Introduction
Deep vein thrombosis (DVT) of the inferior limbs is
one of the most common diseases of the veins and it can develop
into pulmonary embolism (PE) (1,2).
Compared with the use of anticoagulation alone, the use of
thrombolysis for DVT not only breaks down blood clots but also has
other advantages, such as early thrombus removal, reduced risk of
post-thrombotic syndrome (PTS) and avoidance of DVT recurrence
(3,4). Thus, thrombolysis therapy is widely
gaining favor and rapidly becoming popular in the endovascular
treatment of DVT. To date, urokinase remains the most common
fibrinolytic agent selected for use in Asian countries, on the
basis of its economy and predictability for the treatment of DVT.
However, there are certain limitations in its specificity and
efficacy, and some patients do not respond to fibrinolytic therapy
with urokinase (5).
At present, the further therapeutic treatment
options for patients with DVT who do not respond to conventional
fibrinolytic therapy with urokinase remain unclear. This is partly
due to the difficulty of defining ‘unsuccessful’ thrombolysis with
urokinase in this setting, whereas in patients with acute
myocardial infarction or acute stroke, the criteria and
consequences of unsuccessful thrombolysis are well established
(6). In the setting of DVT, the
recovery of clinical manifestations is a straightforward marker of
thrombolytic efficacy, as well as a predictor of in-hospital
course. Furthermore, a lower percentage of thrombotic removal grade
and higher residual thrombotic burden after thrombolytic therapy
have been shown to be adverse long-term outcomes of increased PTS
risk (3). The optimization of early
DVT revascularization could play a pivotal role in improving the
immediate and long-term progression of patients with DVT who do not
respond to thrombolysis with urokinase. However, there are few
structured strategies for the rescue management of unsuccessful
thrombolysis, and the most suitable management strategy for DVT
patients who do not respond to thrombolysis has not been
identified.
The therapeutic options for patients who have
undergone unsuccessful thrombolysis comprise different strategies
including anticoagulation alone, repeat thrombolysis with
recombinant tissue plasminogen activator (rt-PA) and other
therapies. The majority of clinicians follow a conservative
approach of anticoagulation alone and obtain an unsatisfactory
outcome. Thrombolytic therapy is superior compared with
anticoagulation alone (2–4). Alteplase is considered a common
fibrinolytic agent of rt-PA and is used in Europe (5), thus, the present study aimed to
investigate the efficacy and safety of rescue thrombolysis with
alteplase in patients who do not respond to thrombolysis with
urokinase based on a single-center institution.
Materials and methods
Patients
The protocol of the present retrospective study was
approved by the Ethics Committee of Nanjing First Hospital
(Nanjing, China; approval no. KY20140430-01-KS-01). Prior to
therapy, informed consent was obtained from all patients likely to
require endovascular treatment or their immediate family members.
Prior to January 2016, urokinase was the exclusive fibrinolytic
agent used at Nanjing First Hospital, and alteplase was used from
January 2016. All patients had no contraindications to
anticoagulation or thrombolysis therapy (7), and patients who met the following
inclusion criteria were included in the study and received
urokinase thrombolysis during the initial therapeutic encounter:
Age, 18–75 years; proven recent DVT (7) (duration of symptoms <28 days); and a
first-time objectively verified proximal DVT above mid-thigh level.
Unsuccessful thrombolysis with urokinase was defined as a lack of
improvement in the degree of thrombotic removal with a lysis rate
<50% under one of the following three conditions: Two
consecutive venography procedures; administration of >3 million
units of urokinase in total; or >7 days infusion duration.
Patients with a higher risk of bleeding (7), such as those with a fibrinogen level
(FIB) <1.0 g/l, heparin-induced thrombocytopenia (HIT) or a life
expectancy <2 years, were excluded.
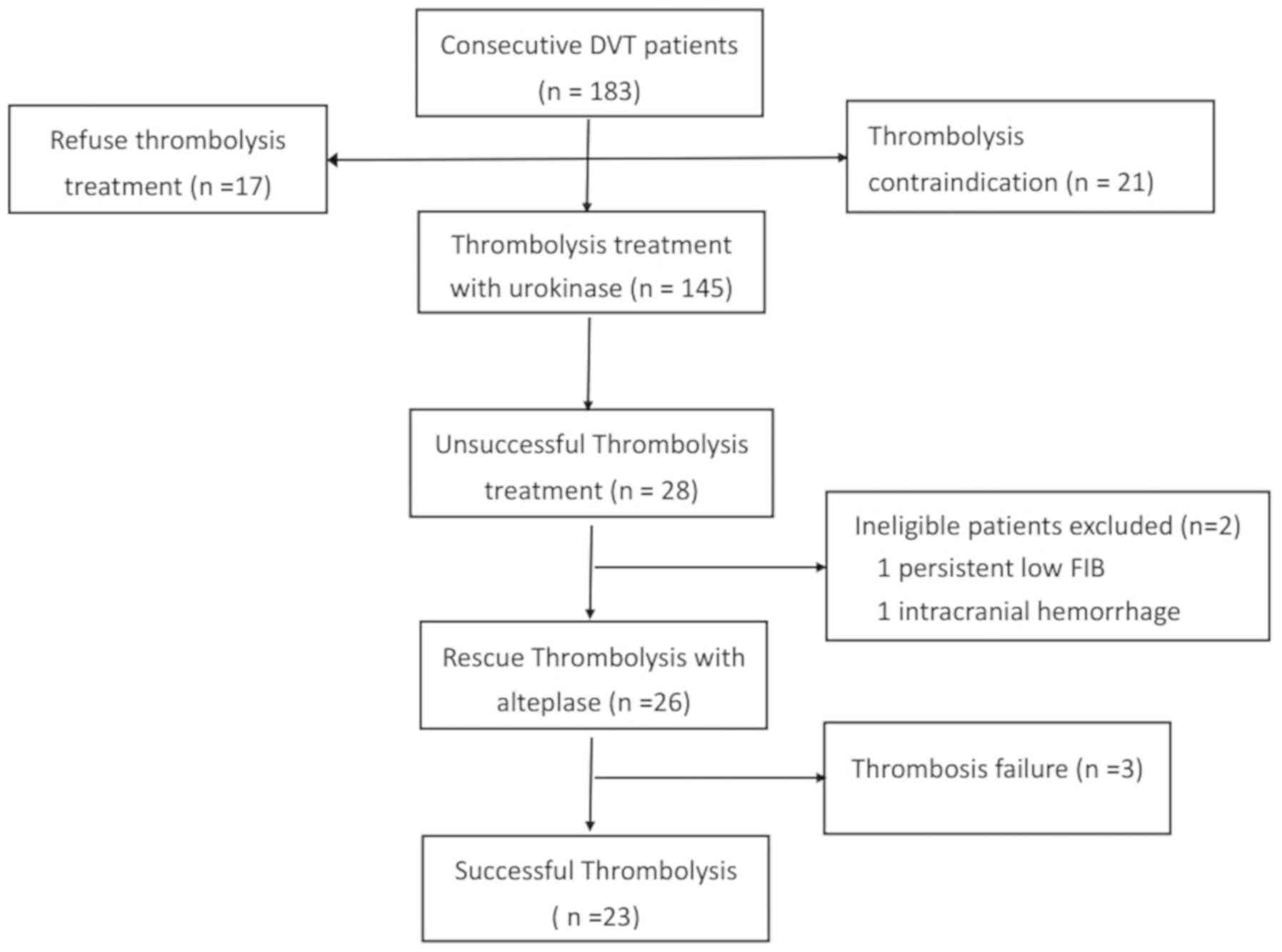
Based on these criteria, 183 consecutive patients
diagnosed with DVT were referred to the hospital between February
2016 and February 2017, and 145 (79.2%) patients were treated with
urokinase thrombolytic therapy in accordance with the institution's
policy. A flowchart of the study is presented in Fig. 1. Thrombolysis with urokinase was
considered unsuccessful in 28 (19.3%) patients. Two ineligible
patients were excluded from the present study as one patient had
persistently low FIB levels (<1.0 g/l) and the other experienced
intracranial hemorrhage following the initial thrombolysis. The
remaining 26 patients, who did not respond to initial urokinase
thrombolytic therapy, received repeat catheter-directed
thrombolysis (CDT) treatment with alteplase. The mean age of the
patients was 50.7±17.2 years (range, 22–75 years), and 14 (53.8%)
patients were female. The onset of symptoms occurred ≤14 days
earlier in ~57.7% of the patients (acute phase), and between 14 and
28 days in the remainder (subacute phase). Locations of the
thrombus were the left lower limb (17 patients) or the right lower
limb (9 patients); none were bilaterally located. Ten (38.4%)
patients had no risk factors for DVT, and 15 patients (50%) had
transient risk factors, such as surgery, trauma, short-term
immobility and pregnancy. Three (11.6%) patients had permanent risk
factors, including cancer and obesity, for DVT (3).
Procedure
The initial diagnosis of DVT was verified by medical
history, physical examination and compression ultrasound. If this
was inconclusive, supplementary venography was also conducted. In
accordance with local routines based on international guidelines
(2,7), anticoagulant treatment was initiated on
the same day with the use of subcutaneous low molecular weight
heparin (LWMH; Hebei Changshan Biochemical Pharmaceutical Co.,
Ltd.) at a bolus dose of 100 U/kg per 12 h (2).
A temporary filter was inserted into the inferior
vena cava (IVC) of patients with an extensive proximal venous
thrombus that was evaluated as potentially life-threatening; the
filter was inserted via the femoral vein of the non-affected leg
prior to the next treatment step (8). Based on the location and distribution
of the thrombus, the visibility of the popliteal vein by
venography, and the tolerance of the patient to the prone position,
a retrograde approach using contralateral femoral vein access or an
anterograde approach via the lateral popliteal vein in the affected
leg was selected. After local anesthesia, a 5–6-French (F) sheath
was inserted into the femoral vein or popliteal vein. Subsequently,
the H1 catheter (Cordis Corporation) and a 0.035-inch smooth
guide-wire (Terumo Corporation) was passed through the thrombus
segment and advanced up to the IVC. Next, the H1 catheter was
exchanged for a multiple side hole catheter (Cook Medical LLC).
Repeat venography from the popliteal vein to the IVC was then
conducted to assess the location and severity of the thrombus and
ensure that the catheter was positioned in the true lumen. For
patients estimated to have an extensive proximal venous thrombus,
adjunctive endovascular techniques, including aspiration
thrombectomy (the use of a syringe to aspirate the thrombus from
the vein via a catheter, device or sheath) or balloon maceration
(the use of an angioplasty balloon to macerate or fragment the
thrombus), were used to enlarge and maintain the venous lumen
patency (7).
Subsequently, a 4–5F perfusion catheter (Uni*Fuse
infusion catheter; AngioDynamics,) of appropriate-length and with
multiple side holes spanning 10–50 cm, was inserted according to
the length of the residual thrombotic segments. Catheter-directed
infusion of urokinase (Livzon Pharmaceutical Group, Inc.) was then
established. According to the patient's body constitution, the
amount of thrombus and blood coagulation status, the specific
dosage regimen given was as follows (9): 25–75 million units of urokinase in 500
ml 0.9% normal saline, at a steady infusion rate of 2–3 million
U/h. The patients were confined to bed with the affected limb
raised at an upward angle of 30° during the administration of the
thrombolytic infusion, and routine blood tests were monitored
daily. Repeat venography examination was systematically performed
to monitor the progression of thrombolysis every 48 h after the
thrombolytic therapy. The position of the perfusion catheter was
adjusted if necessary to ensure that the infusion section of the
catheter was deeply buried in the thrombus. The patients were also
instructed to make ankle joint-toe movements.
When unsuccessful thrombolysis with urokinase was
identified, the thrombolytic therapy was adjusted. Urokinase
infusion through the catheter was discontinued immediately, and the
patients without exclusion criteria were subsequently treated with
a continuous infusion of the rt-PA alteplase (Actilyse®;
Boehringer Ingelheim International GmbH). The alteplase
thrombolytic therapy was administered as 20 mg alteplase in 500 ml
0.9% normal saline via the catheter at an infusion rate of 0.01
mg/kg/h; the maximum rate was ≤1.0 mg/h. Both urokinase and
alteplase were only administered when the FIB level was >1
g/l.
Other comprehensive interventional treatment methods
were the same as reported previously by Chen et al (9) and Shi et al (10). Following CDT, an 8–14-mm diameter
balloon catheter (C.R. Bard, Inc.) angioplasty was performed when
the residual stenosis rate of the iliac vein was assessed to be
>50%. Subsequently, a self-expandable stent (12–14 mm; Luminexx,
CR, Inc.) was deployed in cases with residual stenosis lesion rates
>50% after balloon angioplasty (10). After stent implantation, if the
consecutive venography showed a coarse lumen and relatively slow
blood flow within the lumen of the stent, a perfusion catheter was
maintained to continue thrombolysis for ≥24 h. If thrombolysis
treatment was terminated and patients' symptoms, such as swelling
of the limb, could not be remitted, physical massage treatment
(pressure, 60–80 mmHg) was applied using an air wave pressure
circulation therapy instrument (Power-Q3000; Wonjin Mulsan Co.,
Ltd.) for 30 min each time, once or twice each day. During each
thrombolysis therapy, LMWH therapy overlapped with oral warfarin
therapy was used for 3–5 days. The warfarin dose was adjusted
thereafter to maintain the international normalized ratio at
2.0–3.0 for ≥6 months (11). After
discharge from the hospital, patients were advised to wear
graded-compression stockings with an ankle pressure of 30–40 mmHg
(1 mmHg=0.133 kPa) for ≥2 years (12).
Outcomes and safety
A total thrombus score was calculated independently
through venography by three experienced interventional physicians
before and at the completion of thrombolysis by adding the scores
of seven vein segments (IVC, common iliac vein, external iliac
vein, common femoral vein, proximal and distal segments of femoral
vein, and popliteal vein) (13).
Thrombus scores were determined as follows: 0, vein was patent and
completely free of thrombus; 1, partially occluded vein; and 2,
completely occluded vein (vein lumen completely occluded with
massive thrombus). The score was calculated for each segment,
resulting in a possible total thrombus score of 0–14. The primary
efficacy of thrombolysis was classified based on post-lysis
thrombus scores and the thrombus lysis grade at the completion of
the procedure. The lysis grade was calculated by dividing the
difference between the total pre- and post-lysis thrombus scores by
the pre-lysis score, resulting in grade III (100% lysis with no
residual clots), grade II (50–99% lysis), and grade I (<50%
lysis). Lysis grades II and III (≥50% lysis) were considered
successful outcomes (13,14).
The complications that occurred during CDT included
major complications, such as a reduction of hemoglobin level to 2
g/dl (requiring transfusion of ≥2 units of packed red blood cells),
intracranial hemorrhage, massive hemorrhage of other critical
organs that could lead to death, or PE. Minor complications
included bleeding and hematoma at the puncture site, gross or
microscopic hematuria, mucosal bleeding and other small bleeding
events and infections, which could be controlled by simple
compression or stopping the thrombolysis (14,15).
Statistical analysis
The SPSS statistical package (version 23.0; SPSS
Corp.) was used for all statistical analyses in the present study.
Continuous variables are expressed as the mean ± standard
deviation. Qualitative variables are presented as a percentage.
When assessing the difference between pre-procedural and
post-procedural variables, a paired t-test was used. Significance
for qualitative variables was tested with a Fisher's exact test.
P<0.05 was used to indicate a statistically significant
difference.
Results
Clinical characteristics of patients
and urokinase thrombolysis
All the patients initially showed typical clinical
manifestations of DVT, such as pain, swelling and/or activity
limitation in the affected limb. The clinical characteristics of
the patients in whom the urokinase thrombolysis was unsuccessful
are presented in Table I. After
initial urokinase thrombolytic therapy for 48 h, 16 (61.5%)
patients experienced insoluble initial symptoms, and 5 (19.2%)
patients manifested aggravated swelling in the affected limb.
Subsequent repeat venography showed extension of the clots through
the popliteal femoral vein, iliac-femoral vein and the IVC. There
were 16 (61.5%) patients with no further improvement in thrombotic
removal degree, and the lysis rate of 17 (65.4%) patients remained
<50% despite a total dose of >3 million units of urokinase or
an infusion time >7 days. Additionally, all 11 patients in the
subacute phase (14–28 days) underwent urokinase thrombolytic
therapy for 48 h, but the lysis rate remained <50%. At the end
of unsuccessful urokinase thrombolytic therapy, the mean duration
of the perfusion was 6.09±1.60 days, and the total dose was
(362.5±90.0) ×104 units. The total thrombus scores
calculated before and at the completion of thrombolysis were
7.85±2.40 and 6.19±2.33, respectively. The post-lysis scores were
not significantly decreased compared with those before urokinase
thrombolysis (P>0.05).
 | Table I.Indications for alteplase substitution
of urokinase in 26 patients with DVT. |
Table I.
Indications for alteplase substitution
of urokinase in 26 patients with DVT.
| Conditions after
thrombolysis with urokinase | No. of patients
(%) |
|---|
| Insoluble or
aggravated severe symptoms after 48 h | 16 (61.5) |
| Thrombus
extension |
|
| To the
popliteal-femoral vein | 1 (3.8) |
| To the
iliac-femoral vein | 1 (3.8) |
| To the
IVC | 3 (11.5) |
| Lack of improvement
in thrombotic removal grade, lysis rate <50% | 16 (61.5) |
| >3 million units
or infusion time >7 days, lysis rate <50% | 17 (65.4) |
| Subacute DVT
underwent 48-h CDT, lysis rate <50% | 11 (42.3) |
Rescue thrombolysis with alteplase and
outcomes
All 26 patients subsequently received rescue
thrombolysis with alteplase. The mean duration of the perfusion was
3.36±1.69 days, and the mean total infusion dose was 44.8±22.6 mg.
The mean thrombus scores decreased to 1.19±2.10 at completion. The
post-lysis scores were significantly reduced compared with those at
the end of urokinase thrombolysis (P<0.05). Successful lysis
(grade II/III) of rescue thrombolysis with alteplase was obtained
in 23 (88.5%) patients, and the symptoms of swelling and pain in
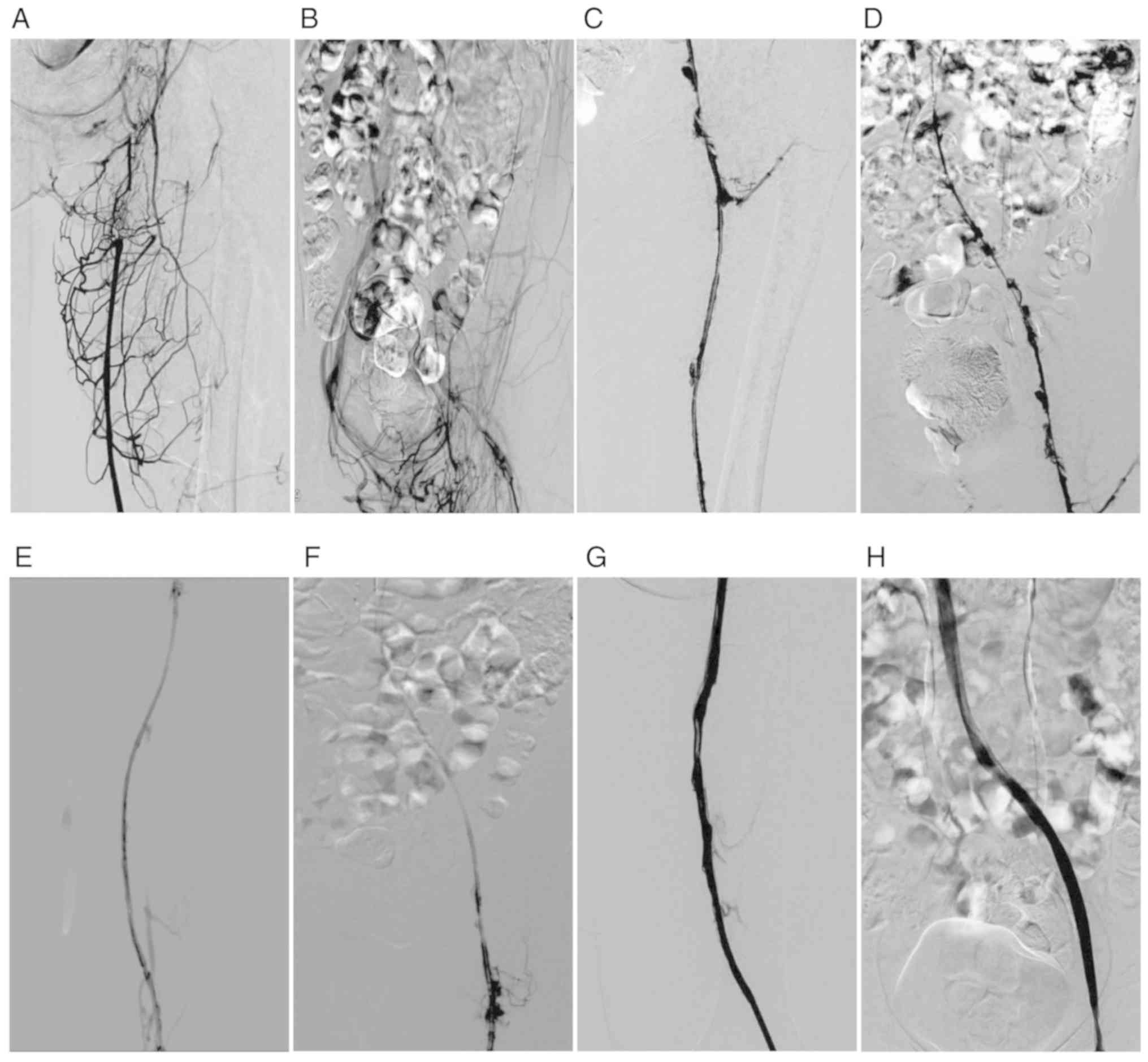
the affected limbs were significantly improved. The venography
results of a typical case are shown in Fig. 2. Based on the symptom duration,
successful lysis was achieved in the majority of patients in the
acute phase (93.3%) and subacute phase (81.8%). No statistically
significant differences were detected between the groups
(P>0.05), and the lysis rates were high in both groups. The
distributions of lysis grade are presented in Table II.
 | Table II.Comparison of rescue thrombolysis
efficacy according to phase of symptom onset. |
Table II.
Comparison of rescue thrombolysis
efficacy according to phase of symptom onset.
|
|
| Lysis grade, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Group | No. of cases | Grade I | Grade II | Grade III | Rate of grade II or
III lysis (%) |
|---|
| Acute phase | 15 | 1 (6.7) | 2 (13.3) | 12 (80) | 93.3 |
| Subacute phase | 11 | 2 (18.2) | 3 (27.3) | 6 (54.5) | 81.8 |
Adjunctive therapies
All 20 patients with DVT evaluated as potentially
life-threatening received a recoverable IVC filter (8 cases used a
Celect™ filter, Cook Group, Inc.; 8 cases used an Optease filter,
Cordis; Cardinal health, Inc.; and 4 cases used an Aegisy™ filter,
LifeTech Scientific Corporation) prior to interventional treatment,
and one underwent mechanical thrombectomy with an AngioJet device
during urokinase thrombolysis. While rescue thrombolysis with
alteplase was discontinued, filters were retrieved from 19 (95.0%)
patients, and one was laid permanently because of superior
paraplegia. Adjunctive angioplasty was performed in 15 patients
(57.7%); only 4 (26.7%) patients underwent dilation with a balloon
in the iliac vein, among whom 2 patients also had balloon
dilatation in the femoral vein. Stents (average diameter 13.5 mm,
range 12–14 mm) were inserted into the iliac veins of 4 (15.4%)
patients.
Safety of rescue thrombolysis with
alteplase
No symptomatic PE or major complications occurred
during ongoing rescue thrombolysis with alteplase. A total of 8
complications were reported, of which 4 (15.7%) were classified as
minor complications. Three of the four bleeding complications were
restricted to bleeding at the puncture site, all of which occurred
in inguinal punctures, and one case of gingival bleeding was
reported. The FIB level of 4 patients (15.7%) was reduced to 1.0
g/l, and in 3 (75%) cases this reduction occurred within the third
day of rescue thrombolysis with alteplase. Thus, the infusion rate
was suspended until the FIB level recovered to >1.0 g/l, and 1
patient was treated with cryoprecipitated antihemophilic factor
transfusion.
Discussion
The risk factors of DVT vary and are largely
dependent upon various conditions of the individual, such as age
(older age, >65 years), obesity, hypertension, metabolic
syndrome, cigarette smoking and protein C or S deficiency, or are
secondary to malignant tumor, major surgery, immobilization,
pregnancy, trauma, oral contraceptives and acute medical illness,
such as pneumonia or congestive heart failure (9). The traditional anticoagulation therapy
for DVT is considered a ‘non-aggressive’ routine approach to avoid
the propagation of clots and prevent symptomatic PE, and can be
reserved for patients with contraindications of thrombolysis
(16). However, anticoagulation
alone depends primarily on the effectiveness of the natural
fibrinolytic system (1). Incomplete
thrombus resolution causes inflammatory responses, provoking severe
compromised valvular competency, resulting in impaired venous
reflux and/or venous insufficiency and ultimately leading to
ambulatory venous hypertension (16). Hence, 70.8% of patients from the
Catheter-directed Venous Thrombolysis in Acute Iliofemoral Vein
Thrombosis (CaVenT) study developed some degree of PTS within 5
years (3). Studies have demonstrated
that additional CDT can accelerate clot lysis, partially or
completely restore the blood flow of tissues and/or organs, relieve
venous hypertension, and in time preserve existing valve function
(3,16). PTS has been demonstrated to achieve
an absolute risk reduction of 28% in 5 years (3). Therefore, CDT is administered as an
‘aggressive’ and effective approach for DVT. Clinically, for fear
of increased hemorrhagic complication risk, the majority of
institutions in China terminate urokinase usage if the total dose
of urokinase exceeds 300–400 units or the FIB level is <1.0 g/l
in patients with a residual thrombus burden. The CaVenT findings
indicate that the percentage of residual thrombus was positively
associated with the incidence of PTS (2,3), thus,
the prognosis of patients who do not respond to thrombolysis with
urokinase appeared to be poor. Thus, it is reasonable for patients
who do not respond to thrombolysis with urokinase to subsequently
receive rescue thrombolysis. No peer-reviewed data exploring the
management of unsuccessful thrombolysis with established urokinase
protocols have been published. Based on this, urokinase was
replaced with alteplase in patients who did not respond to
urokinase in the present study, with the aim of increasing the
successful lysis rate and promoting venous lumen patency.
The successful lysis rates of urokinase and
alteplase reported in multiple central large-scale comparative
clinical trials, such as the National Venous Registry (13) and CaVenT (4), were 83.0 and 88.9%, respectively.
However, because these lysis rates were collected from two
non-uniform studies, some differences were present in the inclusion
criteria. Thus, it is difficult to accurately determine the
therapeutic efficacy of the two fibrinolytic agents on the basis of
these two superficial rates alone. A previous study reported the
successful lysis rate of a low-dose urokinase infusion method as
82.2% (9), which was consistent with
the results of the aforementioned two studies. The lack of success
of thrombolysis with urokinase in this group may be attributable to
several factors. Firstly, reduced pharmacological efficacy of
urokinase may be a contributing factor to unsuccessful
thrombolysis; the specification of urokinase suggests it should be
used within 8 h, at a temperature of 25°C, and if the temperature
is 2–5°C its preservation can be prolonged to 48 h. In the present
study, urokinase was unused for an extended time (~24 h); hence,
its pharmacological efficacy may have been reduced. Secondly,
existing differences in individual sensitivity may also explain
unsuccessful thrombolysis with urokinase. Thirdly, the relatively
poor local clot infiltration effect of urokinase may also be a
factor, especially for partially underlying non-acute phase clots
or abnormalities refractory to thrombolysis with urokinase. Even
so, this result is not applicable to all patients, as a previous
report indicated that urokinase can sometimes be effective for
non-acute phase patients (17).
Furthermore, a lack of positive blood flow into the thrombus,
caused by time-dependent structural changes of the thrombus that
may impede the agent from flowing through the clotted segment where
the partial accumulation of the thrombus is incomplete, can also
lead to unsuccessful thrombolysis. Patients benefitted from timely
relief of the outflow obstruction first and then continued with
thrombolysis; treatment of residual stenosis after thrombolysis
appears to be more reasonable, although it has not been recommended
in the literature. Several factors are associated with the lysis
rate of thrombus, such as the duration of thrombolysis, doses of
lysis-inducing agents and the mode of delivery. However, a previous
study reported that the proportion of patients in which a lysis
rate ≥50% was achieved reached a maximum of 82.8%, when mean doses
of 3–4 million units and an infusion time of <7 days were used
(9). In the present study protocol,
the duration and doses of treatment used in some patients who were
unresponsive to initial thrombolysis might not have reached the
threshold of thrombus lysis. Therefore, extending thrombolysis
beyond this dose and increasing the time frame may improve the
lysis grade; however, it may also result in a higher risk of
bleeding.
During thrombolysis with urokinase, the lysis
thrombus scores pre- (7.85±2.40) and post-thrombolysis (6.19±2.33)
treatment for all 26 patients exhibited no significant difference
(P>0.05). As the CaVenT study indicated, disregarding the
pre-lysis score, the post-lysis score was inversely correlated with
thrombosis degree and patency during follow-up, which was clearly
correlated with a reduction in the frequency of PTS (14). Rescue thrombolysis with alteplase had
a mean duration of 3.36±1.69 days, decreased the lysis thrombus
score to 1.19±2.10 (P<0.05) and achieved a high mean clot
resolution of 88.5% in the in-hospital patients. At the same time,
the clinical symptoms and signs of onset were both ameliorated. It
is predicted that these patients may have a lower incidence of PTS
and better prognosis, but the long-term efficacy requires further
follow-up and investigation. However, all the present findings
indicated that rescue thrombolysis with alteplase might be
considered an effective therapeutic option in patients with
unsuccessful thrombolysis. The highly successful lysis result
observed in patients with rescue thrombolysis may be attributed to
the two circular structures (K region: Ligand binding site) in the
molecular structure of alteplase, which allow it to selectively
bind to a specific lysine site at the thrombus surface, thereby
specifically activating fibrinogen at a local area of the thrombus
and providing selective infiltrative thrombolysis (5). Further sub-analysis, according to the
time of symptom onset in the subgroups, revealed that thrombolysis
with alteplase can obtain satisfactory clinical efficacy in the
subacute phase that is comparable with that in the acute phase
(P>0.05); this may expand the conclusion reported by Sugimoto
et al (18).
The incidences of major and minor bleeding
complications in the thrombolytic procedure have been reported to
be 16.0 and 11.0% for urokinase (13) and 21.7 and 3.3% for alteplase
(2). However, an assessment of the
safety of rescue thrombolysis with alteplase following urokinase
has not been reported in other studies. During the remedial
thrombolysis with alteplase, no symptomatic PE or major bleeding
complications occurred, and the rate of minor bleeding
complications was only 15.7%. Therefore, compared to a previous
report using urokinase alone, additional rescue thrombolysis with
alteplase for unsuccessful thrombolysis with urokinase did not
increase the bleeding risk (9).
However, as the present study was limited by the small number of
cases, this conclusion requires validation in controlled studies.
In addition, 15.7% of patients during rescue thrombolysis with
alteplase experienced reduced FIB (<1.0 g/l) the present study;
however, additional bleeding complications did not increase.
Alteplase has a fast fibrinogen-lowering effect, but due to its
short half-life (4–8 min), it is safe to use (5).
The overall infusion methods of CDT for DVT are
diverse, consisting of continuous infusion (14), pulse-spray infusion (19) and mixed-type infusion (20), comprising a combination of continuous
and pulse-spray infusions To date, there is no convincing clinical
evidence in current guidelines that is superior to others. In
addition, data on the optimal infusion method for rescue
thrombolysis are lacking, and the optimal pattern remains under
debate. Alteplase has a half-life of 4–8 min in the plasma and
decreases the residual circulating fibrinogen levels to <10% of
the initial value for ~20 min, resulting in reduced efficacy
(5). To maintain a sustained drug
concentration, the preferred method in the present study was to
continuously infuse alteplase directly into clots by an infusion
pump through catheters embedded into clots, similar to the CaVenT
study (3). Although current
guidelines have no consensus on the optimal rates and doses of
infusion in thrombolysis with alteplase, the increased experience
of clinical alteplase application has led to a gradual reduction in
the doses of alteplase being administered. Due to the need for
protocols for the use of alteplase in thrombolysis, the Society of
Cardiovascular and Interventional Radiology first published
guidelines recommending the use of alteplase at a ‘low dose’ of ≤2
mg/h (18), and in 2009, the Society
of Interventional Radiology (SIR) and European Cardiovascular
Interventional Radiology (CIR) recommended that the dose should be
decreased to 0.5 mg/h (16). To
date, the current guidelines recommended a weight-based regimen at
the rate of 0.01 mg/kg/h, with a maximum of 1.0 mg/h (10,11).
This recommendation was applied in the present study and resulted
in satisfactory efficacy and safety.
At present, there is a lack of formal large-scale
comparative clinical trials to confirm the time window during which
to carry out rescue thrombolysis, which is important for successful
thrombolysis (14). Based on the
observations of the present study, the following is suggested.
Firstly, if the thrombus is in a non-acute phase and/or patients
have undergone unsuccessful thrombolysis for >48 h, the
sensitivity of the thrombus to urokinase needs to be considered. In
order to shorten the time of thrombolysis and to salvage valve
function, alteplase was substituted for urokinase in the present
study when thrombolysis was continued. The results support the
recommendation of alteplase as the first-line fibrinolytic agent
for patients with subacute DVT. Secondly, if the pharmacoeconomic
factors of alteplase are taken into consideration, urokinase may be
used first for patients who have difficulty affording alteplase,
but the fibrinolytic agent must be changed immediately if there is
no change in the thrombolysis removal grade during two consecutive
venography procedures, especially if the thrombus extends to the
IVC and the longest retrieval time of the IVC filter is near.
Thirdly, if a patient's symptoms exhibit no obvious improvement
within 48 h of the initial thrombolysis with urokinase, or are
further aggravated, alteplase may be used as a remedy.
The conclusions that may be drawn from the present
study are limited because it was a small, retrospective,
non-randomized analysis from a single center, and the causes of
unsuccessful thrombolysis were partially based on a hypothesis from
experience. Nevertheless, to the best of our knowledge, this is the
first study to use alteplase following unsuccessful thrombolysis
with urokinase. Additionally, a gold standard diagnostic method for
unsuccessful thrombolysis is still lacking. The present study was
also constrained by the use of parameters (thrombotic removal
degree, infusion time and infusion dose, which are considered
straightforward markers of thrombolytic efficacy) that were
selected for defining patients with unsuccessful thrombolysis.
Furthermore, the study was limited by the lack of follow-up
outcomes for estimating the long-term efficacy of rescue
thrombolytic therapy. Therefore, it should be regarded as a
preliminary study for assessment of the instant efficacy of
treatment, and further follow-up studies are required.
In conclusion, the aim of the present study was to
accelerate the vein recanalization process in an attempt to save
the valve function and thereby minimize the risks of PTS (21). Rescue thrombolysis with alteplase was
feasible in most patients and led to an effective and secure result
in the management of patients with DVT who did not respond to
initial thrombolysis with urokinase. This may, therefore, be
considered a valid and easy alternative treatment, which may
accelerate the process of recanalization of the vein and lead to a
better in-hospital course. However, further confirmation of the
conclusions using long-term analyses is necessary.
Acknowledgements
Not applicable.
Funding
Funding was received from the Clinical Medicine
Science and Technology Projects of Jiangsu Province, China (grant.
no. BL2014013) and the Nanjing Medical Science Fund, China (grant.
no. YKK14087).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MG, BZ, XH, JG and GC contributed to the conception
and design of the study. MG and BZ were involved in data
collection. MG, BZ and GC contributed to data analysis,
interpretation, statistical analysis and writing the manuscript.
MG, XH and GC revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Nanjing First Hospital (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kearon C: Natural history of venous
thromboembolism. Circulation. 107 (Suppl 1):S122–S130. 2003.
View Article : Google Scholar
|
|
2
|
Kearon C, Akl EA, Ornelas J, Blaivas A,
Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, et
al: Antithrombotic therapy for VTE disease: CHEST guideline and
expert panel report. Chest. 149:315–352. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haig Y, Enden T, Grøtta O, Kløw NE,
Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO,
et al: Post-thrombotic syndrome after catheter-directed
thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up
results of an open-label, randomised controlled trial. Lancet
Haematol. 3:64–71. 2016. View Article : Google Scholar
|
|
4
|
Watson L, Broderick C and Armon MP:
Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst
Rev. Nov 10–2016.(Epub ahead of print). doi:
10.1002/14651858.CD002783.pub3. View Article : Google Scholar
|
|
5
|
Gurman P, Miranda OR, Nathan A, Washington
C, Rosen Y and Elman NM: Recombinant tissue plasminogen activators
(rtPA): A review. Clin Pharmacol Ther. 97:274–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meneveau N, Seronde MF, Blonde MC,
Legalery P, Didier-Petit K, Briand F, Caulfield F, Schiele F,
Bernard Y and Bassand JP: Management of unsuccessful thrombolysis
in acute massive pulmonary embolism. Chest. 129:1043–1050. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vedantham S, Sista AK, Klein SJ, Nayak L,
Razavi MK, Kalva SP, Saad WE, Dariushnia SR, Caplin DM, Chao CP, et
al: Quality improvement guidelines for the treatment of
lower-extremity deep vein thrombosis with use of endovascular
thrombus removal. J Vasc Interv Radiol. 25:1317–1325. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Decousus H, Leizorovicz A, Parent F, Page
Y, Tardy B, Girard P, Laporte S, Faivre R, Charbonnier B, Barral
FG, et al: A clinical trial of vena caval filters in the prevention
of pulmonary embolism in patients with proximal deep-vein
thrombosis. Prévention du Risque d'Embolie Pulmonaire par
Interruption Cave Study Group. N Engl J Med. 338:409–415. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G, Shi W, He X, Lou W, Chen L and Gu
J: Feasibility of continuous, catheter-directed thrombolysis using
low-dose urokinase in combination with low molecular-weight heparin
for acute iliofemoral venous thrombosis in patients at risk of
bleeding. Exp Ther Med. 13:751–758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi WY, Gu JP, Liu CJ, He X and Lou WS:
Endovascular treatment for iliac vein compression syndrome with or
without lower extremity deep vein thrombosis: A retrospective study
on mid-term in-stent patency from a single center. Eur J Radiol.
85:7–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farraj RS: Anticoagulation period in
idiopathic venous thromboembolism. How long is enough? Saudi Med J.
25:848–851. 2004.
|
|
12
|
Kahn SR, Shapiro S, Wells PS, Rodger MA,
Kovacs MJ, Anderson DR, Tagalakis V, Houweling AH, Ducruet T,
Holcroft C, et al: Compression stockings to prevent post-thrombotic
syndrome: A randomised placebo-controlled trial. Lancet.
383:880–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mewissen MW, Seabrook GR, Meissner MH,
Cynamon J, Labropoulos N and Haughton SH: Catheter-directed
thrombolysis for lower extremity deep venous thrombosis: Report of
a national multicenter registry. Radiology. 211:39–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haig Y, Enden T, Slagsvold CE, Sandvik L,
Sandset PM and Kløw NE: Determinants of early and long-term
efficacy of catheter-directed thrombolysis in proximal deep vein
thrombosis. J Vasc Interv Radiol. 24:17–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Buller HR, Davidson BL, Decousus H, Gallus
A, Gent M, Piovella F, Prins MH, Raskob G, van den Berg-Segers AE,
Cariou R, et al: Subcutaneous fondaparinux versus intravenous
unfractionated heparin in the initial treatment of pulmonary
embolism. N Engl J Med. 349:1695–1702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vedantham S, Piazza G, Sista AK and
Goldenberg NA: Guidance for the use of thrombolytic therapy for
thetreatment of venous thromboembolism. J Thromb Thrombolysis.
41:68–80. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao B, Zhang J, Wu X, Han Z, Zhou H, Dong
D and Jin X: Catheter-directed thrombolysis with a continuous
infusion of low-dose urokinase for non-acute deep venous thrombosis
of the lower extremity. Korean J Radiol. 12:97–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugimoto K, Hofmann LV, Razavi MK, Kee ST,
Sze DY, Dake MD and Semba CP: The safety, efficacy, and
pharmacoeconomics of low-dose alteplase compared with urokinase for
catheter-directed thrombolysis of arterial and venous occlusions. J
Vasc Surg. 37:512–517. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang R, Chen CC, Kam A, Mao E, Shawker TH
and Horne MK III: Deep vein thrombosis of lower extremity: Direct
intraclot injection of alteplase once daily with systemic
anticoagulation-results of pilot study. Radiology. 246:619–629.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manninen H, Juutilainen A, Kaukanen E and
Lehto S: Catheter-directed thrombolysis of proximal lower extremity
deep vein thrombosis: A prospective trial with venographic and
clinical follow-up. Eur J Radiol. 81:1197–1202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiengo L, Bucci F, Khalil E and Salvati B:
Original approach for thrombolytic therapy in patients with
Ilio-femoral deep vein thrombosis: 2 years follow-up. Thromb J.
13:402015. View Article : Google Scholar : PubMed/NCBI
|