP and HA collected and analysed the data. IVD, YC, HG, BC and
MS interpreted results of the study. ATD and IVD performed the
statistical analyses, prepared figures, and wrote the paper. All
authors edited and revised manuscript and approved final submission
of manuscript.
Ethics approval and consent to
participate
The study protocol of the present experiment was
reviewed and approved by the Ethics Committee at Ethics Committee
of Gaziantep University (Approval number 2015/193). Written
informed consent was obtained from each patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morin DP, Bernard ML, Madias C, Rogers PA,
Thihalolipavan S and Estes NA III: The state of the art: Atrial
fibrillation epidemiology, prevention, and treatment. Mayo Clin
Proc. 91:1778–1810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Violi F and Loffredo L: Thromboembolism or
atherothromboembolism in atrial fibrillation? Circ Arrhythm
Electrophysiol. 5:1053–1055. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Odutayo A, Wong CX, Hsiao AJ, Hopewell S,
Altman DG and Emdin CA: Atrial fibrillation and risks of
cardiovascular disease, renal disease, and death: Systematic review
and meta-analysis. BMJ. 354:i44822016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lubitz SA, Yin X, Fontes JD, Magnani JW,
Rienstra M, Pai M, Villalon ML, Vasan RS, Pencina MJ, Levy D, et
al: Association between familial atrial fibrillation and risk of
new-onset atrial fibrillation. JAMA. 304:2263–2269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei L, Taffet GE, Khoury DS, Bo J, Li Y,
Yatani A, Delaughter MC, Klevitsky R, Hewett TE, Robbins J, et al:
Disruption of Rho signaling results in progressive atrioventricular
conduction defects while ventricular function remains preserved.
FASEB J. 18:857–859. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kajikawa M, Noma K, Maruhashi T, Mikami S,
Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K,
et al: Rho-associated kinase activity is a predictor of
cardiovascular outcomes. Hypertension. 63:856–864. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demiryürek S, Kara AF, Celik A, Babül A,
Tarakcioglu M and Demiryürek AT: Effects of fasudil, a Rho-kinase
inhibitor, on myocardial preconditioning in anesthetized rats. Eur
J Pharmacol. 527:129–1240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Demiryürek S, Kara AF, Celik A,
Tarakçioğlu M, Bagci C and Demiryürek AT: Effects of Y-27632, a
selective Rho-kinase inhibitor, on myocardial preconditioning in
anesthetized rats. Biochem Pharmacol. 69:49–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hata T, Soga J, Hidaka T, Idei N, Fujii Y,
Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, et al:
Calcium channel blocker and Rho-associated kinase activity in
patients with hypertension. J Hypertens. 29:373–379. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ocaranza MP, Gabrielli L, Mora I, Garcia
L, McNab P, Godoy I, Braun S, Córdova S, Castro P, Novoa U, et al:
Markedly increased Rho-kinase activity in circulating leukocytes in
patients with chronic heart failure. Am Heart J. 161:931–937. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu PY, Chen JH, Lin LJ and Liao JK:
Increased Rho kinase activity in a Taiwanese population with
metabolic syndrome. J Am Coll Cardiol. 49:1619–1624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nohria A, Grunert ME, Rikitake Y, Noma K,
Prsic A, Ganz P, Liao JK and Creager MA: Rho kinase inhibition
improves endothelial function in human subjects with coronary
artery disease. Circ Res. 99:1426–1432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruhashi T, Noma K, Fujimura N, Kajikawa
M, Matsumoto T, Hidaka T, Nakashima A, Kihara Y, Liao JK and
Higashi Y: Exogenous nitric oxide inhibits Rho-associated kinase
activity in patients with angina pectoris: A randomized controlled
trial. Hypertens Res. 38:485–490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Wu X, Li H, Chen H, Wang Y, Li W,
Ding X and Hong X: Increased Rho kinase activity predicts worse
cardiovascular outcome in ST-segment elevation myocardial
infarction patients. Cardiol J. 23:456–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen HC, Chang JP, Chang TH, Lin YS, Huang
YK, Pan KL, Fang CY, Chen CJ, Ho WC and Chen MC: Enhanced
expression of ROCK in left atrial myocytes of mitral regurgitation:
A potential mechanism of myolysis. BMC Cardiovasc Disord.
15:332015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wennerberg K, Forget MA, Ellerbroek SM,
Arthur WT, Burridge K, Settleman J, Der CJ and Hansen SH: Rnd
proteins function as RhoA antagonists by activating p190 RhoGAP.
Curr Biol. 13:1106–1115. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riento K, Villalonga P, Garg R and Ridley
A: Function and regulation of RhoE. Biochem Soc Trans. 33:649–651.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jie W, Andrade KC, Lin X, Yang X, Yue X
and Chang J: Pathophysiological functions of Rnd3/RhoE. Compr
Physiol. 6:169–186. 2016.
|
|
20
|
Yang X, Wang T, Lin X, Yue X, Wang Q, Wang
G, Fu Q, Ai X, Chiang DY, Miyake CY, et al: Genetic deletion of
Rnd3/RhoE results in mouse heart calcium leakage through
upregulation of protein kinase A signaling. Circ Res. 116:e1–e10.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai FC, Chang GJ, Hsu YJ, Lin YM, Lee YS,
Chen WJ, Kuo CT and Yeh YH: Proinflammatory gene expression in
patients undergoing mitral valve surgery and maze ablation for
atrial fibrillation. J Thorac Cardiovasc Surg. 151:1673–1682 e5.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wherlock M and Mellor H: The RhoGTPase
family: A Racs to Wrchs story. J Cell Sci. 115:239–240.
2002.PubMed/NCBI
|
|
23
|
Ji W and Rivero F: Atypical Rho GTPases of
the RhoBTB subfamily: Roles in vesicle trafficking and
tumorigenesis. Cells. 5:E282016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pai SY, Kim C and Williams DA: RacGTPases
in human diseases. Dis Markers. 29:177–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adam O, Frost G, Custodis F, Sussman MA,
Schäfers HJ, Böhm M and Laufs U: Role of Rac1 GTPase activation in
atrial fibrillation. J Am Coll Cardiol. 50:359–367. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loirand G, Sauzeau V and Pacaud P: Small G
proteins in the cardiovascular system: Physiological and
pathological aspects. Physiol Rev. 93:1659–1720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimano M, Shibata R, Inden Y, Yoshida N,
Uchikawa T, Tsuji Y and Murohara T: Reactive oxidative metabolites
are associated with atrial conduction disturbance in patients with
atrial fibrillation. Heart Rhythm. 6:935–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim YH, Lim DS, Lee JH, Shim WJ, Ro YM,
Park GH, Becker KG, Cho-Chung YS and Kim MK: Gene expression
profiling of oxidative stress on atrial fibrillation in humans. Exp
Mol Med. 35:336–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin L, Ying Z and Webb RC: Activation of
Rho/Rho kinase signaling pathway by reactive oxygen species in rat
aorta. Am J Physiol Heart Circ Physiol. 287:H1495–H1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Higashi M, Shimokawa H, Hattori T, Hiroki
J, Mukai Y, Morikawa K, Ichiki T, Takahashi S and Takeshita A:
Long-term inhibition of Rho-kinase suppresses angiotensin
II-induced cardiovascular hypertrophy in rats in vivo: Effect on
endothelial NAD(P)H oxidase system. Circ Res. 93:767–775. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin H, Yin X, Lunetta KL, Dupuis J,
McManus DD, Lubitz SA, Magnani JW, Joehanes R, Munson PJ, Larson
MG, et al: Whole blood gene expression and atrial fibrillation: The
framingham heart study. PLoS One. 9:e967942014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raman K, Aeschbacher S, Bossard M,
Hochgruber T, Zimmermann AJ, Kaufmann BA, Pumpol K, Rickenbacker P,
Paré G and Conen D: Whole blood gene expression differentiates
between atrial fibrillation and sinus rhythm after cardioversion.
PLoS One. 11:e01575502016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gurses KM, Kocyigit D, Yalcin MU, Canpinar
H, Yorgun H, Sahiner ML, Kaya EB, Oto MA, Ozer N, Guc D and Aytemir
K: Monocyte Toll-like receptor expression in patients with atrial
fibrillation. Am J Cardiol. 117:1463–1467. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Düzen IV, Yavuz F, Vuruskan E, Saracoglu
E, Poyraz F, Göksülük H, Candemir B and Demiryürek S: Leukocyte TRP
channel gene expressions in patients with non-valvular atrial
fibrillation. Sci Rep. 7:92722017. View Article : Google Scholar : PubMed/NCBI
|