Introduction
Protei'f cellular processes and is the major
intracellular kinase implicated in transducing extracellular
signals into intracellular events (1). The PKC family has numerous isozymes,
including a number of PKC isotypes that are expressed in mouse
oocytes. PKCδ and λ have been detected at mRNA and protein levels
in prophase I and MII stage oocytes, whereas PKCα, β and ζ have
been detected only at protein level (2). Downs et al (3) reported that PKC γ, λ, µ and ζ are
expressed at mRNA and protein levels in early embryos, whereas PKC
α and δ are expressed only at protein level. Oocyte maturation
involves the activation of various signal transduction pathways.
PKC regulates meiosis I and regulates the progression of meiosis I
in LTXBO oocytes (4). Therefore, PKC
serves an integral role in directing the transformation from egg to
embryo, participating in genome activation in mouse one-cell stage
fertilized embryos. The activity of PKC has also been demonstrated
to increase at fertilization (5). In
addition, the cell cycle resumes in embryos treated with PKC
(6). PKC is thus involved in the
progression of meiosis, oocyte maturation, fertilization and early
embryo development. Evidence suggests that PKC is also a key
regulator of critical cell-cycle transitions during mitosis,
including G1/S and G2/M transitions (7–9). In
different cell types, PKC may promote or inhibit the regulation of
G1/S and G2/M transitions depending on the
timing of PKC activation during the cell cycle and the specific PKC
isoforms involved (10).
The mitosis promoting factor (MPF), consists of the
cell division cycle (Cdc)2/cyclin B complex, and is crucial for
G2/M transition of the cell cycle (11). In addition, it functions as the key
molecule in regulating cell cycle progression during mitosis and
meiosis (12). Prior to mitosis, the
activity of MPF is suppressed by the inhibitory phosphorylation of
Tyr15 and Thr14 residues of Cdc2, the Cdc25 phosphatases and Myt1
kinase (13). The inactive pre-MPF
phosphatase is acted on by the dual specificity of Cdc25 and can be
modified to an active dephosphorylated form (13). Notably, Cdc25B has a central role in
regulating the re-initiation of meiosis in mammalian oocytes
(14). A previous study indicated
that oocytes from mice lacking the Cdc25B gene were unable to
activate MPF and therefore could not resume mitosis, which suggests
the regulatory role of Cdc25B in G2/M transition of the
mammalian cell cycle (15).
A series of experiments have provided evidence that
PKC promotes the maturation in oocytes of Chaetopterus
pergamentaceus by inducing the activity of MPF (16). Previous studies demonstrated that PKC
participates in activating MPF and in developing fertilized mouse
embryos (17,18). The entry into the first mitotic M
phase at the end of the first embryonic cell cycle (one-cell stage
mouse embryo) requires the activation of MPF (19). PKCδ has been observed in the
cytoplasm of zygotes, and the activities of PKC and MPF are high
during M phase (20). Furthermore,
it has been implicated that the major contributor of PKC activity
in mouse embryos derives from that of PKCδ, and MPF is a possible
target substrate for PKCδ (21).
Thus, it was hypothesized that PKCδ controls the cell cycle by
regulating the activity of MPF. Therefore, in the present study,
the role of PKCδ in the regulation of one-cell stage mouse embryos
was explored.
Materials and methods
Animals and reagents
A total of 32 female (age, 4 weeks; weight, 20–24 g)
and 28 male (age, 8–9 weeks; weight, 30–40 g) Kunming
genealogy-specific pathogen-free mice were obtained from the
Department of Laboratory Animals, China Medical University
(Shenyang, China). Mice were housed in environmentally controlled
conditions (20±1°C, 60% relative humidity, with a 12-h light/dark
cycle). All mice had access to food and water ad libitum.
All experiments were performed at China Medical University in
accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals. Reagents, unless otherwise
specified, were from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). The current study was approved by the Ethics Committee of
the China Medical University (Shenyang, China).
Collection and culture of one-cell
stage mouse embryos
Female mice were injected with 10 IU of pregnant
mare's serum gonadotropin (PMSG) and then with 10 IU of human
chorionic gonadotropin (hCG) 48 h later. The following day,
one-cell embryos were collected and placed in M2 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) from the oviducts
of females with a vaginal plug at 20 h post-hCG injection.
Following collection, embryos were maintained in M16 medium (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in a 5%
CO2-humidified incubator. Cell cycle stages
(G1, S, G2 and M phases) were defned as
described reviously (22).
RNA extraction, cDNA synthesis and
reverse transcription- quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from one-cell mouse embryos
at different cell-cycle stages (G1, S, G2 and
M phase) using the QuickPrep MicromRNA Purification kit (GE
Healthcare Life Sciences, Little Chalfont, UK), according to the
manufacturer's protocol. At each stage 100 embryos were collected
and total RNA was reverse transcribed into cDNA using the RevertAid
First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) using random primers, according
to the manufacturer's protocol. A total of 2 µl (≤100 ng) cDNA was
used in a 20-µl qPCR using 2X SYBR Premix Ex TaqII(Takara
Biotechnology Co., Ltd., Dalian, China) with 0.4 µM of each primer.
The following primers were used for qPCR: PKCδ forward,
5′-TCATCTGCGGACTGCAGTTTCTA-3′ and reverse,
5′-CAAAGTCAGCGATCTTGATGTGG-3′; and β-actin forward,
5′-CAACGAGCGGTTCCGATG-3′ and reverse, 5′-GCCACAGGATTCCATACCCA-3′.
The following thermocycling conditions were used for the qPCR:
Initial denaturation at 95°C for 30 sec; 40 cycles of 95°C for 5
sec and 60°C for 20 sec using the Applied Biosystems 7900HT Fast
Real-Time PCR System. PKCδ mRNA levels were quantified using the
2−ΔΔCq method and normalized to the internal reference
gene β-actin (23). All experiments
were repeated at least three times.
Treatment with rottlerin and
observation of mouse embryos
Rottlerin is a natural product derived from
Mallotus philippinesis (24).
This compound has been demonstrated to inhibit PKCδ with greater
selectivity for PKCδ (IC50=3–6 µM) over other PKC
isoforms (25). Rottlerin was
dissolved in dimethyl sulfoxide (DMSO) and diluted with M16 medium
at different concentrations (0, 0.5, 1 and 1.5 µM). Each Zona
pellucida-free one-cell stage embryos (removed with Tyrode's
buffer, pH 2.5) at G1 phase were seeded into 24-well
plates at a density of 25 embryos/well and incubated with various
concentrations (0, 0.5, 1 and 1.5 µM) of rottlerin. Fertilized
embryos in the control group (0 µM rottlerin) were incubated with
the DMSO only in M16 medium. The percentage of egg cleavage was
determined by counting the number of cleaved embryos under a
dissecting microscope, and the activity of MPF was measured 28–35 h
post-hCG injection. Each experiment was repeated at least three
times and the results were statistically analyzed using SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA). Images were
captured using a phase-contrast microscope (magnification, ×600;
DP70; Olympus Corporation, Tokyo, Japan).
RNA interference
To examine the possible function of PKCδ, small
interfering RNAs (siRNAs) specific to PKCδ were used to knockdown
transcript levels in embryos. Control groups included: Non-injected
embryos, and embryos injected with negative control (NC) siRNA. The
fertilized embryos at G1 phase were divided into three
groups: Blank, NC and siRNA group, to which 20 µM siRNAs (10 pl;
GenPharma, Shanghai, China) were microinjected directly into the
cytoplasm of fertilized embryos at G1 phase (20 h
post-hCG injection). Following microinjection, embryos were
cultured in M16 medium for 48 h at 37°C in a 5%
CO2-humidified incubator. Initial control experiments
were undertaken to determine the specificity and efficacy of PKCδ
siRNA to knockdown endogenous PKCδ expression. A total of 4 h
post-microinjection, each group of embryos was processed for
western blotting to assess overall PKCδ protein expression levels.
Transfection efficiency was confirmed using western blot analysis.
The following siRNA duplexes were used: PKCδ siRNA sense,
5′-CCAUGUAUCCUGAGUGGAATT-3′ and antisense,
5′-UUCCACUCAGGAUACAUGGTT-3′; negative control siRNA sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Western blot analysis was performed
following 4 h incubation with siRNA.
In addition, cleavage stage embryos were counted
30–35 h post-hCG injection and MPF activity was measured at
different time points (26–29.5 h).
Plasmid construction and site-directed
mutagenesis
Mouse Cdc25B cDNA was provided by Dr Tony Hunter
(Laboratory of Molecular Biology, The Salk Institute for Biological
Studies, La Jolla, CA, USA). The pBluescript
II/SK-Cdc25B-S96Alanine (pBSK-Cdc25B96A) was obtained by mutating
Ser96 to alanine of Cdc25B by using the site-directed mutagenesis
kit (Stratagene; Agilent Technologies, Inc., Santa Clara, CA, USA).
The aspartic acid residue at position 96 in the pBSK-Cdc25B-S96
(pBSK-Cdc25B96D) was replaced with alanine to form pBSK-Cdc25B96A,
were used as templates for primer design. Primers were designed
using the 96A and 96D templates were as follows: 96A forward,
5′-CACCTCTGAGTGCGCCCTGTCATCTGAG-3′ and reverse,
5′-CTCAGATGACAGGGCGCACTCAGAGGTG-3′ and 96D forward,
5′-ACCTCTGAGTGCGACCTGTCATCTGAGTCCTCA-3′ and reverse,
5′-TGAGGACTCAGATGACAGGTCGCACTCAGAGGT-3′. The above recombinant
plasmids were sequenced to verify correct gene insertion and
successful mutation, and were used as templates for in vitro
transcription.
One-cell stage mouse embryos were divided into five
groups: Blank (only mouse embryos), TE (mouse embryos injected with
TE buffer), Cdc25B-WT (mouse embryos injected with Cdc25B-WT),
Cdc25B-S96A (mouse embryos injected with Cdc25B-S96A) and
Cdc25B-S96D (mouse embryos injected with Cdc25B-S96d).
In vitro transcription
As previously described (26), all pBluescript II/SK constructs were
linearized with XbaI and transcribed in vitro into
5′mRNA for microinjection using the mMESSAGE mMACHINE kit (Ambion;
Thermo Fisher Scientific, Inc.).
Microinjection and morphology
analysis
Various Cdc25B mRNAs were microinjected into the
cytoplasm of one-cell embryos at the G1 or S phase, as
previously described (26). The
typical injection volumes were 5% (10 pl, cytoplasm) and 1% (2 pl,
nuclear) of the total cell volume per egg. mRNAs were diluted to
various concentrations with TE buffer (5 mmol/l Tris-HCL and 0.5
mmol/l EDTA, pH 7.4).
The fertilized embryos at the S phase (22 h post-hCG
injection) were incubated in M16 medium in the presence of
rottlerin (0.5 µM) for 1 h. Subsequently, embryos microinjected
with various Cdc25B mRNAs were cultured in M16 medium with 0.5 µM/l
rottlerin. The rate of embryo cleavage was counted in three
independent experiments under a phase contrast microscope at 30 and
35 h post-hCG injection in the absence or presence of rottlerin.
Morphological analysis was performed by using Image J software
(version 1.46; National Institutes of Health, Bethesda, MD, USA)
and the Sholl Analysis plugin (version 3.4.5; fiji.sc/Sholl), as
previously described (27).
Western blot analysis
Total protein was extracted from treated embryos
using 250 µl radioimmunoprecipitation assay buffer (cat. no. P0013;
Beyotime Institute of Biotechnology, Haimen, China) for 30 min at
4°C. Total protein was quantified using a bicinchoninic acid assay
kit (cat. no. 23225; Thermo Fisher Scientific, Inc.) and an average
200 embryos (40 µg protein/lane) were separated via SDS-PAGE on a
10% gel. The separated proteins were subsequently transferred onto
nitrocellulose membranes and blocked for 1 h at room temperature
with 5% skimmed milk (Blotto; cat. no. sc-2325; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Membranes were incubated
with primary antibodies against PKCδ (1:1,000; cat. no. 610108; BD
Biosciences, San Jose, CA, USA), phospho-PKCδ (Thr505; 1:800; cat.
no. 9374; Cell Signaling Technology, Inc., Danvers, MA, USA),
Cdc25B (1:200; cat. no. sc-5619), Tyr15 of Cdc2 (1:500; cat. no.
sc-24579; both Santa Cruz Biotechnology, Inc.),
phospho-Cdc25B-pSer96 (1:200; cat. no. 11503; Signalway Antibody
LLC, College Park, MD, USA) and β-actin (1:500; cat. no. AF0003;
Beyotime Institute of Biotechnology) overnight at 4°C. Membranes
were washed with PBS for 30 min at room temperature. Following
primary incubation, membranes were incubated with horseradish
peroxidase-conjugated anti-mouse (1:5,000; cat. no. ZB-2305),
anti-goat (1:5,000; cat. no. ZB-2306) or anti-rabbit (1:5,000; cat.
no. ZB-2301; all OriGene Technologies, Inc., Beijing, China)
secondary antibodies. Protein bands were subsequently visualized
using the enhanced chemiluminescence substrate kit (Thermo Fisher
Scientific, Inc.). Protein expression was quantified using Image
Lab analysis software (version 4.1; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The phospho-Cdc25B-pSer96 antibody was raised
in New Zealand white rabbits against the keyhole limpet
hemocyanin-conjugated phosphopeptide.
Immunofluorescence
Immunostaining was performed as previously described
(28). PKCδ was detected using
anti-PKCδ (1:200; cat no. 610398; BD Biosciences) at 4°C overnight.
Subsequently, the enbyors were incubated with fluorescein
isothiocyanate (FITC)-conjugated goat anti-mouse IgG secondary
antibody (1:100; cat. no. AP130F; Chemicon International, Inc.,
Temecula, CA, USA) at room temperature for 2 h. Chromosomes were
labeled with 10 g/ml DAPI (cat. no. P36931; Invitrogen; Thermo
Fisher Scientific, Inc.). For PKCδ and Cdc25B double staining, PKCδ
was detected using anti-PKCδ and FITC-conjugated goat anti-mouse
IgG secondary antibody. Following PKCδ immunostaining, embryos were
washed three times with PBS and Cdc25B was detected using
anti-Cdc25B (1:200; cat. no. sc-326; Santa Cruz Biotechnology,
Inc.) at room temperature for 2 h, followed by tetrarhodamine
isothiocyanate-conjugated rabbit anti-goat IgG secondary antibody
(1:200; cat. no. ab7087; Abcam,) at room temperature for 2 h.
Following secondary incubation, the embryos were stained with DAPI
(10 g/ml) for 10 min at room temperature and observed under a
confocal laser-scanning microscope (magnification, ×400; Leica
Microsystems GmbH, Wetzlar, Germany).
MPF activity assay
MPF kinase activity was measured using a histone H1
kinase assay, as previously described (29). Embryos from each time-point (26–35 h)
post-hCG injection were collected and added to 5 µl collection
buffer (PBS) containing 1 mg/ml polyvinyl alcohol, 5 mM EDTA, 10 mM
Na3VO4, and 10 mM NaF. In total, 25 µl MPF
buffer (54 mM β-glycerophosphate; 14.5 mM p-nitrophenylphosphate;
24 mM 3-(N-morpholino)-propanesulfonic acid, pH 7.2; 14.5 mM
MgCl2; 14.5 mM EGTA; 0.12 mM EDTA; 1 mM dithiothreitol;
2.4 µM PKI; 75 mM genistein (a tyrosine kinase inhibitor); 10 µM
ML-9 (a myosin light chain kinase inhibitor); 1 mg/ml histone H1
(type III-s); and 1 µg/ml each of leupeptin, aprotonin, pepstatin,
chymostatin, and trypsin-chymotrypsin inhibitor.) was subsequently
added to the disrupted cells following three freezing and thawing
steps. The histone H1 kinase reaction was initiated by adding 25 µl
of 20 µCi/ml (γ-32P) ATP (Peking YaHui Biotechnology
Co., Beijing, China) to the sample, which was incubated at 30°C for
10 min. Following this, 25-µl aliquots were spotted onto Whatman
p81 paper, and the reaction was stopped with 5%
H3PO4 solution. Following thorough washing,
the radioactivity on the filter paper was counted with a Beckman
scintillation counter (Beckman Coulter, Inc., Brea, CA, USA).
Statistical analysis
Data are presented as the mean ± SD. Statistical
analyses were performed using SPPS statistical software (version
16.0). All experiments were repeated independently at least three
times. One-way analysis of variance with Tukey's post hoc test was
used to evaluate the difference between groups, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression and subcellular
localization of PKCδ and Cdc25B, and phosphorylation status of
Cdc25B-Ser96 in vivo in one-cell stage mouse embryos
Samples of one-cell stage mouse embryos were
obtained from all four cell cycle phases (G1, S,
G2 and M), and RT-qPCR and western blotting were
performed to identify the mRNA and protein expression levels of
PKCδ. It was observed that PKCδ mRNA was expressed during the
development of one-cell stage embryos. No significant difference in
PKCδ expression was observed among the G1, S and
G2 phases; however, a significant increase in PKCδ
expression was indicated during the M-phase (Fig. 1A). Similar results in protein
expression were also identified with western blotting (Fig. 1B). The activity of PKCδ is promoted
by phosphorylating Thr505 in the activation loop (30). In the present study,
anti-p-PKCδThr505 antibody was used to explore PKCδ kinase activity
(Fig. 1B). Notably, PKCδ
phosphorylated Thr505 (active form of PKCδ) and increased the
expression during M phase. To identify whether Cdc25B-Ser96 was
phosphorylated in vivo, fertilized mouse embryos were
collected at different phases and the phosphorylation status of
Cdc25B-Ser96 was measured using the phosphor-specific antibody. As
indicated in Fig. 1C, higher
expression levels of phosphorylated Cdc25B-Ser96 were observed
during the G2 and M phases compared with expression in
G1 and S phases. Taken together, these results
demonstrated that Cdc25B-Ser96 was phosphorylated during
G2 and M phases in vivo.
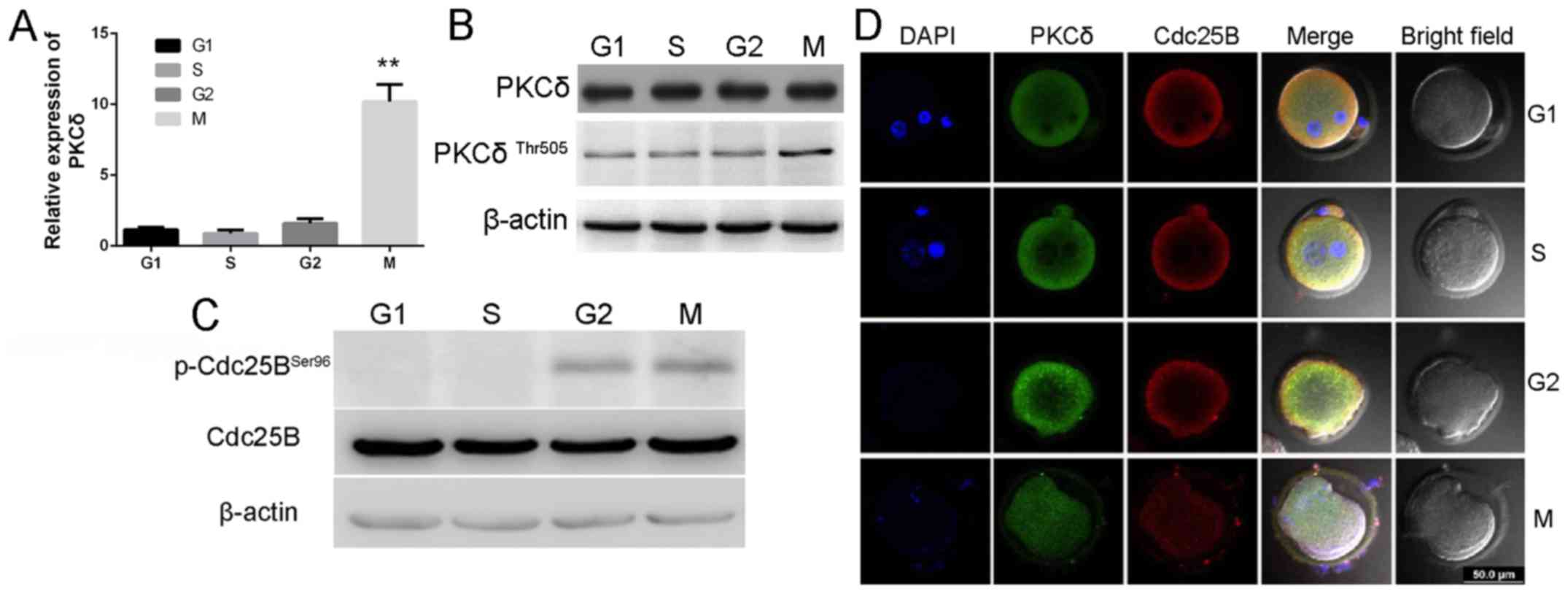 | Figure 1.Expression and subcellular
localization of PKCδ and Cdc25B, and phosphorylation status of
Cdc25B-Ser96 in vivo in mouse one-cell stage embryos. (A)
mRNA expression levels of PKCδ detected by reverse
transcription-quantitative polymerase chain reaction analysis,
which indicated that the expression level of PKCδ was significantly
elevated in M phase. **P<0.01 vs. G1 group. (B and C) Expression
levels of PKCδ, p-PKCδ-Thr505 and p-Cdc25B-Ser96 were detected in
one-cell mouse embryos using western blotting. The data revealed
that the expression levels of PKCδ and p-PKCδ-Thr505 in M phase
were increased. p-Cdc25B-Ser96 expression in G2 and M
phases were increased. (D) Laser scanning confocal microscope
images of PKCδ at G1, S, G2 and M phases;
embryos were double labeled with PKCδ (green), Cdc25B (red) and
DAPI (blue) staining of the DNA. Scale bar, 50 µm. Slightly higher
levels of PKCδ in the cytoplasm of the cortex at G2 and
early M stage were indicated. PKCδ, protein kinase C type δ; Cdc25,
cell division cycle 25; DAPI, 4′,6-diamidino-2-phenylindole. |
Immunofluorescence analysis by laser scanning
confocal microscopy detected a uniform distribution of PKCδ in the
cytoplasm during G1, S, G2 and M phases
(Fig. 1D), with slightly higher
levels in the cortex region during G2 and early M phase.
In addition, PKCδ protein was also detected in the male and the
female pronuclei during the G1 and S phases. These
results provide indirect evidence to support an association between
PKCδ and Cdc25B.
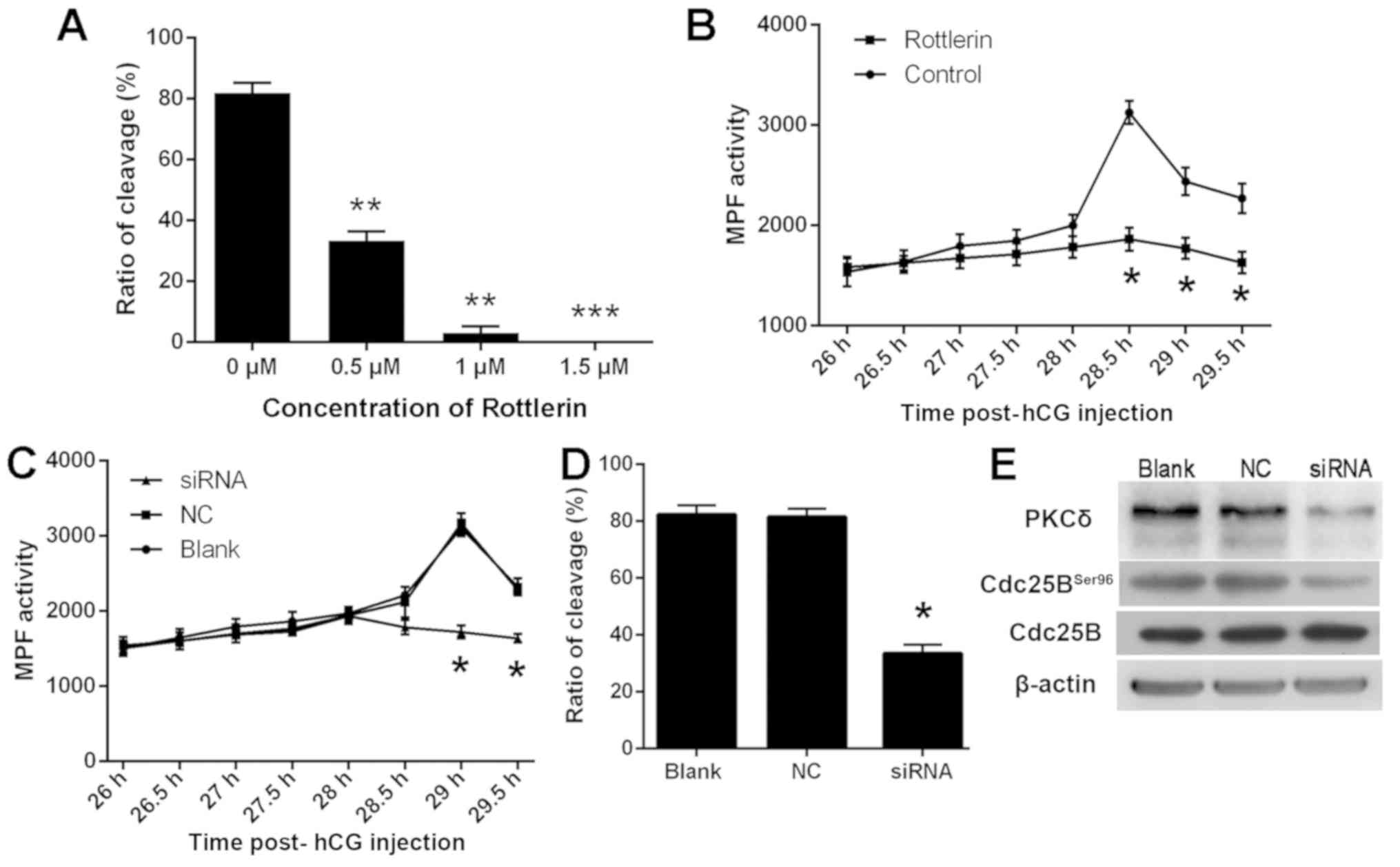
Rotterlin and knockdown of PKCδ
suppresses G2/M transition and inhibits the activity of
MPF in one-cell mouse embryos
To investigate the role of PKCδ on the
G2/M transition, rottlerin, a selective inhibitor of
PKCδ, was used to pretreat one-cell stage mouse embryos (Fig. 2). Embryo cleavage and MPF activity at
different time points were examined. Notably, embryo cleavage was
significantly inhibited when the concentration of rottlerin was
>0.5 µM (Fig. 2A). However, when
the concentration of rottlerin was >0.5 µM, it was considered
toxic to embryos (data not shown). The present results demonstrated
that peak MPF activity was observed 28.5 h post-hCG injection and
MPF activity was significantly increased in the rotterlin-treated
group compared with the control group (Fig. 2B). These data suggest that rottlerin
suppresses G2/M transition in one-cell mouse
embryos.
PKCδ expression was knocked down in G1
phase mouse embryos by microinjecting PKCδ-specific siRNA. The
embryo cleavage was counted 30–35 h post-hCG injection and the
activity of MPF was measured at different time points (Fig. 2C and D). Knockdown of PKCδ expression
significantly inhibited MPF activity 29 h post-hCG injection
(P<0.05; Fig. 2C). In addition,
the rate of embryo cleavage in the siRNA group significantly
decreased compared with NC control group (P<0.05; Fig. 2D). Western blot analysis revealed a
decrease in expression of PKCδ in the siRNA microinjected group
compared with the control groups, indicating that endogenous PKCδ
was effectively inhibited by PKCδ-specific siRNA (Fig. 2E). Additionally, PKCδ siRNA also
suppressed Cdc25B expression (Fig.
2E). The findings concluded that PKCδ serves a role in
G2/M transition in one-cell stage mouse embryos through
phosphorylating Cdc25B on Ser96.
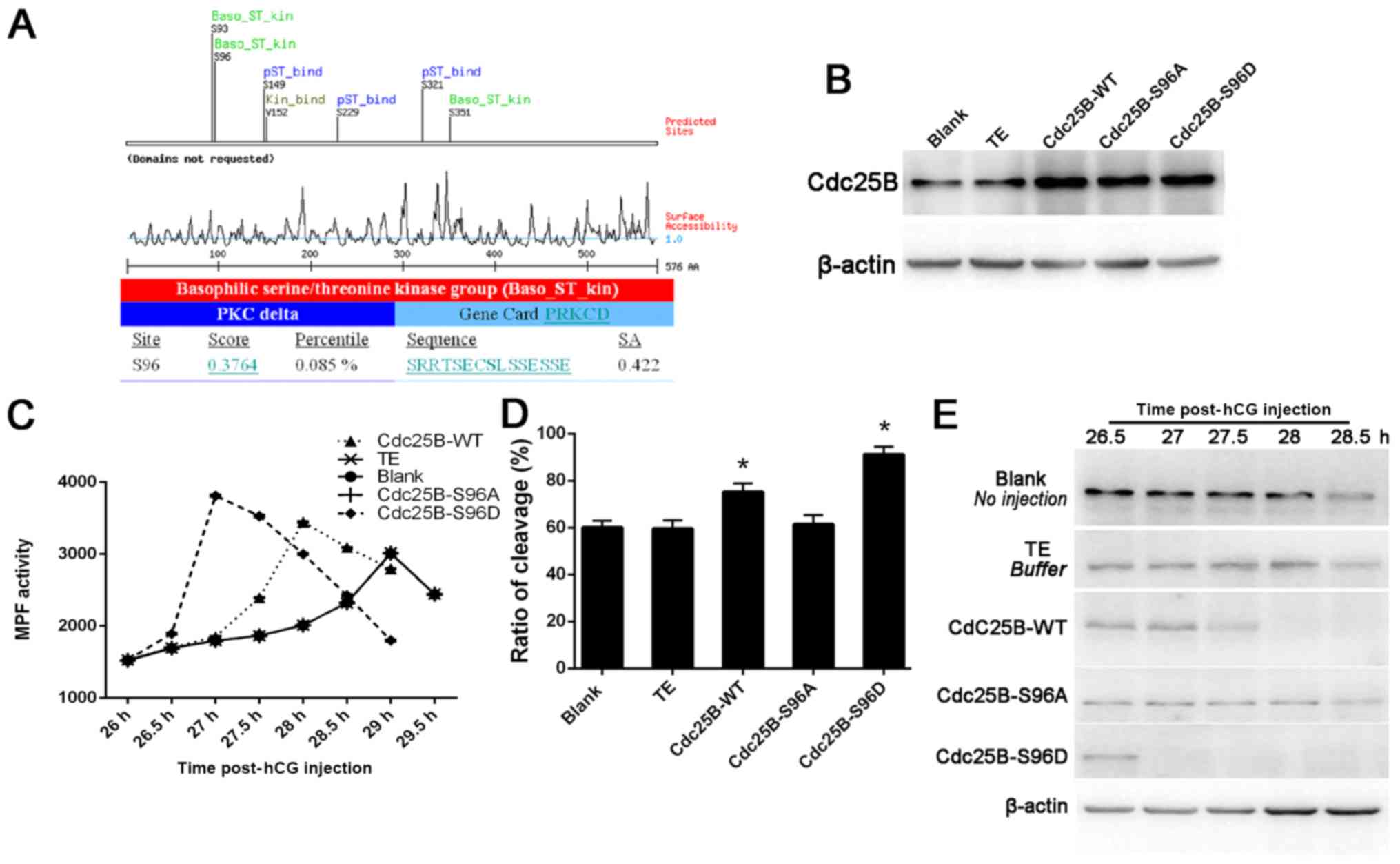
Effects of phosphorylation and
dephosphorylation in one-cell stage mouse embryos
PKCδ phosphorylation sites were predicted using the
Scansite software (scansite.mit.edu). It was revealed that Ser96 on
Cdc25B may be a potential site (Fig.
3A). Thus, a series of Cdc25B mutants were created, in which
alanine and aspartic acid were substituted for a serine residue at
positions 96 (S96A and S96D). The three types of Cdc25B mRNA were
microinjected into mouse embryos and their effects on the
development of early mouse embryos were observed. The results
demonstrated that wild-type and both mutant Cdc25B elevated Cdc25B
expression in mouse embryos (Fig.
3B). In addition, MPF activity in the Cdc25B-S96D was induced
earlier compared with the other groups, reach its peak at 27 h
post-hCG injection amd was the lowest at 29 h post-hCG injection
(Fig. 3C).
As shown in Fig. 3D,
a similar pattern was also observed in the Cdc25B-S96A
mRNA-injected group, and the rate of embryo cleavage was 61.46% at
31 h, which was nearly the same as that in the control groups.
These results suggested that the Cdc25B-96S/D mutant could trigger
one-cell stage mouse embryo division. The cleavage of one-cell
cycle mouse embryos was counted at 26–31 h post-hCG injection, and
the rate of embryo cleavage was calculated at 31 h. therefore, a
significantly higher rate of embryo cleavage was identified in
embryos treated with Cdc25B-WT and Cdc25B-S96D.
Western blotting was used to examine the
phosphorylation status of Cdc2-Tyr15 (Fig. 3E). In the control groups, strong
Cdc2-Tyr15 phosphorylation signals at 26.5–28 h post-hCG injection
were detected, with a weak signal appearing at 28.5 h post-hCG
injection. In the WT group, a weak Cdc2-Tyr15 phosphorylation
signal was observed at 27.5 h and no signal at 28–29 h was
indicated post-hCG injection. In the Cdc25B-S96D group, a weak
Cdc2-Tyr15 phosphorylation signal was detected at 26.5 h, but there
was no signal was identified at 27–29 h post-hCG injection. By
contrast, in the Cdc25B-S96A group, weak Cdc2-Tyr15 phosphorylation
signals were detected 26.5–27.5 h post-hCG injection, but no signal
was detected after 28 h. This data suggests that Cdc25B-S96D can
activate MPF prior to Cdc25B-WT and that Cdc25B-S96D directly
affects the phosphorylation of CDC2-Tyr15 in mouse.
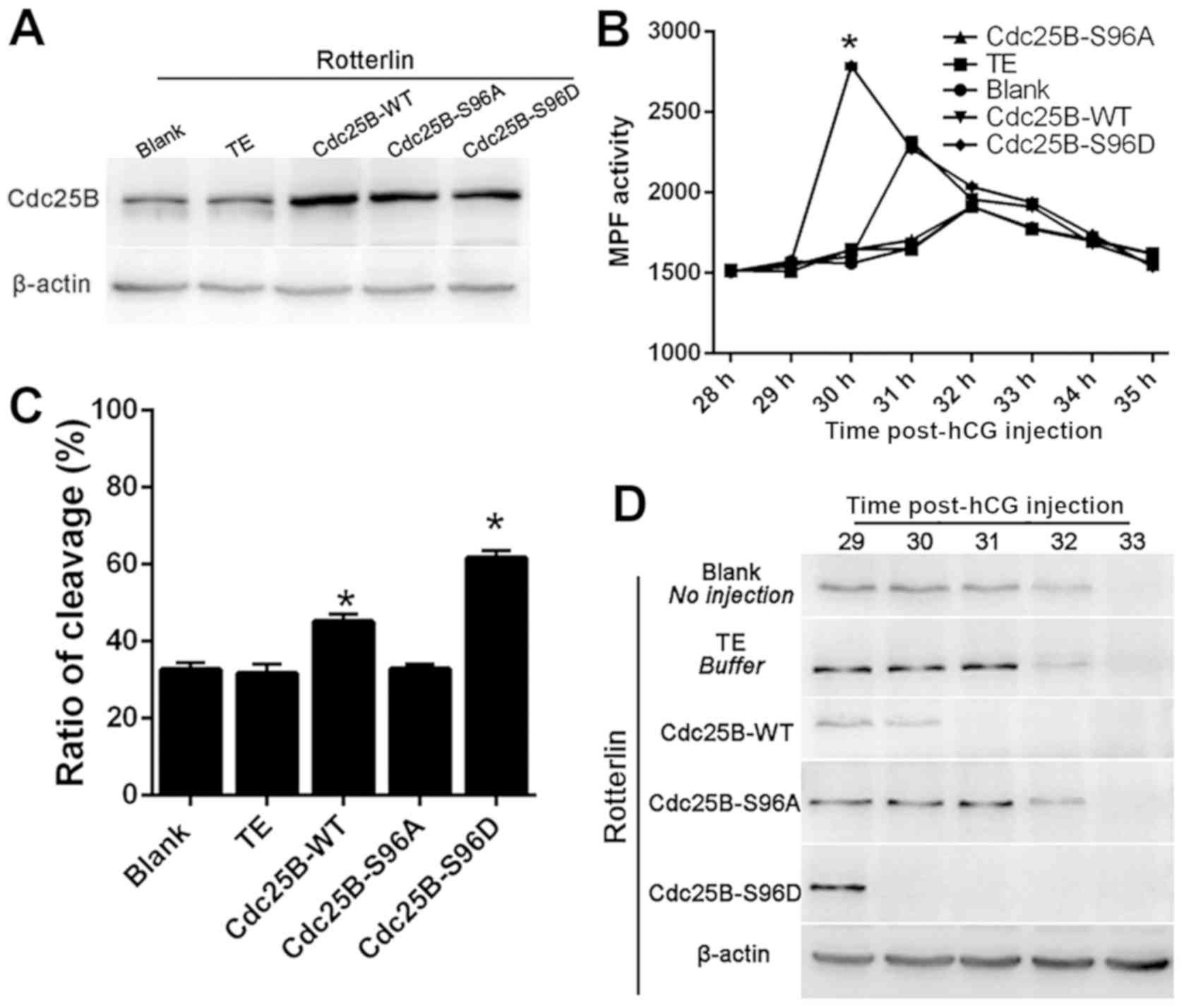
Microinjection of Cdc25B-S96D and
Cdc25B-WT mutants speed mitotic resumption in rottlerin arrested
embryos
To observe the association of PKCδ with Cdc25B,
Cdc25B-WT, two mutant mRNAs (Cdc25B-S96A and Cdc25B-S96D) were
microinjected into fertilized mouse embryos at S stage. Samples
were pre-incubated in M16 medium with 0.5 µM rottlerin for 1 h. All
three microinjected Cdc25B mRNAs were efficiently in
rotterlin-treated mouse fertilized embryos and the level of Cdc25B
expression was subsequently examined. As demonstrated in Fig. 4A, Cdc25B expression was increased in
embryos microinjected with all three mRNAs. MPF activity was
measured between 28–35 h post-hCG injection. MPF activity was
higher in the Cdc25B-S96D and Cdc25B-WT groups between 29–35 h
post-hCG injection compared with the blank and TE groups (Fig. 4B), suggesting that PKCδ inhibition is
responsible for the inhibition of MPF activity. Notably, MPF
activity in the Cdc25B-WT mRNA-injected embryos increased at 31 h
post-hCG injection and gradually decreased thereafter. In the TE
group, MPF activity peaked at 30 h and then declined slowly.
Conversely, MPF activity remained low at 29–35 h post-hCG in the
Cdc25B-S96A group, which was similar to the control groups.
The cleavage of fertilized mouse embryos was
observed at 29–35 h post-hCG injection and the embryo cleavage rate
was calculated at 35 h. The embryo cleavage rate induced by
rottlerin in each group decreased (Fig.
4C). The rate of embryo cleavage was inhibited in the control
and Cdc25B-S96A groups (Fig. 4C)
compared with Cdc25B-WT. The embryo cleavage rates were 32.59%
(blank group), 31.70% (TE-injected group) and 32.84% (Cdc25B-S96A
mRNA-injected group) with no significant differences observed among
the three groups. Furthermore, embryos microinjected with Cdc25B-WT
mRNA entered M phase at 31 h post-hCG injection (data not shown).
In addition, the embryo cleavage rate was significantly increased
(45.14%) compared with the control group. By contrast, embryos
microinjected with Cdc25B-S96D mRNA entered M phase at 29.5–30 h
post-hCG injection, and ~61.69% of embryos had entered the M phase
at 35 h (data not shown). Overall, this data suggests that
microinjection of either Cdc25B-S96D or Cdc25B-WT mRNA could
effectively resume G2 phase arrest induced by rottlerin,
particularly the microinjection of Cdc25B-S96D mRNA.
The phosphorylation status of Cdc2-Tyr15 at 29, 30,
31, 32 and 33 h post-hCG injection was detected following treatment
with rottlerin (Fig. 4D). In the
control groups, strong bands of phosphorylated Cdc2-Tyr15 were
identified at 29–32 h post-hCG injection, demonstrating that MPF
activity was inhibited. In the Cdc25B-WT group, Cdc2-Tyr15
phosphorylation was weakly detected at 30 h post-hCG injection,
which was consistent with the results regarding MPF activity.
However, in the Cdc25B-S96D group, almost no signal was observed at
30–33 h post-hCG injection, which implied that MPF was completely
activated. Notably, the inhibitory phosphorylation signals of
Cdc2-Tyr15 were still observed at 32 h post-hCG injection in the
Cdc25B-S96A mutant group, which was similarly observed in the
control groups. These data suggest that overexpressing either
Cdc25B-S96D or Cdc25B-WT can override the inhibition of rottlerin
in regulating the activity of MPF, particularly the microinjection
of Cdc25B-S96D mRNA. Overall, these data suggested that
microinjection of Cdc25B-S96D and Cdc25B-WT mRNAs could resume the
arrest of mitosis by rottlerin; however, the effects of Cdc25B-S96D
mRNA were more prominent.
Microinjection of Cdc25B-S96D and
Cdc25B-WT mRNAs can resume mitosis following knockdown of PKCδ in
one-cell cycle mouse embryos
Results in Fig. 5A
revealed that the three different microinjected Cdc25B mRNAs were
translated efficiently in mouse fertilized embryos following PKCδ
knockdown. As revealed in Fig. 5B,
MPF activity was low and stable at 29–35 h, and increased slowly at
32 h in the control groups. However, MPF activity in the Cdc25B-WT
mRNA-injected group increased slowly, reached a peak at 31 h
post-hCG injection and then decreased gradually. In the Cdc25B-S96D
mRNA-injected group, MPF activity exhibited a rapid increase at 29
h, peaked at 30 h and then declined slowly. In the Cdc25B-S96A
mRNA-injected group, MPF activity was similar to the levels
detected in the control groups. Data in Fig. 5C suggested that the rate of embryo
cleavage was 31.52% in the non-injected group, 31.88% in the
TE-injected group and 31.01% in the Cdc25B-S96A-injected group, and
there were no significant differences among these groups. However,
50.73% of embryos in the WT mRNA-injected group and 65.31% in the
Cdc25B-S96D group, respectively, entered M phase at 32 and 31 h
post-hCG injection.
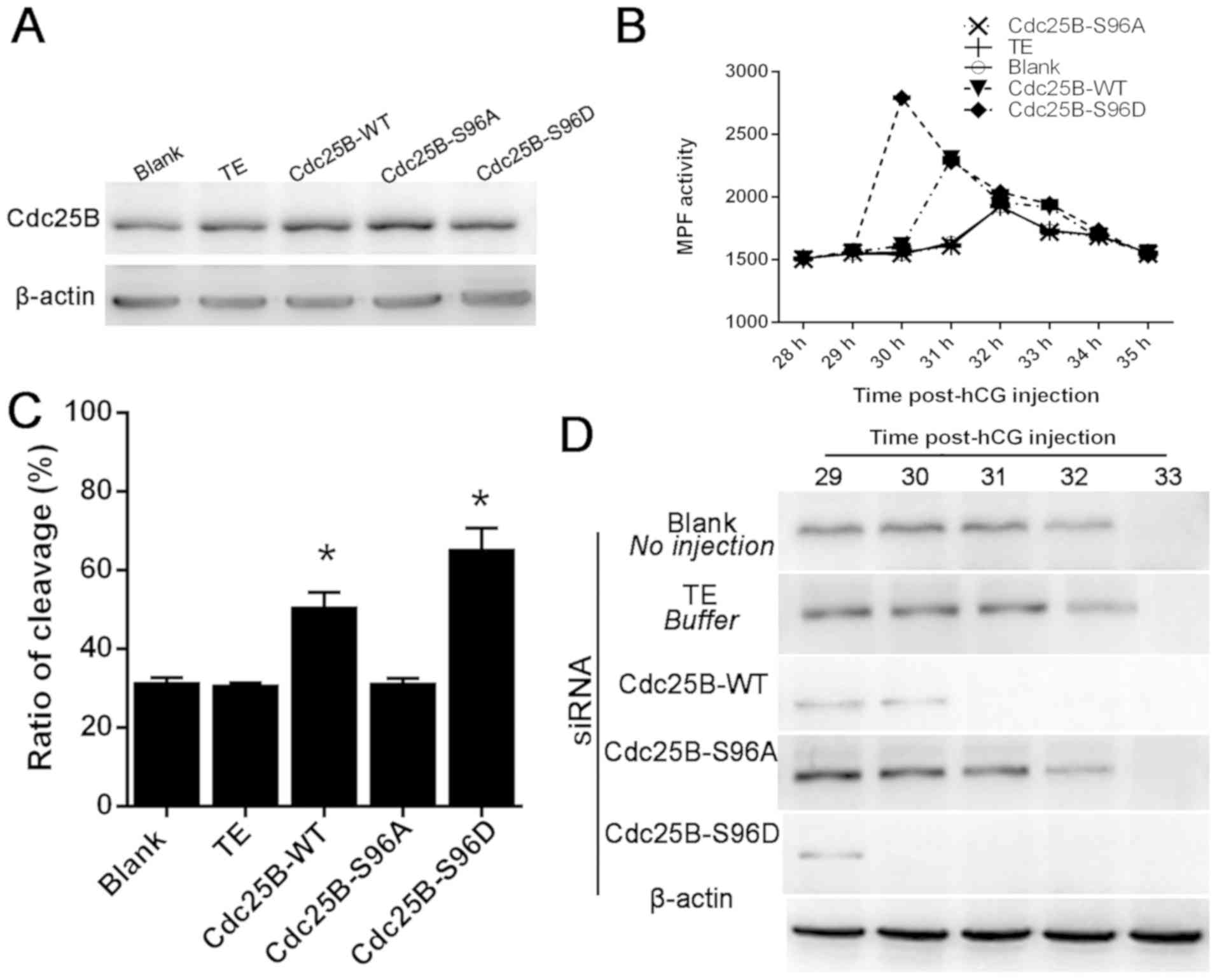 | Figure 5.Cdc25B mRNA microinjection reverses
the inhibitory effect on embryos that were treated with Cdc25B
mRNAs. (A) Western blotting revealed no significant differences in
Cdc25B protein expression levels at 4 h in the PKCδ-silenced groups
following Cdc25B mRNA microinjections, indicating that all of the
microinjected Cdc25B mRNAs were translated efficiently in
fertilized mouse embryos. (B) Activity of MPF 28–35 h post-hCG
injection. Extra time was required for the induction of MPF
activity as the level of PKCδ was limited. (C) Embryo cleavage rate
in cultured mouse embryos injected with various Cdc25B mRNAs at 35
h post-hCG injection. The ratios of cleavage in mouse embryos were
significantly increased following Cdc25B-WT or Cdc25B-96D mRNA
injections. (D) Western blotting of the phosphorylation status of
Cdc2-Tyr15 in the five groups. Embryos were collected at 29, 30,
31, 32 and 33 h post-hCG injection. Results revealed that
Cdc25B-S96D and Cdc25B-WT suppressed the phosphorylation of
Cdc2-Tyr1 after PKCδ siRNA transfection when compared with the TE
group, although the overall reaction time increased. *P<0.05 vs.
TE group. Cdc25, cell division cycle 25; hCG, human chorionic
gonadotropin; MPF, mitosis promoting factor; PKCδ, protein kinase C
type δ; WT, wild-type; TE, Tris-EDTA. |
The phosphorylation status of Cdc2-Tyr15 at 29, 30,
31, 32 and 33 h post-hCG injection was determined (Fig. 5D). In the control groups,
phosphorylated Cdc2-Tyr15 signals were detected at 29–32 h post-hCG
injection and MPF activity was inhibited. In the Cdc25B-WT group,
Cdc2-Tyr15 phosphorylation was weakly detected at 30 h post-hCG
injection, which was consistent with the results regarding MPF
activity. However, in the Cdc25BS96D groups, almost no signal was
detected at 30–33 h post-hCG injection, which implied that MPF was
completely activated. By contrast, the inhibitory phosphorylation
signals of Cdc2-Tyr15 were still observed at 32 h post-hCG
injection in the Cdc25B-S96A mutant group, which was similarly
observed in the control groups. Overall, the data suggested that
microinjection of Cdc25B-S96D and Cdc25B-WT mRNAs could override
the arrest of PKCδ knockdown; however, the effects of Cdc25B-S96D
mRNA were more prominent.
Discussion
Our group has already reported the significant role
of PKC in the development of embryos (17,18). The
PKC family participates in pre-implantation development (3), but the relative importance of each
isozyme still requires further exploration. It is important to
study the functions of specific PKC isotypes and not just the role
of PKC in general since specific PKC isotypes have been
demonstrated to have opposing functions within the same cell
(31). As a member of the PKC super
family, PKCδ is implicated in a significant proportion of the
biochemically measurable PKC increases occurring at fertilization
(32). Notably, Cdc25B is involved
in cell cycle regulation in vascular endothelial cells and the
PKC/Cdc25B signaling pathway negatively regulates G2/M
transition (33). To the best of our
knowledge, the present study explored for the first time the
specific function of the PKCδ/Cdc25B signaling pathway and its
underlying mechanism in one-cell stage mouse embryos.
The present study focused on the expression and
subcellular localization of PKCδ during G1, S,
G2 and M phases in one-cell stage mouse embryos. PKCδ
expression was detected in all four cell cycle phases at mRNA and
protein levels; however, increased mRNA and protein expression
levels were observed in the M phase. The present study extends on
earlier reports by Gangeswaran and Jones (34). In their study, PKCδ expression was
detected in mouse embryos at mRNA and protein levels, but only PKCδ
expression at protein level was demonstrated in mouse embryos.
Location is typically associated with function. Notably, analysis
with confocal microscopy revealed that PKCδ protein not only
persisted in the cytoplasm but also concentrated in the maternal
and paternal pronuclei of early post fertilization zygotes
(G1 and S phase). Using selective inhibitors and siRNAs,
the activity of MPF was inhibited and the effects on the cell cycle
were observed. The present results suggested that PKCδ may be
involved in regulating the development of fertilized mouse
embryos.
Scansite software predicted Cdc25B as a potential
substrate of PKCδ. Scansite predicts whether a candidate site is a
phosphorylation site for a specific kinase or a specific group of
kinases (35). Characterization of
substrates for PKCδ revealed a single basic amino acid close to the
phosphorylation site essential for specific recognition and
phosphorylation (36). Ser96 was
marked among the amino acid sequence of Cdc25B from site 91–100.
Based on the selectivity of PKCδ substrates, an association between
PKCδ and Cdc25B was considered. When either Cdc25B-WT or
Cdc25B-S96D mRNA was overexpressed in early-fertilized mouse
embryos, the mitotic G2/M transition was accelerated.
Specific inhibition of endogenous PKCδ activity was attempted and
the function of Cdc25B was examined. The embryo cleavage rate
induced by rottlerin in each group decreased, and a large number of
embryos microinjected with either Cdc25B-S96D or Cdc25B-WT mRNAs
entered the M phase at 32 h post-hCG injection. Thus, it was
concluded that PKCδ directly phosphorylates Cdc25B Ser96 and
promotes its function during the first cell cycle of mouse
embryos.
Dephosphorylation of specific tyrosine/threonine
residues on Cdc2 is associated with G2/M transition
(37). Hence, in the present study,
the phosphorylation of Tyr15 of Cdc2 was examined during the early
development of mouse fertilized embryos employing
anti-phospho-Tyr15 antibody. In addition, the present study aimed
to elucidate the effects of the Ser96 residue of Cdc25B on MPF
activity. Data revealed that the phosphorylation status of
Cdc2-Tyr15 at different time points in each group was consistent
with MPF activity, whether rottlerin was added or not. The data
suggested that the phosphorylated Cdc25B at Ser96 activates MPF by
directly dephosphorylating Cdc2-pTyr15 in the first mitotic cell
cycle of fertilized mouse embryos. The MPF activity in the TE group
peaked in the Cdc25B-S96D mutant group earlier than in the
Cdc25B-WT and Cdc25B-S96A groups in the presence of rottlerin. MPF
activity remained low at 29–31 h post-hCG injection in the control
groups. However, MPF activity peaked in the Cdc25B-S96D mutant
group, which was 3 h later than in the Cdc25B-S96D mutant group
without rottlerin, suggesting that overexpression of Cdc25B-S96D
mutants may largely overcome G2 arrest induced by
rottlerin.
In the present study, western blotting revealed that
Cdc25B-Ser96 is phosphorylated during G2 and M phases
and dephosphorylated during the G1 and S phases in
vivo. Different phosphorylated sites on Cdc25B give rise to
different functions. Notably, activation of MPF is associated with
Cdc25B (38). In our previous report
(39), when Ser351 was
phosphorylated by PKB MPF was activated, which in turn
dephosphorylated Cdc2-Tyr15. By contrast, phosphorylation on Ser321
by 14-3-3 epsilon caused germinal vesicle breakdown in mouse
oocytes. When 149,321 sites were phosphorylated by PKA, the MPF
activity was inhibited and Cdc2-Tyr15 remained phosphorylated
(40). Taken together, when this
site (Cdc2-Tyr15) is mutated to the phosphorylated residue Asp, the
Cdc25B mutants were activated and the subsequent increase in MPF
activity was indicated.
Ma et al (41)
indicated that the active form of PKCδ (p-PKCδ Thr505) localizes to
the microtubule organizing center that serves a role in meiotic
spindle organization in mammalian oocytes. Following fertilization,
PKCδ is associated with spindle microtubules (42). PKCδ serves a role in spindle
integrity and/or function in embryos (43). Cdc25B accumulation in the cytoplasm
has been correlated with spindle formation, and it has been
suggested that this phosphatase pool located in close vicinity of
the centrosome is responsible for the activation of the cytoplasmic
pool of MPF (44). The
colocalization of PKCδ and Cdc25B produced the same diffuse signal
in one-cell cycle mouse embryos according to immunofluorescence
analysis. Previous results have suggested that active MPF first
appears at centrosomes in prophase, and Cdc25B specifically
activates MPF on centrosomes (45).
Thus, it was speculated that PKCδ is post-translationally modified
in the present study, which was consistent with observations from
Ma et al (41). The
phosphorylation on Cdc25B caused by PKCδ also participates in the
activation of MPF in the centrosomes; however, the underlying
mechanisms are not known.
In conclusion, the present study demonstrated that
PKCδ serves an efficient role in the development of the first cell
stage of mouse embryos by phosphorylating Cdc25B on Ser96. The
results provide novel perspectives for the understanding of the
function of the PKCδ/Cdc25B signaling pathway in the regulation of
early embryo development.
Acknowledgements
The authors would like to thank Dr Tony Hunter
(Laboratory of Molecular Biology, The Salk Institute for Biological
Studies, CA, USA). for kindly providing the full-length mouse
Cdc25B cDNA clone.
Funding
The present work was supported by grants from the
National Nature Science Foundation of China (grant nos. 81270654
and 81370712).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YCL and BZY designed the experiments. YCL performed
the experiments. YCL, XD and BZY collected the data. DDW and MLJ
analyzed the data and YCL validated the analysis. YCL prepared the
manuscript and BZY revised the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of the China Medical University (Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mochly-Rosen D, Das K and Grimes KV:
Protein kinase C, an elusive therapeutic target? Nat Rev Drug
Discov. 11:937–957. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Plusa B, Frankenberg S, Chalmers A,
Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM
and Zernicka-Goetz M: Downregulation of Par3 and aPKC function
directs cells towards the ICM in the preimplantation mouse embryo.
J Cell Sci. 118:505–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Downs SM, Cottom J and Hunzicker-Dunn M:
Protein kinase C and meiotic regulation in isolated mouse oocytes.
Mol Reprod Dev. 58:101–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Viveiros MM, Hirao Y and Eppig JJ:
Evidence that protein kinase C (PKC) participates in the meiosis I
to meiosis II transition in mouse oocytes. Dev Biol. 235:330–342.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma W, Baumann C and Viveiros MM: Lack of
protein kinase C-delta (PKCdelta) disrupts fertilization and
embryonic development. Mol Reprod Dev. 82:797–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalive M, Faust JJ, Koeneman BA and Capco
DG: Involvement of the PKC family in regulation of early
development. Mol Reprod Dev. 77:95–104. 2010.PubMed/NCBI
|
|
7
|
Ouaret D and Larsen AK: Protein kinase C
beta inhibition by enzastaurin leads to mitotic missegregation and
preferential cytotoxicity toward colorectal cancer cells with
chromosomal instability (CIN). Cell Cycle. 13:2697–2706. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poli A, Mongiorgi S, Cocco L and Follo MY:
Protein kinase C involvement in cell cycle modulation. Biochem Soc
Trans. 42:1471–1476. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poli A, Ramazzotti G, Matteucci A, Manzoli
L, Lonetti A, Suh PG, McCubrey JA and Cocco L: A novel
DAG-dependent mechanism links PKCa and Cyclin B1 regulating cell
cycle progression. Oncotarget. 5:11526–11540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Y, Sherman JW, Chen X and Wang R:
Phosphorylation of Cdc25C by AMP-activated protein kinase mediates
a metabolic checkpoint during cell cycle G2/M phase transition. J
Biol Chem. 293:5185–5199. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weimer AK, Biedermann S and Schnittger A:
Specialization of CDK regulation under DNA damage. Cell Cycle.
16:143–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kishimoto T: MPF-based meiotic cell cycle
control: Half a century of lessons from starfish oocytes. Proc Jpn
Acad Ser B Phys Biol Sci. 94:180–203. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaffré M, Martoriati A, Belhachemi N,
Chambon JP, Houliston E, Jessus C and Karaiskou A: A critical
balance between Cyclin B synthesis and Myt1 activity controls
meiosis entry in Xenopus oocytes. Development. 138:3735–3744. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mueller PR, Coleman TR, Kumagai A and
Dunphy WG: Myt1: A membrane-associated inhibitory kinase that
phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science.
270:86–90. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perdiguero E and Nebreda AR: Regulation of
Cdc25C activity during the meiotic G2/M transition. Cell Cycle.
3:733–737. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tiwari M, Gupta A, Sharma A, Prasad S,
Pandey AN, Yadav PK, Pandey AK, Shrivastav TG and Chaube SK: Role
of mitogen activated protein kinase and maturation promoting factor
during the achievement of meiotic competency in mammalian oocytes.
J Cell Biochem. 119:123–129. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi X, Fu W, Zhao Y, Liu Y, Liu Y, Zong Z
and YU B: The effects of PKC on the activation of cdc2 and cdc25 in
one cell stage fertilized mouse eggs. Chin J Biochem Mol Biol.
18:746–749. 2002.
|
|
18
|
Liu Y, et al: The regulation of PKC in
fertilized mouse eggs. Chin J Biochem Mol Biol. 25:619–624.
2000.(In Chinese).
|
|
19
|
Siefert JC, Clowdus EA and Sansam CL: Cell
cycle control in the early embryonic development of aquatic animal
species. Comp Biochem Physiol C Toxicol Pharmacol. 178:8–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu BZ, Zheng J, Yu AM, Shi XY, Liu Y, Wu
DD, Fu W and Yang J: Effects of protein kinase C on M-phase
promoting factor in early development of fertilized mouse eggs.
Cell Biochem Funct. 22:291–298. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gonzalez-Garcia JR, Machaty Z, Lai FA and
Swann K: The dynamics of PKC-induced phosphorylation triggered by
Ca2+ oscillations in mouse eggs. J Cell Physiol. 228:110–119. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Su WH, Feng C, Yu DH, Cui C, Xu
XY and Yu BZ: Polo-like kinase 1 may regulate G2/M transition of
mouse fertilized eggs by means of inhibiting the phosphorylation of
Tyr15 of Cdc2. Mol Reprod Dev. 74:1247–1254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gschwendt M, Müller HJ, Kielbassa K, Zang
R, Kittstein W, Rincke G and Marks F: Rottlerin, a novel protein
kinase inhibitor. Biochem Biophys Res Commun. 199:93–98. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soltoff SP: Rottlerin: An inappropriate
and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci.
28:453–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cui C, Zhao H, Zhang Z, Zong Z, Feng C,
Zhang Y, Deng X, Xu X and Yu B: CDC25B acts as a potential target
of PRKACA in fertilized mouse eggs. Biol Reprod. 79:991–998. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferreira TA, Blackman AV, Oyrer J, Jayabal
S, Chung AJ, Watt AJ, Sjöström PJ and van Meyel DJ: Neuronal
morphometry directly from bitmap images. Nat Methods. 11:982–984.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao J, Liu C, Hou J, Cui C, Wu D, Fan H,
Sun X, Meng J, Yang F, Wang E and Yu B: Ser149 is another potential
PKA phosphorylation target of Cdc25B in G2/M transition of
fertilized mouse eggs. J Biol Chem. 286:10356–10366. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu XY, Zhang Z, Su WH, Zhang Y, Yu YQ, Li
YX, Zong ZH and Yu BZ: Characterization of p70 S6 kinase 1 in early
development of mouse embryos. Dev Dyn. 238:3025–3034. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen EE, Zhu H, Lingen MW, Martin LE, Kuo
WL, Choi EA, Kocherginsky M, Parker JS, Chung CH and Rosner MR: A
feed-forward loop involving protein kinase Calpha and microRNAs
regulates tumor cell cycle. Cancer Res. 69:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou X, Quann E and Gallicano GI:
Differentiation of nonbeating embryonic stem cells into beating
cardiomyocytes is dependent on downregulation of PKC beta and zeta
in concert with upregulation of PKC epsilon. Dev Biol. 255:407–422.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatone C, Delle Monache S, Francione A,
Gioia L, Barboni B and Colonna R: Ca2+-independent protein kinase C
signalling in mouse eggs during the early phases of fertilization.
Int J Dev Biol. 47:327–333. 2003.PubMed/NCBI
|
|
33
|
Oliva JL, Caino MC, Senderowicz AM and
Kazanietz MG: S-Phase-specific activation of PKC alpha induces
senescence in non-small cell lung cancer cells. J Biol Chem.
283:5466–5476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gangeswaran R and Jones KT: Unique protein
kinase C profile in mouse oocytes: Lack of calcium-dependent
conventional isoforms suggested by rtPCR and Western blotting. FEBS
Lett. 412:309–312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Obenauer JC, Cantley LC and Yaffe MB:
Scansite 2.0: Proteome-wide prediction of cell signaling
interactions using short sequence motifs. Nucleic Acids Res.
31:3635–3641. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishikawa K, Toker A, Johannes FJ,
Songyang Z and Cantley LC: Determination of the specific substrate
sequence motifs of protein kinase C isozymes. J Biol Chem.
272:952–960. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strausfeld U, Labbé JC, Fesquet D,
Cavadore JC, Picard A, Sadhu K, Russell P and Dorée M:
Dephosphorylation and activation of a p34 cdc2/cyclin B complex in
vitro by human CDC25 protein. Nature. 351:242–245. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adhikari D and Liu K: The regulation of
maturation promoting factor during prophase I arrest and meiotic
entry in mammalian oocytes. Mol Cell Endocrinol. 382:480–487. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng C, Yu A, Liu Y, Zhang J, Zong Z, Su
W, Zhang Z, Yu D, Sun QY and Yu B: Involvement of protein kinase
B/AKT in early development of mouse fertilized eggs. Biol Reprod.
77:560–568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gaul L, Mandl-Weber S, Baumann P, Emmerich
B and Schmidmaier R: Bendamustine induces G2 cell cycle arrest and
apoptosis in myeloma cells: The role of ATM-Chk2-Cdc25A and
ATM-p53-p21-pathways. J Cancer Res Clin Oncol. 134:245–253. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma W, Koch JA and Viveiros MM: Protein
kinase C delta (PKCδ) interacts with microtubule organizing center
(MTOC)-associated proteins and participates in meiotic spindle
organization. Dev Biol. 320:414–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baluch DP, Koeneman BA, Hatch KR,
McGaughey RW and Capco DG: PKC isotypes in post-activated and
fertilized mouse eggs: Association with the meiotic spindle. Dev
Biol. 274:45–55. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ducibella T and Fissore R: The roles of
Ca2+, downstream protein kinases, and oscillatory signaling in
regulating fertilization and the activation of development. Dev
Biol. 315:257–279. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gabrielli BG, Clark JM, McCormack AK and
Ellem KA: Ultraviolet light-induced G2 phase cell cycle checkpoint
blocks cdc25-dependent progression into mitosis. Oncogene.
15:749–758. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jackman M, Lindon C, Nigg EA and Pines J:
Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat
Cell Biol. 5:143–148. 2003. View
Article : Google Scholar : PubMed/NCBI
|